Abstract
The ability to acquire diverse and abundant forms of iron would be expected to confer a survival advantage in the marine environment, where iron is scarce. Marine bacteria are known to use siderophores and inorganic iron, but their ability to use heme, an abundant intracellular iron form, has only been examined preliminarily. Microscilla marina, a cultured relative of a bacterial group frequently found on marine particulates, was used as a model organism to examine heme uptake. Searches of the genome revealed analogs to known heme transport proteins, and reverse transcription-quantitative PCR analysis of these genes showed that they were expressed and upregulated under iron stress and during growth on heme. M. marina was found to take up heme-bound iron and could grow on heme as a sole iron source, supporting the genetic evidence for heme transport. Similar putative heme transport components were identified in the genomes of diverse marine bacteria. These systems were found in the genomes of many bacteria thought to be particle associated but were lacking in known free-living organisms (e.g., Pelagibacter ubique and marine cyanobacteria). This distribution of transporters is consistent with the hydrophobic, light-sensitive nature of heme, suggesting that it is primarily available on phytoplankton or detritus or in nutrient-rich environments.
Acquisition of adequate iron for bacterial growth is difficult in many environments as a result of the low solubility of Fe(III) and the consequent scarcity of the element or the limited bioavailability of potential iron sources. Ingenious mechanisms to access iron have been developed by bacteria, the most well known of which is the use of siderophores, small iron-binding organic compounds, excreted to scavenge iron (39). Capable of binding various forms of iron, siderophores serve as a general iron acquisition pathway and are employed by bacteria from the human body, where free iron is limited to impede microbial growth, to soils, where iron is abundant but present in inert mineral phases of limited bioavailability (11, 21). However, bacteria also have the ability to directly internalize forms of iron which are abundant in their environment, most notably heme, which is a large pool of iron in organisms and represents a major source of iron for mammalian pathogens (15, 45) and may also be important for some symbiotic bacteria (40). Targeted uptake of abundant iron forms reduces the energy and material costs associated with the production, excretion, and uptake of siderophores.
Bacterial heme uptake is accomplished by using mechanisms similar to siderophore and B12 uptake pathways (45). From the extracellular environment, a TonB-dependent outer membrane receptor selectively transports heme into the periplasmic space. In the periplasm, an ABC transport system consisting of a periplasmic binding protein, a membrane permease, and an ATPase is employed to transport heme into the cytoplasm (28). Once the heme has been internalized, it may be directly incorporated into heme-containing proteins or broken down by a heme oxygenase for storage or use in other forms (47).
In oxic marine environments, minimal inputs of iron combined with the low solubility of Fe(III) lead to subnanomolar concentrations of iron, which limit autotrophic and heterotrophic productivity in portions of the ocean (6, 7, 33). Competition for iron among organisms is consequently expected to be intense, and the ability to access a wide range of iron compounds would be advantageous. Various marine bacteria have been shown to use inorganic iron, produce their own siderophores, and use exogenous siderophores produced by other organisms (18, 34, 52). Some experiments indicate that marine bacteria are able to acquire iron from heme and other porphyrin complexes (52). However, the mechanisms behind heme iron uptake and the generality of its bioavailability to marine bacteria have not been studied in detail despite heme's abundance in biological systems. In particular, because of the localization of phytoplankton iron in heme-rich photosystems, heme could represent as much as 45% of the iron in phytoplankton and form a substantial portion of particulate iron in oceanic systems (17, 46). Bacteria living in association with phytoplankton or those involved in the recycling of particulate organic matter are especially likely to benefit from the ability to access heme iron.
The only marine bacteria in which heme uptake has been studied extensively are pathogenic marine vibrios, including Vibrio cholerae, V. vulnificus, and V. anguillarum (20, 30, 38). The heme uptake systems in these organisms are similar to those studied in phylogenetically diverse bacteria (29, 40, 45), suggesting that such systems could occur in other marine bacteria. However, the relevance of these systems to life in the marine environment is unclear, since heme uptake capabilities may be present in these organisms solely for use during infection.
We used the Flexibacteriaceae family member Microscilla marina, whose genome has been sequenced, as a model organism to study heme uptake. Based on 16S rRNA gene sequences, M. marina is the closest cultured bacterium to a group of organisms associated with the cyanobacterium Trichodesmium (24; E. Mann, unpublished data) and it is a member of the phylum Bacteroidetes, the members of which are abundant on marine particles (8, 9, 41). Therefore, M. marina was considered a good representative of the marine bacteria involved in organic matter remineralization on particles where heme may be a significant source of iron. In addition, we searched the genomes of numerous marine bacteria for heme transport systems in order to assess their distribution, providing insight into the bioavailability and cycling of heme in the marine environment.
MATERIALS AND METHODS
Cell culturing.
M. marina was obtained from the American Type Culture Collection (ATCC 23134) and was maintained in 2216 marine medium (Difco). For experimentation, M. marina was grown in a modified version of the Aquil medium supplemented with peptone and casein, herein referred to as PC medium (18). Filtered (0.2 μm) coastal seawater was supplemented with 1 g/liter bacteriological peptone, 1 g/liter casein hydrolysate, and 400 μM ammonium chloride. Several drops of 50% NaOH were added to adjust the pH to 7.6, and the mixture was then run through a Chelex column to remove trace metals. The mixture was microwave sterilized and allowed to cool overnight. After cooling, K2HPO4 (100 μM), trace metals (50 μM EDTA, 3.9 × 10−8 M Zn2+, 2.3 × 10−7 M Mn2+, 2.5 × 10−8 M Co, and 9.8 × 10−9 M Cu) and appropriate iron sources were added from filter-sterilized stocks. FeCl3 stocks (5 mM and 30 μM) were in 1% HCl and filter sterilized. Fe(III)-heme stocks (1.5 and 5 mM) were made up in 20 mM NaOH, Chelex treated to remove potential contaminating iron, and filter sterilized. It should be noted that although these heme stocks pass through 0.2-μm filters and heme remains in suspension for several months, the stock is likely composed of small heme colloids (3). Heme in PC medium is also expected to be in a small colloidal form (3). The cultures were axenic, and their purity was tested by plating on 2216 agar plates and examination under a microscope. M. marina cells are long filaments, up to 100 μm, and readily distinguished from most contaminants. Growth was monitored by measuring optical density through a 1-cm quartz cell at 600 nm on a spectrophotometer.
Heme uptake.
To synthesize [55Fe(III)]heme, 55FeCl3 (25 μM) was refluxed with equimolar protoporphyrin IX in glacial acetic acid containing 0.1 M sodium acetate for 2 h to insert iron into the porphyrin ring forming Fe(III)-heme (4). The glacial acetic acid solution was diluted to 50% strength with water, and the mixture was then passed through a column of Diaion HP20S resin. Heme was retained on the column, washed with water, and eluted in acetone. The concentration and purity of the [55Fe(III)]heme were determined by UV-Vis spectroscopy by comparison with purchased heme standards (Sigma).
M. marina cultures for uptake experiments were grown in PC medium with 30 nM added FeCl3 to obtain iron-deficient cells and in PC medium with 5 μM added FeCl3 to obtain iron-replete cells. Cultures were harvested by centrifugation during exponential growth and rinsed and resuspended twice in sterile seawater to ensure that all of the culture medium was removed. [55Fe(III)]heme at 5 nM was added to triplicate bottles containing the resuspended M. marina and a single glutaraldehyde (0.01%)-killed control. To monitor uptake of [55Fe(III)]heme, 2-ml samples were taken from each bottle at regular intervals and filtered with a 0.45-μm Durapore filter (Millipore). The filters were washed with Ti-EDTA-citrate wash (22) and filtered seawater, and retained 55Fe was quantified by liquid scintillation counting.
Gene expression.
M. marina growing on different iron sources or at differing iron concentrations was harvested in mid-log phase for analysis of gene expression. Iron-replete cultures in 2216 medium were inoculated in PC medium with 30 nM added FeCl3 and allowed to grow for 24 h, resulting in mild iron stress (data not shown). One milliliter of the mildly stressed culture was then inoculated into 20 ml of PC medium containing 30 nM added FeCl3, 5 μM added FeCl3, or 1.5 μM Fe(III)-heme. After 24 h of growth, cells were harvested by centrifugation and RNA was isolated with TRIzol reagent (Invitrogen) by following the manufacturer's instructions, with the exception that cells were initially incubated with TRIzol at 50°C for 30 min to lyse cells. The isolated RNA was then treated with amplification grade DNase I (Invitrogen), recovered with an RNeasy purification kit (Qiagen), and quantified by using absorbance at 260 and 280 nm measured on a spectrophotometer. Total RNA (200 ng) was reverse transcribed with random hexamer primers to produce cDNA with SuperScript II reverse transcriptase and supplied buffers by following the manufacturer's instructions (Invitrogen). Parallel reactions were run without reverse transcriptase to ensure that there was no significant contaminating DNA.
Real-time quantitative PCR (RT-Q-PCR) was performed on the cDNA to quantify relative transcript amounts on a Stratagene Mx3000P with the Brilliant Sybr green Q-PCR master mix (Stratagene) and gene-specific primers (0.42 μM; Table 1) designed by using the draft genome of M. marina. Temperature profiles for the PCR consisted of an initial 10 min at 95°C, followed by 40 cycles of 95°C for 1 min, 30 s at an annealing temperature several degrees below the melting temperature of the primers (53 to 56°C), and 30 s at 72°C. Five dilutions of genomic DNA were analyzed for each gene to produce a standard curve for quantification of samples based on the crossing of a threshold fluorescence level chosen within the early range of exponential PCR amplification. The genomic DNA was isolated from M. marina cells grown on 2216 medium with a DNeasy kit (Qiagen) and quantified on a spectrophotometer. RNA samples processed without reverse transcriptase had no products or were amplified much later within the run than experimental samples. Melting curve analysis following the PCR and selected analysis of products by gel electrophoresis (2.2% agarose) verified that a single product of the expected length was amplified by each primer set.
TABLE 1.
M. marina genes whose expression was assessed by RT-Q-PCR and the primers used in the analysis
| Gene(s) | Putative function(s) | Accession no. | Forward primer | Reverse primer |
|---|---|---|---|---|
| hmuR | TonB-dependent heme outer membrane receptor | EAY29123 | ACGAGCAAGACCAAAGTGTGT | AGCCTCTACCGACTCCAAGG |
| hmuY | Outer membrane heme binding | EAY29122 | CAGCAAGCGAGGAGTTTC | AATCAAGTGCGAAGGAGGT |
| hmuT | ABC periplasmic binding protein | EAY29124 | TAATTCTCGCCTGGGTAGGG | TCGGTAATGGTTCCGTTTAGC |
| hmuU | ABC permease | EAY29125 | CTATGATGTTGTTGGCAGGT | CTGTAGCAAGCCCAGTAGC |
| hmuV | ABC ATPase | EAY29126 | TACGATTGGGAAGTGGTTC | ACAGTAGTTTGGAGGTGGTG |
| hemS | Heme oxygenase | EAY29127 | ATG GCGAGGCTATTCACAAA | CGTCTGGATGTTCTTCTACCG |
| rhbB | Decarboxylase | EAY27067 | CGACTACTTGAACCCGATAG | CTCGTCTACCATACGCAAAG |
| rhbD and rhbF | Siderophore synthase/acyl coenzyme A transferase | EAY27070 | GTTTGCTTTGTTGTGGTTG | TTGATGCTCTATGGTTTGATT |
| fur | Iron-responsive regulator | EAY31090 | GACTCATTCCTCCGCTCAAA | AATGGCAAACCTTTCCTGAG |
| gyrA | DNA gyrase subunit A | EAY25959 | GGGTATGGTATGTGTGGCAAG | CCTCGGTTTGTAATGCGGTA |
| rpoD | RNA polymerase σ70 | EAY27798 | CTTTGGTCTGAACGGAGAGC | TCGGGAAGTATGTCGCAGTC |
Genomic analyses.
Hidden Markov models (HMMs) of heme uptake protein families were used to search the genomes of M. marina and many additional marine bacteria to identify candidate heme uptake proteins. An HMM for TonB-dependent outer membrane receptors involved in heme transport was built with HMMER (12) by using a training set of functionally characterized heme transporters from diverse bacterial lineages including 19 sequences from gammaproteobacteria, 3 from betaproteobacteria, 2 from alphaproteobacteria, and 2 from Bacteroidetes, which were aligned by using ClustalX (48; see Table S1 in the supplemental material). Threshold levels for considering hits to be putative heme transporters were set at scores of >100 (E value, <1 E-27) for gammaproteobacteria and >50 (E value, <2 E-12) for alphaproteobacteria and Bacteroidetes. To identify the heme transport-associated heme oxygenase, the Pfam HMM HemS (PF05171) was used and proteins with scores of >150 (E value, <1 E-45) were considered putative HemS proteins. For ABC transport components, an insufficient number of characterized heme transporters were available to construct HMMs, and so BLAST searches against the curated Swiss-Prot database were used to identify possible heme transport sequences.
Phylogenetic trees were constructed with the putative heme outer membrane receptors identified from HMM searches of marine bacterial genomes. Marine bacterial sequences and reference genes were aligned by using ClustalX, and Fitch-Margoliash trees based on protein distances were built with PHYLIP 3.6 (14). A bootstrap analysis consisting of 100 replications was conducted, and a consensus tree was generated. 16S rRNA gene trees were constructed in a similar manner by using positions 50 to 1325 of the aligned sequences.
RESULTS
Growth experiments.
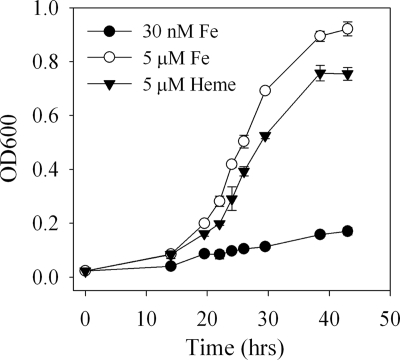
The growth of M. marina was strongly limited by iron at an added inorganic iron concentration of 30 nM (Fig. 1). Addition of 5 μM Fe(III)-heme allowed growth at rates approaching those in medium containing 5 μM inorganic iron. Although in the example shown (Fig. 1) EDTA was present in the medium, no difference in the growth rate on heme was observed when EDTA was present or absent from the medium (μEDTA/μno EDTA ratio = 1.0 ± 0.1, n = 3). Growth on heme as a sole iron source demonstrates that M. marina is capable of accessing heme iron, but multiple mechanisms are possible, including direct uptake of heme, capture of iron through siderophores, and external degradation of heme to release inorganic iron.
FIG. 1.
Growth of M. marina with 5 μM added Fe (iron replete), 30 nM added Fe (iron limited), or 5 μM Fe(III)-heme. OD600, optical density at 600 nm.
Heme uptake.
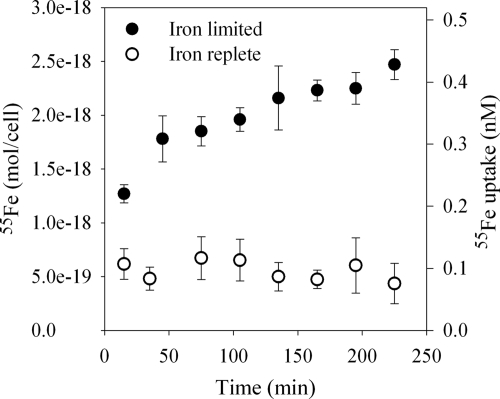
Iron-limited M. marina internalized 55Fe from the [55Fe(III)]heme compound, taking up approximately 10% of the 5 nM 55Fe added over the 4-h time span of the experiment (Fig. 2). Heme uptake began immediately after the compound was added, but rates appeared to decline toward the end of the experiment. In contrast, no active uptake of [55Fe(III)]heme by iron-replete M. marina was observed, though there was significant nonspecific heme binding similar in magnitude to heme binding to glutaraldehyde-killed cells (data not shown).
FIG. 2.
Uptake of [55Fe]heme by M. marina under iron-limited (closed circles) or iron-replete (open circles) conditions.
Putative heme uptake gene cluster.
By HMM and BLAST searches, a cluster of genes was identified in the draft genome of M. marina with similarity to heme uptake genes of other bacteria (Table 2). An HMM search for heme outer membrane receptors identified a sequence (EAY29123) as the best candidate for a heme receptor, though its best BLAST hit in the Swiss-Prot database was a colicin receptor. Lack of conservation in TonB-dependent receptors makes identification of substrate specificity difficult, but an adjacent downstream gene (EAY29122) had similarity to the HmuY heme transport protein of Porphyromonas gingivalis and no significant similarity to other proteins in the P. gingivalis genome or in the Swiss-Prot database (Table 2). In P. gingivalis, hmuY is part of the heme uptake operon though its precise function is unknown (29). Also present in the M. marina gene cluster is an ABC transport system consisting of a periplasmic binding protein (hmuT), an inner membrane permease (hmuU), and an ATPase (hmuV) which powers substrate transport, with best BLAST hits in the Swiss-Prot database to heme transport components of Yersinia pestis (49). Downstream of this ABC transport system is a gene that encodes a protein which BLAST and HMM searches indicate is very similar to the HemS family of proteins often found in heme transport operons and thought to be a heme oxygenase used to free iron from transported heme (47). The colocation of these genes in an operon-like structure provides additional evidence that the genes are involved in heme transport and possibly coregulated.
TABLE 2.
Top BLAST hits of putative M. marina heme transport proteins in the Swiss-Prot database (April 2008)a
| Accession no. | Protein | Highest Swiss-Prot hit | % Identity | % Similarity |
|---|---|---|---|---|
| EAY29122 | HmuY | Porphyromonas gingivalis HmuY | 25 | 38 |
| EAY29123 | HmuR | Escherichia coli CirA, colicin I receptor | 25 | 42 |
| EAY29124 | HmuT | Yersinia pestis HmuT, heme-binding periplasmic protein | 31 | 53 |
| EAY29125 | HmuU | Yersinia pestis HmuU, heme transport permease protein | 40 | 61 |
| EAY29126 | HmuV | Gloeobacter violaceus PCC 7421 HmuV, heme transport ATPase protein | 42 | 61 |
| EAY29127 | HemS | Yersinia pestis HemS, heme degradation protein | 37 | 57 |
Except HmuY, which had no hits in the Swiss-Prot database and instead was searched against the GenBank nonrepetitive database. The best hit with functional annotation is reported for this gene. The genes form a cluster and are listed in the order in which they appear in the genome.
Expression of iron uptake genes.
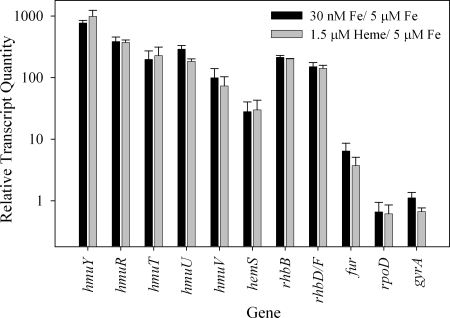
Expression of the putative heme transport genes identified by bioinformatic analyses was examined under differing conditions of iron availability by RT-Q-PCR. Compared to growth under iron-sufficient conditions (5 μM FeCl3), all putative heme transport genes were upregulated 28- to 700-fold under iron stress (30 nM FeCl3; Fig. 3). In M. marina, hmuY was the most strongly upregulated gene examined. A similar result was obtained with P. gingivalis, where the HmuY protein is the major protein upregulated under iron stress (29). Differential expression was observed within operon-like regions of the M. marina putative heme uptake cluster, but similar results have been seen in other heme transport gene clusters (29). The putative heme transport genes were upregulated to similar extents during growth on 1.5 μM Fe(III)-heme (Fig. 3), and transcript levels were also similar (data not shown).
FIG. 3.
Expression of heme transport, siderophore biosynthesis, iron regulation, and housekeeping genes in M. marina under different growth conditions assessed by RT-Q-PCR. Quantities of transcripts during growth with 30 nM Fe (iron limited) or 1.5 μM heme were normalized to transcript quantities under iron-replete conditions (5 μM Fe).
The expression of two putative siderophore biosynthetic genes was also examined for comparison with heme uptake components (Fig. 3; Table 1). M. marina has a complement of siderophore synthesis genes (EAY27067 to EAY27071) which appears to allow for the production of a rhizobactin 1021-like siderophore, and the genes are named in accordance with their homologs in the rhizobactin 1021 biosynthesis pathway (31). rhbB in M. marina has strong similarity to the rhbB gene product (GenBank accession no. AAK65917) of Sinorhizobium meliloti (36% identity, 55% similarity), which decarboxylates a siderophore precursor (31). M. marina also contains a gene with two domains, one of which has strong similarity to rhbF, a siderophore synthase of S. meliloti (AAK65921; 30% identity, 50% similarity). The second domain of the M. marina protein is similar to an acyl coenzyme A transferase involved in rhizobactin 1021 synthesis (rhbD, AAK65919; 25% identity, 46% similarity), suggesting that these genes have been fused in M. marina (5, 31). Both of these genes are upregulated in M. marina under iron stress and when it is growing on heme (Fig. 3).
Three members of the Fur metalloregulatory protein family were identified in the M. marina genome (EAY24380, EAY30621, and EAY31090). Only EAY31090 responded to iron stress or growth on heme, being upregulated under both conditions, which suggests that it may be an iron metalloregulatory protein (Fig. 3) (13).
Transcript quantities were normalized to total RNA, which is predominately rRNA. The essentially constant expression levels of two genes involved in DNA and RNA polymerization and commonly used as controls, rpoD and gyrA (10), confirm that the observed upregulation of putative heme transport genes is not due to changes in the proportion of rRNA relative to mRNA under differing growth conditions (Fig. 3).
Heme uptake genes in marine bacteria.
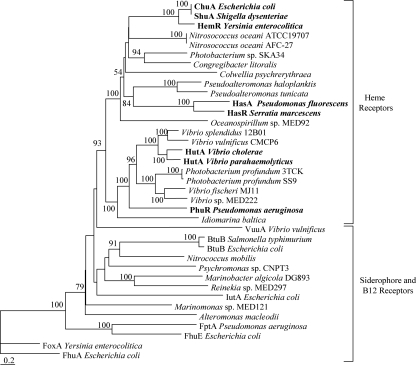
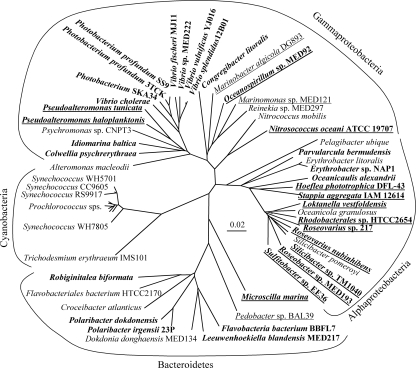
As with M. marina, the genomes of 148 additional marine bacteria were searched for heme transport components. These bacteria are primarily from the water column, though some are from sediments. A representative subset of the results which demonstrates trends in the distribution of putative heme transporters and illustrates their potential phylogenetic diversity is presented here (Fig. 4 and 5; see also Fig. S1 in the supplemental material); for the complete search results, see Table S2 in the supplemental material. Searches with the heme outer membrane receptor HMM yielded some high-scoring sequences in the gamma- and alphaproteobacteria, and additional sequences with intermediate scores which may also be heme transporters were identified (see Table S2 in the supplemental material). The top hits to this HMM from each genome were tested with the Pfam PF00593 model for TonB-dependent outer membrane receptors (see Table S2 in the supplemental material). Sequences which scored above the trusted cutoff threshold on this model, and thus appeared to be TonB-dependent receptors, were used to construct a phylogeny to determine where they clustered in relation to known heme receptors. High-scoring receptors from gammaproteobacteria (e.g., Pseudoalteromonas haloplanktis, Photobacterium profundum 3TCK) and alphaproteobacteria (e.g., Parvularcula bermudensis, Oceanicaulis alexandrii) were closely associated with characterized heme outer membrane receptors with high bootstrap support. These sequences formed a distinct clade from siderophore and B12 outer membrane receptors, strongly suggesting that the genes also code for heme receptors (Fig. 4; see Fig. S1 in the supplemental material). In the alphaproteobacteria, an additional group composed predominately of Roseobacter sequences with intermediate heme outer membrane receptor HMM scores was formed (see Fig. S1 in the supplemental material). These sequences are likely to be heme transporters given their proximity to HemS proteins in the genomes of these roseobacters (see below). Because of the lack of characterized outer membrane receptors in members of the phylum Bacteroidetes, phylogenetic analysis of putative heme transporters in this group was not possible.
FIG. 4.
Phylogenetic tree of TonB-dependent outer membrane receptor protein sequences from representative gammaproteobacteria. Top hits to the heme outer membrane receptor HMM in marine bacteria are included along with reference siderophore, B12, and heme (in bold) outer membrane receptors. Functional labels are placed on sequence groups based on substrate specificity of reference sequences within the group. The tree was constructed from phylogenetic distances by the Fitch-Margoliash method in PHYLIP 3.6. Bootstrap values (from 100 resamplings) are indicated for nodes with values of >50. The scale bar indicates distances in substitutions per site. FhuA from Escherichia coli was used as an outgroup. For the GenBank accession numbers of marine bacterial sequences, see Table S2 in the supplemental material. Reference heme receptors and their GenBank accession numbers are as follows: ChuA from E. coli, AAG44838; ShuA from Shigella dysenteriae, AAC27809; HemR from Yersinia enterocolitica, CAA48250; HasR from Serratia marcescens, CAE46936; HasA from Pseudomonas fluorescens, BAA88490; HutA from V. cholerae, AAF96478; HutA from V. parahaemolyticus, BAC62225; PhuR from Pseudomonas aeruginosa, AAC13289. Reference siderophore receptors and their GenBank accession numbers are as follows: FhuA from E. coli, P06971; FhuE from E. coli, P16869; IutA from E. coli, P14542; FoxA from Y. enterocolitica, Q01674; VuuA from V. vulnificus, AAF28471. Reference B12 receptors and their GenBank accession numbers are as follows: BtuB from Salmonella enterica serovar Typhimurium, P37409; BtuB from E. coli, P06129.
FIG. 5.
Distribution of putative heme transport components (outer membrane receptors and heme oxygenases) with respect to bacterial phylogeny. The tree is an unrooted 16S rRNA gene tree of representative marine bacteria whose genomes were searched for heme uptake components constructed by the Fitch-Margoliash method in PHYLIP 3.6 and bootstrapped with 100 replicates. Positions 50 to 1325 of the 16S rRNA gene with respect to the E. coli sequence were used in this analysis. The scale bar indicates distance in substitutions per site. Organisms which had a good hit to the heme receptor HMM (scores of >100 and E values of <1 E-27 in gammaproteobacteria; scores of >50 and E values of <2 E-12 in alphaproteobacteria and members of the phylum Bacteroidetes) are in bold, and organisms with a HemS-type heme oxygenase (HMM scores of >150 and E values of <1 E-45) are underlined.
Sequences with high similarity to the HemS family of heme transport proteins were identified by using an HMM model in many gamma- and alphaproteobacteria and in two Bacteroidetes members (see Table S2 in the supplemental material). The greater conservation of HemS and lack of affiliated protein families (47) led to clear positive or negative results for HemS HMM searches in each genome. In 20 of the 25 genomes in which a putative hemS gene was identified, the sequence was located adjacent to, or within several genes of, the top hit to the heme outer membrane HMM (for the accession numbers, see Table S2 in the supplemental material). This association provides further evidence that the identified sequences encode proteins involved in a coordinated process, i.e., heme transport. In particular, this finding suggests that the Roseobacter sequences with scores intermediate between the heme outer membrane receptor HMM and adjacent hemS genes are involved in heme transport. In many cases, ABC transport systems were also present in the genome neighborhood, but these transporters were not examined in more detail as it is difficult to determine their substrate specificity.
DISCUSSION
Defining the dominant routes of iron cycling in oceanic ecosystems will allow a better understanding of how this limiting micronutrient controls productivity and ecosystem structure in significant portions of the ocean. One current theory, based on iron uptake studies, is that siderophore-bound iron may be more accessible to marine bacteria, while heme sources might be taken up predominately by eukaryotes, leading to the possibility that the availability of these different forms of iron could modify community structure (23). However, it is well established that many bacteria are capable of using heme, although the presence of heme uptake pathways in marine bacteria have not been well investigated. In this study, the ability of a particle-associated marine bacterium, M. marina, to use heme as an iron source was investigated by physiological and molecular techniques. Finding evidence that this bacterium can transport heme-bound iron, we carried out genomic searches of other marine bacteria to understand the prevalence and ecological role of the heme transport pathway in the marine environment.
Putative heme transport cluster in M. marina.
In the draft genome of M. marina, a cluster of putative heme transport genes, including an outer membrane receptor, an ABC transport system, and a heme oxygenase, was identified based on sequence similarity to known heme transport systems (Table 2). Because TonB-dependent outer membrane receptors and ABC transport proteins are involved in the uptake of many metal chelates, including heme, siderophores, and B12 (45), the presence of additional genes with similarity to proteins specifically involved in heme transport aided the identification of the substrate specificity of the uptake system. These genes encode a heme oxygenase (hemS) and an outer membrane heme-binding protein (hmuY) and may be used to help identify heme transporters in bacterial genomes (see below) or to study organisms responsible for heme uptake in the environment (29, 43, 44, 47).
Analysis of gene expression showed that the putative heme transporter genes are expressed and upregulated under iron-limiting conditions and during growth on Fe(III)-heme as an iron source (Fig. 3). These results are consistent with the putative role of the cluster in heme transport, but there is no evidence for selective upregulation of the cluster during growth on heme. When M. marina is iron limited or growing on Fe(III)-heme, the expression profiles of putative heme transport and siderophore biosynthesis genes are extremely similar, suggesting similar physiological states (Fig. 3). Growth on Fe(III)-heme appears to induce iron stress and an activation of many iron acquisition mechanisms, including heme and siderophore uptake pathways, rather than specific upregulation of heme uptake systems. General responses to iron availability are often regulated by Fur proteins (13). A putative fur gene has been identified and found to be upregulated under iron stress in M. marina, providing a potential mechanism for the observed response (Fig. 3). In some cases, TonB-dependent receptors participate in their own regulation, acting as both transporters and sensors of their substrate (26, 27). However, HmuR in M. marina lacks the N-terminal domain involved in signal transduction, nor are anti-sigma factors or extracytoplasmic function sigma factors involved in signaling located near the putative heme transport cluster (data not shown). This suggests that M. marina cannot sense external heme and must rely on a general iron-responsive element for regulation, consistent with the observed upregulation of multiple iron uptake pathways during growth on Fe(III)-heme.
The genetic evidence that M. marina contains a heme transporter is also supported by physiological studies showing that M. marina is capable of taking up heme-bound iron(III) and can grow on heme as a sole iron source. Uptake of 55Fe from [55Fe(III)]heme was observed under iron-limited conditions but suppressed under iron-replete conditions, showing that uptake was mediated by an iron-regulated pathway and consistent with gene expression patterns of the putative heme uptake cluster (Fig. 2). While internalization of 55Fe from [55Fe(III)]heme does not conclusively demonstrate that the entire heme molecule was transported, the uptake was rapid and immediate, which might not be expected under some alternate explanations for 55Fe uptake such as siderophore uptake. Siderophores would need to be excreted into the uptake medium and extract iron from heme prior to initiation of uptake. M. marina has the ability to grow on Fe(III)-heme as an iron source, and though this may be mediated by multiple iron uptake pathways, it is consistent with the presence of a heme transport system. While genetic manipulations, including knockout experiments, could provide more definitive evidence that the putative heme transport cluster is responsible for heme uptake, such techniques are not currently available for M. marina, nor have they been developed for other, related, marine bacteria. However, the strong sequence similarity of the cluster to known heme transport proteins and its regulatory behavior, in conjunction with physiological evidence demonstrating M. marina's capability to access heme bound iron(III), strongly suggest that the cluster is involved in heme transport.
Distribution of putative heme transport genes in marine bacteria.
Having examined the putative heme transport system in M. marina, we searched the genomes of numerous marine bacteria for related transport systems to better understand the distribution of heme uptake capabilities and the role of heme in the marine iron cycle. Putative heme transport systems were identified in representatives of the gamma- and alphaproteobacteria and the phylum Bacteroidetes based on the identification of a putative heme receptor and, in certain cases, a HemS-type heme oxygenase colocated in the genome (Fig. 5). The most notable feature in the distribution is a lack of heme transporters in known free-living bacteria, including Pelagibacter ubique, Silicibacter pomeroyi, and all marine cyanobacteria (16, 36, 37). In fact, these organisms do not appear to have any TonB-dependent receptors, as no candidate sequences were identified by using the Pfam PF00593 HMM for TonB-dependent outer membrane receptors (data not shown). Putative heme transporters were generally found in bacteria from taxa known to be associated with particles such as vibrios, roseobacters, and members of the phylum Bacteroidetes (8, 9, 19, 41).
The Roseobacter clade, in which the habitats of sequenced representatives have been examined (37), provides a good example of this distribution of heme transporters (Fig. 5). S. pomeroyi, which is thought to be mainly free living (36, 37), lacks putative heme transporters along with other TonB-dependent receptors. In contrast, sulfitobacters and Silicibacter sp. strain TM1040, which have been found associated with dinoflagellates (1, 25), possess putative heme transporters (Fig. 5; see Table S2 in the supplemental material). These putative heme receptors are among the few TonB-dependent receptors in the genomes, and in fact in Silicibacter sp. strain TM1040, the putative heme receptor is the only TonB-dependent receptor, implying that the ability to access heme is especially important for the survival of this bacterium.
Although major advances have been made in understanding the distribution of iron concentrations in the ocean (35), characterization of iron speciation at the molecular level and a mechanistic understanding of iron fluxes have been difficult to achieve as a result of the low concentration and complicated chemistry of iron. Organically complexed iron is known to dominate the dissolved iron pool, and microorganisms must access some component of this iron to maintain observed growth rates (2, 23, 32, 42). The iron acquisition strategies employed by marine microorganisms may provide greater insight into the forms and cycling of oceanic iron, as competition for iron is expected to result in selection of uptake mechanisms capable of accessing iron species which are abundant in an organism's environment. The presence of putative heme transporters in bacteria from taxa which are abundant on particles and their absence in free-living bacteria indicate that heme is primarily available in or near particles but is not present in seawater at significant concentrations in the dissolved form (Fig. 5). This hypothesized distribution is consistent with the hydrophobic, light-sensitive nature of heme, its tendency to form aggregates, and preliminary data on iron-porphyrin distributions in aquatic environments (3, 51, 53). As a major intracellular form of iron, heme may be an important iron source for bacteria living in association with other organisms, although the actual distribution of heme in the ocean has yet to be determined (17). Cell lysis or death may free heme for use by other bacteria, though its light sensitivity and hydrophobicity will likely minimize its importance in the dissolved phase. Particle-associated bacteria play an important role in the marine iron cycle, directly recycling cellular iron forms such as heme from phytoplankton or detritus which might otherwise be lost from the ecosystem due to sinking fluxes (50).
Supplementary Material
Acknowledgments
This work was supported by NSF OCE 0327070 and ACS PRF 38006-G2 (to K.B.) and a National Defense Science and Engineering Graduate Fellowship (to B.M.H.).
We thank the Moore Foundation for sequencing the M. marina genome and Margo Haygood, who initiated and oversaw the genome project. Sheila Podell, Terry Gasterlaand, Christine Anderson, and Chris Dupont assisted with bioinformatic analyses. Information on bacterial taxa associated with Trichodesmium colonies in the field was provided prior to publication by Elizabeth Mann. Assistance with RNA isolation and Q-PCR techniques from Rhona Stuart and Lisa Sudek was much appreciated. Two undergraduates, Joshawna Nunnery and Kristine Lim, helped develop the radiolabeled heme synthesis and conducted preliminary uptake experiments. We are grateful for helpful discussions and advice from Chris Dupont and Brian Palenik.
Footnotes
Published ahead of print on 29 August 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alavi, M., T. Miller, K. Erlandson, R. Schneide, and R. Belas. 2001. Bacterial community associated with Pfisteria-like dinoflagellate cultures. Environ. Microbiol. 3:380-396. [DOI] [PubMed] [Google Scholar]
- 2.Barbeau, K., E. L. Rue, K. W. Bruland, and A. Butler. 2001. Photochemical cycling of iron in the surface ocean mediated by microbial iron(III)-binding ligands. Nature 413:409-413. [DOI] [PubMed] [Google Scholar]
- 3.Bommer, J. C., and P. Hambright. 2002. General laboratory methods for tetrapyrolles, p. 39-67. In A. G. Smith and M. Witty (ed.), Heme, chlorophyll, and bilins: methods and protocols. Humana Press, Totowa, NJ.
- 4.Buchler, J. W. 1975. Static coordination chemistry of metalloporphyrins, p. 157-231. In K. M. Smith (ed.), Porphyrins and metalloporphyrins: a new edition based on the original volume by J. E. Falk. Elsevier, New York, NY.
- 5.Challis, G. L. 2005. A widely distributed bacterial pathway for siderophore biosynthesis independent of nonribosomal peptide synthetases. ChemBioChem 6:601-611. [DOI] [PubMed] [Google Scholar]
- 6.Church, M. J., D. A. Hutchins, and H. W. Ducklow. 2000. Limitation of bacterial growth by dissolved organic matter and iron in the Southern Ocean. Appl. Environ. Microbiol. 66:455-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coale, K. H., K. S. Johnson, S. E. Fitzwater, R. M. Gordon, S. Tanner, F. P. Chavez, L. Ferioli, C. Sakamoto, P. Rogers, F. Millero, P. Steinberg, P. Nightingale, D. Cooper, W. P. Cochlan, M. R. Landry, J. Constantinou, G. Rollwagen, A. Trasvina, and R. Kudela. 1996. A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature 383:495-501. [DOI] [PubMed] [Google Scholar]
- 8.Crump, B. C., E. V. Armbrust, and J. A. Baross. 1999. Phylogenetic analysis of particle-attached and free-living bacterial communities in the Columbia river, its estuary, and the adjacent coastal ocean. Appl. Environ. Microbiol. 65:3192-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLong, E. F., D. G. Franks, and A. L. Alldredge. 1993. Phylogenetic diversity of aggregate-attached vs. free-living marine bacterial assemblages. Limnol. Oceanogr. 38:924-934. [Google Scholar]
- 10.Desroche, N., C. Beltramo, and J. Guzzo. 2005. Determination of an internal control to apply reverse transcription quantitative PCR to study stress response in the lactic acid bacterium Oenococcus oeni. J. Microbiol. Methods 60:325-333. [DOI] [PubMed] [Google Scholar]
- 11.Eaton, J. W., P. Brandt, and J. R. Mahoney. 1982. Haptoglobin: a natural bacteriostat. Science 215:691-693. [DOI] [PubMed] [Google Scholar]
- 12.Eddy, S. R. 1998. Profile hidden Markov models. Bioinformatics 14:755-763. [DOI] [PubMed] [Google Scholar]
- 13.Escolar, L., J. Perez-Martin, and V. deLorenzo. 1999. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181:6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felsenstein, J. 1989. PHYLIP: phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 15.Genco, C. A., and D. W. Dixon. 2001. Emerging strategies in microbial heme capture. Mol. Microbiol. 39:1-11. [DOI] [PubMed] [Google Scholar]
- 16.Giovannoni, S. J., H. J. Tripp, S. Givan, M. Podar, K. L. Vergin, D. Baptista, L. Bibbs, J. Eads, T. H. Richardson, M. Noordewier, M. S. Rappe, J. M. Short, J. C. Carrington, and E. J. Mathur. 2005. Genome streamlining in a cosmopolitan oceanic bacterium. Science 309:1242-1245. [DOI] [PubMed] [Google Scholar]
- 17.Gledhill, M. 2007. The determination of heme b in marine phyto- and bacterioplankton. Mar. Chem. 103:393-403. [Google Scholar]
- 18.Granger, J., and N. M. Price. 1999. The importance of siderophores in iron nutrition of heterotrophic marine bacteria. Limnol. Oceanogr. 44:541-555. [Google Scholar]
- 19.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Bacteria of the gamma subclass Proteobacteria associated with zooplankton in Chesapeake Bay. Appl. Environ. Microbiol. 68:5498-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson, D. P., and S. M. Payne. 1993. Cloning and characterization of the Vibrio cholerae genes encoding the utilization of iron from hemin and hemoglobin. Mol. Microbiol. 7:461-469. [DOI] [PubMed] [Google Scholar]
- 21.Hersman, L., T. Lloyd, and G. Sposito. 1995. Siderophore-promoted dissolution of hematite. Geochim. Cosmochim. Acta 59:3327-3330. [Google Scholar]
- 22.Hudson, R. J. M., and F. M. M. Morel. 1989. Distinguishing between extracellular and intracellular iron in marine-phytoplankton. Limnol. Oceanogr. 34:1113-1120. [Google Scholar]
- 23.Hutchins, D. A., A. E. Witter, A. Butler, and G. W. Luther III. 1999. Competition among marine phytoplankton for different chelated iron species. Nature 400:858-861. [Google Scholar]
- 24.Janson, S., B. Bergman, E. J. Carpenter, S. J. Giovannoni, and K. Vergin. 1999. Genetic analysis of natural populations of the marine diazotrophic cyanobacterium Trichodesmium. FEMS Microbiol. Ecol. 30:57-65. [Google Scholar]
- 25.Jasti, S., M. E. Sieracki, N. J. Poulton, M. W. Giewat, and J. N. Rooney-Varga. 2005. Phylogenetic diversity and specificity of bacteria closely associated with Alexandrium spp. and other phytoplankton. Appl. Environ. Microbiol. 71:3483-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirby, A. E., D. J. Metzger, E. R. Murphy, and T. D. Connell. 2001. Heme utilization in Bordetella avium is regulated by RhuI, a heme-responsive extracytoplasmic function sigma factor. Infect. Immun. 69:6951-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koebnik, R. 2005. TonB-dependent trans-envelope signaling: the exception or the rule. Trends Microbiol. 13:343-347. [DOI] [PubMed] [Google Scholar]
- 28.Köster, W. 2001. ABC transporter-mediated uptake of iron, siderophores, heme and vitamin B12. Res. Microbiol. 152:291-301. [DOI] [PubMed] [Google Scholar]
- 29.Lewis, J. P., K. Plata, F. Yu, A. Rosato, and C. Anaya. 2006. Transcriptional organization, regulation and role of the Porphyromonas gingivalis W83 hmu haemin-uptake locus. Microbiology 152:3367-3382. [DOI] [PubMed] [Google Scholar]
- 30.Litwin, C., and B. L. Byrne. 1998. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: regulation of expression and determination of gene sequence. Infect. Immun. 66:3134-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch, D., J. O'Brien, T. Welch, P. Clarke, P. O Cuiv, J. H. Crosa, and M. O'Connell. 2001. Genetic organization of the region encoding regulation, biosynthesis, and transport of rhizobactin 1021, a siderophore produced by Sinorhizobium meliloti. J. Bacteriol. 183:2576-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macrellis, H. M., C. G. Trick, E. L. Rue, G. Smith, and K. W. Bruland. 2001. Collection and detection of natural iron-binding ligands from seawater. Mar. Chem. 76:175-187. [Google Scholar]
- 33.Martin, J. H., R. M. Gordon, and S. E. Fitzwater. 1990. Iron in Antarctic waters. Nature 345:156-158. [Google Scholar]
- 34.Martinez, J. S., G. P. Zhang, P. D. Holt, H. T. Jung, C. J. Carrano, M. G. Haygood, and A. Butler. 2000. Self-assembling amphiphilic siderophores from marine bacteria. Science 287:1245-1247. [DOI] [PubMed] [Google Scholar]
- 35.Measures, C. I., W. M. Landing, M. T. Brown, and C. S. Buck. 2008. High-resolution Al and Fe data from the Atlantic Ocean CLIVAR-CO2 repeat hydrography A16N transect: extensive linkages between atmospheric dust and upper ocean geochemistry. Global Biogeochem. Cycles 22:GB1005. doi: 10.1029/2007GB003042. [DOI] [Google Scholar]
- 36.Moran, M. A., A. Buchan, J. M. Gonzalez, J. F. Heidelberg, W. B. Whitman, R. P. Kiene, J. R. Henricksen, G. M. King, R. Belas, C. Fuqua, L. Brinkac, M. Lewis, S. Johri, B. Weaver, G. Pai, J. A. Eisen, E. Rahe, W. M. Sheldon, W. Ye, T. R. Miller, J. Carlton, D. A. Rasko, I. T. Paulsen, Q. Ren, S. C. Daugherty, R. T. Deboy, R. J. Dodson, A. S. Durkin, R. Madupu, W. C. Nelson, S. A. Sullivan, M. J. Rosovitz, D. H. Haft, J. Selengut, and N. Ward. 2004. Genome sequence of Silicibacter pomeroyi reveals adaptations to the marine environment. Nature 432:910-913. [DOI] [PubMed] [Google Scholar]
- 37.Moran, M. A., R. Belas, M. A. Schell, J. M. Gonzalez, F. Sun, S. Sun, B. J. Binder, J. Edmonds, W. Ye, B. Orcutt, E. C. Howard, C. Meile, W. Palefsky, A. Goesmann, Q. Ren, I. Paulsen, L. E. Ulrich, L. S. Thompson, E. Saunders, and A. Buchan. 2007. Ecological genomics of marine roseobacters. Appl. Environ. Microbiol. 73:4559-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mouriño, S., C. R. Osorio, and M. L. Lemos. 2004. Characterization of heme uptake cluster genes in the fish pathogen Vibrio anguillarum. J. Bacteriol. 186:6159-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neilands, J. B. 1995. Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem. 45:26723-26726. [DOI] [PubMed] [Google Scholar]
- 40.Nienaber, A., H. Hennecke, and H. M. Fischer. 2001. Discovery of a haem uptake system in the soil bacterium Bradyrhizobium japonicum. Mol. Microbiol. 41:787-800. [DOI] [PubMed] [Google Scholar]
- 41.Pinhassi, J., M. M. Sala, H. Havskum, F. Peters, O. Guadayol, A. Malits, and C. Marrase. 2004. Changes in bacterioplankton composition under different phytoplankton regimens. Appl. Environ. Microbiol. 70:6753-6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rue, E. L., and K. W. Bruland. 1995. Complexation of iron(III) by natural organic ligands in the central North Pacific as determined by a new competitive ligand equilibration/adsoptive cathodic stripping voltammetric method. Mar. Chem. 50:117-138. [Google Scholar]
- 43.Schneider, S., K. H. Sharp, P. D. Barker, and M. Paoli. 2006. An induced fit conformational change underlies the binding mechanism of the heme transport proteobacteria-protein HemS. J. Biol. Chem. 281:32606-32610. [DOI] [PubMed] [Google Scholar]
- 44.Simpson, W., T. Olczak, and C. A. Genco. 2000. Characterization and expression of HmuR, a TonB-dependent hemoglobin receptor of Porphyromonas gingivalis. J. Bacteriol. 182:5737-5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stojiljkovic, I., and K. Hantke. 1992. Hemin uptake system of Yersinia enterocolitica: similarities with other TonB dependent systems in gram-negative bacteria. EMBO J. 11:4359-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strzepek, R. F., and P. J. Harrison. 2004. Photosynthetic architecture differs in coastal and oceanic diatoms. Nature 431:689-692. [DOI] [PubMed] [Google Scholar]
- 47.Suits, M. D. L., G. P. Pal, K. Nakatsu, A. Matte, M. Cygler, and Z. Jia. 2005. Identification of an Escherichia coli O157:H7 heme oxygenase with tandem functional repeats. Proc. Natl. Acad. Sci. USA 102:16955-16960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson, J. M., H. A. Jones, and R. D. Perry. 1999. Molecular characterization of the hemin uptake locus (hmu) from Yersinia pestis and analysis of hmu mutants for hemin and hemoprotein utilization. Infect. Immun. 67:3879-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tortell, P. D., M. T. Maldonado, and N. M. Price. 1996. The role of heterotrophic bacteria in iron-limited ocean ecosystems. Nature 383:330-332. [Google Scholar]
- 51.Vong, L., A. Laes, and S. Blain. 2007. Determination of iron-porphyrin-like complexes at nanomolar levels in seawater. Anal. Chim. Acta 588:237-244. [DOI] [PubMed] [Google Scholar]
- 52.Weaver, R. S., D. L. Kirchman, and D. A. Hutchins. 2003. Utilization of iron/organic ligand complexes by marine bacterioplankton. Aquat. Microb. Ecol. 31:227-239. [Google Scholar]
- 53.White, W. I. 1978. Aggregation of porphyrins and metalloporphyrins, p. 303-339. In D. Dolphin (ed.), The porphyrins. Academic Press, Inc., New York, NY.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.