Abstract
2-Ethyhexyl nitrate (2-EHN) is a major additive of fuel that is used to increase the cetane number of diesel. Because of its wide use and possible accidental release, 2-EHN is a potential pollutant of the environment. In this study, Mycobacterium austroafricanum IFP 2173 was selected from among several strains as the best 2-EHN degrader. The 2-EHN biodegradation rate was increased in biphasic cultures where the hydrocarbon was dissolved in an inert non-aqueous-phase liquid, suggesting that the transfer of the hydrophobic substrate to the cells was a growth-limiting factor. Carbon balance calculation, as well as organic-carbon measurement, indicated a release of metabolites in the culture medium. Further analysis by gas chromatography revealed that a single metabolite accumulated during growth. This metabolite had a molecular mass of 114 Da as determined by gas chromatography/mass spectrometry and was provisionally identified as 4-ethyldihydrofuran-2(3H)-one by liquid chromatography-tandem mass spectrometry analysis. Identification was confirmed by analysis of the chemically synthesized lactone. Based on these results, a plausible catabolic pathway is proposed whereby 2-EHN is converted to 4-ethyldihydrofuran-2(3H)-one, which cannot be metabolized further by strain IFP 2173. This putative pathway provides an explanation for the low energetic efficiency of 2-EHN degradation and its poor biodegradability.
2-Ethyhexyl nitrate (2-EHN) is the nitric ester of 2-ethyl-1-hexanol. It is added at 0.05% to 0.4% to diesel formulations in order to increase the cetane number. As a result of the extensive use of diesel worldwide, the 2-EHN market is about 100,000 tons per year.
Although biodegradability has for a long time been regarded as a relevant characteristic of chemicals, it was only recently incorporated into safety assessments. In the case of fuel oils, large volumes of oxygenates, such as methyl-tert-butyl ether (MTBE), have been added to gasoline since 1992 (19). Because of a lack of knowledge about their biodegradability and insufficient safety regulation, pollution cases resulting from accidental releases occurred in many countries. In the United States, for example, as many as 250,000 sites may have been polluted from leaking underground fuel tanks (35). Poor knowledge of the biodegradation of widely used chemicals may also hide unforeseen concerns relating to the toxicity of metabolic products. For example, the degradation of chlorinated aromatics, such as 4-chlorocatechol, in soil gave rise to the formation of an antibiotic, protoanemonin, which is detrimental to soil microcosms (6).
In case of accidental release of 2-EHN into the environment, the fate and impact of the pollution are unpredictable because of the scarcity of data on 2-EHN biodegradation. Screening tests have been recommended by both the U.S. Environmental Protection Agency (34) and the Organization for Economic Cooperation and Development (24) to evaluate the biodegradability of commercial substances. In this context, the so-called criterion of “ready biodegradability” requires that the tested substance be biodegraded to a level of 60% within 28 days (5). Standard degradation experiments showed that 2-EHN could not be considered readily biodegradable (American Chemistry Council Petroleum Additives Panel High Production Volume Challenge Program final submission for nitric acid, 2-ethylhexyl ester, 2006). It was assumed in this study that 2-EHN was poorly available to microbial communities because of its low water solubility and its high volatility.
In fact, 2-EHN displays both a low vapor pressure, corresponding to about 1.9 mg/liter at 20°C, and a moderate solubility in water (12.6 mg/liter at 20°C). Therefore, 2-EHN is expected to form a separate organic phase in aqueous solution even when present in small amounts. 2-EHN is also a rather hydrophobic molecule, as indicated by a log Ko/w (octonal-water partition coefficient) value of 5.24. Hydrophobic compounds with log Ko/w values in the range of 1 to 5 are often toxic to cells because they insert into the lipid bilayer of the cell membrane, disturbing its integrity and causing cell permeabilization (13, 22).
The backbone of 2-EHN is a branched alkane, a type of molecule that is more resistant to biodegradation than linear alkanes. The metabolism of both linear and branched hydrocarbons by bacteria involves enzymes of the β-oxidation pathway (3). In the case of branched alkanes, their degradation may lead to the formation of β-substituted acyl-coenzyme A intermediates that block β-oxidation (27). Such a metabolic blockage has been encountered during the degradation of terpenoids, such as citronellol, geraniol, and nerol (10, 28). If a quaternary carbon atom occurs at the end of an alkane chain, the result is a molecule quite resistant to microbial attack (18).
In a recent study, microbial communities endowed with the ability to degrade 2-EHN were obtained by enrichment from activated sludge or soil samples (33). The isolation of pure strains able to utilize 2-EHN as a sole source of carbon and energy proved rather difficult. Nevertheless, among several strains of fast-growing mycobacteria previously isolated on other hydrocarbons, some strains, all identified as Mycobacterium austroafricanum, were found to degrade 2-EHN.
In the present study, the kinetics of 2-EHN degradation by selected strains was investigated. M. austroafricanum IFP 2173, which showed the highest rate of degradation, was chosen for further investigation of 2-EHN catabolism. As a means to reduce the expected toxic effect of 2-EHN on bacterial cells and increase its bioavailability in aqueous media, bacterial cultures were mostly carried out in biphasic media. Such biphasic cultures, including a non-aqueous-phase liquid (NAPL) that serves as a solvent for the hydrophobic substrate, have already been implemented to facilitate the degradation of various toxic or recalcitrant compounds (2, 4, 8, 12, 25, 26). A metabolite that accumulated during growth was detected in the culture medium and identified by liquid chromatography-tandem mass spectrometry (LC/MS-MS). Based on our data, a plausible pathway for 2-EHN catabolism by M. austroafricanum IFP 2173 is proposed.
MATERIALS AND METHODS
Microorganisms and culture conditions.
The strains used in this study were M. austroafricanum IFP 2173 (30), isolated on iso-octane; M. austroafricanum IFP 2012 (11) and M. austroafricanum IFP 2015 (15), both isolated on MTBE; and M. austroafricanum C6 (14), M. austroafricanum Spyr_Ge_1, and M. austroafricanum BHF 004 (J. C. Willison, unpublished data), all isolated on pyrene.
The culture medium consisted of a mineral salts solution (7) supplemented with 0.1 g/liter of yeast extract. The carbon source was added after medium sterilization (120°C for 20 min). All cultures were incubated at 30°C with shaking (150 rpm).
Chemicals.
2-EHN (Chemical Abstract Service [CAS] registry number 27247-96-7), 2-ethylhexanol, 2-ethylhexanoic acid, MTBE, decahydronaphthalene, 3-methyldihydrofuran-2(3H)-one, diethyl zinc, and heptamethylnonane (HMN) were obtained from Sigma Aldrich (Saint Quentin Fallavier, France). Mineral salts were from VWR (Fontenay-sous-Bois, France).
Biodegradation experiments.
Biodegradation tests were performed in 120-ml flasks closed with Teflon-coated stoppers and sealed with aluminum caps. Unless otherwise indicated, 4.8 mg of 2-EHN (or 2-ethylhexanol or 2-ethylhexanoic acid) was added to 10 ml of the medium supplemented with 500 μl of 2,2,4,4,6,8,8-HMN. Cultures were adjusted to an optical density at 600 nm of 0.2 using washed pellets of centrifuged precultures grown on Tween 80 (2.5 g/liter) as the sole source of carbon. The degradation rate was monitored by measuring at regular intervals by gas chromatography (GC) the CO2 evolved in the headspace. Residual 2-EHN was estimated as described below in triplicate. Abiotic controls were supplemented with mercuric chloride (0.2 mg/liter), and endogenous controls, lacking a carbon source but containing HMN, were performed under similar conditions.
Analyses of substrate and products.
Cultures grown on 2-EHN were filtered on a polytetrafluoroethylene membrane (0.45 μm), and the cell biomass was determined as dry weight after lyophilization of the cell pellet. When HMN was omitted from the growth medium, the total organic carbon (TOC) was measured on the filtrates using a TOC-5050 carbon analyzer (Shimadzu) according to the European norm NF EN 1484. Residual 2-EHN in the culture filtrate, as well as derived metabolites, was extracted with 10 ml of MTBE containing 0.05% (vol/vol) decahydronaphthalene as an internal standard. After 30 min of shaking and static overnight incubation at 4°C, the solvent extracts were analyzed by GC with flame ionization detection (FID). A Varian 3400 chromatograph (Sugarland) equipped with a CP-Sil Pona CB column (0.25 mm by 50 m) obtained from Chrompack (Raritan, NJ) was used. The carrier gas was helium. The temperatures of the injector and the detector were set at 250 and 280°C, respectively. The column temperature was varied from 100°C to 200°C at 4°C/min and then from 200°C to 259°C at 20°C/min.
Time courses of 2-EHN degradation and metabolite excretion were performed in flasks that were sacrificed at regular time intervals. CO2 in the flask headspace was measured with a Varian 3400 gas chromatograph (Sugarland) equipped with a catharometric detector and a PorapackQ (80/100 mesh, 2 m; Chrompack, Raritan, NJ). The net amount of CO2 produced was determined as the difference between the final quantities found in the test flasks and that found in hydrocarbon-free flasks.
Kinetics of O2 consumption.
Continuous monitoring of substrate oxidation was carried out through measurement of O2 consumption using a respirometer (Sapromat D12-S; Voith, Germany). Flasks containing 250 ml of culture medium and 125 μl of 2-EHN as a carbon source were inoculated with M. austroafricanum IFP 2173 to an optical density at 600 nm of 0.1. Incubation was carried out at 30°C with shaking in the presence or absence of HMN (12.5 ml). Cultures and substrate-free controls were performed in triplicate.
Chemical synthesis of 4-EDF.
4-Ethyldihydrofuran-2(3H)-one (4-EDF) was synthesized according to a published procedure (1). To a three-necked flask containing dry toluene (5 ml), copper(II) trifluoromethanesulfonic acid (0.025 mmol) and triethylphosphite (0.05 mmol) were successively added. The mixture was stirred for 30 min at room temperature to obtain a colorless solution. After it cooled to −20°C, zinc diethyl (5 mmol previously dissolved in hexane) was added, followed by furan-2(5H)-one (5 mmol). The reaction mixture was allowed to warm to 0°C for 6 h and was then incubated at room temperature and monitored by GC. After completion of the reaction, the mixture was hydrolyzed with aqueous 5 N HCl and then extracted with diethyl ether (2 × 15 ml); the organic phase was dried over MgSO4 and concentrated in vacuo. The crude product was purified by column chromatography on SiO2 using a mixture of diethyl ether/pentane [80/20]) as the eluent.
Coupled MS analyses.
GC/MS-MS analysis was carried out under chromatographic conditions identical to those described above for GC-FID. Mass spectra were acquired in the split mode with a time-of-flight mass spectrometer (Tempus TOF MS; Thermo Finnigan).
LC/MS-MS was performed using a high-performance LC system (Alliance 2695; Waters, Guyancourt, France) coupled to a Quattro LC triple-quadrupole mass spectrometer (Micromass, Manchester, United Kingdom) with an electrospray interface. Data were acquired in the positive or negative ionization mode and processed with the MassLynx NT 4.0 system. The electrospray source voltages were as follows: capillary, 3.2 kV; extractor, 2 V; and cone voltage, 22 and 17 V under positive mode. The source block and desolvation gas stream were heated at 120°C and 350°C, respectively. Nitrogen was used as the nebulization and desolvation gas (75 and 350 liters h−1, respectively). For MS-MS, collisional induced dissociation was performed under argon (2.5 × 10−3 mbar) at a collision energy set between 10 and 40 eV.
RESULTS
Time course of 2-EHN biodegradation by selected strains.
The kinetics of 2-EHN biodegradation was studied using a few bacterial strains previously selected from among environmental isolates and collection strains for the ability to attack the compound (33). Most of these strains were identified as members of the genus Mycobacterium. In order to avoid growth inhibition due to 2-EHN toxicity, HMN was added as NAPL to the bacterial cultures, and biodegradation time courses were monitored by measuring the CO2 production in the culture headspace. The biodegradation kinetics were found to vary widely depending on the bacterial strains (data not shown). M. austroafricanum IFP 2173 was the fastest and most efficient of the microorganisms tested, since it produced the largest amount of CO2 (37 μmol per flask) after 13 days of incubation. M. austroafricanum IFP 2173 was also the only strain able to grow on 2-EHN in the absence of HMN (data not shown).
Effect of the 2-EHN supply mode on the biodegradation rate.
The impact of NALP addition on 2-EHN biodegradation by strain IFP 2173 was studied through continuous monitoring of substrate-dependent oxygen consumption by respirometry.
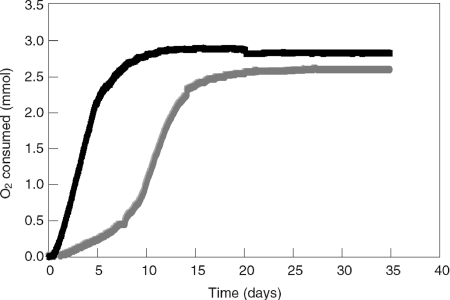
In the culture lacking HMN, O2 uptake started after a lag phase of about 1 day and then increased with time according to a sigmoidal curve (Fig. 1). The maximal growth rate (μmax) could be deduced from the oxygen uptake rate, assuming that the biomass yield remained constant during growth. Over a 9-day period of growth, μmax was calculated to be 0.29 day−1 on average, corresponding to a generation time of 2.4 days. In the HMN-containing culture, the lag phase was shorter, and the O2 uptake became linear after a very short exponential phase (μmax = 0.29 day−1). The maximal rate of O2 uptake was 5.3 mmol/day, and the overall O2 consumption reached a maximum of 2.9 mmol, compared to 2.6 mmol for cells grown without HMN.
FIG. 1.
Effect of a NALP (HMN) on the rate of oxygen consumption by M. austroafricanum IFP 2173. Cultures (250 ml) were grown in the flasks of a respirometer and contained 125 μl of 2-EHN as a carbon source. The cultures were incubated in the presence (black line) or absence (gray line) of HMN (12.5 ml).
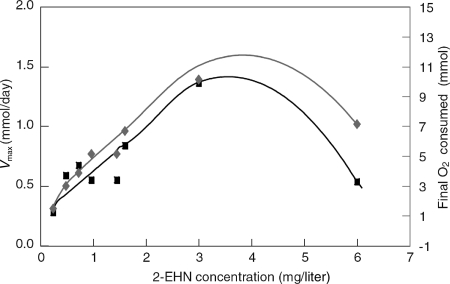
The effect of the 2-EHN concentration on growth was studied in HMN-containing cultures (Fig. 2). The concentration of 2-EHN had little effect on the specific growth rate. During the linear phase of growth, the O2 uptake rate increased proportionally to the 2-EHN concentration in the culture medium up to 3 g/liter. This indicated that the 2-EHN diffusion rate from HMN to the water phase was a limiting factor for bacterial growth. At 2-EHN concentrations higher than 3 g/liter, bacterial growth was inhibited, as indicated by both lower oxygen uptake rates and lower overall O2 consumption. For 2-EHN concentrations lower than 3 g/liter, no residual substrate was detected in the culture medium by the end of growth, and the O2 consumption was roughly proportional to the amount of substrate supplied.
FIG. 2.
Effect of the 2-EHN concentration on oxygen consumption by M. austroafricanum IFP 2173. Biphasic cultures contained a variable concentration of 2-EHN and 12.5 ml of HMN. The maximal rates of O2 uptake or metabolism (Vmax) (▪) and overall O2 consumption ( ) were determined.
) were determined.
Carbon balance of 2-EHN biodegradation by M. austroafricanum IFP 2173.
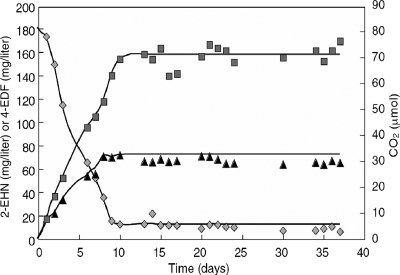
In order to determine the carbon balance of 2-EHN biodegradation, M. austroafricanum IFP 2173 was cultivated in mineral medium lacking HMN to avoid perturbation of TOC measurements by HMN. The culture was stopped when no more CO2 was released, which coincided with the total consumption of 2-EHN (see Fig. 4). The biomass formed, the TOC in the filtered culture medium, and the amount of CO2 released were measured. The carbon recovery as metabolites and cell biomass was calculated by taking into account the elementary compositions of the substrate and products (Table 1). A carbon recovery rate of 92% was obtained for the 2-EHN bioconversion. Carbon converted into biomass (94 mg/liter) and CO2 (165 mg/liter) amounted together to only 33% of the total carbon produced. Accordingly, a high proportion of the substrate-derived carbon was recovered in the clarified culture medium (67%), possibly reflecting metabolite accumulation.
FIG. 4.
Accumulation of 4-EDF during 2-EHN biodegradation. Parallel cultures were carried out in 120-ml flasks and removed at the times indicated for extraction and measurements of 2-EHN ( ) and 4-EDF (▴). CO2 (▪) was measured in a separate culture flask. Residual 2-EHN is the fraction of hydrocarbon that stayed bound to the flask wall and stopper and remained inaccessible to bacteria.
) and 4-EDF (▴). CO2 (▪) was measured in a separate culture flask. Residual 2-EHN is the fraction of hydrocarbon that stayed bound to the flask wall and stopper and remained inaccessible to bacteria.
TABLE 1.
Carbon balance of 2-EHN biodegradation by M. austroafricanum IFP 2173a
| Substrate or product | Mass changeb (mg/liter) | Carbon balance
|
|
|---|---|---|---|
| Carbon changeb (mg/liter) | Carbon recovery (%) | ||
| 2-EHN | 482 | 269 | 0 |
| Cell biomass | 94 | 50c | 19 |
| CO2d | 115 | 31 | 12 |
| TOCe | 165 | 165 | 61 |
| Total products | 92 | ||
Cultures (10 ml) were performed at 30°C in 120-ml flasks.
Considering the whole content of the culture flasks.
The carbon/dry-biomass ratio was assumed to be 52% (17). The dry biomass was determined from 100-ml cultures grown in 1-liter flasks.
CO2 was determined after acidification of the culture.
TOC was measured in the culture fluid after filtration through a 0.22-μm membrane.
Identification of a metabolite excreted in the culture.
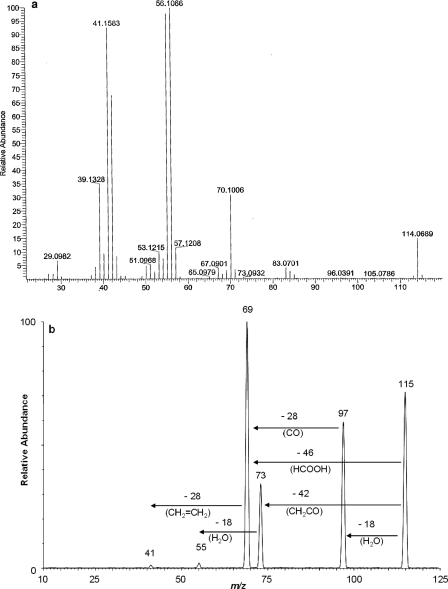
GC-FID analysis of culture fluid extracts performed during 2-EHN degradation experiments revealed a gradual increase in the concentration of an unknown compound with a retention time shorter than that of 2-EHN. This finding suggested that a metabolite might have accumulated during growth and accounted for the substantial level of TOC previously detected in the supernatants of 2-EHN-grown cultures. High-resolution mass spectral analysis of this compound (Fig. 3a) showed that it had a molecular mass of 114.07 Da and the chemical formula C6H10O2. The mass spectrum of this compound did not match any of the spectra currently available in the databases. Nevertheless, a comparison of the LC/MS-MS data of the excreted product with those of 3-methyldihydrofuran-2(3H)-one, a commercially available product, revealed several common fragment ions. The analysis also indicated that the molecule did not contain any carboxylic or hydroxyl groups (Fig. 3b). Taken together, our data indicated that the product of interest might be 4-EDF, which can also be designated β-ethyl-γ-butyrolactone. In order to confirm the structure of the metabolite, the chemical synthesis of 4-EDF was undertaken as described in Materials and Methods (1). The LC/MS-MS characteristics of the synthesized lactone were identical to those of the metabolite, confirming that the product that accumulated in cultures of M. austroafricanum IFP 2173 grown on 2-EHN was 4-EDF.
FIG. 3.
MS characterization of the metabolite produced by strain IFP 2173 upon degradation of 2-EHN. (a) High-resolution electron impact mass spectrum of the accumulated metabolite as obtained by GC-MS analysis. (b) Collisional induced dissociation/MS-MS product ion spectrum of the protonated molecule (MH+) obtained by LC/MS-MS analysis at a collision energy of 10 eV.
The rate of 4-EDF accumulation was assessed by GC-FID analysis of the culture fluid during growth. Figure 4 shows that 4-EDF formation and CO2 release were concurrent with 2-EHN degradation.
Biodegradation of 2-EHN-derived compounds.
As a means to elucidate the pathway of 2-EHN biodegradation by M. austroafricanum IFP 2173, we tested compounds with structures derived from 2-EHN as possible substrates. 2-Ethylhexanol, the primary alcohol resulting from 2-EHN hydrolysis, was biodegraded, yielding 2-ethylhexanoic acid and 4-EDF. 2-Ethylhexanoic acid, the product resulting from 2-ethylhexanol oxidation, was not biodegraded, even in the presence of HMN. This compound is considered to be toxic for most bacteria (21). It should be noted that 2-EHN can be used as a sole nitrogen source by strain IFP 2173, indicating that nitrate is formed, probably as a result of an initial attack on 2-EHN by an esterase (data not shown). 2-EHN biodegradation was also tested in the presence of isooctane, the compound on which M. austroafricanum IFP 2173 was selected. Diauxic growth was observed, with the strain degrading isooctane first and then 2-EHN into 4-EDF (data not shown).
DISCUSSION
2-EHN is a recalcitrant compound that was considered not readily biodegradable according to standard procedures (American Chemistry Council Petroleum Additives Panel High Production Volume Challenge Program final submission for nitric acid, 2-ethylhexyl ester, 2006). However, we demonstrated in the present study that selected strains of mycobacteria were able to slowly utilize 2-EHN as a sole source of carbon under defined culture conditions. The poor biodegradability of 2-EHN might be the consequence of two factors: first, the rare occurrence of microorganisms able to use it as a carbon source, and second, its inhibitory effect on bacterial growth even at a low concentration. 2-EHN inhibition was illustrated by the experiment shown in Fig. 2 and by the lack of growth of all strains tested in HMN-free cultures, except M. austroafricanum IFP 2173. This strain, isolated for its ability to degrade isooctane, a branched alkane (31), demonstrated broad capabilities for hydrocarbon biodegradation (16, 32). Like many members of the Corynebacterium-Mycobacterium-Nocardia group of gram-positive bacteria, it may be resistant to toxic hydrocarbons, thanks to the properties of its cell envelope, which is highly rigid and contains mycolic acids (29). In mycobacteria, mycolic acids are very long fatty acids (C60 to C90) that contribute up to 60% of the cell wall (9). The specific cell wall composition of the M. austroafricanum strains studied here probably accounts for their resistance to 2-EHN.
However, it is unclear whether the unique ability of strain IFP 2173 to grow on 2-EHN without NAPL is due to a cell wall composition slightly different from that of other strains or to some other strain-specific trait.
Biphasic cultures, involving the addition of an inert NAPL like HMN, were found to be critical for 2-EHN biodegradation and bacterial growth. In the HMN-free cultures, the dissolved fraction of 2-EHN represented only a minor part of the substrate supplied, since it partitioned into three distinct phases, i.e., the gas phase, the aqueous phase, and the bulk of insoluble 2-EHN. During the biodegradation process, the uptake of dissolved substrate was counterbalanced by the equilibrium transfer of 2-EHN from the bulk of the substrate (SsubNAPL) to the aqueous substrate (Ssub/aq) according to the following scheme:
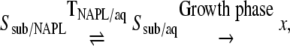 |
where SsubNAPL and Ssub/aq represent the amounts of substrate in the bulk and in the aqueous phases, respectively; x is the cell biomass; and TNAPL/aq is the substrate transfer rate of 2-EHN to the culture medium. In HMN-containing cultures, the dissolved 2-EHN was mainly confined to NAPL. Because of the high hydrophobicity of their cell walls, microbial cells tightly adhered to NAPL, and direct contact was thus the most probable mode of substrate uptake (8, 12). Accordingly, the large NAPL volume (500 μl of HMN versus 5 μl of 2-EHN in the case of the NAPL-free culture), which increased substrate bioavailability, probably accounted for its higher efficiency of assimilation by the microorganisms. Such conditions of substrate delivery were apparently required to promote the growth on 2-EHN of M. austroafricanum strains other than strain 2173.
The biodegradation of 2-EHN by M. austroafricanum IFP 2173 illustrates the remarkable metabolic capabilities of this strain for recalcitrant hydrocarbons. Indeed, it can degrade another methyl branched alkane, 2,2,4-trimethylpentane (31), suggesting that it produces enzymes specific for the degradation of anteiso-alkanes. Nevertheless, our results indicate that the degradation of 2-EHN by strain IFP 2173 is partial and gives rise to the release of an acyl with an ethyl substituent in the beta position. At least two reasons might explain the accumulation of this metabolite: (i) strain IFP 2173 lacks enzymes able to degrade it, and (ii) because of the ethyl group in the beta position, the metabolite might block the enzyme catalyzing the next step in the degradation of branched alkanes.
Considering the high biodegradation potential of strain IFP 2173, it was recently observed that this strain can degrade other xenobiotic compounds structurally related to 2-EHN, such as bis(2-ethylhexyl)phthalate (data not shown), used as a plasticizer (21, 23). The biodegradation of this compound by Mycobacterium sp. strain NK0301 has been reported (20). This bacterium utilized phthalate as a carbon and energy source and left the carbon skeleton of the 2-ethylhexyl moiety intact, releasing it as 2-ethylhexanol or 2-ethylhexanoic acid. In comparison, strain IFP 2173 degraded bis(2-ethylhexyl) phthalate and utilized the 2-ethylhexyl moiety, achieving a higher degree of degradation (data not shown).
The biodegradation of 2-EHN by strain IFP 2173 gave rise to the accumulation of a lactone, which was identified as 4-EDF. The lactone formed by cyclization of a breakdown product, a branched pentanoic acid, which was not metabolized further by the bacteria. The partial degradation of 2-EHN certainly explains the observed slow growth (μmax = 0.29 day−1) and poor growth yield of cultures utilizing this compound as a sole C source.
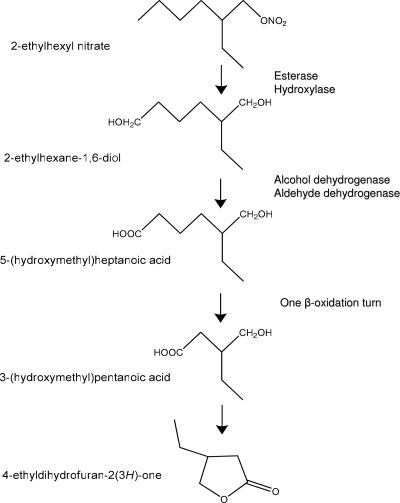
Considering the structure of the intermediate metabolite and the known degradation pathway of n-alkanes (18), we propose for the first time a plausible metabolic pathway for 2-EHN degradation (Fig. 5). The pathway would start by a simultaneous or sequential attack of the molecule on both extremities, with an esterase activity hydrolyzing the nitric ester bond and an oxygenase catalyzing the hydroxylation of the distal methyl group. The involvement of an esterase that would release nitrate was inferred from the observation that strain IFP 2173 utilized 2-EHN as a nitrogen source. The existence in this strain of a hydroxylase active on branched alkane is expected, since it grows on isooctane (31). The intermediate metabolite that would form, 2-ethylpentan-1,6-diol, is proposed to be oxidized to a carboxylic acid in two steps involving successively an alcohol and an aldehyde dehydrogenase. After activation by coenzyme A, the resulting 5-(hydroxymethyl)heptanoic acid would undergo one cycle of classical β-oxidation to give 3-(hydroxymethyl)pentanoic acid, which would spontaneously convert to 4-EDF by cyclization. Since the substrate underwent a single turn of β-oxidation, only two carbon atoms (out of eight in 2-EHN) could reach the tricarboxylic acid cycle, accounting for the low percentage of carbon released as CO2 (12%).
FIG. 5.
Proposed pathway for 2-EHN biodegradation by M. austroafricanum IFP 2173.
The proposed pathway now needs to be assessed experimentally by identifying enzymes involved in 2-EHN degradation. To this end, we have undertaken a proteomic analysis to discover the proteins that are induced upon incubation of strain IFP 2173 with 2-EHN.
Acknowledgments
This work was supported by a Convention Industrielle de Formation par la Recherche (CIFRE) fellowship from the Association Nationale de la Recherche Technique (ANRT) to E. Nicolau and grants from the IFP.
We thank F. Léglise for helpful discussions and J. C. Willison for critical reading of the manuscript.
Footnotes
Published ahead of print on 22 August 2008.
REFERENCES
- 1.Alexakis, A., J. Vastra, and P. Mangeney. 1997. Acceleration of the conjugate addition of diethyl zinc to enones by either Cu(OTf)2 or trivalent phosphorus ligands. Tetrahedron Lett. 38:7745-7748. [Google Scholar]
- 2.Allen, C. C., D. R. Boyd, F. Hempenstall, M. J. Larkin, and N. D. Sharma. 1999. Contrasting effects of a nonionic surfactant on the biotransformation of polycyclic aromatic hydrocarbons to cis-dihydrodiols by soil bacteria. Appl. Environ. Microbiol. 65:1335-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez, H. M. 2003. Relationship betwen beta-oxidation pathway and the hydrocarbon-degradation profile in actinomycete bacteria. Int. Biodeter. Biodeg. 52:35-42. [Google Scholar]
- 4.Ascon-Cabrera, M., and J. M. Lebeault. 1993. Selection of xenobiotic-degrading microorganisms in a biphasic aqueous-organic system. Appl. Environ. Microbiol. 59:1717-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battersby, N. S., D. Ciccognani, M. R. Evans, D. King, H. A. Painter, D. R. Peterson, M. Starkey, et al. 1999. An ‘inherent’ biodegradability test for oil products: description and results of an international ring test. Chemosphere 38:3219-3235. [DOI] [PubMed] [Google Scholar]
- 6.Blasco, R., R. M. Wittich, M. Mallavarapu, K. N. Timmis, and D. H. Pieper. 1995. From xenobiotic to antibiotic, formation of protoanemonin from 4-chlorocatechol by enzymes of the 3-oxoadipate pathway. J. Biol. Chem. 270:29229-29235. [DOI] [PubMed] [Google Scholar]
- 7.Bouchez, M., D. Blanchet, and J. P. Vandecasteele. 1995. Degradation of polycyclic aromatic hydrocarbons by pure strains and by defined strain associations: inhibition phenomena and cometabolism. Appl. Microbiol. Biotechnol. 43:156-164. [DOI] [PubMed] [Google Scholar]
- 8.Bouchez-Naitali, M., H. Rakatozafy, R. Marchal, J. Y. Leveau, and J. P. Vandecasteele. 1999. Diversity of bacterial strains degrading hexadecane in relation to the mode of substrate uptake. J. Appl. Microbiol. 86:421-428. [DOI] [PubMed] [Google Scholar]
- 9.Brennan, P. J., and H. Nikaido. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29-63. [DOI] [PubMed] [Google Scholar]
- 10.Fall, R. R., J. L. Brown, and T. L. Schaeffer. 1979. Enzyme recruitment allows the biodegradation of recalcitrant branched hydrocarbons by Pseudomonas citronellolis. Appl. Environ. Microbiol. 38:715-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francois, A., H. Mathis, D. Godefroy, P. Piveteau, F. Fayolle, and F. Monot. 2002. Biodegradation of methyl tert-butyl ether and other fuel oxygenates by a new strain, Mycobacterium austroafricanum IFP 2012. Appl. Environ. Microbiol. 68:2754-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goswami, P., and H. D. Singh. 1991. Different modes of hydrocarbon uptake by two Pseudomonas species. Biotechnol. Bioeng. 37:1-11. [DOI] [PubMed] [Google Scholar]
- 13.Heipieper, H. J., F. J. Weber, J. Sikkema, H. Keweloh, and J. A. M. de Bont. 1994. Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 12:409-415. [Google Scholar]
- 14.Jouanneau, Y., J. C. Willison, C. Meyer, S. Krivobok, N. Chevron, J. L. Besombes, and G. Blake. 2005. Stimulation of pyrene mineralization in freshwater sediments by bacterial and plant bioaugmentation. Environ. Sci. Technol. 39:5729-5735. [DOI] [PubMed] [Google Scholar]
- 15.Lopes Ferreira, N., H. Maciel, H. Mathis, F. Monot, F. Fayolle-Guichard, and C. W. Greer. 2006. Isolation and characterization of a new Mycobacterium austroafricanum strain, IFP 2015, growing on MTBE. Appl. Microbiol. Biotechnol. 70:358-365. [DOI] [PubMed] [Google Scholar]
- 16.Lopes Ferreira, N., H. Mathis, D. Labbe, F. Monot, C. W. Greer, and F. Fayolle-Guichard. 2007. n-Alkane assimilation and tert-butyl alcohol (TBA) oxidation capacity in Mycobacterium austroafricanum strains. Appl. Microbiol. Biotechnol. 75:909-919. [DOI] [PubMed] [Google Scholar]
- 17.McCarty, P. L. 1972. Energetics of organic matter degradation, p. 91-118. In R. Mitchell (ed.), Water pollution microbiology. Wiley Interscience, New York, NY.
- 18.Mc Kenna, E. J. 1972. Microbial metabolism of normal and branched chain alkanes, p. 73-97. In Degradation of synthetic organic molecules in the biosphere. Proceedings of the San Francisco Conference, Academy of Science, Washington, DC. U.S. Academy of Science, Washington, DC.
- 19.Moran, M. 2007. Occurrence of methyl tert-butyl ether and other fuel oxygenates in source water and drinking water in the United States, p. 57-98. In O. Hutzinger (ed.), The handbook of environmental chemistry: fuel oxygenates, vol. 5. Water pollution. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 20.Nakamiya, K., S. Hashimoto, H. Ito, J. S. Edmonds, A. Yasuhara, and M. Morita. 2005. Microbial treatment of bis(2-ethylhexyl) phthalate in polyvinyl chloride with isolated bacteria. J. Biosci. Bioeng. 99:115-119. [DOI] [PubMed] [Google Scholar]
- 21.Nalli, S., D. G. Cooper, and J. A. Nicell. 2002. Biodegradation of plasticizer by Rhodoccocus rhodochrous. Biodegradation 13:343-352. [DOI] [PubMed] [Google Scholar]
- 22.Neumann, G., N. Kabelitz, A. Zehnsdorf, A. Miltner, H. Lippold, D. Meyer, A. Schmid, and H. J. Heipieper. 2005. Prediction of the adaptability of Pseudomonas putida DOT-T1E to a second phase of a solvent for economically sound two-phase biotransformations. Appl. Environ. Microbiol. 71:6606-6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishioka, T., M. Iwata, T. Imaoka, M. Mutoh, Y. Egashira, T. Nishiyama, T. Shin, and T. Fujii. 2006. A mono-2-ethylhexyl phthalate hydrolase from a Gordonia sp. that is able to dissimilate di-2-ethylhexyl phthalate. Appl. Environ. Microbiol. 72:2394-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Organization for Economic Cooperation and Development. 1993. Guidelines for the testing of chemicals. OCED editions, part 3. Organization for Economic Cooperation and Development, Paris, France.
- 25.Ortega-Calvo, J. J., and M. Alexander. 1994. Roles of bacterial attachment and spontaneous partitioning in the biodegradation of naphthalene initially present in non-aqueous-phase liquids. Appl. Environ. Microbiol. 60:2643-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandrin, T. R., W. B. Kight, W. J. Maier, and R. M. Maier. 2006. Influence of a nonaqueous phase liquid (NAPL) on biodegradation of phenanthrene. Biodegradation 17:423-435. [DOI] [PubMed] [Google Scholar]
- 27.Schaeffer, T. L., S. G. Cantwell, J. L. Brown, D. S. Watt, and R. R. Fall. 1979. Microbial growth on hydrocarbons: terminal branching inhibits biodegradation. Appl. Environ. Microbiol. 38:742-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seubert, W., and E. Fass. 1964. Studies on the bacterial degradation of isoprenoids. IV. The purification and properties of beta-isohexenylglutaconyl-CoA-hydratase and beta-hydroxy-beta-isohexenylglutaryl-CoA-lyase. Biochem. Z. 341:23-34. [PubMed] [Google Scholar]
- 29.Sokolovska, I., R. Rozenberg, C. Riez, P. G. Rouxhet, S. N. Agathos, and P. Wattiau. 2003. Carbon source-induced modifications in the mycolic acid content and cell wall permeability of Rhodococcus erythropolis E1. Appl. Environ. Microbiol. 69:7019-7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solano-Serena, F., R. Marchal, S. Casaregola, C. Vasnier, J. M. Lebeault, and J. P. Vandecasteele. 2000. A Mycobacterium strain with extended capacities for degradation of gasoline hydrocarbons. Appl. Environ. Microbiol. 66:2392-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solano-Serena, F., R. Marchal, S. Heiss, and J. P. Vandecasteele. 2004. Degradation of isooctane by Mycobacterium austroafricanum IFP 2173: growth and catabolic pathway. J. Appl. Microbiol. 97:629-639. [DOI] [PubMed] [Google Scholar]
- 32.Solano-Serena, F., R. Marchal, J. M. Lebeault, and J. P. Vandecasteele. 2000. Selection of microbial populations degrading recalcitrant hydrocarbons of gasoline by monitoring of culture-headspace composition. Lett. Appl. Microbiol. 30:19-22. [DOI] [PubMed] [Google Scholar]
- 33.Solano-Serena, F., E. Nicolau, G. Favreau, Y. Jouanneau, and R. Marchal. Biodegradability of 2-ethylhexyl nitrate (2-EHN), a cetane improver of diesel oil. Biodegradation, in press. [DOI] [PubMed]
- 34.U.S. Environmental Protection Agency. 1982. Chemical fate test guidelines; EPA 560/6-82-003, NTIS PB82-23308. U.S. Environmental Protection Agency, Washington, DC.
- 35.Waul, C. K., E. Arvin, and J. E. Schmidt. 2007. Microbial degradation of MTBE in reactors, p. 213-248. In O. Hutzinger (ed.), The handbook of environmental chemistry: fuel oxygenates, vol. 5. Water pollution. Springer-Verlag, Berlin, Germany. [Google Scholar]