Abstract
Weaned 3- to 4-month-old calves were fasted for 48 h, inoculated with 1010 CFU of Shiga toxin-positive Escherichia coli (STEC) O157:H7 strain 86-24 (STEC O157) or STEC O91:H21 strain B2F1 (STEC O91), Shiga toxin-negative E. coli O157:H7 strain 87-23 (Stx− O157), or a nonpathogenic control E. coli strain, necropsied 4 days postinoculation, and examined bacteriologically and histologically. Some calves were treated with dexamethasone (DEX) for 5 days (3 days before, on the day of, and 1 day after inoculation). STEC O157 bacteria were recovered from feces, intestines, or gall bladders of 74% (40/55) of calves 4 days after they were inoculated with STEC O157. Colon and cecum were sites from which inoculum-type bacteria were most often recovered. Histologic lesions of attaching-and-effacing (A/E) O157+ bacteria were observed in 69% (38/55) of the STEC O157-inoculated calves. Rectum, ileocecal valve, and distal colon were sites most likely to contain A/E O157+ bacteria. Fecal and intestinal levels of STEC O157 bacteria were significantly higher and A/E O157+ bacteria were more common in DEX-treated calves than in nontreated calves inoculated with STEC O157. Fecal STEC O157 levels were significantly higher than Stx− O157, STEC O91, or control E. coli; only STEC O157 cells were recovered from tissues. Identifying the rectum, ileocecal valve, and distal colon as early STEC O157 colonization sites and finding that DEX treatment enhances the susceptibility of weaned calves to STEC O157 colonization will facilitate the identification and evaluation of interventions aimed at reducing STEC O157 infection in cattle.
Enterohemorrhagic Escherichia coli bacteria are a subset of Shiga toxin-producing E. coli (STEC) capable of causing hemorrhagic colitis (bloody diarrhea) and the hemolytic-uremic syndrome in humans. All STEC strains produce one or more Stx proteins (Stx1, Stx2, or a variant of Stx2). STEC O157:H7 (hereafter referred to as STEC O157) is the most frequently reported STEC serotype associated with outbreaks of bloody diarrhea and hemolytic-uremic syndrome in humans worldwide (19, 24). Cattle are important sources of STEC O157. STEC human pathogens are often foodborne, and most human infections for which a source has been identified have been attributed to the consumption of STEC O157-contaminated bovine products or produce and/or water contaminated with bovine manure. Healthy cattle transiently carry STEC serotypes associated with disease in humans and intermittently shed these bacteria into the environment in their feces. Many STEC isolates from healthy cattle share common virulence factors (i.e., Stx and intimin) with STEC that cause disease in humans and with attaching-and-effacing (A/E) E. coli, enteric pathogens that can cause diarrhea in young calves. Dysentery has also been reported in neonatal calves naturally or experimentally infected with non-O157 STEC (10, 31). Although not considered bovine pathogens, STEC O157 strains cause severe (sometimes fatal) diarrhea in experimentally inoculated neonatal calves. STEC-infected colostrum-deprived and colostrum-fed neonatal (<36 h old) calves have high intestinal and fecal levels of STEC O157 and characteristic lesions of bacterial attachment and cytoplasmic effacement (A/E bacteria) in both the large and small intestines (5, 7, 8).
The terminal rectum has been identified as the principal STEC O157 colonization site in naturally and experimentally infected cattle (14, 21, 22). Culturing rectoanal mucosal swab samples is more sensitive than culturing feces for recovering STEC O157 bacteria from naturally and experimentally infected cattle (3, 13, 27).
Our long-term goal is to identify ways to reduce the presence of STEC O157 in cattle in order to reduce food-borne infections in humans. We are using bovine STEC infection models to locate early STEC colonization sites, to identify bacterial and host factors that promote STEC O157 infections in cattle (not necessarily the same as those involved in the pathogenesis of disease in humans), and to evaluate interventions aimed at reducing STEC O157 colonization and shedding in cattle. In this article, we summarize our observations from multiple experiments, involving 55 weaned calves inoculated with STEC O157:H7 strain 86-24 and 9 control calves, performed to establish a reproducible bovine model of acute STEC O157 infection and identify early STEC O157 colonization sites in experimentally inoculated weaned calves (8, 25, 31; unpublished observations). Initial efforts to establish a model in which weaned calves held off feed for 48 h were consistently colonized with high levels of STEC O157 were hampered by the large variations in the susceptibility of weaned calves to experimental STEC infections. This prompted us to add high-dose dexamethasone (DEX) treatment during the peri-inoculation period to determine if exogenous DEX-induced immunosuppression (a method for simulating physiologic stress that enhances the susceptibility of cattle to a variety of bacterial, viral, and protozoal diseases [1]) enhanced the susceptibility of weaned calves to STEC O157 colonization infections (31). For comparison, we also include observations from experiments involving 14 weaned calves inoculated with Shiga toxin-negative E. coli O157 strain 87-23 (n = 6) or STEC O91 strain B2F1 (n = 8).
(A preliminary account of this report was presented at the 86th Annual Meeting of the Conference of Research Workers in Animal Diseases, St. Louis, MO, 4 to 6 December 2005.)
MATERIALS AND METHODS
Bacterial strains and inocula.
The E. coli bacterial strains used in these studies are described in Table 1. STEC strains 86-24 (O157:H7), which produces Stx2 and intimin (9, 15), and B2F1 (O91:H21), which produces the activatable Shiga toxin type 2d (16) but not intimin, are streptomycin-resistant mutants of the original clinical isolates. E. coli O157:H7 strain 87-23 was isolated from the same outbreak as strain 86-24 but lacks Stx1 and Stx2 genes. The E. coli control strain, 123, is resistant to nalidixic acid (9, 15). Stock inocula containing approximately 1010 CFU/ml were prepared and stored at −80°C as previously described (7).
TABLE 1.
E. coli strains used in this study
| Strain (type) | Serotype | eae gene | Shiga toxin gene | Source |
|---|---|---|---|---|
| 86-24 (STEC O157) | O157:H7 | + | stx2 | P. Tarr (7, 15) |
| 87-23a (Stx− O157) | O157:H7 | + | None | P. Tarr (2) |
| B2F1 (STEC O91) | O91:H21 | − | stx2d | M. Karmali (12) |
| 123 (control) | O43:H28 | − | None | NADC (7, 18) |
Isolated from same outbreak as strain 86-24.
Animals.
Weaned calves of various breeds (including Jersey, Hereford, or Hereford × Black Angus crosses), ranging in age from 3 to 5 months, were housed in individual pens in an environmentally controlled facility at the National Animal Disease Center (NADC) in Ames, IA, for at least 2 weeks prior to inoculation and for the duration of the study. Calves were fed a diet of two-thirds grain and one-third hay. To control intestinal nematodes, some of the calves were treated with 1% Ivermectin (1 ml/100 lb) on the day of weaning and 21 to 28 days after the initial treatment, and the cocciodiostat lasalocid was incorporated into the feed. Animal care and all animal experiments were in accordance with the requirements of the NADC Animal Care and Use Committee.
Animal treatments and inoculations.
This report summarizes our observations from a total of 78 weaned calves tested in 17 separate experiments (2 to 8 calves/experiment [Table 2]), some of which have been included in prior reports (6, 8, 25, 31). To enhance their susceptibility to STEC O157 infection, all calves were fasted for 48 h before they were inoculated and 35 calves inoculated with STEC O157 (n = 25), STEC O91 (n = 4), E. coli Stx− O157 (n = 4), or the E. coli control (n = 2) were additionally treated with high-dose DEX (0.25 mg/kg of body weight) by intravenous administration for 5 consecutive days (3 days prior to inoculation, on the day of inoculation, and on the day after inoculation) (1, 4). Peripheral venous blood samples were collected daily from 3 days before the first DEX treatment until the end of the experiment for complete and differential blood counts. Calves were inoculated intrarumenally or orally with 1010 CFU of STEC O157 (55 calves with Stx2+ strain 86-24), Stx− O157 (6 calves with Stx− strain 87-23), STEC O91 (8 calves with Stx 2d+ strain B2F1), or nonpathogenic control E. coli strain (9 calves with control strain 123). All calves were observed twice a day and necropsied at 4 days after inoculation. Fecal samples were collected daily (0 to 4 days postinoculation [p.i.]) and frozen at −80°C for bacteriological culture. At necropsy, sections of tissues indicated in Table 2 were collected aseptically and frozen at −80°C for bacteriological culture. Sections of intestinal tissues, gall bladders, kidneys, livers, and other parenchymal tissues were collected for histopathologic examinations and immunoperoxidase staining (intestinal tissues and gall bladders only).
TABLE 2.
Numbers of calves and tissues evaluated
| E. coli inoculum straina (type) | Treatmentb | No. of calves tested (no. of experiments) | No. of calves sampled for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteriologic studiesc
|
Histologic studiesd
|
||||||||||
| Feces | Distal colon | Cecum | Ileum | Gall bladder | Bile | Distal colon, cecum, and ileum | Ileocecal valve and gall bladder | Rectoanal junction | |||
| 86-24 (STEC O157) | None | 30 (10) | 30 | 30 | 30 | 30 | 15 | 15 | 30 | 21 | 6 |
| DEX | 25 (5) | 25 | 21 | 21 | 21 | 11 | 15 | 25 | 25 | 12 | |
| 87-23 (Stx− O157) | None | 2 (2) | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 |
| DEX | 4 (3) | 4 | 0 | 0 | 0 | 0 | 4 | 4 | 4 | 4 | |
| B2F1 (STEC O91) | None | 4 (2) | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| DEX | 4 (2) | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | |
| 123 (control) | None | 7 (2) | 7 | 7 | 7 | 7 | 0 | 0 | 7 | 4 | 0 |
| DEX | 2 (1) | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | |
As described in Table 1.
Calves were treated with DEX, as described in Materials and Methods, or not treated. All calves were off feed for 48 h before they were inoculated intrarumenally or orally with 1010 CFU of inoculum bacteria.
Number of calves sampled at specified sites for bacteriologic studies. Fecal samples were collected daily (day 0 to 4 days p.i.), and tissue samples were collected 4 days after inoculation for bacteriological examination.
Number of calves sampled at specified sites for histologic studies. Distal colon, cecum, and ileum tissues were collected from all of the calves; ileocecal valve, gall bladder, and rectoanal junction tissues were also collected from some of the calves.
Histologic studies.
Tissues were fixed in neutral buffered 10% formalin for 24 to 48 h, embedded in paraffin, sectioned, and stained with hematoxylin and eosin for routine histology. Inoculum-type bacteria in formalin-fixed intestinal tissues were identified by indirect immunoperoxidase staining (horseradish peroxidase) with goat anti-O157:H7 (7) or by using rabbit anti-O91 or anti-O43 antibodies (E. coli Reference Center, Pennsylvania State University, University Park) as the primary antibody and biotinylated anti-goat or anti-rabbit antibodies (Vector Laboratories, Inc., Burlingame, CA) as secondary antibodies (9).
Bacteriologic examination.
At 4 to 7 days before inoculation, fecal samples were obtained from all calves and screened for inoculum-type bacteria and antibiotic-resistant normal flora by plating on selective media used for quantitating the planned inoculum strain. The numbers of the inoculum-type bacteria recovered from tissues and feces of calves were quantitated on sorbitol MacConkey's agar containing 100 μg/ml streptomycin (strain 86-24), sorbitol MacConkey's agar without antibiotics (strain 87-23), MacConkey's agar containing 100 μg/ml streptomycin (strain B2F1), or MacConkey's agar containing 20 μg/ml nalidixic acid (strain 123). Total coliforms were quantitated on MacConkey agar without antibiotics. To increase selectivity for strain 87-23, which lacked antibiotic selection, samples with high levels of enteric coliforms prior to inoculation were also plated on sorbitol MacConkey's agar supplemented with 2.5 μg/ml potassium tellurite (7). Selected sorbitol-negative isolates (strains 86-24 and 87-23) were tested for O157:H7 antigen by latex agglutination assay (7). Selected coliforms were tested for O91 (strain B2F1) or O43 (strain 123) antigens by colony blot immunoassay using appropriate O-specific sera (9). Gall bladder (1 to 2 g) and bile (5 to 10 ml) samples were also enriched in Trypticase soy broth at 37°C before being cultured on appropriate media. For calculation of group means, samples from which the inoculum-type bacteria were not recovered by direct culture were assigned a value of 1,000 CFU of bacteria/g of sample (the detection limit of the direct plating procedure).
Statistical analyses.
The two-sided Fisher's exact test was used to test the significance of differences between the proportions of samples from DEX-treated and nontreated calves from which inoculum bacteria were recovered or in which A/E O157+ bacteria were detected. The Mann-Whitney test and a two-factor, unequal replication, repeated-measures analysis of variance were used to determine the significance of differences between the numbers of bacteria recovered from DEX-treated and nontreated calves.
RESULTS
Effects of DEX treatment on blood and tissue leukocytes.
All DEX-treated calves exhibited hematologic profiles and histologic lesions consistent with physiologic/corticosteroid stress (1, 23). Beginning the first day following the first DEX treatment and persisting until 3 to 4 days after inoculation, DEX-treated calves' leukograms were characterized by leukocytosis, neutrophilia without left shift, lymphopenia, eosinopenia, and occasionally monocytosis. There was moderate to marked lymphocyte depletion in the Peyer's patches of DEX-treated calves but not of nontreated calves.
Clinical observations and macroscopic lesions.
Four DEX-treated calves experienced watery diarrhea on days 3 and 4 (the feces of one calf contained blood on day 4), after they were inoculated with STEC O157. All other calves remained clinically healthy. The only significant gross lesion was edema of the gall bladder serosa (31), which was seen in 12 of 25 DEX-treated calves inoculated with STEC O157.
Histologic observations.
A/E O157+ bacteria (i.e., intimately attached bacteria associated with effacement of cytoplasm and identified as E. coli O157 by immunohistochemistry) were observed histologically in one or more tissues (rectoanal junction, ileocecal valve, distal colon, cecum, ileum, or gall bladder) from 38 of 55 calves inoculated with STEC O157 (Table 3 and Fig. 1 and 2). At the rectoanal junction, O157+ bacteria were found attached not only to enterocytes but also to squamous epithelial cells (Fig. 1). A/E O157+ bacteria were not found in the distal colon, cecum, ileum, or gall bladder or bile of any calf that was shedding fewer than 105 CFU of inoculum-type STEC O157 bacteria per g of feces at necropsy. However, A/E O157+ bacteria were found in the rectoanal junction or ileocecal valve of six nontreated and two DEX-treated STEC O157-inoculated calves that were shedding <105 CFU of STEC O157 bacteria/g of feces.
TABLE 3.
Recovery of STEC O157 bacteria and detection of A/E O157+ bacteria in samples obtained from weaned calves 4 days after inoculation with STEC O157:H7 strain 86-24
| Type of result | No. of samples positive/no. tested (% positive) fora:
|
P value for DEX vs nontreatedb | ||
|---|---|---|---|---|
| All calves | DEX-treated calves | Nontreated Calves | ||
| Recovery of bacteria from: | ||||
| Feces | 40/54 (74) | 24/25 (96) | 16/29 (55) | <0.01 |
| Distal colon | 24/51 (47) | 15/21 (71) | 9/30 (30) | <0.01 |
| Cecum | 19/51 (37) | 14/21 (67) | 5/30 (17) | <0.01 |
| Ileum | 7/48 (15) | 6/21 (29) | 1/27 (4) | 0.04 |
| Gall bladder | 4/26 (15) | 4/11 (36) | 0/15 (0) | 0.02 |
| Detection of A/E O157+ bacteria in: | ||||
| Any site | 38/55 (69) | 23/25 (92) | 15/30 (50) | <0.001 |
| Distal colon | 22/55 (40) | 18/25 (72) | 4/30 (13) | <0.01 |
| Cecum | 12/55 (22) | 11/25 (44) | 1/30 (3) | <0.01 |
| Ileum | 5/55 (9) | 5/25 (20) | 0/30 (0) | 0.02 |
| Gall bladder | 6/46 (13) | 6/25 (24) | 0/21 (0) | 0.02 |
| Ileocecal tissue | 28/45 (62) | 16/24 (67) | 12/21 (57) | 0.55 |
| Rectoanal junction | 15/18 (83) | 11/12 (92) | 4/6 (67) | 0.25 |
Number of samples from which STEC O157 bacteria were recovered or samples in which A/E O157+ bacteria were detected by histology and immunohistochemistry.
Two-sided P values by Fisher's exact test.
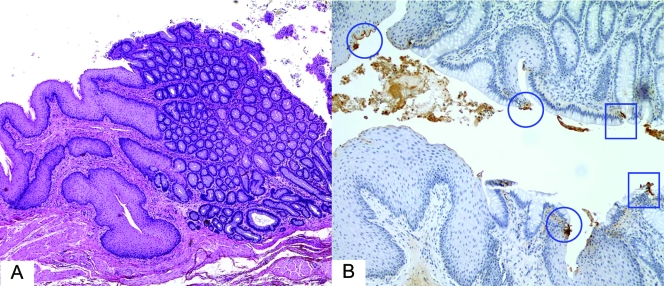
FIG. 1.
STEC O157:H7 binding to rectal squamous and glandular epithelial cells in 4-month-old calves necropsied 4 days after inoculation with STEC O157:H7 strain 86-24. (A and B) Overview of colorectal junction showing squamous epithelium (left) and columnar (glandular) epithelium (right) (A) and immunoperoxidase-stained O157+ bacteria attached to squamous epithelial cells (circles) and columnar epithelial cells (squares) (B).
FIG. 2.
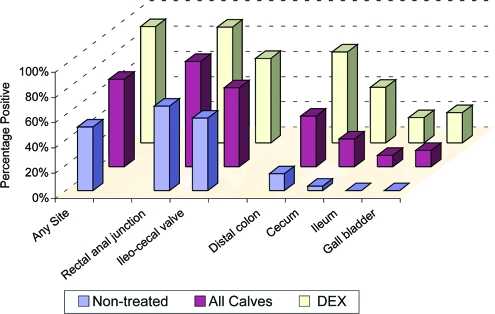
Distribution of O157 + A/E bacteria in tissues obtained from DEX-treated and nontreated weaned calves at 4 days after intrarumenal or oral inoculation with STEC O157:H7 strain 86-24. Values correspond to the proportions of calves positive at any site and proportions of specific tissues examined in which A/E O157+ bacteria were detected by histology and immunostaining with anti-O157:H7 antibodies. (See Table 2 for the numbers of calves and samples in each group.)
As shown in Table 3, A/E O157+ bacteria were found in a higher proportion of DEX-treated calves than of nontreated animals (92% versus 50% [P < 0.01]) inoculated with STEC O157. A/E O157+ bacteria were found in significantly higher proportions of distal colon, cecum, ileum, or gall bladder tissues from DEX-treated animals compared to nontreated animals, and A/E O157+ bacteria were not seen in ileum or gall bladder samples from any nontreated calf (Table 3 and Fig. 2). Rectoanal junction and ileocecal valve tissues were tissues in which A/E O157+ bacteria were most often found, and A/E O157+ bacteria were seen at these sites in similar proportions of DEX-treated and nontreated calves. The rectoanal junction was the only site in which A/E O157+ bacteria were found in 4 of the DEX-treated calves; the ileocecal valve was the only positive site in 11 of the nontreated calves.
A/E O157+ bacteria were also found in the distal colon or ileum (two of six calves) and in rectoanal junction or ileocecal valve tissues (six of six calves) of weaned calves 4 days after they were inoculated with E. coli Stx− O157. No attached STEC O91 bacteria were found in any of the eight calves inoculated with STEC O91, but A/E O157+ bacteria were seen in the rectoanal junctions of two of these calves which were shedding STEC O157 bacteria in their feces before they were inoculated with STEC O91 (see below). No A/E bacteria were seen in any of the nine calves inoculated with E. coli control strain 123.
Recovery of inoculum-type bacteria.
Bacterial culture results are summarized in Table 3 and Fig. 2 and 4. Inoculum-type bacteria were not recovered by direct culture from any of the preinoculation fecal samples. However, rare STEC O157 inoculum-type bacteria (i.e., streptomycin-resistant, sorbitol-negative, O157+ colonies) were recovered from the pre- and postinoculation fecal samples from two of the calves inoculated with the STEC O91 strain B2F1, indicating that they were shedding STEC O157 bacteria before they were inoculated with STEC O91. As noted above, A/E O157+ bacteria were seen in the rectal tissue samples obtained from these two calves at necropsy.
FIG. 4.
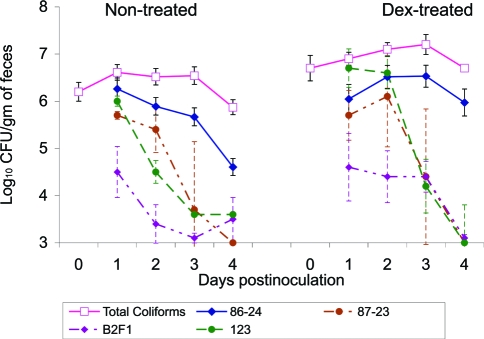
Mean numbers of inoculum-type bacteria and total coliforms recovered from the feces of DEX-treated or nontreated calves 1 to 4 days after inoculation with STEC O157:H7 strain 86-24 (solid lines) and mean numbers of inoculum bacteria from calves inoculated with E. coli Stx− O157:H7 strain 87-23, STEC O91:H21 strain B2F1, or E. coli control strain 123 (broken lines). The mean numbers of total coliforms for all DEX-treated and nontreated calves prior to inoculation are indicated by open squares. Samples from which inoculum bacteria were not recovered were assigned a value of 1,000 CFU/g of sample for the calculation of mean values. Error bars represent ±1 standard error of the mean.
STEC O157 bacteria were recovered by direct culture from the feces (74%), large or small intestinal tissues (47 and 15%, respectively), or gall bladder tissues (15%) collected from calves 4 days after they were inoculated with STEC O157. These bacteria were recovered from higher proportions of the tissues from DEX-treated calves than those from nontreated calves (Table 3). STEC O157 inoculum-type bacteria were recovered from 4 of 11 gall bladders and 3 of 15 bile samples from DEX-treated calves, but not from gall bladder or bile from any of the 15 nontreated calves sampled at these sites. As shown in Fig. 3, the mean numbers of STEC O157 inoculum-type bacteria recovered from feces (2 to 4 days p.i.) and intestines (4 days pi) were significantly higher for all DEX-treated calves (i.e., when negative samples were assigned a value of 1,000) compared to all similarly inoculated nontreated calves. However, the only statistically significant difference between the mean numbers of inoculum-type bacteria in the O157+ samples (i.e., when mean values excluded negative results) in DEX-treated and nontreated calves was the higher number of STEC O157 bacteria in feces from DEX-treated calves at 2 days after inoculation.
FIG. 3.
Mean numbers of inoculum bacteria recovered from feces (1 to 4 days p.i.) and tissues (4 days p.i.) from DEX-treated and nontreated weaned calves inoculated intrarumenally or orally with 1010 CFU of STEC O157:H7 strain 86-24. For the calculation of mean values for “all samples,” samples from which inoculum bacteria were not recovered by direct plating were assigned a value of 1,000 CFU/g of sample. For calculation of mean values for O157+ samples, negative results were excluded. Asterisks identify samples with significant differences (P < 0.05, Mann-Whitney test) between means for DEX-treated and nontreated calves.
The numbers of inoculum-type bacteria recovered from the feces of calves inoculated with Stx− O157, STEC O91, or control strain 123 were consistently lower than the numbers recovered from STEC O157-inoculated calves (Fig. 4). Because the group sizes for Stx− O157-, STEC O91-, and control strain 123-inoculated calves were small and treatment with DEX did not appear to have much of an effect on the fecal levels of these three inoculum types, the results for DEX-treated and nontreated calves in each inoculum group were combined for statistical comparisons with STEC O157. Significantly lower numbers of inoculum-type bacteria were recovered from feces of calves at 1 to 4 days after inoculation with STEC O91, at 3 and 4 days after inoculation with Stx− O157 and at 2 and 3 days after inoculation with control strain 123, compared to the numbers of inoculum-type bacteria recovered from the feces of calves inoculated with STEC O157. None of the fecal or tissue samples from any of the calves inoculated with strains other than STEC O157 contained >104 CFU of inoculum-type bacteria/g of sample at 4 days after inoculation.
The numbers of total fecal coliforms recovered from DEX-treated and nontreated calves are shown in Fig. 4. No significant differences were seen in the numbers of total fecal coliforms recovered from DEX-treated and nontreated calves on day 0 (when DEX-treated calves had been treated with DEX for 3 days and all calves had been off feed for 48 h) or at 1 to 3 days after inoculation. At 4 days after inoculation, the numbers of total coliforms were significantly higher for all DEX-treated calves and for DEX-treated calves inoculated with STEC O157 than those for the respective nontreated calves (P < 0.05). STEC O157 bacteria accounted for 30% of the total number of fecal coliforms recovered from DEX-treated calves but only 5% of those from nontreated calves at 4 days after inoculation.
DISCUSSION
Our findings provide important information about sites where STEC O157:H7 bacteria colonize early (defined by the presence of A/E bacteria or >104 CFU of inoculum-type bacteria/g of tissue at 4 days after inoculation) in experimentally inoculated weaned calves. We showed that STEC O157 bacteria settle and cause characteristic A/E lesions in multiple intestinal sites in weaned calves within 4 days after inoculation, as they do in experimentally inoculated neonatal calves and pigs (9) and other species (7, 20). We identified the rectum, ileocecal valve, and distal colon as sites in calves most likely to contain O157+ A/E bacteria at 4 days after inoculation with STEC O157 and the cecum, ileum, and gall bladder as other sites where these bacteria were found. The most sensitive sites for detecting STEC O157 colonization were the rectoanal junction and ileocecal valve: O157+ A/E bacteria were found at these sites in the greatest number of STEC O157-inoculated calves and even in some calves that had <105 CFU of inoculum/g of feces.
These results extend the evidence that the terminal rectum is an important STEC O157:H7 colonization site in cattle (3, 14, 21). We detected A/E O157+ bacteria in the rectoanal junction of 83% of the calves necropsied 4 days after they were inoculated with STEC O157. The rectoanal junction was the only site where we found A/E O157+ bacteria in the two calves naturally infected with O157+ bacteria before they were inoculated. Our results show that the rectum is not only important as a site where STEC O157 can persist in cattle but is also one of the sites where STEC O157 initially attach and establish infections in cattle. Interestingly, in contrast to what Naylor found in older cattle (21), we did not find STEC O157 bacteria primarily associated with rectal or colonic lymphoid tissues in these early infections in experimentally inoculated weaned calves (results not shown).
We interpreted the presence of A/E O157+ bacteria on the rectoanal mucosa at 4 days following oral inoculation as evidence that STEC O157 colonized the bovine rectoanal junction within the first few days after oral exposure. This argues against the conclusion that recovery of inoculum bacteria from bovine rectoanal mucosal swab samples in the first week after experimental inoculation does not reflect rectal colonization, but rather reflects wash through bacteria (28). Our results also identify the ileocecal valve (62% of samples had A/E O157+ bacteria) and distal colon (47% of samples were culture positive, and 40% had A/E O157+ bacteria) as early STEC colonization sites. Thus, the rectoanal junction, ileocecal valve, and distal colon are early and sensitive colonization sites to monitor when evaluating the efficacy of interventions aimed at preventing STEC O157 infections in cattle.
Treatment of calves with DEX enhanced their susceptibility to STEC O157 infection following experimental inoculation. DEX-treated calves were more likely than nontreated calves to be colonized at 4 days after they were inoculated with STEC O157. The observation that the intestinal and fecal levels of inoculum-type bacteria and the distribution of A/E O157+ bacteria were similar in DEX-treated and nontreated calves which were O157+ (Fig. 2 and 3) indicates that the mechanisms of STEC colonization in DEX-treated calves are similar to those in nontreated calves and validates the use of DEX-treated calves for STEC O157 studies. Treatment with DEX did not increase the susceptibility of experimentally inoculated weaned calves to colonization by Stx− O157 or STEC O91. Different bovine infection models will be needed to evaluate colonization sites and mechanisms of non-O157 STEC.
DEX treatment was not selected because it mimics a specific stress condition but because it has been widely used for enhancing infections with microorganisms and its immunosuppressive effects in cattle have been extensively investigated (1). The specific mechanisms involved in DEX-induced enhancement of susceptibility to STEC infections in cattle are outside the scope of this article, but are the focus of ongoing experiments (17).
The finding that intimin+ STEC O157 bacteria colonized weaned calves better than intimin+ Stx− O157 or intimin− STEC O91 bacteria extends the evidence that Stx and intimin facilitate STEC O157 colonization in cattle (6, 11) but does not rule out the possibility that other differences between these nonisogenic strains also influence colonization. Stx facilitates STEC O157:H7 colonization in orally or intrarumenally dosed ruminant calves (see reference 11 and this study), but it does not appear to influence intestinal colonization in intragastrically dosed neonatal calves (6) or colonization of the terminal rectal mucosa in steers inoculated intrarectally (29). In contrast, intimin is required for STEC O157:H7 colonization in both neonatal and ruminant calves (6, 9) and for rectal colonization after intrarectal inoculation of steers (29).
This study was not designed to evaluate effects of DEX on fecal coliforms in cattle. Total fecal coliform counts were included to allow comparisons of the levels of fecal coliforms in DEX-treated and nontreated calves before they were inoculated and comparisons of the numbers of inoculated strains and total coliforms after inoculations. All of the calves were off feed for 48 h before inoculation, and all were inoculated with 1010 CFU of one of the inoculum strains. Treatment of calves with DEX for 3 days prior to inoculation had no apparent effect on the numbers of total fecal coliforms at the time of inoculation. However, coincident with higher numbers of inoculum bacteria, DEX-treated calves had higher numbers of total fecal coliforms than did nontreated calves at 4 days after inoculation. The observation that the numbers of total coliforms in nontreated calves were lower at 4 days after inoculation than before inoculation extends the evidence that stress due to feed restriction increases the fecal coliforms in cattle (4, 26).
The results of these studies extend the evidence that the susceptibility of cattle to STEC infections is highly variable. Only 55% of nontreated calves shed inoculum bacteria in their feces or had A/E lesions at 4 days after inoculation with STEC O157. Even the high-dose DEX treatments we used in these studies, which increased the frequency and extent of STEC O157 colonization in experimentally inoculated weaned calves, did not result in all calves being equally susceptible to colonization after experimental inoculation with high levels of STEC O157. These studies involved calves of different breeds from different sources. Some of them were colostrum fed or colostrum deprived and raised indoors. (The colostrum-deprived calves were control calves available from earlier experiments; we saw no apparent effects of colostrum deprivation on the parameters we measured.) Some were raised with their dams on pasture, and some were infected with coccidian parasites. We carefully controlled the diets, housing, inocula and inoculation procedures, necropsies, and sample collections. However, the selection of the sites which were sampled was an experimental variable which changed over the course of the experiments included in this study. In previous STEC bovine infection studies at the NADC (5), the spiral colon was the most distal intestinal site sampled. For our initial studies, we extended sampling to include the distal colon. We began including gall bladder and ileocecal valve samples when we introduced DEX treatments. We added rectoanal junction samples after this site was identified as a prominent STEC colonization site in older cattle (21). The addition of ileocecal valves and rectoanal junctions, which were highly sensitive sites for detecting STEC O157, increased the numbers of animals that were positive for A/E O157+ bacteria. One can only speculate on whether inclusion of additional sampling sites, including other sites that contain squamous epithelial cells, will further increase the numbers of positive animals.
Acknowledgments
We thank Matt Inbody, Briony Lachinski, Bryan Wheeler, and the animal caretakers at the NADC for technical assistance.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Published ahead of print on 22 August 2008.
REFERENCES
- 1.Anderson, B. H., D. L. Watson, and I. G. Colditz. 1999. The effect of dexamethasone on some immunological parameters in cattle. Vet. Res. Commun. 23:399-413. [DOI] [PubMed] [Google Scholar]
- 2.CDC. 1995. Outbreak of acute gastroenteritis attributable to Escherichia coli serotype O104:H21 Helena, Montana 1994. MMWR 44:501-503. [PubMed] [Google Scholar]
- 3.Cobbold, R. N., D. D. Hancock, D. H. Rice, J. Berg, R. Stilborn, C. J. Hovde, and T. E. Besser. 2007. Rectoanal junction colonization of feedlot cattle by Escherichia coli O157:H7 and its association with supershedders and excretion dynamics. Appl. Environ. Microbiol. 73:1563-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cray, W. C., Jr., T. A. Casey, B. T. Bosworth, and M. A. Rasmussen. 1998. Effect of dietary stress on fecal shedding of Escherichia coli O157:H7 in calves. Appl. Environ. Microbiol. 64:1975-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean-Nystrom, E. A., B. T. Bosworth, A. D. O'Brien, and H. W. Moon. 1999. Bovine infection with Escherichia coli O157:H7, p. 51-58. In C. S. Stewart and H. J. Flint (ed.), E. coli O157 in farm animals. CAB International, Wallingford, United Kingdom.
- 7.Dean-Nystrom, E. A., B. T. Bosworth, W. C. Cray, Jr., and H. W. Moon. 1997. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect. Immun. 65:1842-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean-Nystrom, E. A., B. T. Bosworth, and H. W. Moon. 1999. Pathogenesis of Escherichia coli O157:H7 in weaned calves. Adv. Exp. Med. Biol. 473:173-177. [DOI] [PubMed] [Google Scholar]
- 9.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunning, R. F., A. D. Wales, G. R. Pearson, E. Done, A. L. Cookson, and M. J. Woodward. 2001. Attaching and effacing lesions in the intestines of two calves associated with natural infection with Escherichia coli O26:H11. Vet. Rec. 148:780-782. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman, M. A., C. Menge, T. A. Casey, W. Laegreid, B. T. Bosworth, and E. A. Dean-Nystrom. 2006. Bovine immune response to Shiga-toxigenic Escherichia coli O157:H7. Clin. Vaccine Immunol. 13:1322-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito, H., A. Terai, H. Kurazono, Y. Takeda, and M. Nishibuchi. 1990. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb. Pathog. 8:47-60. [DOI] [PubMed] [Google Scholar]
- 13.Lim, J. Y., J. Li, H. Sheng, T. E. Besser, K. Potter, and C. J. Hovde. 2007. Escherichia coli O157:H7 colonization at the rectoanal junction of long-duration culture-positive cattle. Appl. Environ. Microbiol. 73:1380-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low, J. C., I. J. McKendrick, C. McKechnie, D. Fenlon, S. W. Naylor, C. Currie, D. G. E. Smith, L. Allison, and D. L. Gally. 2005. Rectal carriage of enterohemorrhagic Escherichia coli O157 in slaughtered cattle. Appl. Environ. Microbiol. 71:93-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKee, M. L., A. R. Melton-Celsa, R. A. Moxley, D. H. Francis, and A. D. O'Brien. 1995. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect. Immun. 63:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melton-Celsa, A. R., S. C. Darnell, and A. D. O'Brien. 1996. Activation of Shiga-like toxins by mouse and human intestinal mucus correlates with virulence of enterohemorrhagic Escherichia coli O91:H21 isolates in orally infected, streptomycin-treated mice. Infect. Immun. 64:1569-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menge, C., and E. A. Dean-Nystrom. 2008. Dexamethasone depletes γδ T cells and alters the activation state and responsiveness of bovine peripheral blood lymphocyte subpopulations. J. Dairy Sci. 91:2284-2298. [DOI] [PubMed] [Google Scholar]
- 18.Moon, H. W., D. K. Sorensen, and J. H. Sautter. 1968. Experimental enteric colibacillosis in piglets. Can. J. Comp. Med. 32:493-497. [PMC free article] [PubMed] [Google Scholar]
- 19.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naylor, S. W., D. L. Gally, and J. C. Low. 2005. Enterohaemorrhagic E. coli in veterinary medicine. Int. J. Med. Microbiol. 295:419-441. [DOI] [PubMed] [Google Scholar]
- 21.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. E. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naylor, S. W., A. J. Roe, P. Nart, K. Spears, D. G. E. Smith, J. C. Low, and D. L. Gally. 2005. Escherichia coli O157:H7 forms attaching and effacing lesions at the terminal rectum of cattle and colonization requires the LEE4 operon. Microbiology 151:2773-2781. [DOI] [PubMed] [Google Scholar]
- 23.Norrman, J., C. W. David, S. N. Sauter, H. M. Hammon, and J. W. Blum. 2003. Effects of dexamethasone on lymphoid tissue in the gut and thymus of neonatal calves fed with colostrum or milk replacer. J. Anim. Sci. 81:2322-2332. [DOI] [PubMed] [Google Scholar]
- 24.Paton, J. C., and A. W. Paton. 1998. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Microbiol. Rev. 11:450-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pohlenz, J. F., and E. A. Dean-Nystrom. 2004. Colonization of Escherichia coli O157:H7 on squamous epithelial cells at the rectal anal junction. Vet. Rec. 155:248. (Letter.) [PubMed] [Google Scholar]
- 26.Rasmussen, M. A., W. C. Cray, Jr., T. A. Casey, and S. C. Whipp. 1993. Rumen contents as a reservoir of enterohemorrhagic Escherichia coli. FEMS Microbiol. Lett. 114:79-84. [DOI] [PubMed] [Google Scholar]
- 27.Rice, D. H., H. Q. Sheng, S. A. Wynia, and C. J. Hovde. 2003. Rectoanal mucosal swab culture is more sensitive than fecal culture and distinguishes Escherichia coli O157:H7-colonized cattle and those transiently shedding the same organism. J. Clin. Microbiol. 41:4924-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng, H., M. A. Davis, H. J. Knecht, and C. J. Hovde. 2004. Rectal administration of Escherichia coli O157:H7: novel model for colonization of ruminants. Appl. Environ. Microbiol. 70:4588-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheng, H., J. Y. Lim, H. J. Knecht, J. Li, and C. J. Hovde. 2006. Role of Escherichia coli O157:H7 virulence factors in colonization at the bovine terminal rectal mucosa. Infect. Immun. 74:4685-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoffregen, W. C., J. F. L. Pohlenz, and E. A. Dean-Nystrom. 2004. Escherichia coli O157:H7 in the gallbladders of experimentally infected calves. J. Vet. Diagn. Investig. 16:79-83. [DOI] [PubMed] [Google Scholar]
- 31.Wray, C., I. McLaren, and G. R. Pearson. 1989. Occurrence of ‘attaching and effacing’ lesions in the small intestine of calves experimentally infected with bovine isolates of verotoxigenic Escherichia coli. Vet. Rec. 125:365-368. [DOI] [PubMed] [Google Scholar]