Abstract
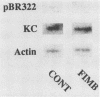
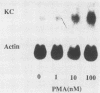
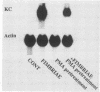
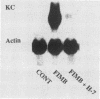
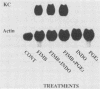
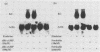
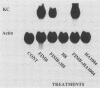
To account for infiltration of the periodontal tissues by neutrophils, the present study was undertaken to examine whether Porphyromonas gingivalis fimbriae, important structures involved in attachment of the bacteria to periodontal tissues, induce gene expression of the neutrophil chemoattractant KC in macrophages. The fimbriae induced expression of the KC gene of mouse peritoneal macrophages in a dose-dependent fashion. The peak of KC gene expression was observed as early as 1 h after initiation of the treatment. However, the gene expression was short lived, with the expression decreasing gradually after 6 h. A nuclear transcriptional assay showed that the fimbriae regulated the KC gene expression at a posttranscriptional level. We observed that the fimbria-induced KC gene expression was not regulated by endogenous or exogenous prostaglandin. Furthermore, forskolin, a potent activator of adenyl cyclase, and dibutyryl cyclic AMP were incapable of inducing KC gene expression of the peritoneal macrophages. H-8 and HA 1004, inhibitors of cyclic nucleotide-dependent protein kinases, had little effect on the fimbria-induced KC gene expression. On the other hand, the fimbria-induced KC gene expression was inhibited markedly by treatment with H-7, a potent inhibitor of protein kinase C. We also observed that phorbol 12-myristate 13-acetate, a specific activator of protein kinase C, induced KC gene expression of peritoneal macrophages in a dose-dependent fashion. In addition, the fimbria-induced KC gene expression was suppressed in the peritoneal macrophages pretreated for 24 h with phorbol 12-myristate 13-acetate. These results suggest that the KC gene expression was mediated through activation of protein kinase C and not through that of cyclic nucleotide-dependent protein kinases. The present study indicates that P. gingivalis fimbriae can induce gene expression of the neutrophil chemotactic factor KC by macrophages via protein kinase C and suggests that this factor may be involved in infiltration of neutrophils into the periodontal tissues of adult periodontal patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attström R. Presence of leukocytes in crevices of healthy and chronically inflamed gingivae. J Periodontal Res. 1970;5(1):42–47. doi: 10.1111/j.1600-0765.1970.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Biesiada E., Chorazy M. Expression of "cell-cycle-dependent" genes in regenerating rat liver. Cell Biol Int Rep. 1988 Jun;12(6):483–492. doi: 10.1016/0309-1651(88)90140-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Bray M. A., Ford-Hutchinson A. W., Smith M. J. Leukotriene B4: an inflammatory mediator in vivo. Prostaglandins. 1981 Aug;22(2):213–222. doi: 10.1016/0090-6980(81)90036-8. [DOI] [PubMed] [Google Scholar]
- Charon J. A., Metzger Z., Hoffeld J. T., Oliver C., Gallin J. I., Mergenhagen S. E. An in vitro study of neutrophils obtained from the normal gingival sulcus. J Periodontal Res. 1982 Nov;17(6):614–625. doi: 10.1111/j.1600-0765.1982.tb01183.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cicco N. A., Lindemann A., Content J., Vandenbussche P., Lübbert M., Gauss J., Mertelsmann R., Herrmann F. Inducible production of interleukin-6 by human polymorphonuclear neutrophils: role of granulocyte-macrophage colony-stimulating factor and tumor necrosis factor-alpha. Blood. 1990 May 15;75(10):2049–2052. [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Bascom C. C., Sipes N. J., Graves-Deal R., Weissman B. E., Moses H. L. Selective inhibition of growth-related gene expression in murine keratinocytes by transforming growth factor beta. Mol Cell Biol. 1988 Aug;8(8):3088–3093. doi: 10.1128/mcb.8.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubravec D. B., Spriggs D. R., Mannick J. A., Rodrick M. L. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6758–6761. doi: 10.1073/pnas.87.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco R. J., Slots J. Host responses in periodontal diseases. J Dent Res. 1984 Mar;63(3):441–451. doi: 10.1177/00220345840630031601. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D. J., Brownlee C., Stiles C. D. Interleukin-1 is a potent regulator of JE and KC gene expression in quiescent BALB/c fibroblasts. J Cell Physiol. 1989 Oct;141(1):154–159. doi: 10.1002/jcp.1041410123. [DOI] [PubMed] [Google Scholar]
- Hanazawa S., Hirose K., Ohmori Y., Amano S., Kitano S. Bacteroides gingivalis fimbriae stimulate production of thymocyte-activating factor by human gingival fibroblasts. Infect Immun. 1988 Jan;56(1):272–274. doi: 10.1128/iai.56.1.272-274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Murakami Y., Hirose K., Amano S., Ohmori Y., Higuchi H., Kitano S. Bacteroides (Porphyromonas) gingivalis fimbriae activate mouse peritoneal macrophages and induce gene expression and production of interleukin-1. Infect Immun. 1991 Jun;59(6):1972–1977. doi: 10.1128/iai.59.6.1972-1977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Nakada K., Ohmori Y., Miyoshi T., Amano S., Kitano S. Functional role of interleukin 1 in periodontal disease: induction of interleukin 1 production by Bacteroides gingivalis lipopolysaccharide in peritoneal macrophages from C3H/HeN and C3H/HeJ mice. Infect Immun. 1985 Oct;50(1):262–270. doi: 10.1128/iai.50.1.262-270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introna M., Bast R. C., Jr, Tannenbaum C. S., Hamilton T. A., Adams D. O. The effect of LPS on expression of the early "competence" genes JE and KC in murine peritoneal macrophages. J Immunol. 1987 Jun 1;138(11):3891–3896. [PubMed] [Google Scholar]
- Koerner T. J., Hamilton T. A., Introna M., Tannenbaum C. S., Bast R. C., Jr, Adams D. O. The early competence genes JE and KC are differentially regulated in murine peritoneal macrophages in response to lipopolysaccharide. Biochem Biophys Res Commun. 1987 Dec 31;149(3):969–974. doi: 10.1016/0006-291x(87)90503-1. [DOI] [PubMed] [Google Scholar]
- Ohmori Y., Hanazawa S., Amano S., Miyoshi T., Hirose K., Kitano S. Spontaneous production of thymocyte-activating factor by human gingival fibroblasts and its autoregulatory effect on their proliferation. Infect Immun. 1987 Apr;55(4):947–954. doi: 10.1128/iai.55.4.947-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori Y., Reynolds E., Hamilton T. A. Modulation of Na+/K+ exchange potentiates lipopolysaccharide-induced gene expression in murine peritoneal macrophages. J Cell Physiol. 1991 Jul;148(1):96–105. doi: 10.1002/jcp.1041480112. [DOI] [PubMed] [Google Scholar]
- Oquendo P., Alberta J., Wen D. Z., Graycar J. L., Derynck R., Stiles C. D. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet alpha-granule proteins. J Biol Chem. 1989 Mar 5;264(7):4133–4137. [PubMed] [Google Scholar]
- Page R. C., Schroeder H. E. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976 Mar;34(3):235–249. [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Stiles C. D. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X. Y., Hamilton T. A., DiCorleto P. E. Lipopolysaccharide-induced expression of the competence gene KC in vascular endothelial cells is mediated through protein kinase C. J Cell Physiol. 1989 Jul;140(1):44–51. doi: 10.1002/jcp.1041400106. [DOI] [PubMed] [Google Scholar]
- Sinden P. R., Walker D. M. Inflammatory cells extracted from chronically inflamed gingiva. J Periodontal Res. 1979 Nov;14(6):467–474. doi: 10.1111/j.1600-0765.1979.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Tiku K., Tiku M. L., Skosey J. L. Interleukin 1 production by human polymorphonuclear neutrophils. J Immunol. 1986 May 15;136(10):3677–3685. [PubMed] [Google Scholar]
- Watanabe K., Konishi K., Fujioka M., Kinoshita S., Nakagawa H. The neutrophil chemoattractant produced by the rat kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem. 1989 Nov 25;264(33):19559–19563. [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]