Abstract
For over 3 decades, sexual development in the human fungal pathogen Cryptococcus neoformans and other fungi has been initiated by growing compatible mating partners on V8 juice medium. Although this medium is an efficient inducer of sexual development, the mechanism by which it promotes the process is unknown. To understand how V8 juice medium induces sexual development, we attempted to purify inducing factors from V8 juice, and we carried out a complete compositional analysis of V8 juice. We discovered that no single factor is responsible for the effects of V8 juice medium. Rather, the unique composition of V8 juice medium provides the proper nutrient composition for inducing and sustaining complete sexual development. Utilizing these findings, we developed a defined V8 (DV8) medium that mimics V8 juice medium in sexual development assays. Then, using DV8 as a tool, we explored the roles that specific molecules play in enhancing sexual development. Surprisingly, we discovered that copper is a key factor, leading to an upregulation of the mating pheromone genes MFa and MFα, both required for the initial steps in sexual development. The utilization of DV8 to investigate the effects of copper on sexual development presented here is an example of how defining the conditions that induce sexual development will advance the study of C. neoformans.
Sexual development in the human fungal pathogen Cryptococcus neoformans is a multistep process that involves recognition of an appropriate mating type partner, cell fusion, initiation of a dikaryotic state, meiosis, and the production of sexual spores (5, 16). Interestingly, the presence of the appropriate mating type partners is essential but not sufficient to initiate sexual development. Appropriate nutritional and environmental conditions must also be present for sexual development to occur (1). However, the mechanisms by which sexual development is initiated are largely unknown.
C. neoformans is unique among human fungal pathogens because it has a well-defined sexual cycle that is readily amenable to genetic manipulation (16). In addition, C. neoformans spores are hypothesized to be infectious (8, 34), which would be consistent with what is known about the infectious forms of other pathogenic fungal species, including Aspergillus fumigatus, Blastomyces dermatitidis, and Coccidioides immitis (13). Indirect evidence suggests that C. neoformans may produce spores in the environment. Environmental sampling following the Cryptococcus outbreak on Vancouver Island, British Columbia, Canada, revealed the presence of Cryptococcus cells that were of a size that was consistent with a spore form (17).
Numerous studies have described the morphological transitions that occur in C. neoformans, starting with mating partner recognition and continuing through meiosis and spore production (1, 10, 15, 18, 19, 28). These studies were largely made possible by the discovery over 30 years ago that solid medium containing 5% V8 juice (Campbell Soup Co.) induces sexual development of C. neoformans (10). Although V8 juice medium is an invaluable tool, the mechanism by which it induces sexual development is unknown. We therefore sought to identify components of V8 juice medium that induce sexual development. Several hypotheses regarding how V8 juice medium induces this process in C. neoformans have been proposed. One prominent hypothesis is that V8 juice medium contains an “inducing factor” from plants that triggers pathways involved in sexual development. Because nitrogen limitation is also known to induce sexual development, a second hypothesis is that V8 juice medium contains low levels of available nitrogen, promoting the induction of sexual development.
In the present study, we used fractionation techniques and inductively coupled plasma/optical emission spectrometry (ICP/OES) to create a defined V8 (DV8) medium based on the chemical composition of V8 juice. This DV8 medium induces sexual development in a manner that is indistinguishable from that of V8 juice medium. DV8 medium was then used to identify components of V8 juice that contributed to the induction of sexual development. We found that sexual development is not initiated by an “inducing factor,” but rather, multiple factors cooperatively create the nutritional conditions required for the induction of sexual development. Interestingly, copper appears to play an important role in this process. The creation of a defined medium with the ability to induce sexual development provides a valuable tool that will shed light on the mechanisms by which environmental conditions may regulate sexual development in C. neoformans and perhaps other fungi.
MATERIALS AND METHODS
Strains and sexual development assays.
All strains used were of the serotype D background. All were handled using standard techniques and media as described previously (29). Crosses were conducted on solid media at room temperature in the dark for 2 to 4 days. Sexual development was evaluated by observing the periphery of test spots on each medium. The mating tester strains used were JEC20 (a) and JEC21 (α) (20). For confrontation assays, strains were streaked after 2 days on yeast extract-peptone-dextrose agar near one another (0.5 to 1 mm apart) on filament agar plates and incubated at room temperature in the dark for 7 days before they were photographed. Fusion assays were carried out by resuspending cells at 10 optical density units at a wavelength of 600 nm/ml and mixing equal densities of two mating partners, wild-type a (JEC20) and α ura5 NEOR (CHY1093). Ten microliters of each mix was spotted onto a core medium plate (4 mM potassium phosphate buffer at pH 6.5, 0.7% dextrose, 0.7% fructose, and 4% agar) containing 0 or 100 μM copper sulfate, and the plates were incubated at room temperature in the dark. After 24 h, the cells were scraped off the plates, resuspended in phosphate-buffered saline, spread on selective plates consisting of synthetic defined medium lacking uracil but containing 200 μg/ml neomycin. The plates were incubated at 30°C for 5 days, and the resulting colonies were counted.
Vegetable juice fractionation and compositional analysis.
For reverse-phase fractionations, 2.5 ml of commercially available vegetable juice (V8; Campbell Soup Co.) was clarified by centrifugation, filtered through a 0.45-μm nylon filter, and adsorbed to a C18 solid-phase extraction cartridge (Alltech). A 10-ml distilled and deionized water wash was eluted and pooled with the flowthrough. Subsequently, 10-ml methanol (high-performance liquid chromatography [HPLC]-grade) and 10-ml ethyl acetate (HPLC-grade) fractions were collected. Each fraction was concentrated via rotary evaporation with vacuum and reconstituted in 200 μl 1% N,N-dimethyl sulfoxide. Solid medium containing 4 mM potassium phosphate buffer (pH 7.0), 0.5% d-glucose, and 4% agar was supplemented with 5% of each fraction collected. Sexual development assays were carried out with each fraction. For total-mineral and heavy metal analyses, filtered V8 juice was subjected to ICP/OES, nitrogen, and carbon analyses at the Soil and Plant Analysis Laboratory, University of Wisconsin—Madison, Verona, WI. The concentrations of glucose and fructose in V8 juice were determined by quantitative HPLC using a refractive index indicator, an Aminex HPX-87H column (Bio-Rad) at 55°C, and 0.005 M H2SO4 as an eluent, with a flow rate of 0.3 ml/min and an injection volume of 20 μl.
Microscopy.
Light microscopy was carried out using a Zeiss Axioplan microscope fitted with a 20× long-working-distance objective and a Nikon Coolpix 5400 camera.
RNA preparation and Northern blotting.
RNA was prepared from C. neoformans cells by using a hot-phenol method (2). Strains were incubated on solid medium for 24 h at room temperature before they were harvested by scraping off the agar surface. Northern blots were carried out according to standard protocols, with 10 μg of total RNA used for each sample. The GPD1 probe was generated by PCR using CHO651 (CGTCGTTGAATCTACCGGTG) and CHO652 (CACCAGCAATGTAAGAGATG). Radiolabeled probes (Decaprime II kit from Ambion) were used in hybridization reactions at 65°C as described previously (14). A CTR4 probe was made by amplifying a 500-bp fragment from genomic DNA, using CHO1920 (CGGTATCTTTTCCTCTGTG) and CHO1921 (GAGCAGCATAATCAAATCCT). Blots were hybridized at 65°C overnight and washed according to standard protocols (2).
RESULTS
Compositional analysis of V8 juice.
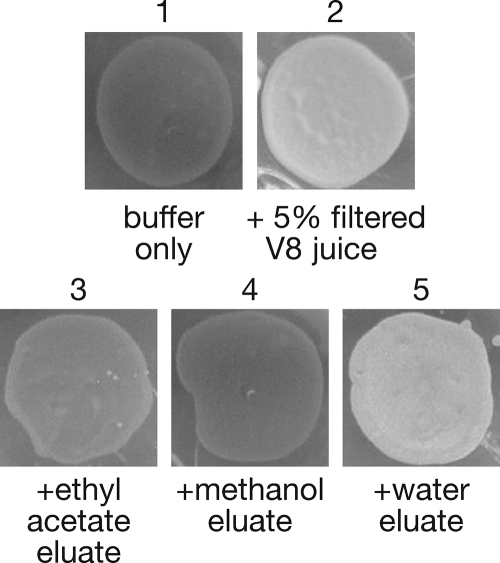
V8 juice medium has traditionally been used to induce sexual development in C. neoformans and other fungi (Fig. 1) (10, 26). However, the mechanism that accounts for this unique property of V8 juice medium is unknown. To understand how V8 juice medium induces sexual development, we first carried out fractionation studies to characterize the general properties of fractions that retained the ability to induce sexual development. C18 reverse-phase solid phase extraction was performed to collect three fractions of V8 juice: a highly polar water fraction, a minimally hydrophobic methanol fraction, and a highly hydrophobic ethyl acetate fraction. We found that the water fraction, but not the methanol or ethyl acetate fraction, induced levels of sexual development comparable to those for V8 juice medium, as evidenced by the formation of white cross spots on plates when viewed macroscopically (Fig. 2). The formation of sexual development filaments results in the white appearance of crosses, rather than black or gray, which correlates with little or no filamentation. This result suggested that the soluble portion of V8 juice was sufficient to induce sexual development and that induction was likely not due to the activity of hydrophobic molecules (e.g., phenolic compounds or lipids).
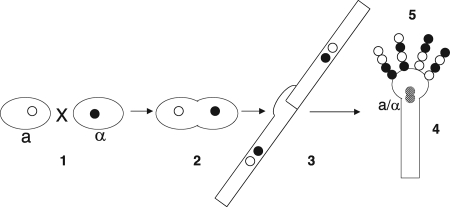
FIG. 1.
Sexual development of C. neoformans. Large ovals represent yeast cells. Black and white circles represent a and α nuclei. (Section 1) Under appropriate environmental conditions, yeast cells of opposite mating types sense one another via pheromones and pheromone receptors. (Section 2) Yeast cells fuse, but their nuclei do not. (Section 3) After fusion, a new developmental program occurs, leading to dikaryotic, filamentous growth. (Section 4) In response to unknown signals, filamentous growth arrests, a basidium is formed, and nuclear fusion takes place. (Section 5) Meiosis and sporulation result in four chains of spores that reside on the surface of the basidium.
FIG. 2.
Sexual development activity fractionates with the soluble portion of V8 juice. Crosses between wild-type a and α strains were placed on agar media containing different HPLC fractions of V8 juice. The macroscopic view reveals white spots, which indicate robust sexual development due to filament formation, whereas dark spots indicate little or no sexual development.
Given that the soluble portion of V8 juice induced sexual development, ICP/OES was performed to determine its composition. The analysis showed that the concentrations of numerous components of soluble V8 juice were generally lower than the concentrations reported by the manufacturer for complete V8 juice (Table 1). For example, the concentrations of iron, calcium, and sodium in soluble V8 juice were 1.3 mg/liter, 80 mg/liter, and 1460 mg/liter, respectively, compared to 3 mg/liter, 250 mg/liter, and 2,458 mg/liter in complete V8 juice (as reported by Campbell Soup Co.). This was not unexpected, given that our analysis was limited to the soluble portion of V8 juice, which did not contain the particulate material present in complete V8 juice.
TABLE 1.
Compositional analysis of filtered V8 juice
| Component | Concn in 100% V8 juice (soluble portion) (mg/liter) | Calculated concn in 5% V8 juice medium (soluble portion)
|
|
|---|---|---|---|
| μMa | % (wt/vol) | ||
| Total minerals | |||
| P | 138 | 223 | |
| K | 1,524 | 1,949 | |
| Ca | 80 | 99 | |
| Mg | 75 | 154 | |
| S | 95 | 148 | |
| B | 0.6 | 2.8 | |
| Al | 0.4 | 0.7 | |
| Na | 1,460 | 3,175 | |
| Heavy metals | |||
| Cd | <0.1 | <0.044 | |
| Co | <0.1 | <0.085 | |
| Cr | <0.1 | <0.096 | |
| Cu | 0.3 | 0.236 | |
| Fe | 1.3 | 1.164 | |
| Mn | 0.4 | 0.364 | |
| Mo | <0.1 | <0.052 | |
| Ni | <0.1 | <0.085 | |
| Pb | 0.2 | 0.048 | |
| Zn | 1.2 | 0.917 | |
| Li | <0.1 | <0.720 | |
| Nitrogen | |||
| Total | 740 | 2,643 | |
| NH4 (inorganic) | 183 | 508 | |
| NO3 (inorganic) | 14 | 11 | |
| Organicb | 543 | 2,124 | |
| Carbon | |||
| Total | 28,380 | 1.4 | |
| d-Glucose | 13,919 | 0.7 | |
| d-Fructose | 14,461 | 0.7 | |
Converted to molar concentrations for ease of comparison with other media.
Equals the difference between the total and the inorganic portions of nitrogen.
The observation that the soluble portion of V8 juice contains 197 mg/liter inorganic nitrogen suggested that sexual development on V8 juice medium (containing ∼0.5 mM inorganic nitrogen) was not likely to be due to severe nitrogen limitation (Table 1). Concentrations of inorganic nitrogen in low-nutrient media of 0.5 mM or higher inhibit Cryptococcus sexual development (P. Ortiz-Bermudez and C. M. Hull, unpublished data). This suggests that with 0.5 mM inorganic nitrogen, V8 juice medium is not nitrogen limiting, and other components contribute to the sexual development-inducing effects of V8 juice medium. In addition, Cryptococcus can utilize some organic nitrogen sources, such as proline and asparagine, likely making the effective concentration of utilizable nitrogen in V8 juice medium even higher than 0.5 mM, further reducing the likelihood that nitrogen starvation is a primary inducing signal during sexual development on V8 juice medium (21).
DV8 medium induces sexual development.
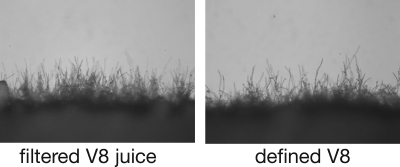
The compositional analysis of soluble V8 juice allowed us to then create a synthetic medium composed entirely of defined components (DV8 medium). The DV8 medium was composed of a carbon source, a nitrogen source, metals, and salts (Table 2). Because our initial fractionation analysis suggested that components in the methanol and ethyl acetate fractions did not induce sexual development, vitamin A, which is present in these fractions, was not included in DV8 medium. To test the ability of DV8 medium to induce sexual development, we carried out sexual development assays in parallel with V8 juice medium. We found that the sexual development process on DV8 is indistinguishable from that on V8 juice medium (Fig. 3). The levels of filamentation for the two media appeared comparable, the filaments were dikaryotic with fused clamp cells, and comparable numbers of basidia and viable spores were produced (data not shown), suggesting that DV8 medium provides all of the components necessary for robust sexual development.
TABLE 2.
Composition of DV8 medium in KH2PO4 buffer, pH 6.5
| Component | Concn in DV8 medium
|
|
|---|---|---|
| μM | % (wt/vol) | |
| Salts and minerals | ||
| KH2PO4 | 3,920 | |
| NaCl | 4,020 | |
| KCl | 1,800 | |
| CaCl2 | 100 | |
| MgCl2 | 134 | |
| H3BO3 | 5 | |
| l-Ascorbic acid | 850 | |
| Metals | ||
| AlKSO4 | 1.900 | |
| CuSO4 | 0.250 | |
| FeSO4 | 0.899 | |
| MnSO4 | 0.355 | |
| ZnSO4 | 0.765 | |
| Nitrogen | ||
| (NH4)2SO4 | 302 | |
| Asparagine | 910 | |
| Carbon | ||
| Glucose | 0.7 | |
| Fructose | 0.7 | |
| Agar | 4 | |
FIG. 3.
DV8 medium mimics V8 juice medium. Crosses between wild-type strains were carried out with agar medium containing 5% filtered V8 juice or defined components determined from a compositional analysis. Panels show the periphery of a cross under ×200 magnification.
Nitrogen, salts, and metals are required for complete sexual development.
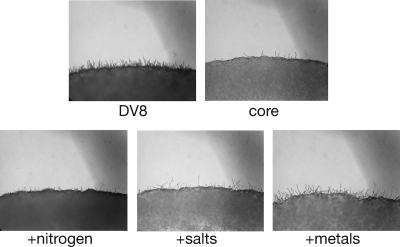
The compositional analysis of V8 juice provided us with valuable information with which to characterize the abilities of specific medium components to induce sexual development. To identify specific factors that facilitate sexual development, we first created a basic “core medium” (4 mM potassium phosphate buffer at pH 6.5, 0.7% dextrose, 0.7% fructose, and 4% agar) that supported cell growth but did not induce sexual development. Core medium was then supplemented with multiple sources of nitrogen, salts, metals, and various combinations of these components at levels found in DV8 medium. We observed that the addition of nitrogen, salts, or metals individually to core medium did not induce wild-type levels of sexual development, i.e., those for DV8 medium, which shows both a dark cell mass, indicating dense filamentation, and filaments at the cross periphery (Fig. 4). The lighter cell mass and limited filamentation observed for core medium represent very weak, if any, sexual development. However, the addition of nitrogen sources (0.3 mM ammonium sulfate and 0.9 mM asparagine) did result in the formation of filaments throughout the colony, illustrated by the dark appearance of the periphery of the cross, as is seen with DV8 medium but not core medium. The addition of 0.9 mM asparagine alone did not induce significant filamentation but did appear to slightly enhance the filamentation induced by the addition of 0.3 mM ammonium sulfate (data not shown). These findings suggest that the inducing effect of nitrogen under these conditions is due primarily to inorganic nitrogen. In contrast, this effect was not observed at the peripheries of crosses containing only salts (4 mM sodium chloride and 1.8 mM potassium chloride) or a cocktail of metals (1.9 μM aluminum-, 0.25 μM copper-, 0.9 μM iron-, 0.36 μM manganese-, 0.77 μM zinc sulfate) where no filaments were visible in the cell mass, and the colony appears relatively light in shade (Fig. 4). Although the addition of metal alone did result in somewhat more filamentation at the cross periphery, no filaments were formed in the cell mass, indicating little sexual development. In fact, robust sexual development (marked filamentation in both the cell mass and the periphery) could be recapitulated only by the addition of nitrogen, salts, and metals together.
FIG. 4.
Sexual development requires nitrogen, salts, and metals. Crosses between wild-type strains were carried out with agar medium containing the full complement of defined components for DV8 medium (DV8), no defined components (core), or defined components tested individually (nitrogen, salts, or metals). The panels show the periphery of a cross under ×100 magnification.
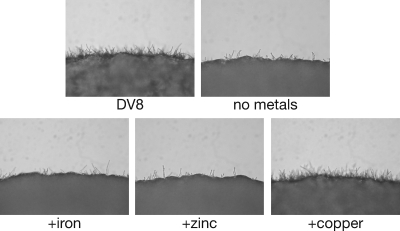
To determine what individual metals might be required for the induction of sexual development, DV8 medium lacking metals was supplemented individually with iron, zinc, or copper sulfate (0.9 μM, 0.77 μM, or 0.25 μM, respectively). The rationale for choosing these metals was that they are known to be important cofactors for many eukaryotic developmental and metabolic processes (6). We found that neither iron, zinc, nor a cocktail containing many metals, including aluminum and magnesium, induced sexual development at concentrations consistent with those found in DV8 medium (Fig. 5 and data not shown). Surprisingly, we found that copper alone restored sexual development in a manner that was indistinguishable from that observed for DV8 medium (Fig. 5) across a large range of concentrations, from 0.25 to 300 μM (data not shown). This result led us to further explore the role of copper in sexual development.
FIG. 5.
Copper induces sexual development in DV8 medium. Crosses between wild-type strains were carried out with agar medium containing the full complement of defined components for DV8 medium, DV8 medium without any metals, or DV8 medium containing only a single metal (iron, zinc, or copper). The panels show the periphery of a cross under ×200 magnification.
Copper induces filament formation and pheromone gene expression.
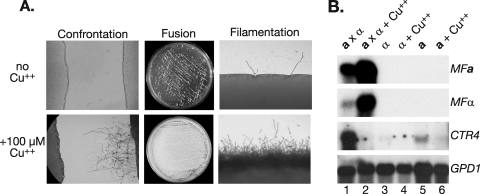
Previous studies have shown that copper is an important cofactor that is required for the activities of numerous C. neoformans enzymes, including laccase and the copper, zinc superoxide dismutase (11, 35). To identify the step in sexual development that is influenced by copper, we carried out confrontation and fusion assays in the presence or absence of copper. The addition of copper enhanced the responses of a and α cells to one another in confrontation assays (Fig. 6A). In fusion assays, the addition of copper resulted in a dramatic increase in the number of fusants (Fig. 6A). An additional quantitative analysis revealed that copper increased the number of fusants by approximately 75% (from 136 to 455), compared to the level for core medium without metals. The numbers of fusants were as follows: for V8 juice medium, 263; for core medium without metals, 136; and for core medium without metals plus 100 μM Cu2+, 455. These results indicate that copper enhances the responses of mating type partners to each other and may additionally enhance cell fusion, resulting in increased filament formation.
FIG. 6.
Copper enhances mate recognition, cell fusion, and filamentation and upregulates pheromone gene expression. (A) Left panels show wild-type a cells (left) and wild-type α cells (right) in confrontation assays in the presence and absence of copper. Magnification, ×200. The middle panels show colonies on petri plates resulting from cell fusion in the presence and absence of copper. The right panels show the peripheries of crosses between wild-type cells in the presence and absence of copper. Magnification, ×200. (B) Northern blot on RNA from strains under a variety of conditions after 24 h of incubation: Lane 1, wild-type cross on DV8 medium without metals; lane 2, wild-type cross on DV8 with 100 μM copper; lane 3, haploid α on DV8 medium; lane 4, haploid α on DV8 with 100 μM copper; lane 5, haploid a on DV8 medium; lane 6, haploid a on DV8 medium with 100 μM copper. The probes are listed to the right of each panel.
Previous studies have shown that mating pheromones (MFa and MFα) are important during the early stages of the C. neoformans sexual development process (22, 28). Therefore, we carried out Northern blot analysis to determine if the presence of copper differentially regulated the mating pheromone genes (MFa and MFα). We observed that this was indeed the case; the addition of copper dramatically increased the transcript levels of MFa and MFα in sexual crosses but not haploid cells (Fig. 6B). In addition, we assessed the effect of copper on the levels of CTR4, a known copper-responsive gene repressed in the presence of copper (25, 33). As expected, the addition of copper abolished detectable CTR4 transcript levels (Fig. 6B). There were no differences between the levels of the control gene GPD1 for any of the samples analyzed. These results demonstrate that copper mediates a direct effect on pheromone levels, which likely accounts for its ability to induce robust sexual development.
DISCUSSION
For more than 3 decades, V8 juice medium has been used to study sexual development in C. neoformans and other fungi (10, 26). Numerous hypotheses have been postulated to explain the mechanism by which V8 juice medium accomplishes this task, but none has been rigorously tested. One prominent hypothesis has been that V8 juice medium contains an “inducing factor” from plants that is responsible for inducing sexual development. In the present study, we describe the creation of a DV8 medium based on the composition of V8 juice. Using this medium, we have demonstrated for the first time that inducing conditions in V8 juice are dependent on the stoichiometry of macro- and micronutrients, not the activity of an “inducing factor” or severe nitrogen limitation.
The availability of a defined medium with properties indistinguishable from those of V8 juice medium provides an excellent tool with which to elucidate the molecular mechanisms involved in the initiation of sexual development. A significant limitation of V8 juice medium has been the heterogeneous nature of the medium and batch-to-batch variation in its ability to induce sexual development. DV8 medium overcomes these limitations and allows for the selective analysis of specific components and combinations of components for sexual development. We anticipate that DV8 medium may also be useful for the study of development in other fungi. Numerous fungal species undergo sporulation on V8 juice medium, including the plant pathogens Colletotrichum gloeosporioides and Cochliobolus carbonum and some Candida species (4, 30, 32).
Sexual development in fungi in several systems has been studied in detail (12). For many fungi, the processes of sexual development and spore formation are initiated in response to reduced nitrogen availability. For example, Neurospora crassa, Schizosaccharomyces pombe, and Ustilago maydis all initiate sexual development in response to nitrogen limitation (3, 23, 31). A notable exception to this trend is the model yeast species Saccharomyces cerevisiae, which undergoes sexual development under nitrogen-rich conditions (29). Given that the majority of fungi studied undergo sexual development in response to nitrogen starvation, we were surprised to discover that V8 juice medium was not particularly nitrogen limiting. In fact, the inorganic nitrogen concentration in V8 juice medium was approximately 0.8 mM, which was sufficient to sustain vegetative growth and sexual development. The observation that C. neoformans does not require nitrogen starvation to induce sexual development is consistent with previous studies reporting that C. neoformans undergoes sexual development on pigeon guano agar, a nitrogen-sufficient medium (24). Our results further confirm that abundant nitrogen would not prevent C. neoformans from undergoing sexual development in presumably nitrogen-rich habitats from which it has been isolated (e.g., pigeon guano and Eucalyptus plants) (7, 9).
The discovery that copper was important for the induction of sexual development was unexpected. Copper has not previously been implicated as a nutritional factor important for the regulation of C. neoformans sexual development. Copper is, however, an important cofactor for many metabolic processes in C. neoformans, including the production of melanin (35). Equally surprising was the wide range of concentrations at which copper mediated this effect (0.25 μM to 300 μM). For most eukaryotes, copper homeostasis is tightly regulated and very little free copper is available. In fact, an abundance of copper is toxic for most organisms (27). These results suggest the interesting possibility that the presence of copper in the ecological niche of C. neoformans could potentially promote sexual development.
Equally interesting was the observation that copper dramatically enhanced the transcription of the C. neoformans pheromone genes. This suggests a mechanism in which copper enhances sexual development through the regulation of pheromone genes (MFa and MFα), potentially increasing pheromone production and subsequent responses between mating partners. Studies are currently under way to determine the molecular mechanism(s) by which copper mediates this response.
Acknowledgments
We thank R. Borchardt and K. Klein for laboratory support, J. Ekena for technical assistance, and M. Botts, E. Kruzel, B. Stanton, and M. Staudt for helpful discussions and critical comments on the manuscript. We also thank the University of Wisconsin—Madison Soil and Plant Analysis Laboratory for assistance with compositional analyses and R. Rodriguez and T. Jeffries for their assistance with carbon sugar analyses.
This work was supported by R01 no. AI064287, awarded to C.M.H. by the National Institutes of Health, and a Career Award in the Biomedical Sciences, awarded to C.M.H. by the Burroughs Wellcome Fund.
Footnotes
Published ahead of print on 5 September 2008.
REFERENCES
- 1.Alspaugh, J. A., R. C. Davidson, and J. Heitman. 2000. Morphogenesis of Cryptococcus neoformans, p. 217-238. In J. F. Ernst and A. Schmidt (ed.), Dimorphism in human pathogenic and apathogenic yeasts, vol. 5. Contributions to Microbiology. Karger, Basel, Switzerland. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F., R. Brent, R. Kingston, D. Moore, J. Seidman, J. Smith, and K. Struhl (ed.). 1997. Current protocols in molecular biology, vol. 2. John Wiley and Sons, Inc., Boston, MA.
- 3.Banuett, F., and I. Herskowitz. 1994. Morphological transitions in the life cycle of Ustilago maydis and their genetic control by the a and b loci. Exp. Mycol. 18:247-266. [Google Scholar]
- 4.Brysch-Herzberg, M. 2004. Metschnikowia kunwiensis comb. nov., the teleomorph of Candida kunwiensis. FEMS Yeast Res. 4:605-607. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, DC.
- 6.De Freitas, J., H. Wintz, J. H. Kim, H. Poynton, T. Fox, and C. Vulpe. 2003. Yeast, a model organism for iron and copper metabolism studies. Biometals 16:185-197. [DOI] [PubMed] [Google Scholar]
- 7.Denton, J. F., and A. F. Di Salvo. 1968. The prevalence of Cryptococcus neoformans in various natural habitats. Sabouraudia 6:213-217. [PubMed] [Google Scholar]
- 8.Ellis, D. H., and T. J. Pfeiffer. 1990. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. Lancet 336:923-925. [DOI] [PubMed] [Google Scholar]
- 9.Ellis, D. H., and T. J. Pfeiffer. 1990. Natural habitat of Cryptococcus neoformans var. gattii. J. Clin. Microbiol. 28:1642-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erke, K. H. 1976. Light microscopy of basidia, basidiospores, and nuclei in spores and hyphae of Filobasidiella neoformans (Cryptococcus neoformans). J. Bacteriol. 128:445-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton, A. J., and M. D. Holdom. 1997. Biochemical comparison of the Cu,Zn superoxide dismutases of Cryptococcus neoformans var. neoformans and Cryptococcus neoformans var. gattii. Infect. Immun. 65:488-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heitman, J. 2007. Sex in fungi: molecular determination and evolutionary implications. ASM Press, Washington, DC.
- 13.Heitman, J., S. G. Filler, J. E. Edwards, and A. P. Mitchell (ed.). 2006. Molecular principles of fungal pathogenesis. ASM Press, Washington, DC.
- 14.Hull, C. M., M.-J. Boily, and J. Heitman. 2005. Sex-specific homeodomain proteins Sxi1α and Sxi2a coordinately regulate sexual development in Cryptococcus neoformans. Eukaryot. Cell 4:526-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hull, C. M., R. C. Davidson, and J. Heitman. 2002. Cell identity and sexual development in Cryptococcus neoformans are controlled by the mating-type-specific homeodomain protein Sxi1α. Genes Dev. 16:3046-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hull, C. M., and J. Heitman. 2002. Genetics of Cryptococcus neoformans. Annu. Rev. Genet. 36:557-615. [DOI] [PubMed] [Google Scholar]
- 17.Kidd, S. E., Y. Chow, S. Mak, P. J. Bach, H. Chen, A. O. Hingston, J. W. Kronstad, and K. H. Bartlett. 2007. Characterization of environmental sources of the human and animal pathogen Cryptococcus gattii in British Columbia, Canada, and the Pacific Northwest of the United States. Appl. Environ. Microbiol. 73:1433-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon-Chung, K. J. 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68:821-833. [PubMed] [Google Scholar]
- 19.Kwon-Chung, K. J. 1975. A new genus, Filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia 67:1197-1200. [PubMed] [Google Scholar]
- 20.Kwon-Chung, K. J., J. C. Edman, and B. L. Wickes. 1992. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 60:602-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Littman, M. L. 1958. Capsule synthesis by Cryptococcus neoformans. Trans. N. Y. Acad. Sci. 20:623-648. [DOI] [PubMed] [Google Scholar]
- 22.McClelland, C. M., J. Fu, G. L. Woodlee, T. S. Seymour, and B. L. Wickes. 2002. Isolation and characterization of the Cryptococcus neoformans MATα pheromone gene. Genetics 160:935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson, M. A., and R. L. Metzenberg. 1992. Sexual development genes of Neurospora crassa. Genetics 132:149-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen, K., A. L. De Obaldia, and J. Heitman. 2007. Cryptococcus neoformans mates on pigeon guano: implications for the realized ecological niche and globalization. Eukaryot. Cell 6:949-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ory, J. J., C. L. Griffith, and T. L. Doering. 2004. An efficiently regulated promoter system for Cryptococcus neoformans utilizing the CTR4 promoter. Yeast 21:919-926. [DOI] [PubMed] [Google Scholar]
- 26.Pitt, J. I., and M. W. Miller. 1968. Sporulation in Candida pulcherrima, Candida Reukaufii, and Chlamydozyma species: their relationships with Metschnikowia. Mycologia 60:663-685. [Google Scholar]
- 27.Salas, S. D., J. E. Bennett, K. J. Kwon-Chung, J. R. Perfect, and P. R. Williamson. 1996. Effect of the laccase gene, CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 184:377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen, W. C., R. C. Davidson, G. M. Cox, and J. Heitman. 2002. Pheromones stimulate mating and differentiation via paracrine and autocrine signaling in Cryptococcus neoformans. Eukaryot. Cell 1:366-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherman, F. 1991. Getting started with yeast, p. 3-21. In C. Guthrie and G. R. Fink (ed.), Methods in enzymology, vol. 194. Academic Press, Inc., San Diego, CA. [DOI] [PubMed] [Google Scholar]
- 30.Slade, S. J., R. F. Harris, C. S. Smith, J. H. Andrews, and E. V. Nordheim. 1987. Microplate assay for Colletotrichum spore production. Appl. Environ. Microbiol. 53:627-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugimoto, A., Y. Iino, T. Maeda, Y. Watanabe, and M. Yamamoto. 1991. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev. 5:1990-1999. [DOI] [PubMed] [Google Scholar]
- 32.Tonukari, N. J., J. S. Scott-Craig, and J. D. Walton. 2000. The Cochliobolus carbonum SNF1 gene is required for cell wall-degrading enzyme expression and virulence on maize. Plant Cell 12:237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waterman, S. R., M. Hacham, G. Hu, X. Zhu, Y. D. Park, S. Shin, J. Panepinto, T. Valyi-Nagy, C. Beam, S. Husain, N. Singh, and P. R. Williamson. 2007. Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J. Clin. Investig. 117:794-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickes, B. L., M. E. Mayorga, U. Edman, and J. C. Edman. 1996. Dimorphism and haploid fruiting in Cryptococcus neoformans: association with the α-mating type. Proc. Natl. Acad. Sci. USA 93:7327-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williamson, P. R. 1997. Laccase and melanin in the pathogenesis of Cryptococcus neoformans. Front. Biosci. 2:e99-e107. [DOI] [PubMed] [Google Scholar]