Abstract
A bacteriophage cocktail (designated ECP-100) containing three Myoviridae phages lytic for Escherichia coli O157:H7 was examined for its ability to reduce experimental contamination of hard surfaces (glass coverslips and gypsum boards), tomato, spinach, broccoli, and ground beef by three virulent strains of the bacterium. The hard surfaces and foods contaminated by a mixture of three E. coli O157:H7 strains were treated with ECP-100 (test samples) or sterile phosphate-buffered saline buffer (control samples), and the efficacy of phage treatment was evaluated by comparing the number of viable E. coli organisms recovered from the test and control samples. Treatments (5 min) with the ECP-100 preparation containing three different concentrations of phages (1010, 109, and 108 PFU/ml) resulted in statistically significant reductions (P = <0.05) of 99.99%, 98%, and 94%, respectively, in the number of E. coli O157:H7 organisms recovered from the glass coverslips. Similar treatments resulted in reductions of 100%, 95%, and 85%, respectively, in the number of E. coli O157:H7 organisms recovered from the gypsum board surfaces; the reductions caused by the two most concentrated phage preparations were statistically significant. Treatment with the least concentrated preparation that elicited significantly less contamination of the hard surfaces (i.e., 109 PFU/ml) also significantly reduced the number of viable E. coli O157:H7 organisms on the four food samples. The observed reductions ranged from 94% (at 120 ± 4 h posttreatment of tomato samples) to 100% (at 24 ± 4 h posttreatment of spinach samples). The data suggest that naturally occurring bacteriophages may be useful for reducing contamination of various hard surfaces, fruits, vegetables, and ground beef by E. coli O157:H7.
Escherichia coli O157:H7 is an important food-borne pathogen responsible for ca. 62,000 food-borne cases of disease/year in the United States alone (29), at an annual cost estimated by the USDA (13) of ca. 0.7 billion dollars. E. coli O157:H7 infections often lead to bloody diarrhea and, occasionally, to a type of kidney failure called hemolytic-uremic syndrome (HUS). Most of the illnesses have been associated with eating undercooked, contaminated ground beef; however, contaminated fruits and vegetables are currently increasingly implicated as sources of E. coli O157:H7 infections (3, 39). For example, the most recent multistate outbreak of E. coli O157:H7 illness in the United States occurred in 2006 and was associated with consumption of spinach contaminated by the bacterium. One hundred ninety-nine people in 26 states were infected with the outbreak strain, of whom 102 (51%) were hospitalized, 31 (16%) developed HUS, and 3 (1.5%) died (http://www.cdc.gov/foodborne/ecolispinach/100606.htm).
In addition to the potential hazards associated with the consumption of naturally contaminated foods, foodstuffs also may be used as a delivery system for pathogenic bacteria (i.e., bioterrorism). Although terrorist attacks via food contamination have so far been relatively limited (perhaps the best known food-related terrorist attack in the United States was the intentional contamination of restaurant salad bars by Salmonella enterica serovar Typhimurium) (42), the possibility is a real and serious threat (46). The potential impact on human health of such deliberate sabotage of food could be devastating, and it may be estimated by extrapolation from the many documented examples of unintentional outbreaks of food-borne disease. For example, an outbreak of Salmonella serovar Typhimurium infection in Illinois during 1985 affected ca. 170,000 people (34), and an E. coli O157:H7-caused outbreak of food-borne illness in Japan, due to contaminated radish sprouts served in school lunches, affected ca. 8,000 children (some of whom died) (31).
Decontaminating fruits, vegetables, and ground meat presents considerable challenges. The two most common strategies used to limit the growth of bacteria on fruits and vegetables are washing with water and washing with solutions of various antibacterial chemicals. Those approaches are generally effective, e.g., dipping lettuce leaves and broccoli florets in a solution of calcium hypochlorite reduces E. coli contamination by 1.9 to 2.8 logs and by 1.7 to 2.5 logs CFU/g, respectively (6). However, the extensive use of chemical sanitizers has led to various bacteria developing resistance to them, which has resulted in a decline in the sanitizers' efficacy (16, 26, 32). Moreover, many of the currently available chemical sanitizers may damage foods, and many, if not all, of these sanitizers also adversely affect the environment. Decontaminating ground beef presents similar challenges. Gamma irradiation of beef, approved by the U.S. FDA in 1997, produces about a 1,000-fold reduction in the number of viable aerobic bacterial contaminants. However, it is expensive and requires batch processing. In addition, it is not specific (i.e., it kills all microorganisms in the treated foods, most of which are benign and may be beneficial), and it alters the taste of the treated products, especially when the highest (>4.5 kGy), most effective gamma irradiation levels are used (9, 43).
In a somewhat different but related context, contamination of buildings, equipment, food processing facilities, and other facilities of strategic importance by pathogenic bacteria also remains a serious problem, which is compounded by the possibility that these areas may be intentionally contaminated by virulent bacteria by bioterrorists. Decontamination of such facilities also presents considerable challenges because of the increased resistance of many potentially pathogenic bacteria to traditional sanitizers, including hypochlorous acid (the active form of hypochlorite sanitizers) and benzalkonium chloride (a quaternary ammonium sanitizer) (17). Also, many chemical sanitizers are corrosive and toxic and, therefore, are unacceptable for treating foods or surfaces that come into direct contact with foods. Thus, new and novel approaches are needed to aid the prevention of diseases caused by natural or intentional dissemination of pathogenic bacteria on various building materials or by the ingestion of various foods intentionally or accidentally contaminated by E. coli O157:H7. Ideally, such approaches will be effective, safe, and economical. Lytic bacteriophages may provide one such approach.
Bacteriophages/phages are viruses that are natural predators of bacteria, and they have potent bactericidal activity against their specifically targeted bacterial species or strains. Recently, the possibility of using phages to reduce the concentration of bacterial pathogens in and on various foods has been gaining attention. Several authors (8, 26, 27, 44) reported that treatment with bacteriophages significantly reduced the levels of major food-borne pathogens in various foods, with reduction levels ranging from 1.8 to 4.6 logs compared to those of untreated or placebo-treated controls (those and several other relevant studies were reviewed recently [20, 37]) In 2006, the Food and Drug Administration (FDA) approved a Listeria monocytogenes-specific phage preparation (LMP-102) for use as an antimicrobial agent against L. monocytogenes contamination of ready-to-eat foods (16a). LMP-102 is the first phage-based preparation to be approved for a food safety application by a Western regulatory agency. Also, shortly afterward, the FDA approved the generally recognized as safe (GRAS) designation for another L. monocytogenes-specific phage for application with cheese and other foods (http://www.cfsan.fda.gov/∼rdb/opa-g218.html).
The results of preliminary studies indicate that bacteriophages also are effective in managing E. coli O157:H7 infections in various settings. For example, Kudva et al. (24) reported that E. coli O157:H7-specific phages were effective in lysing the bacterium in vitro. Also, although the phages' ability to reduce the bacterium's concentration in foods was not determined, the authors suggested that the phages might be useful as a biocontrol agent for E. coli O157:H7. More recently, O'Flynn et al. (33) observed that a mixture of three E. coli O157:H7-specific phages eliminated E. coli O157:H7 from the surfaces of seven of nine specimens of sliced, experimentally contaminated beef. Also, E. coli O157:H7-specific phages were effective in reducing the concentrations of E. coli O157:H7 (i) in the gastrointestinal tracts of mice (40) and ruminants (36) and (ii) on stainless steel coupons (35). The goal of the studies described in this communication was to determine whether treatment with E. coli O157:H7-specific phages significantly reduces the number of viable E. coli O157:H7 cells on experimentally contaminated (i) inanimate surfaces that mimic those of various types of building materials and (ii) foods with various textures.
MATERIALS AND METHODS
Phage preparation.
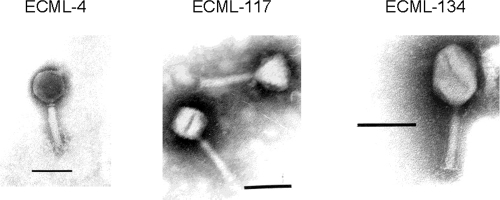
The phage preparation used for our studies is a cocktail, designated ECP-100, of E. coli O157:H7-specific bacteriophages. ECP-100 is a pH 7.0 to 7.5, clear to opalescent, odorless liquid (specific gravity ca. 1.008) containing three lytic phages, ECML-4, ECML-117, and ECML-134, isolated from fresh and salt water environments in phosphate-buffered saline (PBS; pH 7.4). ECML-4, ECML-117, and ECML-134 are members of the family Myoviridae (Fig. 1) (1), and their genomes have been fully sequenced and determined to contain 157,308 bp (202 open reading frames [ORFs]), 66,854 bp (103 ORFs), and 166,783 bp (157 ORFs), respectively (A. Sulakvelidze, unpublished data). The three phages were mixed with sterile PBS (pH 7.4) to prepare three versions of ECP-100 with viable phage concentrations of ca. 108, 109, and 1010 PFU/ml.
FIG. 1.
Electron micrographs of the three phages in ECP-100. Bars = 0.1 μm.
Determining target range and specificity of ECP-100.
The target range of ECP-100 and its three component phages was determined by characterizing the components' ability to lyse (i) 111 strains of E. coli O157:H7, (ii) 76 strains of E. coli not possessing the O157:H7 serotype, and (iii) 20 strains of bacterial species other than E. coli. Lytic activity was detected with a classical spot-testing technique (4), by incubating bacterial lawns spotted with aliquots of diluted phage preparations (104 PFU/ml) and examining the lawns for zones of lysis.
Bacterial strains.
A total of 111 E. coli O157:H7 isolates were obtained from various research and public health laboratories in the United States and from the enterohemorrhagic Escherichia coli 1 (EHEC-1) reference collection maintained by the Microbial Evolution Laboratory at Michigan State University (http://www.shigatox.net/). A total of 76 E. coli strains not possessing the O157:H7 serotype were obtained from the ECOR Reference Collection (72 strains) and from the EHEC-1 reference collection (4 strains) maintained by the Microbial Evolution Laboratory. A total of 20 strains of four bacterial species other than E. coli were used for the studies. They included (i) five strains of Staphylococcus aureus (ATCC strains 14458 and 49775 and Sa295, Sa296, and Sa297 from Intralytix's bacterial strain collection), (ii) five strains of L. monocytogenes (ATCC 13932, ATCC 19116, ATCC 19117, ATCC 19118, and ATCC 35152), (iii) five strains of various Salmonella serotypes (ATCC 6962, ATCC 10719, ATCC 13076, ATCC 13311, and ATCC 15480), (iv) and five strains of Pseudomonas aeruginosa (ATCC strains 10145 and 27853 and Pa27, Pa28, and Pa42 from Intralytix's bacterial strain collection). All strains were stored at −80°C in 70% LB broth-30% glycerol.
The three E. coli O157:H7 strains used in the hard-surface decontamination studies were EHEC-4, EHEC-6, and Ec0157. The three E. coli O157:H7 strains used in the food studies were nalidixic acid-resistant mutants of EHEC strains. (i) Ec229 was developed from the EHEC-5 strain (also known as 2886-75; this strain was responsible for the first known U.S. case of disease caused by E. coli O157:H7 infection); (ii) Ec230 was developed from the EHEC-8 strain (also known as G5101, a human isolate); and (iii) Ec231 was developed from the EHEC-2 strain (also known as 93-111; this EHEC-2 strain was responsible for an outbreak in Washington state in 1993). The original EHEC strains were selected for nalidixic acid resistance at Intralytix, by serially passaging the original isolates on LB agar plates supplemented with increasing concentrations of nalidixic acid. Each strain underwent ≤8 serial passages before it was determined to be nalidixic acid resistant at a concentration of 25 μg/ml. After the three mutants underwent passaging, their Intralytix strain designations, as indicated above, were assigned (i.e., Ec229, Ec230, and Ec231). All six strains used to experimentally contaminate foods and surfaces during our studies were preselected for their susceptibility to ECP-100 (i.e., they were susceptible to ECP-100). For the efficacy studies, the strains were grown (37 ± 2°C, 24 to 48 h) separately, diluted 100- or 1,000-fold, and mixed in equal concentrations just prior to the beginning of the studies. The levels of E. coli O157:H7 contamination of each test matrix prior to treatment with ECP-100 are indicated in the relevant sections of Results.
Test matrices.
The studies examining the ability of ECP-100 to decontaminate inanimate surfaces experimentally contaminated by E. coli O157:H7 utilized glass coverslips and gypsum boards (both ca. 25 by 25 mm) as prototypes of various hard and porous building materials, respectively. After matrices were cleaned with 70% ethanol and rinsed with deionized water, they were placed in glass petri dishes (one item/dish) and sterilized in an oven (≥2 h, 160 to 170°C) or an autoclave (≥30 min) with a drying cycle (≥10 min). The matrices were cooled to room temperature and used immediately, or they were stored at room temperature until use.
The studies examining the ability of ECP-100 to decontaminate foods contaminated by E. coli O157:H7 utilized ground beef and three fruits and vegetables with different surface characteristics: smooth (tomato), rough (spinach), and complex (broccoli). Tomatoes, spinach, and broccoli purchased at a local supermarket were rinsed immediately prior to use with copious amounts of tap water, followed by rinsing with 70% ethanol. The purpose of this pretreatment was to reduce the number of bacteria that naturally contaminate produce surfaces and whose presence could have complicated the interpretation of our results. The ground beef (also purchased at a local supermarket) was not washed or pretreated prior to the studies.
Phage application.
Aliquots (100 μl) of ECP-100 were applied on the tops of areas of the hard surfaces and foods contaminated by a mixture of three strains of E. coli O157:H7. The foods were treated by applying ECP-100 with a spray gun (Basic spray gun, model no. 250-2; Badger Air-Brush Co., Franklin Park, IL) precalibrated to deliver the designated volume (100 μl) in ca. 4 s. The amount of ECP-100 applied per gram of the E. coli-contaminated foods was ca. 20 μl/g of broccoli and ca. 2 ml/500 cm2 of surface area for each remaining food, corresponding to ca. 9 μl/g for the tomato samples, ca. 90 μl/g for the spinach samples, and ca. 1.9 μl/g for the ground beef samples.
General design of studies utilizing E. coli O157:H7-contaminated hard surfaces.
Five experimental groups were examined during the studies, with the two hard matrices (glass coverslips and gypsum boards). Samples of all groups were tested in triplicate. All matrices were pretreated with skim milk to “dirty” their surfaces, to mimic real-life settings where surfaces are often covered with dried organic matter. Briefly, aliquots (10 μl) of a solution of dried skim milk (5% [wt/vol]) were applied to the matrices, after which they were stored (in a laminar flow biosafety hood) at room temperature for 20 to 30 min or until completely dry. After drying, the matrices in group 1 (the “dry control”) were contaminated by E. coli but not treated with phage or PBS. The matrices in group 2 (the PBS control or “nonactive control”) were contaminated by E. coli and treated with PBS, and groups 3, 4, and 5 were composed of matrices contaminated by E. coli and treated with ECP-100 preparations containing three different concentrations of phages, 108 PFU/ml, 109 PFU/ml, and 1010 PFU/ml, respectively.
The matrices were contaminated by aliquots (10 μl) of a 1:1:1 mixture of three E. coli O157:H7 strains (EHEC-4, EHEC-6, and Ec0157, ca. 1 × 107 CFU/ml) applied to their surfaces, after which they were dried (at room temperature for 15 to 25 min) in a laminar flow biosafety hood to ensure that the inocula were completely dried. After the drying step, aliquots (0.1 ml) of the appropriate ECP-100 preparation or PBS were applied on the top of the E. coli mixture used to contaminate the matrices, the matrices were stored for 5 min at room temperature, and the excess ECP-100 was removed. After the excess ECP-100 and buffer were removed, the test and control matrices were mixed gently (30 s) in separate conical tubes (50-ml capacity) containing peptone water (20 ml), three serial 10-fold dilutions (10−1, 10−2, and 10−3) of the mixtures were prepared in peptone water, the undiluted and diluted mixtures were immediately passed through separate membrane filters (0.45-μm pore size; Nalgene), and the filters were washed with PBS (20 ml) to remove unattached phages. The washed filters were placed (upside down) in separate petri dishes containing sorbitol MacConkey agar supplemented with cefixime and rhamnose (CR-SMAC; Remel, Lenexa, KS), and the number of recovered E. coli was enumerated by counting the colonies that grew on the filters during incubation (37°C, 24 to 48 h). Because the entire 20-ml peptone water solution was filtered through the Nalgene membrane filters, the resulting counts represented the total CFU recovered from each of the tested hard surfaces. Another control group (the “neutralizer control”) was used to verify the removal of unattached phages, as explained below.
The neutralizer control was used to verify that the filtration and filter-washing steps described above effectively removed most of the free phages from the filters and that the data accurately represented the reduction in the number of viable E. coli O157:H7 cells resulting from a 5-min interaction with ECP-100. That is, the results were not appreciably affected by the lytic activity of free phages (phages that did not attach to the bacteria during the 5-min contact time) during the filters' overnight incubation on CR-SMAC agar. After aliquots (0.1 ml) of ECP-100 were applied to the E. coli-contaminated glass coverslips and gypsum boards, the matrices were stored for 5 min at room temperature (i.e., the contact/interaction time mentioned in the preceding paragraph for ECP-100 and the E. coli-contaminated matrices), and excess ECP-100 was removed. Each of the phage-treated matrices was mixed gently (30 s) in separate conical tubes (50-ml capacity) containing peptone water (20 ml), the mixtures were passed through membrane filters (0.45-μm-pore size; Nalgene), and the filters were washed with PBS (20 ml). The washed filters were placed in separate conical tubes (50-ml capacity) containing peptone water (20 ml), and the E. coli-contaminated matrices from the neutralizer control group were added to the appropriate tubes. After the tubes were gently vortexed for 30 s, three serial 10-fold dilutions (10−1, 10−2, and 10−3) of the mixtures were passed through membrane filters (0.45-μm-pore-size “neutralizer filters”; Nalgene). After the neutralizer filters were washed with PBS (20 ml), each neutralizer filter was placed (upside down) on CR-SMAC agar in a separate petri dish, and the number of recovered E. coli was enumerated by counting the colonies that grew on the filters after incubation (37°C, 24 to 48 h). The observation that the concentrations of viable E. coli O157:H7 recovered from the PBS and neutralizer control groups were not significantly different would support the idea that the filtration step effectively removed free phage (i.e., unattached to bacteria) from the Nalgene filters after the specified contact time between the E. coli-contaminated matrices and the ECP-100 phage. However, the observation that the number of E. coli O157:H7 recovered from the PBS control was significantly higher than that recovered from the neutralizer control would demonstrate that the filtration step did not remove free phages after contact between the contaminated matrices and the ECP-100 phage.
General design of studies utilizing E. coli O157:H7-contaminated foods.
After triplicate samples of each matrix were weighed, aliquots of the mixture of strains Ec229, Ec230, and Ec231 were applied (using a pipette) to the matrix surfaces, and the contaminated samples were stored at room temperature (20 to 22°C) for 60 min. Except for broccoli, the bacterial challenge was performed based on the foods' surface areas; thus, the number of CFU of E. coli applied per gram of food varied (for specific E. coli concentrations, refer to Results). After samples were stored for 60 min, ECP-100 was applied to the contaminated samples' surfaces (the same volume of sterile PBS was applied to the control samples), and all of the samples were covered with plastic (Saran) wrap and stored at 10°C, after which they were tested at three time points posttreatment (24 h, 120 h, and 168 h). At 24 h posttreatment, the first set of triplicate samples was placed into sterile plastic bags containing peptone water (5 ml/bag) and thoroughly mashed and suspended using a rolling pin. Aliquots (0.1 ml and 0.3 ml) of the suspensions were plated in separate petri dishes containing CR-SMAC supplemented with nalidixic acid (25 mg/ml), the plates were incubated (35 ± 2°C, 16 to 24 h), and the CFU/g of sample was calculated after the colonies were counted, as follows: total CFU/bag (actual CFU/0.1 × 10 × 5)/weight of sample analyzed. The number of residual ECP-100 phages in the samples also was determined. The bacteria in aliquots of the suspensions were removed by membrane filtration (0.22-μm pore size), and the concentrations of phages in the filtered preparations were determined with a standard plaque-counting technique (4). The number of PFU/g of sample was calculated after the plaques were counted, as follows: total PFU/bag (actual PFU/0.1 × 10 × 5)/weight of sample analyzed. The second set and third sets of samples were assayed for residual E. coli and phages at 120 h and 168 h posttreatment, respectively.
Studies of all foods were performed in the same manner, except for three food-specific modifications. First, for the studies with tomatoes, 2 ml (instead of 5 ml) of peptone water was used per bag (corresponding adjustments were made in the formula to calculate the CFU/g and PFU/g). Second, for the studies with ground beef, E. coli-contaminated and phage-treated samples were ground with a meat grinder (catalog no.168610; Northern Industrial Tools, Burnsville, MN), aliquots of the ground beef samples to be analyzed were weighed and placed in sterile plastic bags containing peptone water (125 ml/bag), and the ground beef samples were thoroughly mashed in the bags before their CFU/g were calculated. Third, due to spoilage during storage at 10°C, the E. coli-contaminated and phage-treated ground beef samples were assayed at only one time point (24 h).
Statistical analyses.
Statistical analyses were performed with GraphPad InStat (version 3.05) software and GraphPad Prism (version 4.0) software (GraphPad Software, San Diego, CA). One-way analysis of variance and Tukey-Kramer multiple comparisons tests were used to determine whether treatment with ECP-100 significantly reduced the number of viable E. coli O157:H7 on glass, gypsum, broccoli, tomato, and spinach surfaces contaminated by the bacterium. Only one time point (24 h) was analyzed for the ground beef samples. Thus, an unpaired t test was used to determine whether ECP-100 significantly reduced the number of viable E. coli O157:H7 contaminating that matrix. A P value of <0.05 indicated a statistically significant difference between the results obtained with the ECP-100-treated matrices and that of the control matrices.
RESULTS
Lytic spectrum (target range) of ECP-100 and its three component phages.
ECP-100 lysed 100 (90%), and ECML-4, ECML-117, and ECML-134 lysed 78 (70%), 97 (87%), and 72 (65%), respectively, of the 111 strains of E. coli O157:H7 in our collection. The three phages' lytic potency was noticeably less against the non-O157:H7 isolates of E. coli we examined. ECML-4 and ECML-117 each lysed only one strain (ECOR-11 and ECOR-53, respectively), and ECML-134 lysed only 18 (24%) of the 76 non-O157:H7 isolates of E. coli. Neither ECP-100 nor its three component phages lysed any of the 20 strains of the four other bacterial species (S. aureus, L. monocytogenes, Salmonella serovar Typhimurium, and P. aeruginosa) we tested.
Reduction in E. coli recovery from ECP-100-treated hard surfaces.
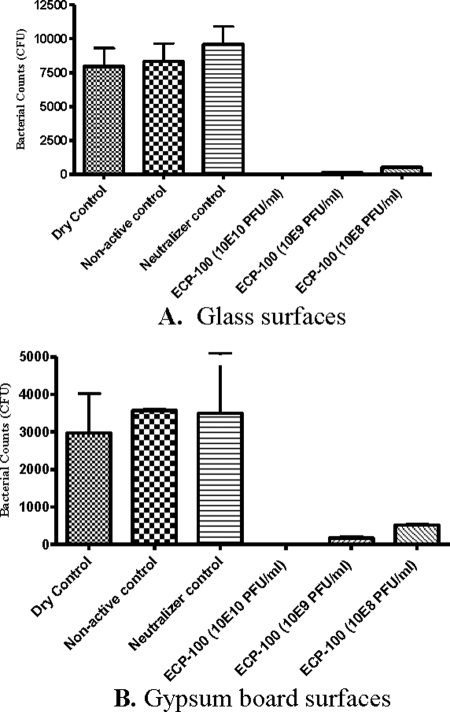
Treatment of the experimentally contaminated hard matrices with ECP-100 significantly reduced (P = <0.05) the number of viable E. coli organisms recovered from them (Fig. 2 and Table 1). The number of E. coli organisms recovered from the contaminated glass coverslips and gypsum boards not treated with ECP-100 or PBS (group 1, the “dry control”) was not significantly different from that recovered from samples treated with PBS (group 2, the “nonactive control”). Also, the recovery of E. coli from the group 1 matrices was not significantly different from that observed for the neutralizer controls.
FIG. 2.
Reduction of E. coli contamination of glass coverslips and gypsum boards treated with ECP-100 (based on triplicate samples; bars, standard errors of the means). Nonactive control, PBS-treated matrices; dry control, untreated matrices; neutralizer control, control for the absence of free phages on the Nalgene filters after rinsing with PBS. The 10E10, 10E9, and 10E8 PFU/ml format is used to convey phage concentrations of 1010, 109, and 108 PFU/ml, respectively.
TABLE 1.
Reduction in the amount of E. coli O157:H7 recovered or at a given time point as a result of treatment with ECP-100
| Matrix and no. of PFU/ml or mean length of incubation (h) ± SDa | ECP-100 vs PBS treatment
|
P valueb | Significantly different | |
|---|---|---|---|---|
| Fold reduction | % Reduction | |||
| Glass slides | ||||
| 1010 | 8,333 | 99.99 | 0.05 | Yes |
| 109 | 52 | 98 | 0.05 | Yes |
| 108 | 16 | 94 | 0.05 | Yes |
| Gypsum slides | ||||
| 1010 | 3,567 | 100 | 0.05 | Yes |
| 109 | 20 | 95 | 0.05 | Yes |
| 108 | 7 | 85 | 0.05 | No |
| Broccoli | ||||
| 24 ± 4 | 188 | 99.5 | 0.05 | Yes |
| 120 ± 4 | 106 | 99 | 0.05 | Yes |
| 168 ± 4 | 31 | 97 | 0.05 | Yes |
| Tomatoes | ||||
| 24 ± 4 | 117 | 99 | 0.05 | Yes |
| 120 ± 4 | 16 | 94 | 0.05 | Yes |
| 168 ± 4 | 22 | 96 | 0.05 | Yes |
| Spinach | ||||
| 24 ± 4 | 1,769 | 100 | 0.05 | Yes |
| 120 ± 4 | 242 | 99.6 | 0.05 | Yes |
| 168 ± 4 | 91 | 99 | 0.05 | Yes |
| Red meat | ||||
| 24 ± 4 | 19 | 94.5 | 0.05 | Yes |
SD, standard deviation.
A P value of <0.05 was considered statistically significant.
Treating the contaminated glass coverslips with ECP-100 preparations containing three different phage concentrations (1010, 109, and 108 PFU/ml) produced a 8,333-fold (99.99%), 52-fold (98%), and 16-fold (94%) reduction, respectively, in the number of E. coli organisms recovered from the coverslips (compared to that from PBS-treated controls) (Fig. 2A). In each case, the observed reduction was statistically significant (P = <0.05). The differences between the results yielded by the three dilutions of the ECP-100 preparations were not significant (P = >0.05).
Treatment of the contaminated gypsum boards with the three phage preparations containing 1010, 109, and 108 PFU/ml elicited a 3,567-fold (100%), 20-fold (95%), and 7-fold (85%) reduction, respectively, in the number of recovered E. coli organisms (compared to that from the PBS-treated controls) (Fig. 2B). The reductions produced by the two highest phage concentrations were statistically significant (P = <0.05) (Table 1). The differences between the results yielded by the two highest phage concentrations were not significant (P = >0.05).
Reduction in E. coli recovery from ECP-100-treated broccoli.
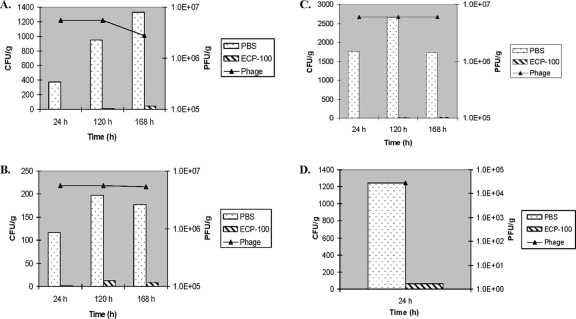
Prior to phage treatment, broccoli samples (average weight of 14.1 g) were experimentally contaminated with ca. 710 CFU of E. coli O157:H7/gram. ECP-100 application significantly reduced (P = <0.05) the concentration of viable E. coli organisms on broccoli by ca. 99.5%, 99%, and 97% during storage (10°C) for 24 h, 120 h, and 168 h, respectively (Fig. 3A and Table 1). The minor variations in ECP-100's efficacy at the three time points posttreatment were not significant. The initial concentrations of ECP-100's phages declined significantly (ca. 50%; P = <0.05) during storage for 120 h and 168 h (Fig. 3A).
FIG. 3.
Reduction of E. coli contamination of (A) broccoli, (B) tomato, (C) spinach, and (D) ground beef treated with ECP-100 (based on mean values; phage titers are shown in logarithmic scale). The 1.0E + 7, 1.0E + 6, etc., PFU/g format is used to convey phage concentrations of 107, 106, etc., PFU/g, respectively.
Reduction in E. coli recovery from ECP-100-treated tomato slices.
Prior to phage treatment, tomato samples (average surface area, 35 cm2; average weight, 15.4 g) were experimentally contaminated with ca. 650 CFU of E. coli O157:H7/gram. ECP-100 application significantly reduced (P = <0.05) the concentration of viable E. coli organisms on tomato slices by ca. 99%, 94%, and 96% during storage (10°C) for 24 h, 120 h, and 168 h, respectively (Fig. 3B and Table 1). The minor variation in ECP-100's efficacy at the three time points posttreatment was not significant. The initial concentration of ECP-100's phages did not change significantly over the study's duration (Fig. 3B).
Reduction in E. coli recovery from ECP-100-treated spinach.
Prior to phage treatment, spinach samples (average surface area, 80 cm2; average weight, 3.6 g) were experimentally contaminated with ca. 14,000 CFU of E. coli O157:H7/gram. ECP-100 application significantly reduced (P = <0.05) the concentration of viable E. coli on spinach by ca. 100% during storage (10°C) for 24 h and 120 h, and by ca. 99% during storage for 168 h (Fig. 3C and Table 1). The minor variation in ECP-100's efficacy at the three time points posttreatment was not significant. The initial concentration of ECP-100's phages did not change significantly over the study's duration (Fig. 3C).
Reduction in E. coli recovery from ECP-100-treated ground beef.
Prior to phage treatment, ground beef samples (average surface area, 137 cm2; average weight, 294 g) were experimentally contaminated with ca. 3,400 CFU of E. coli O157:H7/gram. ECP-100 application significantly reduced (two-tailed P value = 0.0072) the concentration of viable E. coli organisms on/in ground beef by ca. 95% during storage (10°C) for 24 h (Fig. 3D and Table 1). The effect of longer storage/phage exposure times (120 h and 168 h) was not examined because of the ground beef's spoilage at those time intervals.
Sensitivity of surviving E. coli O157:H7 to ECP-100 phages.
Viable E. coli O157:H7 cells were not recovered from gypsum board and spinach treated with ECP-100 preparations containing 1010 PFU/ml and 109 PFU/ml, respectively, by 24 h posttreatment (Table 1). However, small numbers of bacteria were recovered from all other samples (including hard surfaces and foods), albeit at significantly lower levels than from phage-untreated control samples. We tested surviving bacteria in randomly selected colonies (ca. five colonies from various samples) to determine whether they were mutants resistant to ECP-100's phages. All of the surviving bacterial colonies we examined were sensitive to lysis by ECP-100.
DISCUSSION
ECP-100 against E. coli O157:H7.
The three phages (ECML-4, ECML-117, and ECML-134) contained in ECP-100 are in the family Myoviridae (Fig. 1), which includes nonenveloped, double-stranded DNA phages with contractile tails (2). ECP-100's three component phages have strong lytic activity against their targeted E. coli O157:H7 strains, as documented by their ability to form clear lysis zones when low concentrations (104 PFU/ml) of them are spotted onto bacterial lawns of their hosts. Moreover, each phage has a broad spectrum of lytic activity against E. coli O157:H7. The three phages' lytic spectra overlap noticeably; i.e., many of the E. coli O157:H7 strains we tested were lysed by two or all three of the phages (data not shown). However, some strains were lysed only by one of the three phages. Thus, by combining the three phages into one cocktail, we increased the target range of the ECP-100 preparation, which lysed 90% of the E. coli O157:H7 strains examined. In addition (as discussed later in Discussion), combining two or more phages lytic for the same bacterial strains also may improve the long-term potency of the phage preparation by reducing the emergence of phage-resistant mutants of E. coli O157:H7.
ECP-100 is specific for E. coli O157:H7, and it lysed only 24% of the 76 non-O157:H7 E. coli strains in our collection, and it did not lyse any of the strains of the four other bacterial species we examined. Our data support the previous observation (24) that E. coli O157:H7-specific phage lytic activity is extremely species specific and fairly serotype specific. The specificity may have some important practical implications; e.g., the consumption of ECP-100 (by eating foods on which it has been applied) is unlikely to alter the microbial balance of the gastrointestinal tract. In this regard, the administration of E. coli O157:H7-specific phages has been reported (S. J. Bach, R. P. Johnson, Y. Wang, and T. A. McAllister. Presented at the Canadian Society of Animal Science, Edmonton, Alberta, Canada, 20 to 23 July 2004) not to alter natural rumen fermentation. Also, Bruttin and Brussow (12) recently observed that the oral administration of T4 phage (an E. coli-specific phage in the family Myoviridae) to human volunteers did not (i) decrease total fecal E. coli counts, (ii) lead to noticeable phage replication in the commensal E. coli population, and (iii) trigger adverse events in any of the volunteers enrolled in the study.
Another safety-associated concern about therapeutic applications of bacteriophages is the possibility that they may carry and introduce toxin-encoding genes or other undesirable genes into bacterial strains/species via phage-mediated transduction (26, 38). In this regard, ECP-100's three component phages are lytic/virulent rather than transducing phages, and full-genome sequence analyses (A. Sulakvelidze, unpublished data) revealed that they do not contain bacterial toxin-encoding genes nor antibiotic resistance-encoding genes.
Phage treatment of inanimate hard surfaces contaminated by E. coli O157:H7.
Bacteriophages have been used to decontaminate bacterially contaminated hospital rooms in the Soviet Union (A. Meiphariani, personal communication). However, with the exception of one recent study (35) that examined the value of using an E. coli O157:H7-specific bacteriophage to kill wild-type and rpoS-deficient cells of E. coli O157:H7 attached to coupons, rigorous scientific data demonstrating the efficacy of phage treatment for reducing E. coli O157:H7 contamination of inanimate hard surfaces is not readily available. The results of our studies support the idea that E. coli O157:H7-specific lytic bacteriophages significantly reduce contamination of inanimate hard surfaces by E. coli O157:H7, even when the bacteria are surrounded by dried organic matter. Indeed, the effectiveness of reduction observed for our studies was as high as 100% with glass slides and gypsum boards treated with the most concentrated (1010 PFU/ml) phage preparation and remained significant (P = <0.05) (ca. 98% reduction on glass slides and 95% reduction on gypsum boards) with a 10-fold-diluted (109 PFU/ml) ECP-100 preparation (Fig. 2 and Table 1). The glass matrix was easier to decontaminate than was the gypsum matrix: all three dilutions of ECP-100 significantly reduced the levels of E. coli O157:H7 on glass surfaces, but the reduction was statistically significant only on gypsum board surfaces treated with the two most concentrated phage preparations (Table 1).
During our studies, we applied only a relatively small volume of ECP-100 to the E. coli O157:H7-contaminated glass and gypsum surfaces (100 μl/ca. 625 mm2 of surface area). In all instances, the degree of decontamination appeared to directly correlate with the total phage concentration in the ECP-100 preparation used (Table 1). Although the differences between various effective concentrations of ECP-100 were not statistically significant, the numerically superior reduction of E. coli counts we observed with the more concentrated phage preparations suggests that it may be possible to further improve the effectiveness of phage treatment by increasing the phage concentration in ECP-100 and/or by using larger volumes of ECP-100 per unit of surface area. This idea is supported, as discussed below, by data obtained from our studies examining ECP-100's efficacy in reducing E. coli O157:H7 contamination of various foods and by recent studies (19, 27) with other bacteriophages.
The normal lytic cycle of a bacteriophage, from the phage's attachment to the release of progeny phage from the lysed host bacterium, requires about 20 to 40 min (28). Thus, since we used only a 5-min contact between ECP-100 and the contaminated hard surfaces, the significant reductions in E. coli O157:H7 counts we observed in our studies were not likely to be the endpoints of the full lytic process on the hard surfaces examined but were, rather, the result of initial adsorption of the phage particles to the bacterial membrane and subsequent lysis of the bacteria. In this context, although the entire lytic cycle requires approximately 40 min, most lytic phage-infected bacteria are “doomed” at less than 1 min postinjection of phage DNA (28).
The neutralizer control was designed to confirm that appreciable amounts of ECP-100's phages were not left on the PBS-washed membrane filters after the 5-min contact time between ECP-100 and the E. coli-contaminated glass and gypsum matrices. Samples in the neutralizer control group were processed similarly to the samples in other control and test groups, and the number of viable E. coli cells was enumerated in the same manner. If appreciable amounts of phages remained attached to the membrane filters after they were washed with PBS, the phages would infect additional E. coli and reduce the viable counts. The rationale for the neutralizer control did not require the removal of all phages from the filters. Rather, its protocol determined whether enough free phages were removed so that the remaining phages did not affect the recovery of E. coli in the samples; i.e., the data are comparable to those obtained from the nonactive-ingredient control (i.e., the PBS control). We found that the number of E. coli organisms recovered from the PBS-treated control and that of the neutralizer control were not significantly different, which indicated that the PBS washing step appropriately removed ECP-100's phages from the filters. Therefore, the observed reduction in the number of viable E. coli organisms was due to the effect of ECP-100's phages during the 5-min contact time rather than to new infections by free phages (i.e., phages not attached to the bacteria during the 5-min contact time) in the filters on which the bacteria were grown overnight and enumerated the next day.
The phage-to-bacterium contact time may be critical for some applications but not for others. For example, it may not be crucial when phages are used to decontaminate food processing plants, because phages can be left on surfaces for a prolonged period of time (e.g., overnight) and have maximal efficacy via the classical phage lysis cycle. However, short exposure times may be important for various other applications, e.g., when rapid decontamination of buildings intentionally contaminated by a specific bacterial pathogen is required. In this context, and, as discussed above, the “bacterial cell-doomed” phase of the classical lytic cycle occurs within ≤1 min postinfection. Thus, in theory, contact times shorter than the 5-min interval used in our studies may be also effective. Additional studies that characterize the effect of various amounts of phages and contact times are required to examine the validity of that possibility.
Phage treatment of foods.
We treated four different E. coli O157:H7-contaminated foods with only one version of ECP-100, the preparation with the lowest phage concentration that significantly reduced (P = <0.05) E. coli O157:H7 contamination of both glass and gypsum matrices (i.e., 109 PFU/ml). That preparation also significantly reduced contamination of the foods by E. coli O157:H7 (Table 1). The reduction ranged from 94% to 100% (Fig. 3 and Table 1) compared to the PBS-treated control samples, which is comparable to prior results obtained by other investigators. For example, O'Flynn et al. (33) recently reported that treatment with a phage cocktail containing three E. coli O157:H7-specific phages eliminated E. coli O157:H7 from seven of nine beef surfaces examined.
The population of phages we applied to food samples remained about the same over the duration of our studies, with the exception of the phage population applied to broccoli, which declined by ca. 50% during storage for 120 h and 168 h (Fig. 3). A low pH may deleteriously affect the ability of some phages to persist and exert their antibacterial activity in some foods; e.g., levels of Salmonella serovar Typhimurium- and L. monocytogenes-specific phages have been reported (26, 27) to decline rapidly in fresh-cut apple slices (pH ca. 4.2) but not in fresh-cut honeydew melon slices (pH 5.8). However, since broccoli's pH (ca. 6.30 to 6.52) is significantly less acidic than that of apples and it is similar to the pH of other foods examined during our current studies (http://www.cfsan.fda.gov/∼comm/lacf-phs.html), an acidic pH seems unlikely to be solely responsible for the decline of phage concentrations in broccoli. Further studies are needed to determine why some phages persist better in some foods than in others.
In real-life settings, a very high level of contamination of foods by E. coli O157:H7 is unlikely. In fact, many outbreaks of disease caused by E. coli O157:H7 have been elicited by less than 20 E. coli O157:H7 CFU/g of food and, often, with less than 1 CFU/g of food (30). Therefore, the challenge dose of E. coli O157:H7 that we used to contaminate food samples was several-hundred-fold to several-thousand-fold higher than those commonly found in outbreak-associated foods. In our studies, the degree of reduction in viable E. coli counts in the phage-treated foods appeared to be correlated with the phage concentration used: the lower bacteria-to-phage ratio resulted in a larger reduction in E. coli counts in all foods, with the exception of tomatoes (Table 1). Similar data were also reported earlier by other investigators (19, 27). Thus, using ECP-100 in real-life settings, where natural bacterial contamination is lower than that used in our studies, may be even more effective than it was during the laboratory studies described in this report. Large-scale field trials are required to confirm or refute that possibility.
Phage resistance.
The emergence of phage-resistant bacterial mutants has been suggested to be a potential problem that might hinder the efficacy of phage treatment (reviewed in references 20 and 37). However, the results of numerous studies suggest that phage resistance is not a very frequent event and does not deleteriously affect the efficacy of phage treatment. For example, Capparelli et al. (14) recently estimated that the frequency of resistance to S. aureus-specific phages ranged from 1.3 × 10−8 ± 4.16 to 1.3 × 10−9, which is, indeed, a rare event and is less than that of resistance arising against many antibiotics. In addition, O'Flynn et al. (33) recently reported that the emergence frequency of phage-resistant mutants was very low when beef samples experimentally contaminated by E. coli O157:H7 were treated with their phage preparation and that it did not hinder the preparation's efficacy. The study used a cocktail of three phages, which may have decreased the risk of selecting for phage-resistant mutants. In that regard, several authors (37, 38, 41) have proposed that using phage preparations containing several different phages lytic for the same bacterial species will reduce the likelihood of selecting mutants resistant to the preparations. One possible explanation for this phenomenon is that since various phages attach to different receptors on bacteria, mutations in one phage receptor would not be expected to alter the mutant's susceptibility to another phage that attaches to a different receptor on the bacterial cell membrane (41). Although there is at least one study (15) in which a single phage was used successfully (and the emergence of phage-resistant mutants was not detected) to treat foods contaminated by L. monocytogenes, the majority of previous studies utilized phage cocktails, and none reported the efficacy-hindering emergence of phage-resistant mutants (37). In agreement with these reports, we did not detect the emergence of phage-resistant mutants of E. coli O157:H7 when we tested randomly selected surviving colonies from various food samples for their susceptibility to ECP-100. Therefore, it seems unlikely that the emergence of phage-resistant mutants will be an insurmountable problem for practical applications in the environmental decontamination and food safety areas. Although there is little doubt that phage-resistant mutants will emerge eventually because of “selective pressure” provided by phage applications or because of natural shifts in bacterial populations (25), phage-based intervention strategies hold much promise. Phages are extremely ubiquitous in nature, and the rapid ability to isolate new phages can be used to update phage cocktails and make them effective against emerging phage-resistant mutants (37).
Bacteriophages are the most abundant organisms on this planet; e.g., 1 ml of nonpolluted water has been reported (7) to contain approximately 2 × 108 PFU of phages, and the total number of phages on Earth has been estimated to be in the range of 1030 to 1032 (10, 11). Bacteriophages are consumed daily by humans via the various foods they eat and water they drink. For example, phages have been commonly isolated from a wide range of food products, including ground beef, pork sausage, chicken, farmed freshwater fish, common carp and marine fish, oil sardines, raw skim milk, and cheese (5, 18, 20-23, 45). Indeed, the daily ingestion of phages may be an important natural strategy for replenishing the phage population in the gastrointestinal tract and for regulating the colon's microbial balance. Therefore, the approach of using bacteriophages to reduce contamination of foods by bacterial pathogens may be one of the most environmentally friendly and natural approaches for reducing the incidence of food-borne disease. Also, phages may be useful for decontaminating food processing plants and other buildings and facilities naturally or intentionally contaminated by pathogenic bacteria. The data presented in this report provide additional support for the idea that E. coli O157:H7-specific phages can be useful in preventing disease caused by that food-borne bacterium as long as it is susceptible to phage and suggest that a phage-based approach may be warranted against other virulent bacteria, including those of high bioterrorism importance, e.g., class A bacterial pathogens (http://www.bt.cdc.gov/bioterrorism/).
Acknowledgments
We thank Thomas Whittam (STEC Center, Michigan State University) for providing the reference EHEC strains and Lee Harrison (University of Pittsburgh) for providing E. coli O157:H7 strains from his collection. Hans-Wolfgang Ackermann is gratefully acknowledged for analyzing the phages by electron microscopy. We thank Amiran Meiphariani, Eliava Institute of Bacteriophage, for discussions about using bacteriophages to decontaminate hospital facilities in the Soviet Union. Arnold Kreger is acknowledged for editorial assistance.
T.A., M.L., and A.S. hold an equity stake in Intralytix, Inc., a Maryland corporation involved in the development of therapeutic phage preparations.
The U.S. Environmental Protection Agency through its Office of Research and Development partially funded and collaborated in the research described in the manuscript (hard-surface decontamination) under contract no. EP-06-C-000325 (to A.S.). Additional funding was provided by Intralytix, Inc., and by an SBIR award, W911QY-07-C-0125, from the U.S. Army (to A.S.).
Footnotes
Published ahead of print on 22 August 2008.
REFERENCES
- 1.Ackermann, H. W., and L. Berthiaume. 1995. Atlas of virus diagrams. CRC Press, Boca Raton, FL.
- 2.Ackermann, H. W., and M. S. DuBow. 1987. Viruses of prokaryotes. General properties of bacteriophages. CRC Press, Boca Raton, FL.
- 3.Ackers, M. L., B. E. Mahon, E. Leahy, B. Goode, T. Damrow, P. S. Hayes, W. F. Bibb, D. H. Rice, T. J. Barrett, L. Hutwagner, P. M. Griffin, and L. Slutsker. 1998. An outbreak of Escherichia coli O157:H7 infections associated with leaf lettuce consumption. J. Infect. Dis. 177:1588-1593. [DOI] [PubMed] [Google Scholar]
- 4.Adams, M. H. 1959. Methods of study bacterial viruses, p. 443-519. Bacteriophages. Interscience Publishers, Ltd., London, United Kingdom.
- 5.Atterbury, R. J., P. L. Connerton, C. E. Dodd, C. E. Rees, and I. F. Connerton. 2003. Isolation and characterization of Campylobacter bacteriophages from retail poultry. Appl. Environ. Microbiol. 69:4511-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrsing, J., S. Winkler, P. Franz, and R. Premier. 2000. Efficacy of chlorine for inactivation of Escherichia coli on vegetables. Postharvest Biol. Technol. 19:187-192. [Google Scholar]
- 7.Bergh, O., K. Y. Borsheim, G. Bratbak, and M. Heldal. 1989. High abundance of viruses found in aquatic environments. Nature 340:467-468. [DOI] [PubMed] [Google Scholar]
- 8.Bigwood, T., J. A. Hudson, C. Billington, G. V. Carey-Smith, and J. A. Heinemann. 2008. Phage inactivation of foodborne pathogens on cooked and raw meat. Food Microbiol. 25:400-406. [DOI] [PubMed] [Google Scholar]
- 9.Brito, M. S., A. L. C. H. Villavicencio, and J. Jorge Mancini-Filho. 2002. Effects of irradiation on trans fatty acids formation in ground beef. Radiat. Phys. Chem. 63:337-340. [Google Scholar]
- 10.Brussow, H., and R. W. Hendrix. 2002. Phage genomics: small is beautiful. Cell 108:13-16. [DOI] [PubMed] [Google Scholar]
- 11.Brussow, H., and E. Kutter. 2005. Phage ecology, p. 129-163. In E. Kutter and A. Sulakvelidze (ed.), Bacteriophages: biology and application, 1st ed. CRC Press, Boca Raton, FL.
- 12.Bruttin, A., and H. Brussow. 2005. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob. Agents Chemother. 49:2874-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buzby, J. C. 2001. Children and microbial foodborne illness. Food Rev. 24:32-37. [Google Scholar]
- 14.Capparelli, R., M. Parlato, G. Borriello, P. Salvatore, and D. Iannelli. 2007. Experimental phage therapy against Staphylococcus aureus in mice. Antimicrob. Agents Chemother. 51:2765-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlton, R. M., W. H. Noordman, B. Biswas, E. D. de Meester, and M. J. Loessner. 2005. Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 43:301-312. [DOI] [PubMed] [Google Scholar]
- 16.Chesney, J. A., J. W. Eaton, and J. R. Mahoney, Jr. 1996. Bacterial glutathione: a sacrificial defense against chlorine compounds. J. Bacteriol. 178:2131-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Code of Federal Regulations. Revised 1 April 2008. Title 21, Part 172, Sec. 172.785. 3:77-78. U.S. Government Printing Office, Washington, DC.
- 17.Davidson, P. M., and M. A. Harrison. 2002. Resistance and adaptation to food antimicrobials, sanitizers, and other process controls. Food Technol. 56:69-78. [Google Scholar]
- 18.Gautier, M., A. Rouault, P. Sommer, and R. Briandet. 1995. Occurrence of Propionibacterium freudenreichii bacteriophages in Swiss cheese. Appl. Environ. Microbiol. 61:2572-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goode, D., V. M. Allen, and P. A. Barrow. 2003. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 69:5032-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greer, G. G. 2005. Bacteriophage control of foodborne bacteria. J. Food Prot. 68:1102-1111. [DOI] [PubMed] [Google Scholar]
- 21.Hsu, F. C., Y. S. Shieh, and M. D. Sobsey. 2002. Enteric bacteriophages as potential fecal indicators in ground beef and poultry meat. J. Food Prot. 65:93-99. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy, J. E., Jr., C. I. Wei, and J. L. Oblinger. 1986. Methodology for enumeration of coliphages in foods. Appl. Environ. Microbiol. 51:956-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy, J. E. J., J. L. Oblinger, and G. Bitton. 1984. Recovery of coliphages from chicken, pork sausage, and delicatessen meats. J. Food Prot. 47:623-626. [DOI] [PubMed] [Google Scholar]
- 24.Kudva, I. T., S. Jelacic, P. I. Tarr, P. Youderian, and C. J. Hovde. 1999. Biocontrol of Escherichia coli O157 with O157-specific bacteriophages. Appl. Environ. Microbiol. 65:3767-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenski, R. E. 1984. Coevolution of bacteria and phage: are there endless cycles of bacterial defenses and phage counterdefenses? J. Theor. Biol. 108:319-325. [DOI] [PubMed] [Google Scholar]
- 26.Leverentz, B., W. S. Conway, Z. Alavidze, W. J. Janisiewicz, Y. Fuchs, M. J. Camp, E. Chighladze, and A. Sulakvelidze. 2001. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit: a model study. J. Food Prot. 64:1116-1121. [DOI] [PubMed] [Google Scholar]
- 27.Leverentz, B., W. S. Conway, M. J. Camp, W. J. Janisiewicz, T. Abuladze, M. Yang, R. Saftner, and A. Sulakvelidze. 2003. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 69:4519-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathews, C. K. 1994. An overview of the T4 developmental program, p. 1-8. In J. D. Karam (ed.), Molecular biology of bacteriophage T4. ASM Press, Washington, DC.
- 29.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng, J., M. P. Doyle, T. Zhao, and S. Zhao. 2001. Enterohemorrhagic Escherichia coli, p. 193-213. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers, 2nd ed. ASM Press, Washington, DC.
- 31.Mermin, J. H., and P. M. Griffin. 1999. Public health in crisis: outbreaks of Escherichia coli O157:H7 infections in Japan. Am. J. Epidemiol. 150:797-805. [DOI] [PubMed] [Google Scholar]
- 32.Mokgatla, R. M., V. S. Brozel, and P. A. Gouws. 1998. Isolation of Salmonella resistant to hypochlorous acid from a poultry abattoir. Lett. Appl. Microbiol. 27:379-382. [DOI] [PubMed] [Google Scholar]
- 33.O'Flynn, G., R. P. Ross, G. F. Fitzgerald, and A. Coffey. 2004. Evaluation of a cocktail of three bacteriophages for biocontrol of Escherichia coli O157:H7. Appl. Environ. Microbiol. 70:3417-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan, C. A., M. K. Nickels, N. T. Hargrett-Bean, M. E. Potter, T. Endo, L. Mayer, C. W. Langkop, C. Gibson, R. C. McDonald, R. T. Kenney, et al. 1987. Massive outbreak of antimicrobial-resistant salmonellosis traced to pasteurized milk. JAMA 258:3269-3274. [PubMed] [Google Scholar]
- 35.Sharma, M., J. H. Ryu, and L. R. Beuchat. 2005. Inactivation of Escherichia coli O157:H7 in biofilm on stainless steel by treatment with an alkaline cleaner and a bacteriophage. J. Appl. Microbiol. 99:449-459. [DOI] [PubMed] [Google Scholar]
- 36.Sheng, H., H. J. Knecht, I. T. Kudva, and C. J. Hovde. 2006. Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants. Appl. Environ. Microbiol. 72:5359-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sulakvelidze, A., and P. Barrow. 2005. Phage therapy in animals and agribusiness, p. 335-380. In E. Kutter and A. Sulakvelidze (ed.), Bacteriophages: biology and applications. CRC Press, Boca Raton, FL.
- 38.Sulakvelidze, A., and E. Kutter. 2005. Bacteriophage therapy in humans, p. 381-436. In E. Kutter and A. Sulakvelidze (ed.), Bacteriophages: biology and application. CRC Press, Boca Raton, FL.
- 39.Swinbanks, D. 1996. Outbreak of E. coli infection in Japan renews concerns. Nature 382:290. [DOI] [PubMed] [Google Scholar]
- 40.Tanji, Y., T. Shimada, H. Fukudomi, K. Miyanaga, Y. Nakai, and H. Unno. 2005. Therapeutic use of phage cocktail for controlling Escherichia coli O157:H7 in gastrointestinal tract of mice. J. Biosci. Bioeng. 100:280-287. [DOI] [PubMed] [Google Scholar]
- 41.Tanji, Y., T. Shimada, M. Yoichi, K. Miyanaga, K. Hori, and H. Unno. 2004. Toward rational control of Escherichia coli O157:H7 by a phage cocktail. Appl. Microbiol. Biotechnol. 64:270-274. [DOI] [PubMed] [Google Scholar]
- 42.Torok, T. J., R. V. Tauxe, R. P. Wise, J. R. Livengood, R. Sokolow, S. Mauvais, K. A. Birkness, M. R. Skeels, J. M. Horan, and L. R. Foster. 1997. A large community outbreak of salmonellosis caused by intentional contamination of restaurant salad bars. JAMA 278:389-395. [DOI] [PubMed] [Google Scholar]
- 43.Wheeler, T. L., S. D. Shackelford, and M. Koohmaraie. 1999. Trained sensory panel and consumer evaluation of the effects of gamma irradiation on palatability of vacuum-packaged frozen ground beef patties. J. Anim. Sci. 77:3219-3224. [DOI] [PubMed] [Google Scholar]
- 44.Whichard, J. M., N. Sriranganathan, and F. W. Pierson. 2003. Suppression of Salmonella growth by wild-type and large-plaque variants of bacteriophage Felix O1 in liquid culture and on chicken frankfurters. J. Food Prot. 66:220-225. [DOI] [PubMed] [Google Scholar]
- 45.Whitman, P. A., and R. T. Marshall. 1971. Isolation of psychrophilic bacteriophage-host systems from refrigerated food products. Appl. Microbiol. 22:220-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO. 2002. Terrorist threats to food: guidance for establishing and strengthening prevention and response systems. World Health Organization, Geneva, Switzerland.