Abstract
Despite the ubiquity of ammonium in geothermal environments and the thermodynamic favorability of aerobic ammonia oxidation, thermophilic ammonia-oxidizing microorganisms belonging to the crenarchaeota kingdom have only recently been described. In this study, we analyzed microbial mats and surface sediments from 21 hot spring samples (pH 3.4 to 9.0; temperature, 41 to 86°C) from the United States, China, and Russia and obtained 846 putative archaeal ammonia monooxygenase large-subunit (amoA) gene and transcript sequences, representing a total of 41 amoA operational taxonomic units (OTUs) at 2% identity. The amoA gene sequences were highly diverse, yet they clustered within two major clades of archaeal amoA sequences known from water columns, sediments, and soils: clusters A and B. Eighty-four percent (711/846) of the sequences belonged to cluster A, which is typically found in water columns and sediments, whereas 16% (135/846) belonged to cluster B, which is typically found in soils and sediments. Although a few amoA OTUs were present in several geothermal regions, most were specific to a single region. In addition, cluster A amoA genes formed geographic groups, while cluster B sequences did not group geographically. With the exception of only one hot spring, principal-component analysis and UPGMA (unweighted-pair group method using average linkages) based on the UniFrac metric derived from cluster A grouped the springs by location, regardless of temperature or bulk water pH, suggesting that geography may play a role in structuring communities of putative ammonia-oxidizing archaea (AOA). The amoA genes were distinct from those of low-temperature environments; in particular, pair-wise comparisons between hot spring amoA genes and those from sympatric soils showed less than 85% sequence identity, underscoring the distinctness of hot spring archaeal communities from those of the surrounding soil system. Reverse transcription-PCR showed that amoA genes were transcribed in situ in one spring and the transcripts were closely related to the amoA genes amplified from the same spring. Our study demonstrates the global occurrence of putative archaeal amoA genes in a wide variety of terrestrial hot springs and suggests that geography may play an important role in selecting different assemblages of AOA.
Microorganisms carry out a variety of respiratory processes involving nitrogen species, and these processes are important forces in controlling the form and fate of inorganic nitrogen in nature. Despite recent advances in our understanding of nitrogen cycling activities in soils, fresh and marine waters, and sediments (9, 11, 15, 16, 19, 24), knowledge gaps in high-temperature ecosystems have been slow to fill. In particular, chemolithotrophic ammonia oxidation had not been known to occur at high temperatures until recently (5, 10, 18, 30, 37, 40). In retrospect this is amazing, since ammonium has been observed as the major source of inorganic nitrogen in most geothermal springs, and in some sites, concentrations of NH4-N reach ∼50 mM (31, 42). Consequently, once exposed to earth surface conditions (e.g., 20% O2), spring waters are in disequilibrium with respect to the ammonia/nitrite and ammonia/nitrate redox couples, such that the oxidation of ammonium is reasonably exergonic (14, 35).
In one study, a single phylotype of group 1.1b crenarchaeota, “Candidatus Nitrososphaera gargensis,” was implicated in the near-stoichiometric oxidation of ammonia to nitrite in a 46°C ammonia-oxidizing enrichment (10). The organism was shown to fix carbon in the presence of ammonia, yet this activity was inhibited at high ammonia concentrations. Genes and transcripts predicted to encode two subunits of the ammonia monooxygenase, amoA and amoB, were shown to group within the cluster of archaeal ammonia monooxygenases typically found in soils and sediments. In another study, a highly purified enrichment of “Candidatus Nitrosocaldus yellowstonii” was shown to mediate the conversion of ammonia to nitrite in the absence of fixed carbon at 74°C, significantly increasing the known upper temperature for ammonia oxidation (5). This organism branched basally to the radiation of mesophilic crenarchaeota on the 16S rRNA gene tree and was shown to possess linked amoA and amoB genes. Similar amoA genes were amplified using PCR from hot spring sediment samples from a variety of Yellowstone springs (5). In culture, the predominant membrane lipid of “Candidatus Nitrosocaldus yellowstonii” was the glycerol dialkyl glycerol tetraether (GDGT) crenarchaeol, confirming a thermophilic source for crenarchaeol in hot spring environments (28, 29, 34, 44). Finally, a third study coupled rate measurements of complete nitrification by the 15NO3− isotope pool dilution approach in two 84 to 85°C hot springs in Iceland, with the identification of crenarchaeol in one hot spring (30). Ammonia oxidation rates were limited by the ammonia supply, and the complete oxidation of ammonia to nitrate indicated that yet-unidentified thermophilic nitrite-oxidizing microorganisms also exist. In addition, amoA gene fragments were PCR amplified and sequenced from 14 springs in Iceland and Kamchatka, from 38 to 97°C and pH 2.5 to 7, representing four amoA phylotypes. In summary, this recent work suggests the importance of ammonia oxidation in geothermal systems; however, the abundance and distribution of ammonia-oxidizing organisms in these environments are not well known (10, 18; our unpublished data).
In this study, we used previously established PCR primers (7) to perform an extensive survey of putative archaeal amoA genes from a large number of physicochemically diverse hot springs in the United States (the Great Basin and Yellowstone National Park), China (Tengchong), and Russia (Kamchatka). Our results demonstrate that archaeal amoA genes are ubiquitous in geothermal systems and greatly extend the number and diversity of amoA gene alleles recovered from high-temperature habitats. At least some of these genes are transcribed in situ, and amoA sequences from hot springs are demonstrated to be different from those found in sympatric soils. Although some amoA gene types are found in hot springs on disparate continents, suggesting cosmopolitanism of those types, the majority of amoA gene assemblies present in hot springs were strongly correlated with geographic locations and not temperature or chemical measurements performed in this study.
MATERIALS AND METHODS
Site description, water chemistry, and sample collection.
A total of 21 hot spring samples were analyzed for archaeal amoA genes, which included seven from the Great Basin and six from Yellowstone National Park (United States), five from Tengchong (China), and three from Kamchatka (Russia). At each location, water pH, temperature, and total dissolved solids (TDS) were determined using a Hach pH meter equipped with a pH and temperature probe and a TDS probe or a YSI conductivity meter and a WTW 330 pH meter. Calibration of the pH meter was performed at ambient temperature (∼25°C), and measurements of pH were expressed as pH25°C. Sulfate and hydrogen sulfide were determined at the spring using Hach kits by following the manufacturer's instructions. Nitrate and ammonium were either measured using Hack kits in the field (Great Basin, Yellowstone, and Kamchatka) or determined colorimetrically in the laboratory using samples preserved with HgCl2 (Tengchong). After chemical measurements in the field, the mat or mat-containing sediment was collected in sterile polypropylene tubes or plastic bags using a sterilized spoon. Samples from Great Basin and Yellowstone springs were immediately frozen on dry ice for transportation to the laboratory. Because dry ice was not available in Tengchong and Kamchatka, samples from those sites were transported on wet ice. In the laboratory, samples were stored at −80°C until analysis of DNA or RNA.
DNA extraction, PCR amplification, cloning and sequencing, and phylogenetic analysis.
Genomic DNA was extracted from 5 g (wet weight) of mat or sediment material by using an Ultraclean Mega Prep soil DNA kit (MO Bio Laboratory, Inc., Solana Beach, CA). The precipitated DNA was purified by gel electrophoresis plus minicolumn preparation of a Wizard DNA clean-up system (Promega, Madison, WI). Archaeal amoA gene fragments (approximately 635 bp) were amplified in a 9700 thermal cycler (Perkin-Elmer, Waltham, MA) using the primer pair Arch-amoAF (5′ STAATGGTCTGGCTTAGACG 3′) and Arch-amoAR (5′ GCGGCCATCCATCTGTATGT 3′) (7). PCR cycling was performed by following the method of Francis et al. (7), with initial denaturation at 95°C for 15 min. In order to avoid potential sample biases and to obtain enough PCR product for cloning, three replicated amplifications were carried out for each sample. The combined PCR products were purified by cutting out the appropriate band from a low-melting-point agarose gel (0.8%). The PCR products were purified from the gel using a QIA Quick gel extraction kit (Qiagen, Valencia, CA). The purified DNA was ligated with the pCR vector from TA-cloning kit, and competent Escherichia coli cells were transformed according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Forty to fifty randomly chosen colonies per sample were analyzed for insert archaeal amoA gene sequences. Plasmid DNA containing inserts of the archaeal amoA gene were prepared using a QIAprep Spin miniprep kit (Qiagen, Valencia, CA). Sequencing reactions were carried out by following instructions provided in an ABI BigDye Terminator v. 3.1 kit (Applied Biosystems, Foster City, CA). The archaeal amoA gene sequences were determined with an ABI 3100 automated sequencer. Sequences were typically 600 to 700 bp long.
The sequences were aligned using CLUSTALX 1.83 and compared with reference sequences from the database. Phylogenetic trees were constructed using ARB maximum likelihood (AxML), neighbor joining (Kimura correction), and maximum parsimony with a heuristic search (21). Sequences were assigned to operational taxonomic units (OTUs) by using the nearest-neighbor algorithm in DOTUR at percent identity differences of ≤2% or ≤5% (33). This algorithm adds a query sequence to an existing OTU whenever that sequence is within the specified percent identity level of an existing OTU. Otherwise, a new OTU is created.
RNA extraction, RT-PCR analysis of archaeal amoA gene expression, and construction of cDNA tree.
Samples for reverse transcription (RT)-PCR were collected from the Eagleville hot spring (California) using RNase-free vials and saved immediately on dry ice. Total RNA was extracted using an RNeasy mini kit (Qiagen, Valencia, CA) combined with ballistic cell disruption by vortexing with 0.1-mm sterile glass beads. Purified RNA (5 μl) was reverse transcribed in separate reactions using random hexamers or the archaeon-specific amoA reverse primer (Arch-amoAR) and an iScript Select cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA). Double-stranded cDNA was amplified with archaeon-specific amoA primers (Arch-amoAF and Arch-amoAR) and a HotStarTaq Plus PCR kit (Qiagen, Valencia, CA). PCR products were analyzed by agarose gel electrophoresis (1.0%) with a 1.0-kb molecular size marker. A clone library was constructed for the cDNA sequences from RT-PCR using the same procedure described above.
Statistical analysis.
Rarefaction and richness, including the nonparametric richness estimators Chao1 and ACE and the Shannon diversity index, were calculated using DOTUR (33). A phylogenetic tree derived from the alignment used for Fig. 1 but restricted to amoA gene sequences recovered in this study was analyzed by using the PCA, Cluster Environments, and Jackknife Environment Clusters analyses in UniFrac (20). For Jackknife Environment Clusters, all analyses were run with 1,000 permutations, counting abundance weights. As recommended, “the number of sequences to keep” was set to 15 to account for 75% of the smallest clone library, as suggested previously (20). All three analyses use the UniFrac metric, which measures the distances between communities based on the lineages they contain. In all analyses, each clone library was treated as a community. Environment cluster trees were projected using Tree Explore (38).
FIG. 1.
Maximum likelihood phylogeny of archaeal amoA sequences from DNA libraries and related sequences from hot springs, marine settings, and soils. Nodes with filled circles were supported by maximum likelihood, parsimony, and distance analyses; nodes with open circles were supported by two of the three methods. OTUs (2% percent identity difference) are color coded for springs from different locations. Sample names consist of a two- or three-letter code (see footnote to Table 1) followed by the temperature at the sampling location. Numbers following names of type clones for each OTU are indicated in parentheses.
Nucleotide sequence accession numbers.
The amoA gene sequences reported in this study have been deposited in GenBank. The accession numbers are EU553345 to EU553450 for hot spring archaeal DNA, EU553451 to EU553471 for soil archaeal DNA, and EU553472 to EU553476 for hot spring archaeal cDNA.
RESULTS
Water chemistry.
Temperature, pH, and TDS and nitrate, nitrite, total ammonia, and total sulfide concentrations in the bulk spring water were concurrently determined for all springs (Table 1). Temperature ranged from 41°C to 86°C, and pH ranged from 3.4 to 9.0. Hot springs sampled in the Great Basin were circumneutral to alkaline and included both sodium chloride-dominated springs in the Great Boiling Springs system and sodium bicarbonate-dominated springs in Eagleville and Surprise Valley. Tengchong springs were also circumneutral or alkaline. Springs sampled in Yellowstone National Park included both bicarbonate-buffered circumneutral or alkaline springs in the Mammoth Hot Springs (CSB74), Sylvan Springs (AJ41), the outflow of Yellowstone RCN feature GSSGNN025, and Mirror Plateau geothermal regions (JCS82) and acidic springs in the Norris Geyser Basin (PB75, MG85, and WG66). Samples from Kamchatka were collected from two slightly acidic hot springs (BLS51 and JV73) in the East Thermal Fields and one alkaline spring (GV73) in the Geyser Valley (Table 1).
TABLE 1.
Temperature, water chemistry, and sequencing information for 21 hot spring samples from which archaeal amoA genes were amplifieda
| Sample (GPS location) | Temp (°C) | pH | ΣNH3 (μM) | NO2− (μM) | NO3− (μM) | ΣS2− (μM) | n | OTU
|
H′
|
Chao1
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2% | 5% | 2% | 5% | 2% | 5% | ||||||||
| EV41b (41°12′34.6′′N, 120°03′24.4′′W) | 41.0 | 9.0 | <0.5 | <0.2 | 3.17 | <0.3 | 43 | 2 | 2 | 0.7 | 0.6 | 8 | 3 |
| EV41cDNAb (same as EV41) | 41.0 | 9.0 | <0.5 | <0.2 | 3.17 | <0.3 | 34 | 4 | 4 | 0.8 | 0.8 | 4 | 4 |
| GBS74b (40°39′45.1′′N, 119°21′59.0′′W) | 74.4 | 6.6 | 16.8 | <0.2 | 15.87 | 0.60 | 45 | 2 | 1 | 0.2 | 0.0 | 4 | ND |
| GBS45b (40°39′32.5′′N, 119°21′58.0′′W) | 45.7 | 7.6 | 7.4 | <0.2 | 6.35 | 0.40 | 44 | 6 | 3 | 0.7 | 0.5 | 6 | 3 |
| SV86b (41°32′02.5′′N, 120°04′22.0′′W) | 86.0 | 6.4 | 8.9 | 0.43 | 3.17 | 3.90 | 29 | 6 | 4 | 1.0 | 0.6 | 12 | 5 |
| SV69b (outflow channel of SV86) | 69.2 | 6.2 | 6.3 | <0.2 | 1.59 | 0.10 | 46 | 4 | 4 | 0.8 | 0.5 | 5 | 3 |
| SV60b (outflow channel of SV86) | 60.4 | 6.0 | 3.7 | <0.2 | 1.59 | 0.10 | 45 | 3 | 2 | 0.7 | 0.2 | 3 | 2 |
| SV42b (outflow channel of SV86) | 42.0 | 8.8 | 0.5 | <0.2 | 1.59 | 0.40 | 29 | 3 | 3 | 1.0 | 0.6 | 3 | 2 |
| AJ41c (44°42′01.9′′N, 110°45′55.6′′W) | 41.0 | 8.4 | 66.5 | 0.50 | 106.40 | 1.24 | 44 | 14 | 11 | 2.2 | 2.1 | 51 | 17 |
| PB75c (44°43′43.9′′N, 110°42′12.0′′W) | 75.0 | 3.5 | 68.0 | ND | <0.7 | 1.30 | 39 | 9 | 7 | 1.2 | 1.0 | 12 | 8 |
| JCS82c (44°44′21.4′′N, 110°19′28.2′′W) | 82.0 | 6.2 | 5,722.0 | ND | 9.60 | 20.0 | 41 | 6 | 5 | 1.0 | 1.0 | 9 | 6 |
| MG85c (44°43′27.5′′N, 110°42′20.2′′W) | 85.0 | 4.4 | 158.0 | ND | <0.7 | 30.0 | 39 | 6 | 5 | 1.3 | 1.3 | 8 | 5 |
| CSB74c (44°54′29.1′′N, 110°24′2.42′′W) | 74.0 | 8.2 | 1,167.0 | ND | <0.7 | 290.0 | 42 | 4 | 3 | 0.4 | 0.3 | 7 | 3 |
| WG66c (44°43′43.3′′N, 110°42′11.3′′W) | 66.0 | 3.4 | 74.0 | ND | <0.7 | <0.3 | 34 | 10 | 9 | 1.7 | 1.7 | 13 | 12 |
| LS75d (24°56′59.2′′N, 98°26′16.4′′E) | 75.3 | 7.6 | 8.8 | 0.88 | <0.7 | ND | 44 | 6 | 6 | 1.7 | 1.2 | 9 | 5 |
| BSA59d (24°57′01.2′′N, 98°26′11.3′′E) | 59.3 | 7.5 | 40.5 | 1.13 | 33.02 | ND | 35 | 6 | 5 | 1.3 | 1.2 | 9 | 6 |
| BSB44d (within 5 m of BSA59) | 43.6 | 7.5 | 8.6 | 2.24 | 27.03 | ND | 40 | 8 | 7 | 1.7 | 1.6 | 11 | 8 |
| WM77d (24°57′03.6′′N, 98°26′08.0′′E) | 77.0 | 7.7 | 14.7 | 1.33 | 0.79 | ND | 43 | 6 | 4 | 1.0 | 0.9 | 5 | 4 |
| LP52d (24°57′03.0′′N, 98°26′15.5′′E) | 52.4 | 8.2 | 0.7 | 1.35 | 0.60 | ND | 33 | 11 | 9 | 2.2 | 1.9 | 15 | 10 |
| BLS51e | 50.5 | 6.0 | 1,105.3 | ND | ND | 5.88 | 41 | 8 | 6 | 1.5 | 0.8 | 12 | 6 |
| GV66e | 66.0 | 8.0 | ND | ND | ND | ND | 35 | 7 | 6 | 1.9 | 0.9 | 9 | 6 |
| JV73e (54°30′02.5′′N, 160°00′27.3′′E) | 72.6 | 6.0 | 1,368.4 | ND | ND | 2.94 | 21 | 2 | 1 | 1.2 | 0.7 | 2 | 1 |
The diversity data are based on DNA clone libraries. In addition, a cDNA library was constructed for Eagleville. n, number of clones; ND, not determined; H′, Shannon index. The numbers of OTUs presented in the table were calculated using nucleic acid sequences.
Samples from the Great Basin. EV, Eagleville; GBS, Great Boiling Springs; SV, Surprise Valley. The two-digit number following each sample indicates the temperature of the spring location from which the sample was collected. The same is true for all other locations.
Samples from Yellowstone National Park. AJ, outflow of Yellowstone RCN feature GSSGNN025; PB, Porcelain Basin; JCS, Joseph's Coats Springs; MG, Monarch Geyser; CSB, Calcite Spring “B” in outflow channel; WG, Whirligig Geyser.
Samples from Tengchong, China. LS, Little Spring; BSA, Bridge Spring A; BSB, Bridge Spring B; WM, Wu Ming; LP, Little Pond 2A.
Samples from Kamchatka, Russia. BLS, Burliyashi D; GV, Geyser Valley; JV, Jenn's Vent 3.
The Great Basin hot springs had low concentrations of ammonia (<0.5 to 17 μM) and hydrogen sulfide (<0.3 to 4 μM); both are possible electron donors for chemolithotrophs. Concentrations of ammonia were significantly higher in Yellowstone (68 to 5,700 μM), Tengchong (∼1 to 40 μM), and Kamchatka (∼1,100 to 1,370 μM) springs, and so were concentrations of hydrogen sulfide (Table 1). Nitrite was only detected in the source water of the Surprise Valley spring (0.43 μM) (Table 1) in the Great Basin, but it was significantly higher (0.9 to 2.2 μM) in Tengchong hot springs (Table 1). Similarly high nitrite concentrations have also been observed in other Yellowstone National Park and Kamchatka hot springs (35; also data not shown). Other inorganic electron donors such as arsenite, Fe(II), CH4, H2, and thiosulfate also occur variably in these systems (14, 26, 35-37).
Occurrence and diversity of archaeal amoA genes in hot springs.
Nearly complete putative archaeal amoA gene fragments were obtained from DNA extracted directly from environmental samples by PCR using primers specific for archaeal amoA genes. A total of 812 clones were sequenced from environmental DNA from the 21 hot spring samples. OTUs were calculated separately for each spring using the nearest-neighbor algorithm (33) based on percent identity differences of 2% or 5% (Table 1). The Chao1 diversity estimator, which is a conservative estimator of species richness, estimated between 1 and 17 amoA OTUs per spring at a 5% identity difference (Table 1), which is comparable to the archaeal amoA richness at 5% reported for estuarine sediments (5 to 21 OTUs) and corals (2 to 31 OTUs), respectively (3, 4). The Shannon Index (H′), which takes into account both species richness and evenness, ranged from 0.24 to 2.07 at a 5% identity difference, which was also similar to the range reported for corals, 0.69 to 2.3 (4). Both the Chao1 diversity estimator and the Shannon diversity index correlated significantly with high nitrate concentrations (Table 2). In addition, the Shannon diversity index correlated strongly with nitrite concentration. Neither was highly correlated with ammonia concentration or even the molar ratio of oxidized inorganic nitrogen species (nitrate plus nitrite) to ammonia; therefore, the diversity and evenness of ammonia oxidizers in a geothermal environment appears to be independent of the concentration of source ammonia.
TABLE 2.
Statistical analysis of physicochemical parameters and amoA gene diversity
| Parameter | Pearson moment correlationa
|
|||||
|---|---|---|---|---|---|---|
| OTU
|
Chao1
|
H′
|
||||
| 2% | 5% | 2% | 5% | 2% | 5% | |
| Temp (°C) | −0.21 | −0.21 | −0.33 | −0.23 | −0.25 | −0.24 |
| pH | −0.13 | −0.13 | 0.13 | −0.12 | 0.00 | −0.05 |
| ΣNH3 | −0.07 | −0.07 | −0.07 | −0.07 | −0.09 | −0.07 |
| NO2− | 0.52 | 0.57 | 0.14 | 0.40 | 0.63* | 0.69* |
| NO3− | 0.55* | 0.50* | 0.88* | 0.70* | 0.45* | 0.47* |
| NO2− + NO3− | 0.53* | 0.49* | 0.87* | 0.69* | 0.39* | 0.49* |
| NO2− + NO3−/ΣNH3 | 0.22 | 0.26 | 0.19 | 0.24 | 0.33 | 0.35 |
| ΣS2− | −0.13 | −0.12 | −0.08 | −0.16 | −0.34 | −0.21 |
Pearson moment correlation (r) was determined by using the following equation:  . Asterisks denote a P of <0.05, which is typically regarded as significant, as determined by Excel function TDIST from the t value given by the following equation:
. Asterisks denote a P of <0.05, which is typically regarded as significant, as determined by Excel function TDIST from the t value given by the following equation: 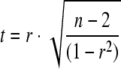 . The number of samples is given by n.
. The number of samples is given by n.
Phylogeny of archaeal amoA genes in hot springs.
Phylogenetic trees based on amoA sequences were constructed to compare hot spring amoA sequences to each other and to those recovered from other habitats (Fig. 1). The amoA genes from hot springs were diverse, with up to 41% nucleotide sequence divergence, but they all branched within the two known major clusters of archaeal amoA sequences: cluster A, with subclusters A1 to A4, and cluster B (3, 4) (Fig. 1). Clone libraries from the different geographic regions contained very different quantities of the major subclusters (Fig. 2). Cluster A, which is typically found in sediments and water, both marine and fresh (3, 4), included 84% of the hot spring clones and four major sequence groups, A1 to A4. Within Cluster A, the hot spring sequences were most closely related to amoA sequences from sediments as opposed to sequences from water columns.
FIG. 2.
Pie charts of the relative abundance of amoA gene clusters in clone libraries from hot springs in different geographic regions.
Group A1 was a large monophyletic clade that appeared to be exclusive to geothermal habitats, including 48% of the clones from this study as well as several clones from an Austrian thermal spring (40). Group A1 sequences were found in hot springs in the Great Basin, Tengchong, and Yellowstone, ranging from 41 to 86°C. However, amoA sequences from the three geothermal regions were not distributed evenly within the group. One clade, designated A1.1, comprised 82% of all amoA sequences from Great Basin springs, whereas two sister clades, together designated A1.2, included 80% of sequences from Tengchong. Clade A1 sequences were 91 to 97% identical to amoA sequences recovered from 46°C, pH 8.0 deep fracture water sampled from an Austrian thermal spring (40) but less than 85% identical to amoA sequences from the marine environment (3, 4).
Groups A2 and A3 branched from within large numbers of sequences recovered from cooler aquatic habitats, both marine and fresh, and “Candidatus Nitrosopumilus maritimus.” These two groups were comprised mainly of amoA sequences from Kamchatka hot springs, together making up 85% of all sequences from that region. However, these two groups also included the majority of sequences from one Great Basin spring, GBS45. A2 and A3 amoA sequences were only recovered from temperatures ranging from 45 to 73°C and 45 to 66°C, respectively, suggesting that organisms harboring these amoA alleles were not hyperthermophiles.
Group A4 was recovered almost exclusively from Yellowstone National Park springs, ranging from 41 to 85°C. Within A4, one OTU alone (AJ41-Clone15) accounted for 73% of Yellowstone National Park amoA sequences and was recovered from each of the six Yellowstone National Park springs that were included in the study.
Cluster B, which includes sequences from soil and sediment habitats (3, 4), included only 16% of the hot spring amoA sequences; however, each of the four geographic regions and nearly the full range of pHs and temperatures were represented. Although only one OTU was recovered from more than one region, no overt biogeographical pattern was evident among different OTUs. One major OTU from Tengchong was closely related to clone D07 from a 49°C, pH 6.4 geothermal mine adit (37); however, the majority of OTUs from cluster B were related to sequences recovered from geothermally unimpacted soils or sediments (Fig. 1). None of the OTUs from this study were closely related to the amoA gene sequence of “Candidatus Nitrosocaldus yellowstonii” (identity less than 71% [5]), which formed a clade that was separate from cluster A and cluster B, or to “Candidatus Nitrososphaera gargensis,” which branched within cluster B.
Geographic distribution of hot spring amoA genes.
Among the 39 hot spring amoA OTUs amplified from DNA extractions, five were recovered from springs on more than one continent (Fig. 1). The wide geographical range of these OTUs shows that these populations are not distinct, at least at the 2% amoA difference level. However, the phylogenetic structure of OTUs in cluster A suggested that there was a nonrandom geographical distribution of these alleles. Furthermore, even in cluster B, almost all sequences from any one OTU were particular to springs in one geothermal region. To examine the phylogenetic structure more rigorously, derivatives of the phylogenetic tree were analyzed by using UniFrac (20) as three different data sets: cluster A sequences alone (Fig. 3A), cluster B sequences alone (Fig. 3B), and all hot spring sequences (Fig. 3C). For cluster A sequences, both Cluster Environments and PCA analyses separated the communities of ammonia-oxidizing archaea (AOA) into clearly delineated biogeographic clusters, with the exception of Great Basin hot spring GBS45, which grouped with Kamchatka hot springs. The nodes representing the otherwise biogeographic groups were supported by high Jackknife values (>90%). Parallel analyses with cluster B only or with all hot spring amoA gene sequences produced no such biogeographic groups and weakly supported biogeographical clusters, respectively. The geographic clusters of cluster A amoA gene sequences were not due to pH or temperature differences between the geothermal systems. For example, Yellowstone National Park springs included a wide range of pH values and temperatures yet they all hosted similar cluster A amoA alleles (Fig. 1 and 3; Table 1).
FIG. 3.
Trees based on the UniFrac metric of cluster A archaeal amoA gene diversity, including sequence abundance data. Circles represent Jackknife support for the monophyly at that node. Solid circles, >90%; open circles, >70%. Results of principal-component analysis on cluster A were consistent with the UPGMA analysis (unweighted-pair group method using average linkages; data not shown). (A) Cluster A sequences only. (B) Cluster B sequences only. (C) All hot spring sequences.
Archaeal amoA expression in the Eagleville spring, CA.
To evaluate the expression of putative archaeal amoA genes in the hot spring environment, RT-PCR was performed on an environmental sample collected from the Eagleville hot spring (EV41). EV41 was chosen based on prior evidence of archaeal amoA genes in an ammonia-oxidizing enrichment culture containing crenarchaeota (42°C, pH 9.0) established from this spring (our unpublished data). RT-PCR products were recovered from EV41, and a total of 34 clones were sequenced from the cDNA library of the RT-PCR product (Table 1). Three of the four cDNA groups, including 82% of the cDNA sequences, belonged to cluster A1, which circumscribed the dominant amoA gene OTUs found in Great Basin springs. Furthermore, two of these cDNAs were identical to the two amoA OTUs recovered from EV41 (Fig. 4). The third cDNA OTU in cluster A1 (EVcDNA-Clone23) and a single OTU in cluster B (EVcDNA-Clone20) were distinct from any amoA gene sequence, perhaps representing less-abundant but active AOA. Together, the two unique cDNA OTUs (EVcDNA-Clone20 and EVcDNA-Clone23) added to a total of 41 OTUs of amoA gene sequences (39 from DNA sequences) for all samples analyzed.
FIG. 4.
Maximum likelihood phylogeny of archaeal amoA sequences from hot spring samples (DNA and cDNA) and soils (DNA only) at Eagleville and Surprise Valley. Nodes with filled circles were supported by maximum likelihood, parsimony, and distance analyses; nodes with open circles were supported by two of the three methods. OTUs (2% percent identity difference) are color coded for hot springs and soils.
Comparison of hot spring amoA genes with those from sympatric soil.
To examine the possibility that some amoA alleles from this study represent contaminants from nearby soil communities, topsoil samples that appeared to be geothermally unimpacted were collected from within 10 meters of the sources of Eagleville (EV41) and Surprise Valley (SV86) hot springs and used as substrates for DNA extraction and amoA gene PCR and sequencing. Whereas 97% of hot spring sequences from Eagleville and Surprise Valley grouped within cluster A1 (Fig. 1 and 4), every amoA sequence recovered from nearby soils grouped within cluster B (Fig. 1 and 4). Within cluster B, the seven DNA sequences (represented by SV86-Clone24/SV42-Clone10 and SV60-Clone8) and the six cDNA sequences (represented by EVcDNA-Clone20) amplified from hot spring sediments were distinct and distant (<85% similarity) from those recovered from sympatric soils (Fig. 4). These results indicate that Eagleville and Surprise Valley hot springs have distinct AOA community compositions compared with surrounding soils.
DISCUSSION
Phylogenetic structure of archaeal amoA alleles in hot spring sediments and mats.
Archaeal amoA genes recovered from hot springs in this study belonged to two distinct clusters. The amoA clade represented by “Candidatus Nitrosocaldus yellowstonii” and related sequences from Yellowstone National Park hot springs (5) were not detected. It is possible that these organisms were present below the limit of detection. Alternatively, the PCR primers might not match the target sites. Therefore, it is likely that the putative AOA revealed in this study underestimate the in situ diversity. Furthermore, none of these putative ammonia monooxygenases have been characterized biochemically, so their function in chemolithotrophic ammonia oxidation in situ remains somewhat speculative.
In each of the four geothermal regions examined, the predominant clones belonged to cluster A (84% of sequences total), which is the dominant type found in marine and estuarine sediments and waters (3, 7), whereas only 16% of sequences belonged to cluster B, which to date has been found primarily in geothermally unimpacted soils and sediments (19, 39). The distribution of sequences within amoA cluster A was not random with respect to geographical location. Four different clades within cluster A emerged from the studies of amoA alleles recovered from hot springs: A1 to A4. Cluster A1 appears to be exclusive to geothermal habitats. This sequence type was geographically widespread, yet it was dominant only among clones from Great Basin and Tengchong hot springs (Fig. 2). It is possible that these genes derive from archaea within the Great Basin hot spring crenarchaeal cluster I (GBSHSC1), which was designated to describe the dominant archaeal 16S rRNA genes recovered from several 56 to 67°C spring systems in the Great Basin, including Surprise Valley (13). The Surprise Valley and Eagleville samples that were the source for cluster A1.1 amoA alleles also contained a preponderance of crenarchaeol relative to other GDGTs (28, 29, 44). However, in the absence of a pure or highly enriched culture of GBSHSC1, amoA gene alleles from these springs cannot be linked definitively to either 16S rRNA phylotypes or particular membrane lipids.
Biogeography of archaeal amoA in hot springs.
Continental hot springs are logical focal points for biogeography studies because surface expressions of geothermal waters are disjunctive, have uneven geographical distribution, and are therefore “island-like.” The most careful biogeography studies of the hot spring microflora by Whitaker et al. (41) and Papke et al. (27) have revealed highly nonrandom distributions of thermophiles. Strains of Sulfolobus islandicus are globally distributed in springs of appropriate pH and temperature, yet populations are clearly endemic, even within different geothermal regions of Yellowstone National Park (41). However, analyses of third-position nucleotides for several coding genes were required to resolve these populations. Conversely, Papke et al. (27) showed that one major 16S rRNA gene type of cyanobacteria (A/B lineage) was endemic to hot springs of North America. Other 16S rRNA gene lineages were present in springs on multiple continents, yet different lineages dominated in springs on different continents and internal transcribed sequence groups were nonrandomly distributed with respect to geography.
This study uncovered a nonrandom geographical distribution of archaeal amoA sequences, particularly within cluster A. This pattern was broadly similar to observations by Francis et al. (7) for marine sediment samples in which the majority of archaeal amoA sequences were unique to particular sampling locations. On the other hand, marine pelagic AOA seem to be much more cosmopolitan, with respect to sequences of both the 16S rRNA gene (22) and the amoA gene (7).
It is intriguing that Great Basin spring GBS45 has an AOA community comprised of amoA sequences similar to some from Kamchatka hot springs (clusters A2 and A3 and cluster B) (Fig. 1). Nevertheless, with the exception of a few identical sequences within the OTU represented by BLS51-Clone27 (Fig. 1), the related sequences from GBS45 and Kamchatka are all distinct, and phylogenetic analysis within these OTUs shows a biogeography-specific structure. This suggests that these AOA have diverged due to allopatry and are on separate evolutionary trajectories. The striking similarity between the AOA in GBS45 and Kamchatka hot springs suggests that the broad-phylogenetic-scale biogeographic clustering uncovered in this study may be susceptible to sampling bias and suggests that thermophilic AOA populations are controlled by a yet-unidentified geochemical parameter that, itself, is not distributed randomly with respect to geography.
Environmental factors affecting AOA.
Considerable efforts have been made by a number of research groups to understand how environmental variables may affect the diversity, abundance, and activity of archaea in low-temperature soil or aquatic environments (1, 7, 11, 12, 17, 19, 23-25, 32, 39, 43). So far, patterns between environmental variables and genetic properties have not been demonstrated. Although the types of archaea harboring amoA genes require further elucidation, geothermal systems offer advantages for linking genetic attributes with geochemical parameters, given their relative simplicity. In the current study, a positive correlation between amoA gene diversity and nitrate and nitrite concentrations was noted. A similar observation was made by Murray et al. (23), who noted a correlation between nitrite concentration and archaeal abundance in the Santa Barbara Channel. Since nitrite and nitrate are the products of ammonia and nitrite oxidation, respectively, these correlations are reflective of nitrification activity and/or potential rather than predictive.
Distinctness and ecological importance of thermophilic AOA.
It has previously been suggested that archaeal 16S rRNA genes distantly related to the Thermoprotei and the archaeal lipid crenarchaeol owe their presence in continental hot springs to contamination from adjacent communities of soil archaea (6, 34). The discovery of thermophilic AOA containing crenarchaeol notwithstanding (5, 10), the possibility remains that some of the amoA alleles recovered in this study may derive from soil AOA, particularly those in cluster B, which is ubiquitous in soils and sediments (3, 4, 8). However, the distinction in AOA community structure between Eagleville hot spring and adjacent soil samples suggests that thermophily has arisen, or been lost, multiple times throughout the evolution of cluster B AOA. Furthermore, the dominance in hot spring sediments of cluster A amoA alleles, which are not found in soils, suggests that the majority of amoA alleles recovered in this study are from indigenous thermophiles.
Given the apparent ubiquity of AOA in continental hot springs (this study; 5, 30), the ubiquity of ammonia in the source water of hot springs, and the thermodynamic favorability of ammonia oxidation in these systems (2, 14, 35), ammonia oxidation may be a major source of energy flux through hot spring ecosystems. This hypothesis is supported by the recent measurements of high ammonia oxidation rates in two Iceland hot springs (30). Because ammonia-oxidizing bacteria have not been detected as widely as AOA in the hot spring environments (10, 18), AOA may play a dominant role in the nitrogen cycle in these environments. The nitrite produced by these organisms (5, 10) does get oxidized to nitrate even at 85°C (30). Although Nitrospira spp. have been cultivated up to 60°C (18), it is unclear which organisms oxidize nitrite to nitrate at higher temperatures. Future research needs to couple in situ measurements with experiments to identify important community members that may be contributing to various N transformations, including denitrification or nitrogen fixation.
In summary, our study demonstrates the global occurrence of putative AOA in sediments and microbial mats in a wide variety of terrestrial hot springs. Most of the putative AOA communities appear to be structured largely by geographic locations even in Yellowstone National Park, where the geochemical diversity of geothermal systems is exceptional. However, the similarity in AOA communities between one Great Basin spring (GBS45) and those in Kamchatka provides evidence that geographically separated springs can have similar AOA communities.
Acknowledgments
We thank David and Sandy Jamieson and Suzy Jackson for their great hospitality and support during our sampling expedition in the Great Basin. Thanks are due to Doug Crowe and Paul Schroeder as well as colleagues from the Microbiology Institute of the Russian Academy of Sciences (Anna Perevalova, Nikolai Pimenov, and Tatyana Sokolova) and the Institute of Volcanology (Gennadii A. Karpov) for field support during sampling of Kamchatka hot springs. We thank Ann Pearson and her undergraduate students from Harvard University for helping with sampling and Minhan Dai of Xiamen University for analyses of ammonia, nitrate, and nitrate from the hot springs in Tengchong, China. Kyle Costa at UNLV helped with analysis of amoA gene sequences.
Funding for this research was provided by the National Science Foundation to C.L.Z., J.W., and C.S.R. (MCB-0348180), B.P.H. (MCB-0546865), and E.L.S. (EAR-0525561). C.R. was funded with NIH grant P20 RR-01464 from the INBRE program of the National Center for Research Resources. W.P.I. appreciates support for this research from the Thermal Biology Institute (NASA project NAG5-8807).
Footnotes
Published ahead of print on 1 August 2008.
REFERENCES
- 1.Adair, K., and E. Schwartz. 2008. Evidence that ammonia-oxidizing archaea are more abundant than ammonia-oxidizing bacteria in soils along an elevation gradient in northern Arizona, USA. Microb. Ecol. doi: 10.1007/s00248-007-9360-9. [DOI] [PubMed]
- 2.Amend, J. P., and E. L. Shock. 2001. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 25:175-243. [DOI] [PubMed] [Google Scholar]
- 3.Beman, J. M., and C. A. Francis. 2006. Diversity of ammonia-oxidizing archaea and bacteria in the sediments of a hypernutrified subtropical estuary: Bahia del Tobari, Mexico. Appl. Environ. Microbiol. 72:7767-7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beman, J. M., K. J. Roberts, L. Wegley, F. Rohwer, and C. A. Francis. 2007. Distribution and diversity of archaeal ammonia monooxygenase genes associated with corals. Appl. Environ. Microbiol. 73:5642-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Torre, J. R., C. B. Walker, A. E. Ingalls, M. Könneke, and D. A. Stahl. 2008. Cultivation of a thermophilic ammonia-oxidizing archaeon synthesizing crenarchaeol. Environ. Microbiol. 10:810-818. [DOI] [PubMed] [Google Scholar]
- 6.DeLong, E. F. 1998. Everything in moderation: archaea as ‘non-extremophiles.’ Curr. Opin. Genet. Dev. 8:649-654. [DOI] [PubMed] [Google Scholar]
- 7.Francis, C. A., K. J. Roberts, J. M. Beman, A. E. Santoro, and B. B. Oakley. 2005. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 102:14683-14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis, C. A., J. M. Beman, and M. M. M. Kuypers. 2007. New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation. ISME J. 1:19-27. [DOI] [PubMed] [Google Scholar]
- 9.Geets, J., N. Boon, and W. Verstraete. 2006. Strategies of aerobic ammonia-oxidizing bacteria for coping with nutrient and oxygen fluctuations. FEMS Microbiol. Ecol. 58:1-13. [DOI] [PubMed] [Google Scholar]
- 10.Hatzenpichler, R., E. V. Lebedeva, E. Spieck, K. Stoecker, A. Richter, H. Daims, and M. Wagner. 2008. A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc. Natl. Acad. Sci. USA 105:2134-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He, J., J. Shen, L. Zhang, Y. Zhu, Y. Zheng, M. Xu, and H. Di. 2007. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 9:2364-2374. [DOI] [PubMed] [Google Scholar]
- 12.Herfort, L., S. Schouten, B. Abbas, M. J. W. Veldhuis, M. J. L. Coolen, C. Wuchter, J. P. Boon, G. J. Herndl, and J. S. S. Damste. 2007. Variation in spatial and temporal distribution of Archaea in the North Sea in relation to environmental variables. FEMS Microbiol. Ecol. 62:242-257. [DOI] [PubMed] [Google Scholar]
- 13.Huang, Z., J. Wiegel, J. Zhou, B. Hedlund, and C. L. Zhang. 2007. Molecular phylogeny of uncultivated crenarchaeota in Great Basin hot springs of moderately elevated temperature. Geomicrobiol. J. 24:535-542. [Google Scholar]
- 14.Inskeep, W. P., G. G. Ackerman, W. P. Taylor, M. Kozubal, S. Korf, and R. E. Macur. 2005. On the energetics of chemolithotrophy in nonequilibrium systems: case studies of geothermal springs in Yellowstone National Park. Geobiology 3:297-317. [Google Scholar]
- 15.Jetten, M. S., I. Cirpus, B. Kartal, L. van Niftrik, K. T. van de Pas-Schoonen, O. Sliekers, S. Haaijer, W. van der Star, M. Schmid, J. van de Vossenberg, I. Schmidt, H. Harhangi, M. van Loosdrecht, J. Gijs Kuenen, H. Op den Camp, and M. Strous. 2005. 1994-2004: 10 years of research on the anaerobic oxidation of ammonium. Biochem. Soc. Trans. 33:119-123. [DOI] [PubMed] [Google Scholar]
- 16.Könneke, M., A. E. Bernhard, J. R. de la Torre, C. B. Walker, J. B. Waterbury, and D. A. Stahl. 2005. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543-546. [DOI] [PubMed] [Google Scholar]
- 17.Lam, P., M. M. Jensen, G. Lavik, D. F. McGinnis, B. Müller, C. J. Schulbert, R. Amann, B. Thamdrup, and M. M. Kuypers. 2007. Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proc. Natl. Acad. Sci. USA 104:7104-7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebedeva, E. V., M. Alawi, C. Fiencke, B. Namsaraev, E. Bock, and E. Spieck. 2005. Moderately thermophilic nitrifying bacteria from a hot spring of the Baikal rift zone. FEMS Microbiol. Ecol. 54:297-306. [DOI] [PubMed] [Google Scholar]
- 19.Leininger, S., T. Urich, M. Schloter, L. Schwark, J. Qi, G. W. Nicol, J. I. Prosser, S. C. Schuster, and C. Schleper. 2006. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806-809. [DOI] [PubMed] [Google Scholar]
- 20.Lozupone, C., M. Hamady, and R. Knight. 2006. UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics. doi: 10.1186/1471-2105-7-371. [DOI] [PMC free article] [PubMed]
- 21.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, A. Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jacob, W. Förster, I. Brettske, S. Gerber, A. W. Ginart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. König, T. Liss, R. Lüβmann, M. May, B. Nonhoff, B. Reichel, R. Strhlow, A. Stamatkis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massana, R., E. F. DeLong, and C. Pedros-Alio. 2000. A few cosmopolitan phylotypes dominate planktonic archaeal assemblages in widely different oceanic provinces. Appl. Environ. Microbiol. 66:1777-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray, A. E., K. Y. Wu, C. L. Moyer, D. M. Karl, and E. F. DeLong. 1999. Evidence for circumpolar distribution of planktonic Archaea in the Southern Ocean. Aquat. Microb. Ecol. 18:263-273. [Google Scholar]
- 24.Nicol, G. W., and C. Schleper. 2006. Ammonia-oxidising Crenarchaeota: important players in the nitrogen cycle? Trends Microbiol. 14:207-212. [DOI] [PubMed] [Google Scholar]
- 25.Nicol, G. W., G. Webster, L. A. Glover, and J. I. Prosser. 2004. Differential response of archaeal and bacterial communities to nitrogen inputs and pH changes in upland pasture rhizosphere soil. Environ. Microbiol. 6:861-867. [DOI] [PubMed] [Google Scholar]
- 26.Nordstrom, D. K., J. W. Ball, and R. B. McCleskey. 2005. Ground water to surface water: chemistry of thermal outflows in Yellowstone National Park, p. 143-162. In W. P. Inskeep and T. R. McDermott (ed.), Geothermal biology and geochemistry in Yellowstone National Park, vol. 1. Montana State University, Bozeman, MT. [Google Scholar]
- 27.Papke, R. T., N. B. Ramsing, M. M. Bateson, and D. M. Ward. 2003. Geographical isolation in hot spring cyanobacteria. Environ. Microbiol. 5:650-659. [DOI] [PubMed] [Google Scholar]
- 28.Pearson, A., Z. Huang, A. E. Ingalls, C. S. Romanek, J. Wiegel, K. H. Freeman, R. H. Smittenberg, and C. L. Zhang. 2004. Nonmarine crenarchaeol in Nevada hot springs. Appl. Environ. Microbiol. 70:5229-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson, A., Y. Pi, W. Zhao, W. Li, Y.-L. Li, W. Inskeep, E. Bonch-Osmolavskaya, C. S. Romanek, S. Li, and C. L. Zhang. 2008. Factors controlling the distribution of archaeal tetraethers in terrestrial hot springs. Appl. Environ. Microbiol. 74:3523-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reigstad, L. J., A. Richter, H. Daims, T. Ulrich, L. Schwark, and C. Schleper. 2008. Nitrification in terrestrial hot springs of Iceland and Kamchatka. FEMS Microbiol. Ecol. 64:167-174. [DOI] [PubMed] [Google Scholar]
- 31.Reysenbach, A.-L., and E. Shock. 2002. Merging genomes with geochemistry at hydrothermal ecosystems. Science 296:1077-1082. [DOI] [PubMed] [Google Scholar]
- 32.Santoro, A. E., C. A. Francis, N. R. de Sieyes, and A. B. Boehm. 2008. Shifts in the relative abundance of ammonia-oxidizing bacteria and archaea across physicochemical gradients in a subterranean estuary. Environ. Microbiol. 10:1068-1079. [DOI] [PubMed] [Google Scholar]
- 33.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schouten, S., M. T. van der Meer, E. C. Hopmans, W. I. Rijpstra, A. L. Reysenbach, D. M. Ward, and J. S. S. Damsté. 2007. Archaeal and bacterial glycerol dialkyl glycerol tetraether lipids in hot springs of Yellowstone National Park. Appl. Environ. Microbiol. 73:6181-6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shock, E. L., M. Holland, D. R. Meyer-Dombard, and J. P. Amend. 2005. Geochemical sources of energy for microbial metabolism in hydrothermal ecosystems: Obsidian Pool, Yellowstone National Park, USA, p. 95-112. In W. P. Inskeep and T. R. McDermott (ed.), Geothermal biology and geochemistry in Yellowstone National Park, vol. 1. Montana State University, Bozeman, MT. [Google Scholar]
- 36.Spear, J. R., J. J. Walker, T. M. McCollom, and N. R. Pace. 2005. Hydrogen and bioenergetics in the Yellowstone geothermal ecosystem. Proc. Natl. Acad. Sci. USA 102:2555-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spear, J. R., H. A. Barton, C. E. Robertson, C. A. Francis, and N. R. Pace. 2007. Microbial community biofabrics in a geothermal mine adit. Appl. Environ. Microbiol. 73:6172-6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 39.Treusch, A. H., S. Leininger, A. Kletzin, S. C. Schuster, H. P. Klenk, and C. Schleper. 2005. Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ. Microbiol. 7:1985-1995. [DOI] [PubMed] [Google Scholar]
- 40.Weidler, G. W., M. Dornmayr-Pfaffenhuemer, F. W. Gerbl, W. Heinen, and H. Stan-Lotter. 2007. Communities of Archaea and Bacteria in a subsurface radioactive thermal spring in the Austrian Central Alps, and evidence of ammonia-oxidizing Crenarchaeota. Appl. Environ. Microbiol. 73:259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitaker, R. J., D. W. Grogan, and J. W. Taylor. 2003. Geographic barriers isolate endemic populations of hyperthermophilic archaea. Science 301:976-978. [DOI] [PubMed] [Google Scholar]
- 42.Windman, T., N. Zolotova, F. Schwandner, and E. L. Shock. 2007. Formate as an energy source for microbial metabolism in chemosynthetic zones of hydrothermal ecosystems. Astrobiology 7:873-890. [DOI] [PubMed] [Google Scholar]
- 43.Wuchter, C., B. Abbas, M. J. L. Coolen, L. Herfort, J. van Bleijswijk, P. Timmers, M. Strous, E. Teira, G. J. Herndl, and J. J. Middelburg. 2006. Archaeal nitrification in the ocean. Proc. Natl. Acad. Sci. USA 103:12317-12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, C. L., A. Pearson, Y.-L. Li, G. Mills, and J. Wiegel. 2006. Thermophilic temperature optimum for crenarchaeol synthesis and its implication for archaeal evolution. Appl. Environ. Microbiol. 72:4419-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]






