Abstract
A phylogenetic comparison of microbial communities in hypersaline evaporites was conducted on crusts from Guerrero Negro, Mexico, and Lindsey Lake, New Mexico, using culture-independent rRNA gene sequence analysis. Many sequences were shared between evaporites, which suggests that similar environments select for specific microbial lineages from a global metacommunity.
Hypersaline environments, although commonly considered “extreme” (8, 13), harbor diverse microbial communities (6, 16, 17). Most microbiological characterizations of hypersaline environments have focused on microbial mat communities found in solar crystallizers, such as Guerrero Negro, Mexico (6, 12, 17), Solar Lake, Sinai, Egypt (5), and Camargue, France (18). In the final stages of evaporative crystallization, deposits that consist primarily of halite (NaCl) and gypsum (CaSO4·2H2O) develop in brine containing at least 130 g/liter NaCl. The crystal lattice structure of these deposits (evaporites) can facilitate channeling of light relatively deeply into the crusts (∼1 to 2 cm or more) and create niches for halophilic photosynthetic communities.
Microbial communities within evaporites (endoevaporites) frequently appear as colorful strata due to organisms with various photosynthetic and radiation-protective pigments. Although some microbial characterizations of evaporitic crusts have been conducted, culture-independent, sequence-based analyses have been reported only recently (16, 17). This study builds upon previous results through the analysis of microbial communities in crusts from two different hypersaline environments, the use of statistical analyses to understand how microbial communities are structured within evaporites, and the analysis of geographical effects on sequence distribution.
Evaporite crust samples were collected from pond 9 of the Exportadora de Sal, SA, saltern in Guerrero Negro, Baja California Sur, Mexico, and from Lindsey Lake, located near Carlsbad, NM. Crust samples from Guerrero Negro were collected in November 2005 (GNR05) and March 2006 (GNR06), while the Lindsey Lake sample was collected in April 2003. The GNR05 sample was taken from the southwestern corner of pond 9, while the GNR06 sample was collected from the southeastern corner. Layers of crusts were separated aseptically and stored at −80°C or at 4°C for immediate processing. Details of evaporite samples are described in Table S1 in the supplemental material.
DNA was extracted from isolated layers either by phenol-chloroform extraction combined with a bead-beating protocol (3) or by use of a PowerSoil extraction kit from Mobio, Inc. (Carlsbad, CA). 16S rRNA genes were amplified using universal and domain-specific primers, resulting in 1,172 gene sequences. Phylogenetic analyses were conducted to understand the relatedness of specific sequences, and the statistical programs UniFrac (7) and DOTUR (14) were used to compare the microbial community structures of the different endoevaporites (see the supplemental material for detailed methods).
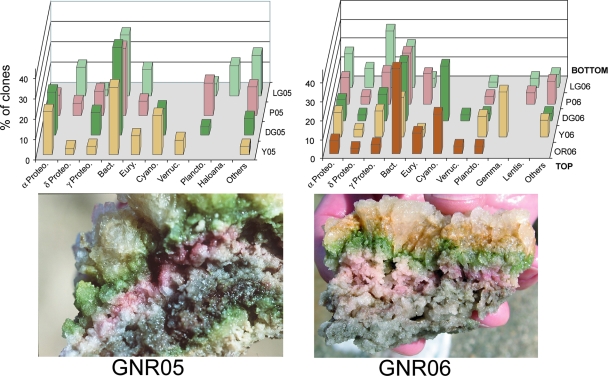
The goal of this study was to obtain an overview of the microbial community composition of brine-saturated communities by using culture-independent 16S rRNA gene sequence analysis. The Lindsey Lake endoevaporites are much less developed than those from Guerrero Negro, with limited overall microbial diversity (see Table S1 in the supplemental material); therefore, the majority of the microbial community discussion will focus on the endoevaporites from Guerrero Negro. The results indicate that the Guerrero Negro communities show relatively low phylum level diversity (Fig. 1). Phylogenetic trees of representative sequences from the domains Bacteria (see Fig. S1A in the supplemental material) and Archaea (see Fig. S1B in the supplemental material) show close relationships between many of the sequences obtained in this study and sequences obtained from cultured halophiles, an endoevaporite in Israel (16), the Guerrero Negro microbial mat (6), and comparable environments in additional studies. These results show at the broad scale that hypersaline environments select for similar phylogenetic types (phylotypes) of organisms.
FIG. 1.
Three-dimensional bar graphs showing the community composition of endoevaporitic crusts from GNR05 and GNR06, using universal primers. Bars from each layer represent the percentages of clones in that particular library. The following abbreviations are used: Proteo., Proteobacteria; Bact., Bacteroidetes; Cyano., Cyanobacteria; Plancto., Planctomycetes; Verruc., Verrucomicrobia; Lentis., Lentisphaerae; Eury., Euryarchaeota; Gemma., Gemmatimonadetes; Haloana., Haloanaerobiales. “Others” indicates pooled divisions that represent <1% of the library. Layers of each endoevaporite are abbreviated by the color (OR, orange; Y, yellow; DG, dark green; P, pink; LG, light green) and the year the sample was collected (05, 2005; 06, 2006). The brown layer from GNR06 is not shown. (Photograph of GNR06 crust from NASA [courtesy of Brad Bebout].)
In general, bacterial sequences dominated universal primer libraries from each endoevaporite, while no microbial eucaryotes were observed in any of the libraries. Of the 227 archaeal sequences obtained from endoevaporitic communities in this study, no sequences grouped with crenarchaeaota. These results are consistent with other studies of brine environments (2, 16, 17) in which both eucaryotes and crenarchaeotes are rare or absent.
Shallow layers in Guerrero Negro endoevaporites contained many sequences closely related to known phototrophic microbes. Cyanobacteria (primarily Euhalothece) and Ectothiorhodospiraceae sequences were prevalent in the libraries from shallow layers. However, the dominant phylum present in sequences from shallow layers was Bacteroidetes. Many Bacteroidetes sequences showed >96% sequence identity and related phylogeny to Salinibacter ruber (an aerobic, halophilic, heterotrophic microorganism) (10). These phylotypes are closely related to sequences from an endoevaporite community in Israel (16), a gypsum crust in France (11), and the north arm of the Great Salt Lake, Utah (John Spear, unpublished) (see Fig. S2 and Table S2 in the supplemental material).
Deeper layers in the Guerrero Negro crusts revealed a wide variety of phylotypes, including those phylogenetically similar to Chromatium spp. (pink layers), Desulfohalobiaceae (light green layers), Desulfobacteraceae (light green layers), Salinivibrio (brown layers), and Idiomarinaceae (light green layers). Sequences associated with known photosynthetic microbes were absent in the deepest regions of the crusts (light green and brown layers), indicating that this may be the limit of light penetration.
The statistical comparative software program UniFrac (7) showed that the microbial community composition of each layer was significantly distinct from that of each adjacent layer (P < 0.03) and that layers with the same pigment shared between years generally clustered (see Fig. S3 in the supplemental material) when the two endoevaporites from Guerrero Negro were compared. Overall, the total community from Guerrero Negro was significantly different from the community from Lindsey Lake, as was expected (P < 0.03). These differences are likely due to geographic isolation, where history of separation can overwhelm similar environmental stresses in selection for microbial community structure (4).
To determine the distribution of phylotypes in endoevaporites, sequences from Guerrero Negro, Lindsey Lake, and a community from Eilat, Israel (16), were binned into operational taxonomic units (OTUs) based on a shared-sequence threshold (97% identity); the sequences found to be shared between OTUs in pairwise comparisons of environments were compiled. The results show that although the endoevaporites from Guerrero Negro and Eilat show large-scale geographical separation, the two systems contain many sequences from shared OTUs (Fig. 2; also see Table S3 in the supplemental material). The percentage of overlapping sequences from shared OTUs is relatively high, especially considering the low sampling effort conducted; for comparison, the GN05 and GN06 evaporites shared only 36.6% ± 1.1% of sequences from common OTUs. And although the Guerrero Negro and Lindsey Lake endoevaporites showed shared sequences from common OTUs (Fig. 2; also see Table S3 in the supplemental material), the Lindsey Lake and Eilat endoevaporites showed almost no overlap (Fig. 2). The causative forces behind the observation of phylotypes shared between Guerrero Negro and Eilat are difficult to determine with 16S rRNA gene data alone. However, based on the results of this study, factors such as similar geochemical parameters (1, 15) and/or common dispersion mechanisms (e.g., ocean transport) (9) may select for specific microbial lineages from a global metacommunity in these systems.
FIG. 2.
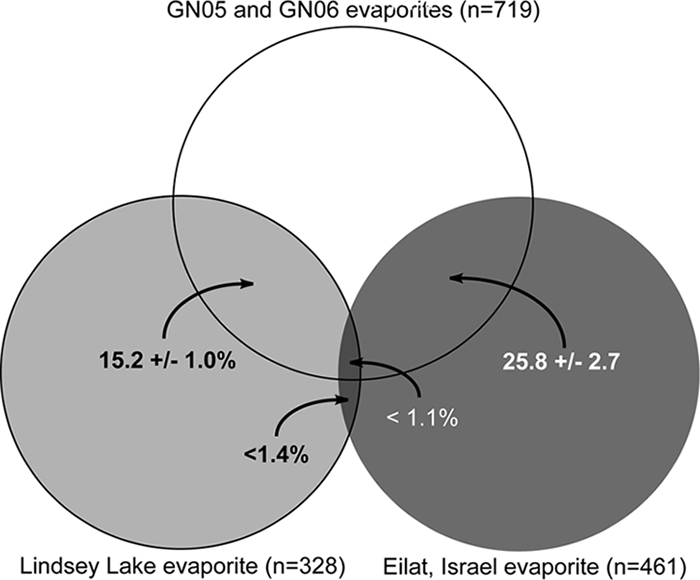
Venn diagram showing average shared-sequence similarities for three endoevaporites. Similarity is presented as a percentage and a corresponding 95% confidence range where applicable.
Nucleotide sequence accession numbers.
A total of 1,172 sequences were generated from all sampled environments; all sequences were submitted to GenBank under accession numbers EF105550 to EF106721.
Supplementary Material
Acknowledgments
We thank the Exportadora de Sal of Guerrero Negro, Baja California Sur, Mexico, for access to sampling locations. Crust samples from Lindsey Lake were collected and graciously provided by Jeff Gillow, Denver, CO. Many thanks go to the Guerrero Negro team from NASA-Ames, including Moira Doty, Jason Smith, Brad Bebout, Dave DesMarais, Tori Hoehler, Mary Hogan, and Kendra Turk. We thank Leah Feazel and Kirk Harris from the Pace laboratory for assistance in operating the MegaBACE 1000. Additional thanks go to Kirk Harris and Jill Tomaras for helpful comments regarding the manuscript and Erik Sahl for programming assistance.
Funding for this work was provided by an NSF microbiology postdoctoral fellowship and a NASA-ASTEP grant to J.R.S. and an astrobiology grant to N.R.P.
Footnotes
Published ahead of print on 29 August 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Canfield, D. E., K. B. Sørensen, and A. Oren. 2004. Biogeochemistry of a gypsum-encrusted microbial ecosystem. Geobiology 2:133-150. [Google Scholar]
- 2.Cytryn, E., D. Minz, R. S. Oremland, and Y. Cohen. 2000. Distribution and diversity of Archaea corresponding to the limnological cycle of a hypersaline stratified lake (Solar Lake, Sinai, Egypt). Appl. Environ. Microbiol. 66:3269-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dojka, M. A., P. Hugenholtz, S. K. Haack, and N. R. Pace. 1998. Microbial diversity in a hydrocarbon- and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl. Environ. Microbiol. 64:3869-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes Martiny, J. B., B. J. M. Bohannan, J. H. Brown, R. K. Colwell, J. A. Fuhrman, J. L. Green, M. C. Horner-Devine, M. Kane, J. A. Krumins, C. R. Kuske, P. J. Morin, S. Naeem, L. Øvreås, A.-L. Reysenbach, V. H. Smith, and J. T. Staley. 2006. Microbial biogeography: putting microorganisms on the map. Nat. Rev. 4:102-112. [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen, B. B., and N. P. Revsbech. 1983. Photosynthesis and structure of benthic microbial mats: microelectrode and SEM studies of four cyanobacterial communities. Limnol. Oceanogr. 28:1075-1093. [Google Scholar]
- 6.Ley, R. E., J. K. Harris, J. Wilcox, J. R. Spear, S. R. Miller, B. M. Bebout, J. A. Maresca, D. A. Bryant, M. L. Sogin, and N. R. Pace. 2006. Unexpected diversity and complexity of the Guerrero Negro hypersaline microbial mat. Appl. Environ. Microbiol. 72:3685-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lozupone, C., M. Hamady, and R. Knight. 2006. UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. Bioinformatics 7:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madigan, M. T. 2003. Anoxygenic phototrophic bacteria from extreme environments. Photosynth. Res. 76:157-171. [DOI] [PubMed] [Google Scholar]
- 9.Massana, R., E. F. DeLong, and C. Pedrós-Alió. 2000. A few cosmopolitan phylotypes dominate planktonic archaeal assemblages in widely different oceanic provinces. Appl. Environ. Microbiol. 66:1777-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mongodin, E. F., K. E. Nelson, S. Daugherty, R. T. DeBoy, J. Wister, H. Khouri, J. Weidman, D. A. Walsh, R. T. Papke, G. S. Perez, A. K. Sharma, C. L. Nesbø, D. MacLeod, E. Bapteste, W. F. Doolittle, R. L. Charlebois, B. Legault, and F. Rodriguez-Valera. 2005. The genome of Salinibacter ruber: convergenece and gene exchange among hyperhalophilic Bacteria and Archaea. Proc. Natl. Acad. Sci. USA 102:18147-18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mouné, S., P. Caumette, R. Matheron, and J. C. Willison. 2003. Molecular sequence analysis of prokaryotic diversity in the anoxic sediments underlying cyanobacterial mats of two hypersaline ponds in Mediterranean salterns. FEMS Microbiol. Ecol. 44:117-130. [DOI] [PubMed] [Google Scholar]
- 12.Nübel, U., M. M. Bateson, M. T. Madigan, M. Kühl, and D. M. Ward. 2001. Diversity and distribution in hypersaline microbial mats of bacteria related to Chloroflexus spp. Appl. Environ. Microbiol. 67:4365-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothschild, L. J., L. J. Giver, M. R. White, and R. L. Mancinelli. 1994. Metabolic activity of microorganisms in evaporites. J. Phycol. 30:431-438. [DOI] [PubMed] [Google Scholar]
- 14.Schloss, P. D., and J. Handelsman. 2005. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl. Environ. Microbiol. 71:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shumilin, E., M. Grajeda-Muñoz, N. Silverberg, and D. Sapozhnikov. 2002. Observations on trace element hypersaline geochemistry in surficial deposits of evaporation ponds of Exportadora de Sal, Guerrero Negro, Baja California Sur, Mexico. Mar. Chem. 79:133-153. [Google Scholar]
- 16.Sørensen, K. B., D. E. Canfield, A. P. Teske, and A. Oren. 2005. Community composition of a hypersaline endoevaporitic microbial mat. Appl. Environ. Microbiol. 71:7352-7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spear, J. R., R. E. Ley, A. B. Berger, and N. R. Pace. 2003. Complexity in natural microbial ecosystems: The Guerrero Negro experience. Biol. Bull. 204:168-173. [DOI] [PubMed] [Google Scholar]
- 18.Wieland, A., J. Zopfi, M. Benthien, and M. Kühl. 2005. Biogeochemistry of an iron-rich hypersaline microbial mat (Camargue, France). Microb. Ecol. 49:34-49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.