Abstract
Removing endotoxins is an important target in the pharmaceutical industry and in clinical practice. A filter introduced into an intravenous line prevents microbiological contamination, but to date no filters have retained bacterial endotoxins. In our study, we assayed a new-generation filter which is able to capture endotoxins from solutions.
Endotoxins are lipopolysaccharides (LPS) which constitute the main component of the outer membranes of gram-negative bacteria. Endotoxins at high doses are pathogenic molecules which act as potent activators of the immune system. Monocytes and macrophages, following LPS stimulation, release mediators with powerful biological and pyrogenic activities. Moreover, LPS interact with the vascular endothelium and stimulate the complement and blood coagulation pathways (1, 6).
LPS are formed by three fractions, known as lipid A, core oligosaccharide, and O-antigen (6, 7). Since LPS are partially phosphorylated, the P group confers the net negative charge.
Removing undesirable endotoxins from aqueous solutions is an important aim in the pharmaceutical industry and in clinical practice. Indeed, intravenous (i.v.) therapy is an integral part of modern patient care and is used in the clinical management of the treatment of more than a quarter of hospitalized patients. Unfortunately, i.v. systems also provide a direct route for microorganisms to enter the bloodstream (4). The introduction of a 0.22-μm filter membrane into the i.v. line prevents inadvertent microbial (bacterial, fungal, and yeast) contamination in i.v. fluids from reaching the patient (3, 8). The removal of bacterial endotoxins from liquids is often difficult. The conventional heat sterilization of liquids and filtration with microporous membrane filters, which kill or remove whole bacterial cells, do not eliminate bacterial endotoxins (10, 11). Endotoxins can be eliminated by heating for long periods at elevated temperatures, while the depyrogenation of heat-sensitive biological materials is not feasible. Endotoxins can be removed using ion exchange resins, activated carbon (5), or asbestos-containing filters (9).
Due to the difficulty in removing endotoxins by conventional methods (10), the production of innovative filters able to retain endotoxins is an important goal for the pharmaceutical industry and for medicine. A new filter, known as Speedflow Positive and equipped with a HI-FLO polyethersulfone (PES) 0.2-μm-pore-size positively charged membrane that electrostatically attracts and/or retains endotoxins, has been produced by GVS SpA (Zola Predosa, Italy) and is referred to hereinafter as filter A. This filter is a modification of a standard filter, known as Speedflow (GVS) and referred to hereinafter as filter C, which guarantees superior flow rate performance and protection against contamination. These new-generation filters (both A and C) are formed by two opposing layers of hydrophilic membranes in a small package; it guarantees mechanical resistance with pump applications, total safety from air embolism, the elimination of large globules in liquids with lipids, and the removal of drug precipitates.
In this study, the two GVS filters A and C were comparatively assayed with two additional filters, B and D, purchased from Pall SpA, Milano, Italy. Filter B is an adult-size 10-cm2 positively charged PES membrane with 0.2-μm pores, and filter D is an adult-size 10-cm2 standard PES membrane with 0.2-μm pores.
The endotoxin retention assay was carried out with an apparatus which consisted of a test filter, sterile tubing sets, Luer lock outlet connections, a peristaltic pump, and nonpyrogenic glass reservoirs. The depyrogenation cycle was set at 200°C for 4 h.
Pyrotell Limulus amoebocyte lysate (LAL) lot no. S05-398 (Associates of Cape Cod [ACC], MA), with a sensitivity of 0.03 endotoxin units (EU)/ml, was used for the gel clot method LAL test. The control standard endotoxin (CSE [lot no. 100; ACC]) was from Escherichia coli strain 0113. The accuracy of the gel clot LAL test was established by the range of endotoxin concentrations determined as indicated by the manufacturer (ACC). However, the exact endotoxin concentrations are not known. Nevertheless, the data obtained in our experiments are consistent with the purpose of our work. The reproducibility of the results obtained with the gel clot LAL test was ensured. There was no variability in the results; indeed, we obtained the same data by repeating the experiments 20 times. Results from four representative experiments are shown in Table 1.
TABLE 1.
Endotoxin measurements by the LAL testa
| Solution | Filtration time (h) | Amt (EU/ml) of endotoxins in (charged) filter A sample:
|
Amt (EU/ml) of endotoxins in (charged) filter B sample:
|
Amt (EU/ml) of endotoxins in (noncharged) filter C sample:
|
Amt (EU/ml) of endotoxins in (noncharged) filter D sample:
|
||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | ||
| CS | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8 | 1 | 0.5 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 24 | 1 | 0.5 | 1 | 1 | 0.5 | 1 | 1 | 1 | |
| 48 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 72 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | 1 | |
| 96 | 1 | 1 | 1 | 1 | 0.5 | 1 | 1 | 1 | |
| FF | 0 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | 0.125 | 0.03 |
| 8 | <0.03 | <0.03 | <0.03 | <0.03 | 0.5 | 1 | 0.25 | 0.5 | |
| 24 | <0.03 | <0.03 | <0.03 | <0.03 | 1 | 0.5 | 1 | 1 | |
| 48 | <0.03 | <0.03 | <0.03 | <0.03 | 2 | 8 | 1 | 1 | |
| 72 | 0.03 | <0.03 | <0.03 | <0.03 | 0.5 | 0.5 | 2 | 1 | |
| 96 | <0.03 | <0.03 | <0.03 | <0.03 | 0.5 | 2 | 1 | 1 | |
| Conclusionb | R | R | R | R | NR | NR | NR | NR | |
Results for 2 samples representative of the 20 samples analyzed per filter are shown. Filters A and C were obtained from GVS, and filters B and D were obtained from Pall.
R, the filter retained endotoxins; NR, the filter did not retain endotoxins.
Although high endotoxin retention rates (from 1 EU/ml to 106 EU/ml) are demanded for membranes used in environmental microbiology, the removal of endotoxins in the range of 0.03 EU/ml to 1 EU/ml is applied in biomedical fields and in clinical practice, where the concentrations used to assess filters are approximately 1 to 2 EU/ml. Indeed, we tested the filters at 1 EU since this is the clinical practice standard for the application of interest (i.v. filtration of clinical solutions). For this reason, our work was focused mostly on the development of a method with replicable results to characterize endotoxin retention at such low levels.
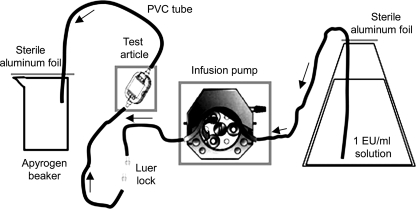
CSE challenge solution (CS) was prepared by dissolving lyophilized CSE in nonpyrogenic water to yield a concentration of 1 EU/ml in 10 liters. To confirm the concentration, a sample of CS was collected and evaluated by the LAL test. The endotoxin retention test was carried out using the following apparatus: a silicon tube edge, attached to the filter, was inserted into the 10-liter reservoir with the 1-EU/ml CSE solution and threaded through an infusion pump; the polyvinyl chloride tube edge terminated in a nonpyrogenic vessel (Fig. 1). The pump was set up to start at 80 ml/h. The first filtrate fluid (FF) was collected at the beginning of the assay, while subsequent samples of FF and CS which had not passed through the filter were collected after 8, 24, 48, 72, and 96 h of filtration and then tested for endotoxin concentrations by the gel clot method LAL test. All test results were deemed to be valid as the CS yielded the same endotoxin concentration measurement (1 EU/ml) in all the experiments. The endotoxin measurements performed on the filtrates from filters A and B consistently indicated that the amounts of endotoxin which had passed through the filters during the infusion experiment time (96 h) were less than 0.03 EU/ml. On the contrary, the filtrates from filters C and D showed amounts of endotoxin similar to that in the CS (1 EU/ml). From these assay results, we can infer that only filters equipped with a positively charged membrane, filters A and B, were able to retain the endotoxin. Indeed, filters with a standard membrane, filters C and D, did not retain the LPS. This result obtained with purified E. coli CSE (ACC) extends the work by Schindler and Dinarello (10). Gerba and Hou (2) demonstrated that increasing the net positive charge on filters results in the enhancement of endotoxin retention. Nevertheless, Gerba and Hou (2) obtained good results with depth filters but not with membrane filters.
FIG. 1.
Apparatus used to carry out the test: an infusion pump was used to test each tubing set and filter, with a 1-EU/ml CSE solution. PVC, polyvinyl chloride.
In this study, we have demonstrated high levels of endotoxin retention using an innovative membrane filter equipped with a positively charged membrane, which electrostatically attracts and/or retains the endotoxins. It is believed that endotoxin retention is mediated by electrostatic interaction forces. Endotoxins easily pass through the 0.2-μm pores of noncharged membrane filters, in which size exclusion is the only retention mechanism. Since endotoxins are negatively charged, the positively charged membrane mounted in our filter may aid the removal of endotoxins, even though these molecules are smaller than the pore size of the filter. The endotoxin retention capabilities of filters equipped with a positively charged membrane indicate that the filters can be safely used for periods of up to 4 days. For this reason, together with endotoxin retention, this filter allows a reduction in therapy cost and treatment time.
Acknowledgments
This work was supported by grants from the SPINNER Consortium, which includes the Emilia-Romagna Region, Italy; the Ministry of Labor and Social Politics and the Ministry of Universities and Research, Rome, Italy; and the European Union, Brussels, Belgium.
We thank Georgia Gili for her excellent assistance in revising the manuscript.
Footnotes
Published ahead of print on 22 August 2008.
REFERENCES
- 1.Baron, S. (ed.) 1996. Medical microbiology, 4th ed. University of Texas Medical Branch, Galveston. [PubMed]
- 2.Gerba, C. P., and K. Hou. 1985. Endotoxin removal by charge-modified filters. Appl. Environ. Microbiol. 50:1375-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes, C. J., R. B. Kundson, R. K. Ausman, and C. W. Walter. 1980. Potential hazards associated with microbial intravenous therapy. J. Clin. Microbiol. 12:725-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorente, L., R. Santacreu, M. M. Martin, A. Jimenez, and M. L. Mora. 2006. Arterial catheter-related infection of 2,949 catheters. Crit. Care 10:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maitra, S. K., T. T. Yoshikawa, L. B. Guze, and M. C. Schotz. 1981. Properties of binding of Escherichia coli endotoxin to various matrices. J. Clin. Microbiol. 13:49-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petsch, D., and F. B. Anspach. 2000. Endotoxin removal from protein solutions. J. Biotechnol. 76:97-119. [DOI] [PubMed] [Google Scholar]
- 7.Pohlman, T. H., R. S. Munford, and J. M. Harlan. 1987. Deacylated lipopolysaccharide inhibits neutrophil adherence to endothelium induced by lipopolysaccharide in vitro. J. Exp. Med. 165:1393-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roe, J. A., and D. Smith. 1995. Filtration and infection control. Pall Corporation, East Hills, NY. http://www.pall.com/34445_6472.asp.
- 9.Rossitto, J. 1979. A solution to the asbestos problem. Pharm. Technol. 2:39-55. [Google Scholar]
- 10.Schindler, R., and C. A. Dinarello. 1989. A method for removing interleukin- and tumor necrosis factor-inducing substances from bacterial cultures by ultrafiltration with polysulfone. J. Immunol. Methods 116:159-165. [DOI] [PubMed] [Google Scholar]
- 11.Sweadner, K. J., M. Forte, and L. L. Nelse. 1977. Filtration removal of endotoxin (pyrogens) in solution in different states of aggregation. Appl. Environ. Microbiol. 34:382-385. [DOI] [PMC free article] [PubMed] [Google Scholar]