Abstract
Neuronal communication is mediated by Ca2+-triggered fusion of transmitter-filled synaptic vesicles with the presynaptic plasma membrane. Synaptotagmin I functions as a Ca2+ sensor that regulates exocytosis, whereas soluble N-ethylmaleimide–sensitive factor attachment protein (SNAP) receptor (SNARE) proteins in the vesicle and target membrane assemble into complexes that directly catalyze bilayer fusion. Here we report that, before the Ca2+ trigger, synaptotagmin interacts with SNARE proteins in the target membrane to halt SNARE complex assembly at a step after donor vesicles attach, or dock, to target membranes. This results in fusion complexes that, when subsequently triggered by Ca2+, drive rapid, highly efficient lipid mixing. Ca2+-independent interactions with SNAREs also predispose synaptotagmin to selectively penetrate the target membrane in response to Ca2+; we demonstrate that Ca2+–synaptotagmin must insert into the target membrane to accelerate SNARE-catalyzed fusion. These findings demonstrate that Ca2+ converts synaptotagmin from a clamp to a trigger for exocytosis.
Membrane fusion in cells requires protein machines to overcome energy barriers and regulatory mechanisms to ensure that fusion occurs at the correct time and place1. SNARE proteins are believed to form the core of a membrane fusion complex that is conserved throughout phylogeny. SNAREs on transport vesicles (v-SNAREs) assemble with cognate SNAREs on target membranes (t-SNAREs), forming trans-SNARE complexes that are essential for membrane fusion in vivo 2 and sufficient to catalyze fusion of proteoliposomes in vitro 3. However, neither the precise means by which SNAREs mediate the merger of lipid bilayers, nor the molecular mechanisms that regulate the fusion complex, is understood.
A highly specialized membrane fusion pathway occurs in presynaptic nerve terminals. Synaptic vesicles are loaded with neurotransmitters and docked to the presynaptic plasma membrane. Upon depolarization, Ca2+ influx through voltage-activated channels triggers fusion of docked synaptic vesicles, releasing transmitters into the synaptic cleft, where they bind and activate postsynaptic receptors4. This fusion pathway shows two striking specializations: it is strictly regulated by Ca2+, and it occurs with faster kinetics than any other known membrane fusion event5–7. These properties are crucial for the rapid point-to-point communication that underlies neuronal function.
Synaptic vesicle exocytosis is mediated by the v-SNARE synaptobrevin 2 (syb), and the t-SNAREs syntaxin 1A (syx) and SNAP-25. In addition, the Ca2+ binding protein synaptotagmin I (syt) is crucial for rapid evoked transmitter release; disruption of syt virtually abolishes the fast component of synaptic transmission in mice and Drosophila melanogaster 8. Spontaneous release persists in syt-null neurons, indicating that syt is not required for fusion per se but, rather, has a regulatory role, perhaps controlling SNARE function9–12.
The cytoplasmic domain of syt harbors two Ca2+ binding modules, termed C2A and C2B13, which, upon binding Ca2+, penetrate membranes that harbor anionic phospholipids such as phosphatidylserine14–17. The interaction of syt with phosphatidylserine-harboring membranes is crucial during regulated fusion18, but whether syt functions by penetrating the vesicle or the target membrane remains unresolved15,19,20.
The tandem C2-domains of syt also mediate Ca2+-independent and Ca2+-promoted interactions with the t-SNAREs syx and SNAP-25 (refs. 11,21,22), providing a bridge between the putative Ca2+ sensor for synaptic vesicle exocytosis and the core of the synaptic vesicle fusion apparatus. In support of this model, the cytoplasmic domain of syt renders fusion between reconstituted t-SNARE and v-SNARE vesicles sensitive to Ca2+ (refs. 10,12,20,23,24). Current goals are to increase the speed and efficiency of reconstituted fusion and to use this model system to determine directly the step-by-step series of events that culminates in rapid, regulated exocytosis.
Indeed, numerous questions remain concerning the precise mechanism by which syt regulates SNARE-catalyzed membrane fusion. Does syt regulate the structure and function of SNARE proteins before the Ca2+ signal, perhaps by priming the fusion machinery or acting as a fusion clamp? Which membrane—target or vesicle—does Ca2+–syt penetrate to stimulate fusion, and how is this choice made? Here we address these questions using a reconstituted fusion assay that employs t-SNAREs and syt I from rat and v-SNAREs from mice, in conjunction with electrophysiological analysis of syt I–null neurons from mice. The reconstitution experiments described here incorporated a new feature: a preincubation step in which syt was added to a mixture of t-SNARE and v-SNARE vesicles before the addition of Ca2+. This allowed us to study the possible regulatory functions of syt, both before and after the Ca2+ signal, within a single fusion reaction.
First, we show that, before the addition of Ca2+, syt acts as a ‘clamp’ and efficiently inhibits SNARE-catalyzed membrane fusion. We note that others have reported that complexin, a regulatory protein required for synaptic transmission, clamps reconstituted SNARE-catalyzed membrane fusion and that Ca2+–syt relieved this clamp to yield a small burst of fusion23. However, a Ca2+-independent clamping role for syt could not be ruled out in ref. 23 because samples lacking complexin, but containing syt during the Ca2+-free preincubation period, were not included. Here we demonstrate that, before the Ca2+ trigger, syt alone can function as a fusion clamp and this function is due to syt’s ability to halt SNARE complex assembly at a previously unidentified intermediate stage. Thus, apo-syt (Ca2+-free syt) allows partial assembly of trans-SNARE complexes that are poised to mediate rapid membrane fusion in response to Ca2+. In support of the Ca2+-independent clamping role of syt, we demonstrate that the frequency of spontaneous neurotransmitter release (minis) is elevated in dissociated cultures of hippocampal neurons from syt I knockout mice. Finally, we show that Ca2+-independent interactions with t-SNAREs serve to steer the Ca2+-triggered membrane penetration activity of syt to the target membrane; this interaction with the target membrane is a crucial step during Ca2+-activated fusion. These experiments reveal key sites of action of syt and demonstrate that syt switches from a clamp to an accelerator of fusion in response to Ca2+.
RESULTS
Dual roles of syt: clamp and accelerator of fusion
In presynaptic boutons, synaptic vesicles dock to release sites at resting intracellular Ca2+ concentration ([Ca2+]i) and rapidly fuse with the plasma membrane upon Ca2+ influx. The lag time between the rise in Ca2+ and the postsynaptic response can be as brief as 60–200 µs5,6. Therefore, regulated fusion machines are likely to partially pre-assemble before the Ca2+ signal. We recapitulated the early Ca2+-independent preassembly step using the minimal protein complement for Ca2+-triggered membrane fusion—neuronal SNAREs and syt10. We then determined the impact of this step on subsequent Ca2+-triggered fusion.
The v-SNARE syb was reconstituted into one population of vesicles containing membrane-anchored fluorescence resonance energy transfer (FRET) donor-acceptor pairs, whereas the t-SNAREs syx and SNAP-25 were reconstituted into another population of unlabeled vesicles3. When mixed together, vesicle fusion results in dilution of FRET pairs from the v-SNARE vesicles into the bilayer of unlabeled t-SNARE vesicles and is monitored as an increase in the fluorescence of the FRET donor due to dequenching. A 9:1 ratio of t-SNARE to v-SNARE vesicles is used to allow for several rounds of fusion3. Addition of the cytoplasmic domain of syt increases the rate and extent of fusion in this minimal system in response to Ca2+ (refs. 10,23,24). However, in earlier studies, the effect of preincubating syt with SNARE-bearing vesicles before Ca2+ addition had not been examined.
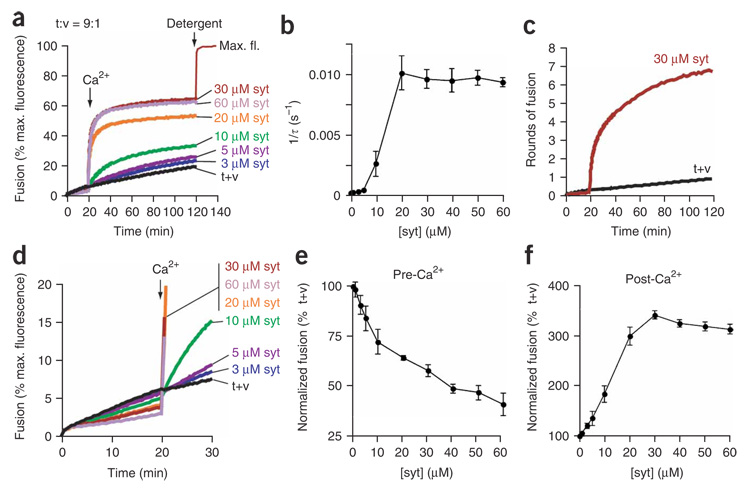
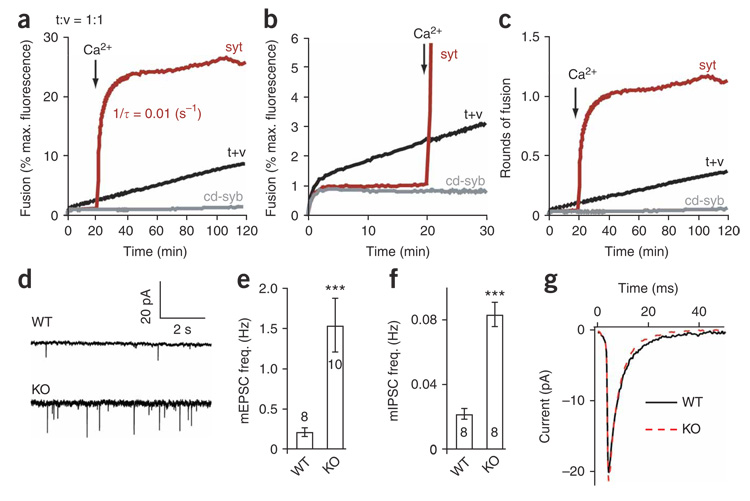
Preincubation of the cytoplasmic domain of syt with t-SNARE and v-SNARE vesicles in the absence of Ca2+ had marked effects on SNARE-catalyzed fusion. Subsequent addition of Ca2+ triggered rapid (τ = ~100 s at 30 µM syt), robust fusion (Fig. 1a,b). More than 60% of the maximum possible lipid-mixing signal was obtained, which equates to seven rounds of fusion (Fig. 1c). The regulated fusion in these experiments now occurs on the seconds, rather than the minutes time scale, and approaches the kinetics of secretion from PC12 cells25; fusion was also more efficient than in previous reports3,10. Preincubation of apo-syt with t-SNARE and v-SNARE vesicles helped prime SNAREs for fast, efficient fusion (Supplementary Fig. 1 online). In contrast, control experiments revealed that a 20-min incubation of syt with t-SNARE vesicles (before addition of cognate v-SNARE vesicles) had no effect on the rate or extent of Ca2+- triggered fusion (Supplementary Fig. 1). These experiments implicate trans-SNARE complexes as critical targets for the action of apo-syt.
Figure 1. Reconstitution of rapid, efficient, Ca2+-triggered membrane fusion.
(a) Reconstituted membrane fusion assays were carried out using a 9:1 t-SNARE:v-SNARE vesicle ratio. Data were plotted as a percentage of the maximum donor fluorescence intensity obtained by adding detergent to samples at t = 120 min. The syt concentration was varied as indicated; t+v denotes fusion reactions lacking syt. Ca2+ was added to a final concentration of 1 mM at t = 20 min. (b) Curves from a were well fitted by a double-exponential function. Rate constants of the major component (amplitude >70%) were plotted as a function of syt concentration and reached a maximum at 20 µM syt. (c) Fluorescence signals were converted to rounds of fusion52. t-SNAREs and v-SNAREs alone mediated one round of fusion (t+v); a maximum of seven rounds of fusion were obtained when 30 µM syt was also included. (d) Expansion of the first 30 min from a. (e,f) Quantification of apo-syt inhibition and Ca2+–syt stimulation of fusion. The average fluorescence signal obtained for each syt concentration at t = 20 min (pre-Ca2+; e) or at t = 120 min (post-Ca2+; f) was plotted as a percentage of the extent of fusion obtained by t+v. Data represent the mean ± s.e.m., and representative traces are shown from ≥3 independent trials.
Closer inspection of the lipid-mixing signal during the Ca2+-free preincubation step revealed syt-mediated, dose-dependent inhibition of SNARE-catalyzed fusion; 60 µM syt reduced fusion by > 60% when compared to samples lacking syt (Fig. 1d,e). These data suggest that syt has distinct roles in regulating fusion before and after the Ca2+ trigger (Fig. 1e,f). It seems that syt allows initiation of trans-SNARE pairing, but prevents further assembly until arrival of the Ca2+ signal.
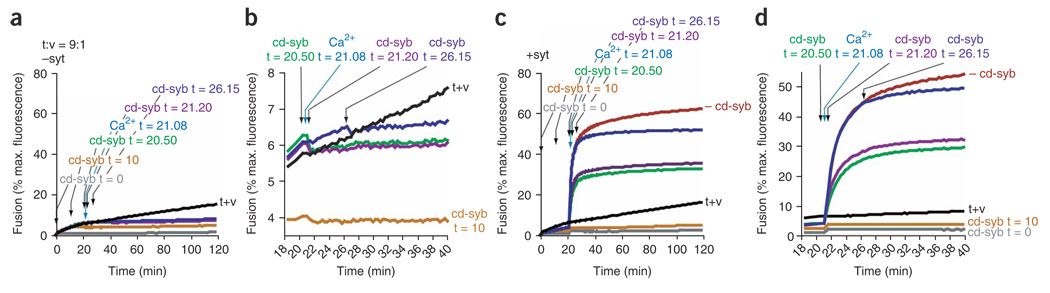
We explored the mechanism by which syt clamps fusion using an acute inhibition experiment in which the cytoplasmic domain of synaptobrevin (cd-syb) was added to fusion reactions at different time points (Fig. 2). In the absence of syt, addition of cd-syb immediately inhibited membrane fusion (Fig. 2a,b and Supplementary Fig. 2 online). In contrast, preincubation of syt with t-SNARE and v-SNARE vesicles, before the Ca2+ signal, delayed the onset of cd-syb–mediated inhibition during Ca2+-activated fusion (Fig. 2c,d and Supplementary Fig. 2). These data indicate that trans-SNARE pairing had been initiated, but was stalled at an intermediate stage of assembly. Thus, apo-syt has a clamping function that may aid in stable docking between t-SNARE and v-SNARE vesicles.
Figure 2. Functional trans-SNARE pairing is arrested by apo-syt.
(a) The cytoplasmic domain of synaptobrevin (cd-syb; 10 µM) and 1 mM Ca2+ were added to t-SNARE– and v-SNARE–mediated fusion reactions (t+v) without syt (−syt) at the indicated times. A 9:1 t-SNARE:v-SNARE vesicle ratio was used. (b) Expansion of data from a. (c) Syt (+syt, 30 µM) was added to t+v and experiments were carried out as in a. Ca2+ was added to trigger syt-stimulated fusion at t = 21 min, 8 s. (d) Expansion of data from c showing that the presence of syt delayed the onset of cd-syb–mediated inhibition whether syt was added just before (t = 20 min, 50 s), during (t = 21 min, 20 s) or minutes after (t = 26 min, 15 s) the Ca2+ trigger. Representative traces are shown from ≥3 independent trials.
Syt synchronizes vesicle-vesicle membrane fusion
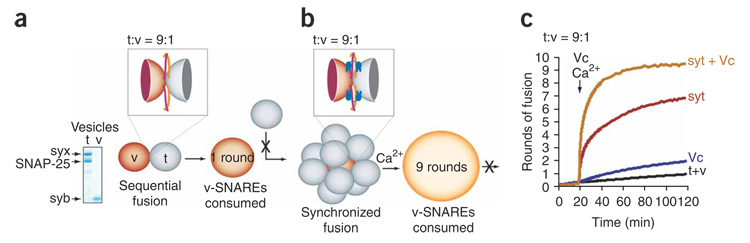
The burst of efficient fusion observed in our experiments is unlikely to occur without a prior docking step, given that we reconstitute physiological densities of v-SNAREs per vesicle26 with ~45 copies of syb in the correct orientation (that is, outside-out), whereas t-SNARE vesicles harbor ~65 outside-out syx–SNAP-25 heterodimers reconstituted at a ~1:1 molar ratio (Fig. 3a). Without docking and synchronized fusion of numerous vesicles, the first round of fusion would sequester all v-SNAREs owing to the excess number of t-SNAREs, leaving no free v-SNAREs to participate in subsequent rounds of fusion (Fig. 3a; sequential fusion model). Accordingly, the upper limit for attainable rounds of fusion would be determined by the number of t-SNARE vesicles that can dock to a single v-SNARE vesicle and fuse simultaneously (Fig. 3b; synchronized, parallel fusion model). At a 9:1 t-SNARE:v-SNARE vesicle ratio, a maximum of nine rounds of fusion can occur, but only in the synchronized fusion model. The results in Figure 1 rule out the sequential fusion model, because seven rounds of fusion were observed. Moreover, when using a combination of syt and another established stimulator of membrane fusion, Vc, a small peptide derived from the C-terminal half of syb (residues 57–92)27, we obtained the maximum nine complete rounds of fusion when using a 9:1 t-SNARE:v-SNARE vesicle ratio (Fig. 3c). Because 7–9 rounds of triggered fusion would not be obtained in the absence of a clamp (that is, apo-syt), these data further support the synchronized fusion model (Fig. 3b).
Figure 3. Apo-syt enables docking of multiple vesicles by arresting fusion; Ca2+–syt then triggers synchronous fusion.
(a) When the number of SNAREs per t-SNARE vesicle (t, gray) equals or exceeds the number of v-SNAREs per vesicle (v, brown), sequential fusion between the two results in a larger vesicle, leaving no free v-SNAREs available for subsequent rounds of fusion. Box shows initial trans-SNARE pairing between t-SNAREs and v-SNAREs, which serves to dock vesicles together in the reduced-fusion system32. Left, t-SNARE and v-SNARE vesicles analyzed by SDS-PAGE and stained with Coomassie blue. Optical densities of the syx and SNAP-25 bands were 117 arbitrary units (a.u.) and 111 a.u., respectively, indicating a ~1:1 syx:SNAP-25 molar ratio. (b) The inhibitory activity of apo-syt (blue) stalls SNARE-mediated fusion after initiation of trans-SNARE pairing. This prevents the low efficiency, sequential pathway described in a and allows efficient docking of multiple t-SNARE vesicles to a single v-SNARE vesicle. Ca2+ then triggers rapid, synchronous fusion of all docked vesicles. (c) Fusion reactions were carried out as described for Figure 1. All samples contained t-SNARE and v-SNARE vesicles (t+v), with or without syt (30 µM) and Ca2+ (1 mM). In addition, some reactions included the Vc peptide (20 µM), which is a C-terminal peptide derived from synaptobrevin (residues 57–92)27; Vc and Ca2+ were added at t = 20 min. Representative traces are shown from ≥3 independent trials.
Syt and the Vc peptide synergize to accelerate fusion
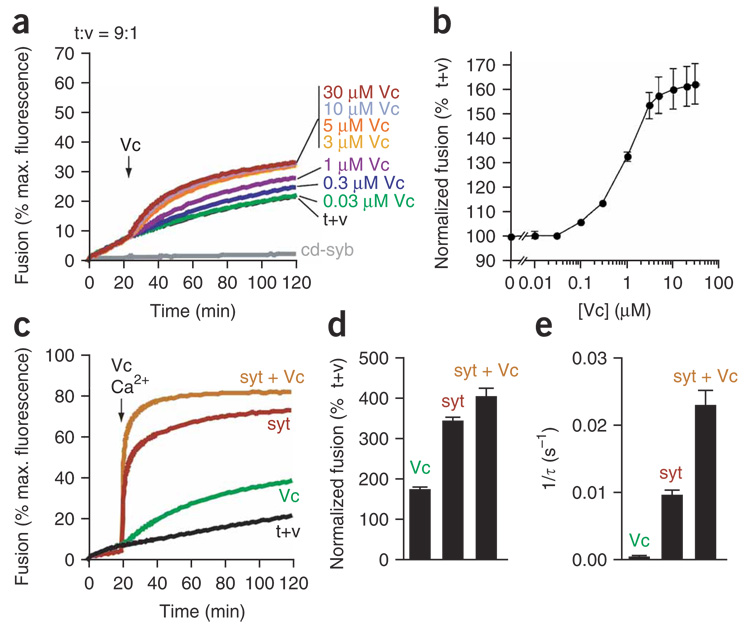
When added together, syt and the Vc peptide increased the extent of SNARE-catalyzed fusion four-fold (Fig. 4a–d), which is only slightly above the 3.5-fold increase obtained by Ca2+–syt alone. However, syt and the Vc peptide synergized to more than double the rate of fusion (τ = ~43 s), as compared to Ca2+–syt alone (τ = ~100 s) (Fig. 4e). We observed synergy between Ca2+–syt and the Vc peptide regardless of when we added the Vc peptide to the reactions (data not shown). The underlying basis for this synergy is unclear; however, both the Vc peptide and syt interact directly with t-SNAREs. The Vc peptide might prevent t-SNARE aggregation and/or assembly into off-pathway complexes27,28, thus allowing t-SNAREs to efficiently accept an incoming v-SNARE. Ca2+–syt can also directly activate t-SNAREs, perhaps by acting as a chaperone, to influence the folding of t-SNAREs with each other and with an incoming v-SNARE12. In another study, a syb fragment, similar to the Vc peptide, did not synergize with syt during fusion20. However, the experimental paradigm in that study was markedly distinct from ours and cannot be directly compared.
Figure 4. Syt and the Vc peptide synergize to increase the rate of SNARE-catalyzed fusion.
(a) Fusion reactions were carried out using a 9:1 t:v-SNARE vesicle ratio. Increasing concentrations of the Vc peptide were added to reactions at t = 20 min. t+v indicates fusion reactions lacking the Vc peptide. The cytoplasmic domain of synaptobrevin (cd-syb, 10 µM) was added to t+v at t = 0 min to inhibit SNARE-mediated fusion. Representative traces are shown from ≥4 independent trials. (b) The extent of fusion obtained for each concentration of the Vc peptide at t = 120 min was plotted as a percentage of the extent of fusion obtained by t+v. Data are represented as the mean ± s.e.m. from ≥4 independent trials. (c) Fusion was monitored in the presence of the Vc peptide (20 µM), syt (30 µM) or a combination of both (syt + Vc). Syt, t-SNARE vesicles and v-SNARE vesicles were combined at t = 0 min, as in Figure 1, and the Vc peptide and Ca2+ were added at t = 20 min. Representative traces from ≥3 independent trials are shown. (d) The extent of fusion after 120 min from c was normalized to the extent of fusion obtained by t+v. Data are represented as the mean ± s.e.m. from ≥3 independent trials. (e) Curves from c were fitted using a double-exponential function, and rates for the fast component were plotted. Data are represented as mean ± s.e.m. from ≥3 independent trials.
A syt switch enables highly efficient membrane fusion
The synchronous fusion model above predicts that the rate of fusion should be independent of the number of attached/docked t-SNARE and v-SNARE vesicles. To test this, we studied fusion under single-turnover conditions using a 1:1 ratio of t-SNARE and v-SNARE vesicles. Apo-syt inhibited nearly all SNARE-mediated fusion (Fig. 5a,b); a greater degree of inhibition was observed under single-turnover conditions owing to the larger syt:t-SNARE ratio. Addition of Ca2+ triggered one complete round of fusion (Fig. 5c). As predicted by the synchronous fusion model, the time constant for syt-regulated fusion under single-turnover conditions (τ = ~100 s; Fig. 5a) was the same as under multiple vesicle–fusion conditions. These data further demonstrate that syt can coordinate rapid, synchronized fusion of single, or multiple, docked vesicles.
Figure 5. Dual function of syt in the regulation of SNARE-catalyzed fusion.
(a) Fusion reactions were carried out as in Figure 1 except that a 1:1 t-SNARE:v-SNARE vesicle ratio was used. Identical rates were obtained for both the 1:1 and 9:1 t-SNARE:v-SNARE vesicle ratios using optimal syt concentrations (9:1 = 30 µM, 1:1 = 10 µM). The cytoplasmic domain of syb (cd-syb, 10 µM) was added to t+v to inhibit SNARE-mediated fusion. Representative traces are shown from ≥3 independent trials. (b) Expansion of the first 30 min from a. (c) During Ca2+–syt-regulated fusion, all vesicles rapidly completed one round of fusion. In reactions lacking Ca2+–syt, <50% of vesicles completed a round of fusion in 120 min (t+v). (d) mEPSCs were recorded from dissociated wild-type (WT) and Syt1 knockout (KO) neurons. (e,f) Mini frequency, at excitatory (e) and inhibitory (f) synapses, is significantly increased in syt I KO neurons. The number of cells analyzed is indicated. The number of active synapses per mm was the same for WT and KO neurons (Supplementary Fig. 3). Significance (***) was determined using the Student’s t-test; P < 0.001. (g) Mini amplitude (WT = 18.05 ± 1.5 pA, KO = 21.12 ± 0.97 pA) and quantal size (WT = 123.80 ± 20.60 fC, KO = 142.22 ± 25.5 fC) were unaffected in the knockouts.
Under single vesicle and multiple vesicle–fusion conditions, fusion reactions approached 100% efficiency, addressing concerns that only a small population of highly fusogenic vesicles (possibly having high curvature, or excess copies of SNARE proteins) gives rise to the lipid-mixing signal. Second, these data help alleviate concerns that fusion was trapped at a hemifusion end point. Using either the 9:1 or 1:1 conditions, reactions that dead-end in a hemifused state, in which only the outer leaflets mixed, would yield a maximum of ~60% (rather than 50%, because the outer leaflet harbors more lipid) of the expected rounds of fusion. Therefore, if 100% of the vesicles in our fusion reactions are involved in a fusion event, hemifusion would be marked by ~0.6 rounds of fusion using a 1:1 vesicle ratio, and ~5.4 rounds of fusion using a 9:1 vesicle ratio. The fact that we obtain 1 and 9 rounds of fusion, respectively, rules out the occurrence of appreciable levels of dead-end hemifusion.
Syt as a fusion clamp in hippocampal neurons
If apo-syt functions as a clamp, the frequency of spontaneous synaptic vesicle fusion events (minis) should increase in Syt1 −/− neurons. Although most studies indicate that the mini frequency is elevated at the neuromuscular junction of syt-null D. melanogaster 8, this remains an unresolved issue for dissociated mouse neurons in culture (for example, ref. 29 versus ref. 30). We therefore carried out electrophysiological analysis of dissociated hippocampal neurons from wild-type and Syt1 −/− mice. Tetrodotoxin (TTX) and 1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA-AM) were used in our analysis to block sodium channels and chelate internal Ca2+ to prevent evoked neurotransmitter release. In the absence of increases in [Ca2+]i, excitatory and inhibitory mini frequencies increased by factors of six and four, respectively, in Syt1 −/− neurons (Fig. 5d–f), indicating that apo-syt can act as a negative regulator of SNARE-catalyzed fusion in situ. All other properties of minis remained the same in Syt1 −/− neurons as compared to wild type (for example, mini amplitude, total charge and number of active boutons; Fig. 5g and Supplementary Fig. 3 online).
Syt acts upon lipids and SNAREs in the target membrane
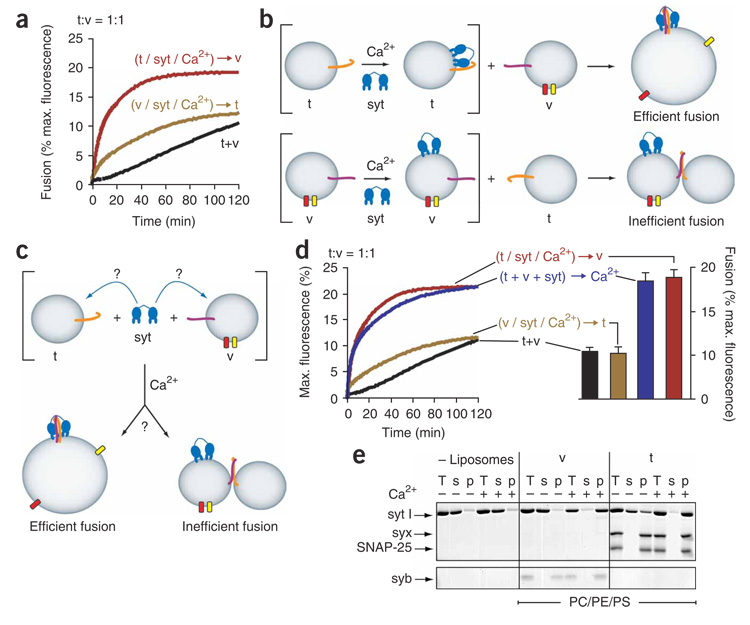
We used the in vitro fusion assay to determine the site of action of Ca2+–syt. In the presence of Ca2+, two Ca2+ binding loops in each C2 domain of syt penetrate into membranes that harbor anionic phospholipids (for example, phosphatidylserine), resulting in high-affinity complexes (K d = ~0.7 nM)15,16. In essence, Ca2+–syt becomes stably bound to membranes and does not dissociate substantially, even on relatively long time scales (E.R.C., unpublished observations). In cells, phosphatidylserine is present in both the vesicle and target membranes. Therefore, in principle, syt could bind and penetrate either membrane in response to Ca2+ (ref. 31). We performed control experiments using two different conditions in our reconstituted system, in which both t-SNARE and v-SNARE vesicles contain phosphatidylserine, to determine whether syt works more efficiently when bound to one membrane or the other (Fig. 6a,b). In the first condition, we mixed syt with t-SNARE vesicles and ‘pinned’ syt onto the t-SNARE membrane by adding Ca2+ to the samples. We then added v-SNARE vesicles and monitored fusion. In the second condition, we mixed syt with v-SNARE vesicles and pinned syt onto the v-SNARE membrane by adding Ca2+ to the samples. We then added t-SNARE vesicles and monitored fusion. Pinning syt onto t-SNARE vesicles resulted in robust fusion, whereas pinning syt onto v-SNARE vesicles largely abrogated fusion (Fig. 6a). We note that a low level of fusion was observed when syt had been pinned to the v-SNARE membrane; syt might be able to stimulate fusion to a limited degree at this site, or perhaps a minor fraction of syt was exchanged between v-SNARE and t-SNARE membranes in this experiment. These results reveal that the t-SNARE membrane is the relevant target for the stimulatory action of Ca2+–syt. It should be noted that a nonsaturating concentration of syt (1 µM) was used for these experiments to ensure that most of the syt was pinned to only one vesicle population, leaving little excess syt to bind cognate vesicles (Fig. 6). Use of a suboptimal syt concentration, in conjunction with the protocol used for these experiments (that is, lack of a Ca2+-free preincubation step with both t-SNARE and v-SNARE vesicles (Supplementary Fig. 1b)), accounts for the slower rates of fusion in Figure 6.
Figure 6. Syt must act on t-SNARE membranes to stimulate fusion.
(a) 1 µM syt was incubated with t-SNARE (t/syt/Ca2+) or v-SNARE (v/syt/ Ca2+) vesicles in the presence of 1 mM Ca2+, which pins syt to the respective vesicle membrane, leaving little free syt in solution. At t = 0 min, cognate vesicles without syt were added (t-SNARE:v-SNARE vesicle = 1:1) and fusion monitored. Samples lacking syt (t+v) were also included. (b) Illustration depicting the outcome of the experiment in a. Above, syt-stimulated fusion when prepinned to t-SNARE vesicles (t). Below, syt fails to efficiently stimulate fusion when prepinned to v-SNARE vesicles (v). Fusion is depicted by an increase in vesicle size and separation of donor (yellow) and acceptor (red) FRET pairs. (c) Syt might selectively prebind (‘steer’) to either t-SNARE or v-SNARE vesicles in the absence of Ca2+. (d) Results of pinning experiments, carried out as in a. In addition, syt was added to a mixture of t-SNARE and v-SNARE vesicles (t + v + syt) in the absence of Ca2+ at t = 0 min; Ca2+ (1 mM) was added after 15 s. Fusion was monitored and the extent of fusion at t = 120 min was plotted (error bars represent s.e.m. from ≥3 independent trials). (e) Cosedimentation of syt with t-SNARE or v-SNARE vesicles harboring 55% phosphatidylcholine (PC), 30% phosphatidylethanolamine (PE) and 15% phosphatidylserine (PS). All components were at the same concentration as used for fusion reactions in a and d. Equal fractions of samples prepared in the presence (+) and absence (−) of Ca2+ before centrifugation (total, T), as well as the supernatant (s) and pellet (p) collected after centrifugation, were analyzed by SDS-PAGE and stained with Coomassie blue. Representative fusion traces and a representative gel are shown from ≥3 independent trials. A full-length gel is presented in Supplementary Figure 4.
Although the control experiments identified the membrane that syt must act on to stimulate fusion, they did not reveal whether and, if so, how syt chooses the t-SNARE membrane without being artificially pinned onto it. Therefore, to mimic the in situ condition, we performed a series of experiments in which syt had not been pinned onto either membrane with Ca2+. Instead, apo-syt was incubated with a 1:1 mixture of t-SNARE and v-SNARE vesicles, containing identical amounts of phosphatidylserine, for 15 s before addition of Ca2+ (Fig. 6c). Under these conditions, syt was allowed to ‘choose’ which vesicle it adsorbed to, if at all, in the absence of Ca2+. Addition of Ca2+ to these samples triggered syt-stimulated fusion that was similar to the rate and extent of fusion observed when syt had been pinned to the t-SNARE membrane (Fig. 6d). Apparently, during the Ca2+-free preincubation step, syt was recruited to the t-SNARE membrane by virtue of Ca2+-independent interactions with syx–SNAP-25, thereby predisposing syt to penetrate the target membrane, and not the vesicle membrane, in response to Ca2+. As a control, the experiment was repeated using 0.5 µM syt (Supplementary Fig. 4 online). The same outcome was observed as with 1 µM syt but with lower levels of fusion, demonstrating that the assay would have detected ‘loss’ of syt due to adsorption onto the v-SNARE vesicles, had this occurred (quantification and additional controls are provided in Supplementary Fig. 4).
In the absence of Ca2+, t-SNAREs serve to steer syt toward the target membrane (Fig. 6). Indeed, cosedimentation assays revealed a substantial amount of syt–t-SNARE binding in the absence of Ca2+ (Fig. 6e) under conditions identical to the fusion assays in Figure 6d. Weak Ca2+-independent interactions are not as readily detected using coflotation assays in which complexes slowly dissociate as samples move through gradients10,12,19. Densitometry revealed that a minimum of 64% of syt adsorbs to t-SNARE membranes in the absence of Ca2+. Therefore, in the fusion assay, if the remaining 36% distributes evenly between t-SNARE and v-SNARE membranes on Ca2+ addition, 82% of syt will bind to the t-SNARE membrane.
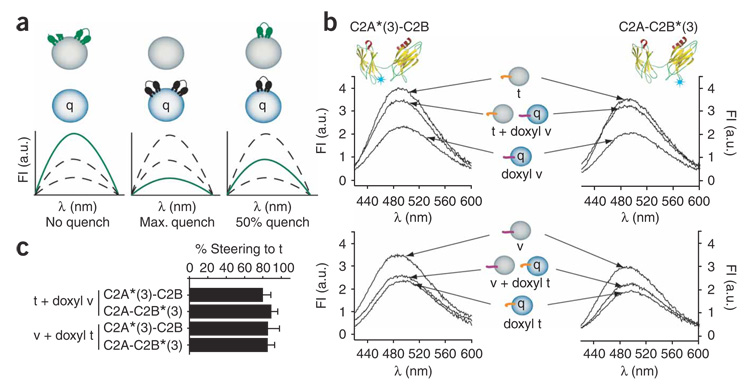
To test directly the t-SNARE steering model and determine whether Ca2+–syt selectively penetrates the target membrane, we made use of syt that harbored fluorescent probes in membrane-penetration loops of either C2A or C2B. We prepared t-SNARE and v-SNARE vesicles using phosphatidylcholine that harbored a doxyl group on the seventh carbon of one acyl chain. In this system, when syt penetrates the lipid bilayer, the fluorescent probes in syt come into contact with membrane-embedded doxyl groups, quenching their fluorescence. Steering can be assessed by comparing fluorescence signals obtained using quenching versus nonquenching vesicles. The experimental approach is shown in Figure 7a.
Figure 7. t-SNAREs predispose syt to penetrate the target membrane in response to the Ca2+ signal. AEDANS probes were placed on the Ca2+ binding loops of syt. Emission of these probes increases upon Ca2+-triggered penetration of syt into membranes15.
(a) Schematic representation of the experiment shown in b. AEDANS-labeled syt was added to a 1:1 mixture of blank vesicles (gray) and fluorescence-quenching vesicles (blue, q). Upon the addition of Ca2+, if syt exclusively penetrates blank vesicles, a maximum AEDANS fluorescence signal will be observed (green syt, left). If syt exclusively penetrates quenching vesicles, minimal AEDANS fluorescence will be observed (black syt, middle). If syt distributes equally between blank and quenching vesicles, an intermediate level of AEDANS fluorescence will be observed (right). Fl (a.u.), fluorescence in arbitrary units. (b) Above, model of syt-C2A-C2B (yellow) showing the location of AEDANS probes (blue star) on either loop 3 of C2A (C2A*(3)-C2B) or loop 3 of C2B (C2A-C2B*(3)). We added 0.5 µM AEDANS-syt to blank t-SNARE vesicles (t), quenching v-SNARE vesicles (doxyl v) or a 1:1 mixture of both (t + doxyl v). Spectra were obtained in 0.2 mM EGTA and 1 mM Ca2+. Spectra collected in EGTA were normalized across samples to obtain a correction factor. Below, experiments were repeated using the reciprocal set of vesicles. Representative traces are shown from ≥3 independent trials. (c) Quantification of data shown in b. Data are represented as mean ± s.e.m.
Incubation of Ca2+–syt with t-SNARE vesicles, or quenching v-SNARE vesicles, yielded the maximum and minimum fluorescence signals, respectively (Fig. 7b, above). When syt was incubated with equal amounts of both populations of vesicles in the absence of Ca2+, subsequent addition of Ca2+ resulted in a fluorescence signal that closely mirrored the signal obtained when syt penetrated t-SNARE vesicles alone (Fig. 7b, above). The reciprocal experiment, in which t-SNARE vesicles contained doxyl quenchers, revealed the same preference of syt for t-SNARE membranes (Fig. 7b, below). Indeed, when given a ‘choice’, 80–90% of the syt penetrated the bilayer that harbored t-SNAREs (Fig. 7c). These data provide additional evidence for a model in which t-SNAREs steer the membrane penetration activity of syt away from the synaptic vesicle membrane and toward the target membrane, where syt fulfills its function as a dual regulator of SNARE-catalyzed fusion.
DISCUSSION
Using a defined, reconstituted system consisting of liposomes and only four proteins, neuronal SNAREs and syt, we have directly addressed the sequence of events that culminate in rapid, synchronous, Ca2+-triggered membrane fusion. To mimic physiological conditions, syt was preincubated with t-SNARE and v-SNARE vesicles at 37 °C before the Ca2+ signal. This preincubation step altered the fusion reaction as compared to earlier assays in which all components were mixed simultaneously on ice (before the reaction was warmed to 37 °C to allow fusion)10. During preincubation, progression toward fusion was arrested by syt before the Ca2+ trigger. Thus, we reconstituted a clamping function for apo-syt, consistent with electrophysiological measurements showing that loss of syt enhances the frequency of minis in neurons (Fig. 5d–g). Timing experiments, in which cd-syb was added to fusion reactions either before, during or after Ca2+, indicate that the syt clamp allowed initiation of SNARE-complex assembly, but stalled this process at an intermediate stage. Indeed, interactions between t-SNAREs and v-SNAREs rapidly mediate vesicle docking in vitro 32, and in our experiments apo-syt seems to trap a late-arrested trans-SNARE complex intermediate at a key junction along the membrane fusion pathway—after trans-SNARE pairing, but before the onset of fusion. Syx, perhaps by forming similar complexes in vivo, mediates vesicle docking in neurons and neuroendocrine cells33,34.
Thus, apo-syt holds the fusion apparatus in a cocked, ‘off ’ state, allowing for efficient vesicle attachment or docking of one, or several, target vesicles. Further support for this idea stems from our finding that apo-syt favors synchronized fusion (Fig. 3b) over sequential fusion (Fig. 3a) of t-SNARE and v-SNARE vesicles. Indeed, we observed the same rate of fusion when using a 9:1 t-SNARE:v-SNARE vesicle ratio or when using single-turnover conditions (1:1 t-SNARE:v-SNARE vesicle ratio), as predicted by the synchronized fusion model.
Others have reconstituted rapid fusion under single-turnover conditions by deleting the Habc domain of syx and adding the Vc peptide28 or Ca2+–syt20. For example, one study reported a 1.25-fold increase in fluorescence in just under 60 s28. Using full-length syx plus syt and the Vc peptide, we reached the same fluorescence increase (after the Ca2+ trigger) in ~15 s (Supplementary Fig. 5 online). The greater speed and efficiency of our fusion reactions might be due to the use of t-SNARE complexes having a 1:1 syx:SNAP-25 ratio, carrying out fusion at 37 °C rather than 30 °C, and the Ca2+-free incubation step of syt with v-SNARE and t-SNARE vesicles. We have not yet attained the maximum rate of fluorophore dilution that can be measured in our experimental set-up (τ = ~5.9 s, determined by measuring the rate of the donor fluorescence increase after adding detergent to (t+v) fusion reactions). Attaining such rates is likely to require the presence of additional regulatory proteins, including nSec1 (the neuronal ortholog of Sec1) and complexin35.
It has been proposed that syt collaborates with complexins, a family of soluble proteins that bind SNARE complexes36, to regulate SNARE-mediated fusion. The question of whether complexins have positive37,38 or negative roles23,39–42 during exocytosis has yet to be resolved. Furthermore, the molecular mechanism by which syt and complexin might work together to regulate SNARE-mediated fusion is an open issue, as it is still unclear whether syt and complexin bind SNAREs in a simultaneous or competitive manner36,40. In the present study, we demonstrate that syt is a dual regulator of membrane fusion in the complete absence of complexin.
The means by which Ca2+ converts syt from a clamp to an accelerator of fusion has yet to be fully elucidated, but this function involves the ability of Ca2+–syt to penetrate membranes15,19 and act on SNARE proteins10,12. Here we demonstrated that Ca2+-promoted fusion is a direct outcome of syt’s ability to penetrate the bilayer of the target membrane rather than the vesicle membrane. These experiments also revealed that syt is directed to selectively penetrate the target membrane by virtue of its Ca2+-independent interaction with t-SNAREs, analogous to what has been proposed for the interaction between syt and phosphatidylinositol–4,5-bisphosphate (PIP2)15. Together, several steering factors, such as t-SNAREs and PIP2, would be expected to increase the likelihood that syt is directed to its proper site of action.
Our finding, that syt acts by penetrating the target membrane and cannot efficiently stimulate fusion if it penetrates the v-SNARE membrane provides insight into the functional architecture of the regulated fusion complex. We note that two earlier studies attempted to address this question by omitting phosphatidylserine from either the v-SNARE or t-SNARE membrane, but conflicting results were obtained19,20. Importantly, phosphatidylserine is present on both the vesicle and target membrane in vivo, so the phosphatidylserine-omission experiments do not provide insight into which membrane syt will penetrate in situ. The pinning and steering experiments described here (Fig. 6 and Fig. 7) reveal that t-SNAREs help direct the membrane penetration activity of syt toward the target membrane.
Penetration of syt into target membranes might drive localized buckling to facilitate fusion18,24, as predicted by early studies suggesting that protein factors operate to pull the plasma membrane toward the vesicle membrane to initiate exocytosis43 at localized sites of contact44. Distortion of the planar target membrane into a nipple-like structure would facilitate local contact between bilayers destined to fuse45.
In summary, we have uncovered previously unknown, crucial functions for syt–t-SNARE interactions, before the Ca2+ trigger, that allow for stable vesicle docking and set the stage for rapid, synchronous vesicle fusion in response to Ca2+. However, the effect of Ca2+ on the functional relationship between syt and SNAREs, other than increasing the affinity of binding11, remains somewhat obscure. Ca2+–syt pinned to t-SNARE membranes is likely to influence the folding of the C-terminal regions of t-SNAREs and v-SNAREs, thereby aiding SNARE-complex assembly by promoting zippering of SNAREs into four-helix bundles18,46. In this role, Ca2+–syt–phosphatidylserine would operate in a manner analogous to a molecular chaperone; indeed, Ca2+–syt can drive the folding of SNAP-25 onto membrane-embedded syx12. Alternatively, or in conjunction with this model, the Ca2+–syt–phosphatidylserine complex might alter the physical relationship between assembled SNARE complexes and the bilayer to drive fusion-pore opening and expansion18,47,48. Further study of the positive role of Ca2+–syt in the acceleration of fusion will require the design of reporter systems that can be used to monitor local structural transitions in SNARE proteins in real time during membrane fusion.
METHODS
Plasmids and protein purification
cDNA encoding rat Syt113 was provided by T.C. Südhof (University of Texas Southwestern Medical Institute) and the Asp374 mutation was corrected by substitution with a glycine residue49. We used the cytoplasmic domain of syt, residues 96–421, throughout this study because we have been unable to obtain sealed, well-behaved v-SNARE vesicles that contain nonaggregated full-length syt. The cytoplasmic domain of syt was subcloned into a pTrc-His vector (Invitrogen Life Technologies), expressed in Escherichia coli and purified as described12. Proteins were eluted in wash buffer with 500 mM imidazole and dialyzed overnight against 25 mM HEPES, pH 7.4, 100 mM KCl, 10% (w/v) glycerol, 1 mM DTT (Buffer A). For steady-state fluorescence-quenching experiments, we expressed and purified syt as described16. The lone native Cys277 was replaced with alanine, and a single cysteine was introduced in loop 3 of C2A (F234C; designated C2A(3)-C2B), C2B (I367C; designated C2A-C2B(3)).
Plasmids to generate recombinant full-length mouse syb 2 (pTW2) and the cytoplasmic domain of syb (cd-syb, residues 1–94; pET-rsybCD), were provided by J.E. Rothman (Columbia University), and proteins were expressed and purified as described10. To generate full-length t-SNARE heterodimers, a cDNA encoding full-length rat SNAP-25 (ref. 50; provided by M.C.Wilson (University of New Mexico) was subcloned into pRSFDuet-1 (Novagen) using the EcoRI and NotI sites; full-length syntaxin 1A cDNA51 (provided by R.H. Scheller, Genentech) was subcloned into a downstream site via NdeI and KpnI restriction sites. This construct is designated pRSFDuet-1 SNAP-25+syx 1. All constructs were confirmed by DNA sequencing.
The t-SNARE heterodimer was expressed and purified as described10. Vc, a C-terminal peptide of synaptobrevin (residues 57–92)27, was synthesized by the University of Wisconsin-Madison Biotechnology Center. Protein concentration of all recombinant proteins was determined using SDS-PAGE and staining with Coomassie brilliant blue using BSA as a standard. Optical densities were calculated using a UVP BioImaging System and Labworks software.
Preparation of SNARE-bearing vesicles
All lipids were obtained from Avanti Polar Lipids. Reconstitution of v-SNARE and t-SNARE vesicles was carried out as previously described10, with the addition of 1-palmitoyl-2-oleoyl-phospha-tidylethanolamine (PE) to the lipid mix. Briefly, v-SNAREs were reconstituted using a lipid mix composed of 27% (mol/mol) PE, 55% (mol/mol) 1-palmitoyl, 2-oleoyl phosphatidylcholine (PC), 15% (mol/mol) 1,2-dioleoyl phosphatidylserine (PS), 1.5% (mol/mol) N-(7-nitro-2-1,3-benzoxadiazol-4-yl)-1,2-dipalmitoyl phosphatidylethanolamine (NBD-PE, donor) and 1.5% (mol/mol) N-(lissamine rhodamine B sulfonyl)-1,2-dipalmitoyl phosphatidyl-ethanolamine (Rhodamine-PE, acceptor). t-SNAREs were reconstituted in 30% (mol/mol) PE, 55% (mol/mol) PC and 15% (mol/mol) PS. v-SNARE (syb) and t-SNARE (SNAP-25 + syx 1) vesicles were reconstituted to give a minimum of ~60 copies and ~95 copies (yielding ~45 and ~65 copies in the correct orientation) per vesicle, respectively, as described10. These copy numbers result in a minimum t-SNARE concentration in the fusion assay of ~4.5 µM and 0.5 µM, for t-SNARE:v-SNARE vesicle ratios of 9:1 and 1:1, respectively. For steady-state fluorescence experiments, as shown in Figure 7, NBD-PE and Rhodamine-PE were replaced with unlabeled PE. In the case of quenching vesicles, all phosphatidylcholine was replaced with 1-palmitoyl-2-stearoyl-(7-doxyl)-phosphatidylcholine.
Reconstituted membrane fusion assays
Fusion assays were carried out as described previously10 with three modifications: inclusion of PE in reconstituted vesicles, slightly different densities of SNARE proteins and a Ca2+-free preincubation step, as detailed in the text and below. Briefly, each 75-µl reaction consisted of 45 µl or 5 µl purified t-SNARE vesicles (t-SNARE:v-SNARE liposome ratio 9:1 and 1:1, respectively) and 5 µl of purified, NBD- and Rhodamine-PE–labeled v-SNARE vesicles plus 0.2 mM EGTA in 25 mM HEPES, pH 7.4, 100 mM KCl, 1 mM DTT. All components of the fusion reaction were prewarmed to 37 °C for 15 min separately or in combination as indicated before being mixed together at t = 0 min. Fusion was monitored as an increase in NBD fluorescence using a Biotek SynergyHT plate reader and Gen5 software with data acquisition every 8–15 s during rapid Ca2+-induced fusion, and every 1.5–2 min during slower phases. After 2 h, 0.5% (w/v) n-dodecylmaltoside (Roche Applied Science) was added to maximally dequench the NBD fluorescence, yielding a maximum fluorescence signal. Raw fluorescence was converted to percentage of the maximum fluorescence and plotted or converted to rounds of fusion as described10,52. Syt, Vc and 1 mM free Ca2+ were included as indicated in the figures. Ca2+ was added at t = 20 min using injectors controlled by Gen5 software. Vc was added manually at t = 20 min by ejecting the plate for 10 s and resuming the run after addition. Fusion traces were well fitted by a double-exponential function. Rate constants (1/τ) for the fast component (amplitude >70%) were plotted, as this represents the major component of each trace and our focus is on reconstituting fast fusion.
Hippocampal neuron cultures
Hippocampi of newborn pups (postnatal day 0) from heterozygous Syt1-deficient mouse matings were isolated as described53 in accordance with the guidelines of the US National Institutes of Health, as approved by the Animal Care and Use Committee of the University of Wisconsin-Madison. Tails were kept for genotyping, and electrophysiological recordings from Syt1 knockout and wild-type littermate neuron cultures were compared. Dissociated neurons, for mini recordings, were plated at 25,000–50,000 cells per cm2 on 12-mm coverslips in Neurobasal media supplemented with B27 and Glutamax (Gibco/Invitrogen). Neurons were allowed to mature for 11–12 d before being used for electrophysiological recordings.
Electrophysiology
Whole-cell patch-clamp recordings were made from dissociated hippocampal neurons in culture. For recording excitatory currents, the pipette solution contained 130 mM K-gluconate, 1 mM EGTA, 5 mM sodium phosphocreatine, 2 mM magnesium ATP, 0.3 mM sodium GTP, 10 mM HEPES, pH 7.3 (290 mOsm). For recording inhibitory currents, the pipette solution contained 135 mM CsCl2, 1 mM EGTA, 2 mM magnesium ATP, 0.3 mM sodium GTP, 10 mM HEPES, pH 7.3 (290 mOsm). Cells were continuously perfused with extracellular solution consisting of 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM EGTA, 10 mM glucose and 10 mM HEPES, pH 7.3 (300 mOsm), with 0.1 mM picrotoxin, 50 µM AP-V, 25 µM BAPTA-AM, 30 µM cyclopiazoic acid (CPA) and µ1 mM TTX. Cells were loaded with BAPTA-AM and CPA for 30 min. When recording miniature inhibitory postsynaptic currents (mIPSCs), picrotoxin in the extracellular solution was replaced with 20 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to block α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor responses. Picro-toxin was dissolved in ethanol, AP-V was dissolved in NaOH, and BAPTA-AM and CPA were dissolved in DMSO. All drugs were obtained from Sigma-Aldrich. Neurons were voltage clamped at −70 mV with an EPC-10/2 amplifier (HEKA Electronics). Only cells with series resistance of <15 MΩ, with 70–80% of this resistance compensated, were analyzed. Currents were acquired using PATCH-MASTER software (HEKA), filtered at 2.9 kHz, and digitized at 5 kHz. Data were analyzed using MiniAnalysis software (Synaptosoft), Clampfit (Molecular Devices Corporation) and Igor (WaveMetrics). All experiments were carried out at room temperature. Methods for counting active synapses are detailed in Supplementary Methods online.
Steady-state fluorescence measurements
Steady-state fluorescence measurements were made at 24 °C using a PTI QM-1 fluorometer with Felix software (Photon Technology International). AEDANS (5-({2-[(iodoacetyl)amino] ethyl}amino)naphthalene-1-sulfonic acid) was excited at 336 nm and emission spectra were collected from 420 nm to 600 nm (5-nm slits). Emission spectra were corrected for blank, dilution and instrument response. Labeled syt (0.5 µM) was mixed with 80 µl of t-SNARE or v-SNARE vesicles or a 1:1 mixture of both (40 µl each) in a total volume of 600 µl. Spectra for each condition were obtained before (0.2 mM EGTA) and after the addition of 1 mM Ca2+. The concentrations of all components were identical to those used in the fusion assays (Supplementary Fig. 4a). Methods for fluorescent labeling of syt and quantification of syt steering can be found in Supplementary Methods.
Supplementary Material
Note: Supplementary information is available on the Nature Structural & Molecular Biology website.
ACKNOWLEDGMENTS
We thank members of the Chapman laboratory, W. Tucker, J.M. Edwardson and M. Jackson for helpful discussions. This work was supported by the Howard Hughes Medical Institute and by grants from the American Heart Association and US National Institutes of Health.
References
- 1.Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 2.Jahn R, Scheller RH. SNAREs–engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 3.Weber T, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 4.Katz B. The Release Of Neural Transmitter Substances. Springfield: Thomas; 1969. [Google Scholar]
- 5.Llinas R, Steinberg IZ, Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys. J. 1981;33:323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabatini BL, Regehr WG. Timing of neurotransmission at fast synapses in the mammalian brain. Nature. 1996;384:170–172. doi: 10.1038/384170a0. [DOI] [PubMed] [Google Scholar]
- 7.Sun JY, Wu LG. Fast kinetics of exocytosis revealed by simultaneous measurements of presynaptic capacitance and postsynaptic currents at a central synapse. Neuron. 2001;30:171–182. doi: 10.1016/s0896-6273(01)00271-9. [DOI] [PubMed] [Google Scholar]
- 8.Koh TW, Bellen HJ, Synaptotagmin Ia. a Ca2+ sensor for neurotransmitter release. Trends Neurosci. 2003;26:413–422. doi: 10.1016/S0166-2236(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Kim-Miller MJ, Fukuda M, Kowalchyk JA, Martin TF. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron. 2002;34:599–611. doi: 10.1016/s0896-6273(02)00671-2. [DOI] [PubMed] [Google Scholar]
- 10.Tucker WC, Weber T, Chapman ER. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 2004;304:435–438. doi: 10.1126/science.1097196. [DOI] [PubMed] [Google Scholar]
- 11.Bai J, Wang CT, Richards DA, Jackson MB, Chapman ER. Fusion pore dynamics are regulated by synaptotagmin*t-SNARE interactions. Neuron. 2004;41:929–942. doi: 10.1016/s0896-6273(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 12.Bhalla A, Chicka MC, Tucker WC, Chapman ER. Ca2+-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 2006;13:323–330. doi: 10.1038/nsmb1076. [DOI] [PubMed] [Google Scholar]
- 13.Perin MS, Fried VA, Mignery GA, Jahn R, Sudhof TC. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 14.Chapman ER, Davis AF. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 1998;273:13995–14001. doi: 10.1074/jbc.273.22.13995. [DOI] [PubMed] [Google Scholar]
- 15.Bai J, Tucker WC, Chapman ER. PIP2 increases the speed-of-response of synaptotagmin and steers its membrane penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- 16.Hui E, Bai J, Chapman ER. Ca2+-triggered simultaneous membrane penetration of the tandem C2-domains of synaptotagmin I. Biophys. J. 2006;91:1767–1777. doi: 10.1529/biophysj.105.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrick DZ, Sterbling S, Rasch KA, Hinderliter A, Cafiso DS. Position of synaptotagmin I at the membrane interface: cooperative interactions of tandem C2 domains. Biochemistry. 2006;45:9668–9674. doi: 10.1021/bi060874j. [DOI] [PubMed] [Google Scholar]
- 18.Chapman ER. How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 2008;77:615–641. doi: 10.1146/annurev.biochem.77.062005.101135. [DOI] [PubMed] [Google Scholar]
- 19.Bhalla A, Tucker WC, Chapman ER. Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion. Mol. Biol. Cell. 2005;16:4755–4764. doi: 10.1091/mbc.E05-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein A, Radhakrishnan A, Riedel D, Fasshauer D, Jahn R. Synaptotagmin activates membrane fusion through a Ca2+-dependent trans interaction with phospholipids. Nat. Struct. Mol. Biol. 2007;14:904–911. doi: 10.1038/nsmb1305. [DOI] [PubMed] [Google Scholar]
- 21.Chapman ER, Hanson PI, An S, Jahn R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J. Biol. Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- 22.Schiavo G, Stenbeck G, Rothman JE, Sollner TH. Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the plasma membrane t-SNARE, SNAP-25, can explain docked vesicles at neurotoxin-treated synapses. Proc. Natl. Acad. Sci. USA. 1997;94:997–1001. doi: 10.1073/pnas.94.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaub JR, Lu X, Doneske B, Shin YK, McNew JA. Hemifusion arrest by complexin is relieved by Ca2+-synaptotagmin I. Nat. Struct. Mol. Biol. 2006;13:748–750. doi: 10.1038/nsmb1124. [DOI] [PubMed] [Google Scholar]
- 24.Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- 25.Earles CA, Bai J, Wang P, Chapman ER. The tandem C2 domains of synaptotagmin contain redundant Ca2+ binding sites that cooperate to engage t-SNAREs and trigger exocytosis. J. Cell Biol. 2001;154:1117–1123. doi: 10.1083/jcb.200105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Melia TJ, et al. Regulation of membrane fusion by the membrane-proximal coil of the t-SNARE during zippering of SNAREpins. J. Cell Biol. 2002;158:929–940. doi: 10.1083/jcb.200112081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pobbati AV, Stein A, Fasshauer D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science. 2006;313:673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- 29.Geppert M, et al. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 30.Pang ZP, Sun J, Rizo J, Maximov A, Sudhof TC. Genetic analysis of synaptotagmin 2 in spontaneous and Ca2+-triggered neurotransmitter release. EMBO J. 2006;25:2039–2050. doi: 10.1038/sj.emboj.7601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai J, Earles CA, Lewis JL, Chapman ER. Membrane-embedded synaptotagmin penetrates cis or trans target membranes and clusters via a novel mechanism. J. Biol. Chem. 2000;275:25427–25435. doi: 10.1074/jbc.M906729199. [DOI] [PubMed] [Google Scholar]
- 32.Melia TJ, You D, Tareste DC, Rothman JE. Lipidic antagonists to SNARE-mediated fusion. J. Biol. Chem. 2006;281:29597–29605. doi: 10.1074/jbc.M601778200. [DOI] [PubMed] [Google Scholar]
- 33.de Wit H, Cornelisse LN, Toonen RF, Verhage M. Docking of secretory vesicles is syntaxin dependent. PLoS ONE. 2006;1:e126. doi: 10.1371/journal.pone.0000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammarlund M, Palfreyman MT, Watanabe S, Olsen S, Jorgensen EM. Open syntaxin docks synaptic vesicles. PLoS Biol. 2007;5:e198. doi: 10.1371/journal.pbio.0050198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wojcik SM, Brose N. Regulation of membrane fusion in synaptic excitation-secretion coupling: speed and accuracy matter. Neuron. 2007;55:11–24. doi: 10.1016/j.neuron.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 36.McMahon HT, Missler M, Li C, Sudhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- 37.Reim K, et al. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- 38.Xue M, et al. Distinct domains of complexin I differentially regulate neurotransmitter release. Nat. Struct. Mol. Biol. 2007;14:949–958. doi: 10.1038/nsmb1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–680. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- 40.Tang J, et al. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 41.Itakura M, Misawa H, Sekiguchi M, Takahashi S, Takahashi M. Transfection analysis of functional roles of complexin I and II in the exocytosis of two different types of secretory vesicles. Biochem. Biophys. Res. Commun. 1999;265:691–696. doi: 10.1006/bbrc.1999.1756. [DOI] [PubMed] [Google Scholar]
- 42.Huntwork S, Littleton JT. A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat. Neurosci. 2007;10:1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- 43.Monck JR, Fernandez JM. The exocytotic fusion pore. J. Cell Biol. 1992;119:1395–1404. doi: 10.1083/jcb.119.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozlov MM, Chernomordik LV. The protein coat in membrane fusion: lessons from fission. Traffic. 2002;3:256–267. doi: 10.1034/j.1600-0854.2002.030403.x. [DOI] [PubMed] [Google Scholar]
- 45.Marrink SJ, Mark AE. The mechanism of vesicle fusion as revealed by molecular dynamics simulations. J. Am. Chem. Soc. 2003;125:11144–11145. doi: 10.1021/ja036138+. [DOI] [PubMed] [Google Scholar]
- 46.Chen YA, Scales SJ, Patel SM, Doung YC, Scheller RH. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 1999;97:165–174. doi: 10.1016/s0092-8674(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 47.Davis AF, et al. Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron. 1999;24:363–376. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- 48.Jackson MB, Chapman ER. Fusion pores and fusion machines in Ca2+-triggered exocytosis. Annu. Rev. Biophys. Biomol. Struct. 2006;35:135–160. doi: 10.1146/annurev.biophys.35.040405.101958. [DOI] [PubMed] [Google Scholar]
- 49.Desai RC, et al. The C2B domain of synaptotagmin is a Ca2+-sensing module essential for exocytosis. J. Cell Biol. 2000;150:1125–1136. doi: 10.1083/jcb.150.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bark IC, Wilson MC. Human cDNA clones encoding two different isoforms of the nerve terminal protein SNAP-25. Gene. 1994;139:291–292. doi: 10.1016/0378-1119(94)90773-0. [DOI] [PubMed] [Google Scholar]
- 51.Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 52.Parlati F, et al. Rapid and efficient fusion of phospholipid vesicles by the α-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc. Natl. Acad. Sci. USA. 1999;96:12565–12570. doi: 10.1073/pnas.96.22.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gitler D, et al. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J. Neurosci. 2004;24:11368–11380. doi: 10.1523/JNEUROSCI.3795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary information is available on the Nature Structural & Molecular Biology website.