Summary
Cryptococcus neoformans is a human fungal pathogen that survives exposure to stresses during growth in the human host, including oxidative and nitrosative stress, high temperature, hypoxia, and nutrient deprivation. There have been many genes implicated in resistance to individual stresses. Notably, the catalases do not have the expected role in resistance to external oxidative stress, but specific peroxidases appear to be critical for resistance to both oxidative and nitrosative stresses. Signal transduction through the HOG1 and calcineurin/calmodulin pathways has been implicated in the stress response. Microarray and proteomic analyses have indicated that the common responses to stress are induction of metabolic and oxidative stress genes, and repression of genes encoding translational machinery.
Introduction
The ubiquitous environmental fungus, Cryptococcus neoformans, can cause morbid meningioencephalitis in the mammalian host [1]. The disease progresses after inhalation into the lung, followed by evasion of the innate immune system, replication within phagocytes and tissues, and systemic dissemination. Stressors include those that impede growth, such as temperature, pH, anoxia and nutrient deprivation, and those that are potentially toxic, such as reactive oxidative, nitrosative and chlorinating species. It is unlikely that the stress response pathways were developed specifically for survival in a mammalian host, but are the result of stress that the fungus encounters in its primary ecological niche. How C. neoformans is able to survive the diverse stressors it encounters within the host is the focus of research over the last three years.
Substantial progress has been made in the field of stress response, but the regulation and mechanisms behind the overall stress responses are not fully understood. Research has continued to utilize studies of individual genes and pathways, but the recent sequencing of the genome [2•] has led to the development of C. neoformans-specific microarrays and the application of proteomic techniques. These research strategies have contributed to the field by providing genome-wide data that has led to hypotheses about global responses, such as translation repression, metabolic changes, or cell wall rearrangements. This review will discuss the genes that are important for stress resistance, the signal transduction pathways that have been implicated in stress response, and the trends revealed by genome-wide studies (see Figure 1).
Figure 1.
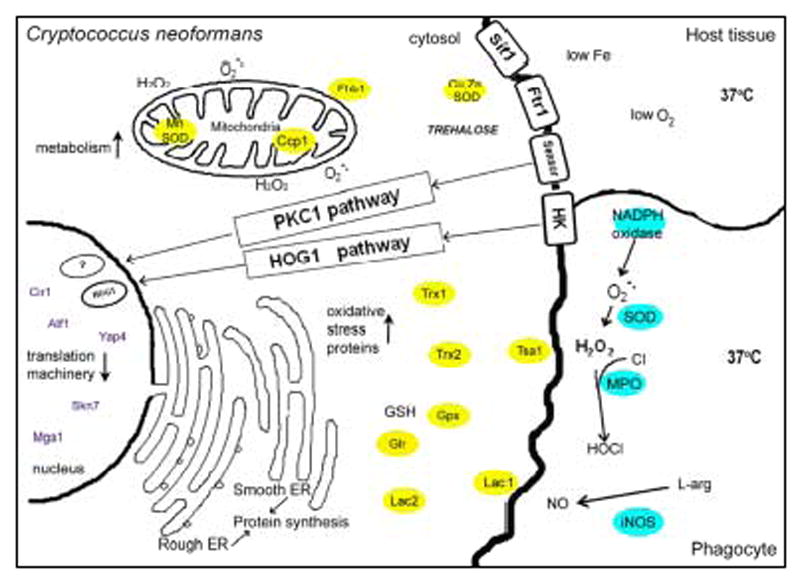
A schematic of the stresses that C. neoformans encounters in the human host, and the genes and pathways involved in the stress responses. The C. neoformans proteins are defined in the text.
Increased temperature is likely the first stressor that C. neoformans encounters after it gains entry to the host
Two diverse temperature resistance mechanisms employed by C. neoformans include the apparent protective properties of the sugar, trehalose, and the mechanistic link between antioxidant protection and the adaptation to growth at human body temperature.
Accumulation of the disaccharide trehalose in S. cerevisiae has been shown to prevent denaturation of important proteins as well as to aid heat-shock protein chaperones in re-naturation by preventing aggregation of denatured proteins [3]. In C. neoformans , null mutants of trehalose-6-phosphate synthase (TPS1) and trehalose-6-phosphate phosphatase (TPS2) revealed they are both required for growth at 37°C, but the protective mechanism of trehalose remains to be elucidated. As expected, these Ts mutants were avirulent in mammalian infection models, however the tps1Δ strain also had attenuated virulence in a C. elegans model at 25°C, suggesting that trehalose biosynthesis has a role in virulence independent from its role in growth at 37°C [4].
Recent data implies that higher temperatures may stimulate the rate of mitochondrial respiration, leading to an increase of production of superoxide. Mitochondrial manganese superoxide dismutase (SOD), which promotes the rapid degradation of superoxide into hydrogen peroxide and O2, has been suggested to augment adaptation of C. neoformans to human host body temperature [5] by regulating steady-state concentrations of oxygen radicals in the mitochondria and contributing to the integrity of the electron transport chain.
Resistance to reactive oxidative and nitrosative species is important for successful colonization of mammalian hosts
C. neoformans is phagocytosed by alveolar macrophages during the initial stages of infection, and must protect itself from reactive nitrogen and oxygen intermediates. Activated alveolar macrophages can produce up to 14mM hydrogen peroxide and 57μM nitric oxide (NO) [6], however C. neoformans can prevent macrophage activation to avoid exposure to these reactive molecules. The inhibition of activation is mediated by the extracellular polysaccharide capsule [7]. C. neoformans does not appear to be unusually resistant to reactive oxidative and nitrosative stresses, compared to other fungi. However, resistance is clearly important for survival in mammalian hosts, since impairment of several resistance pathways reduces survival in macrophages and attenuates virulence. Furthermore, these pathways are upregulated by oxidative and nitrosative stresses.
Cu,Zn superoxide dismutase has been shown to be important for resistance to oxygen radicals generated by epinephrine and for growth in macrophages [8]. Unexpectedly, catalases do not seem to play a major role in detoxification of exogenous reactive molecules [9••], since deletion of the entire family of catalases has no effect on resistance to oxidative stress or virulence. Instead, several peroxidases have critical roles in resistance to oxidative stress and, in some cases, overlap with resistance to nitrosative stress.
The glutathione system, which ultimately depends on the pools of reduced glutathione (GSH) maintained by glutaredoxin, is critical for resistance to oxidative and nitrosative stress. Glutathione peroxidase (GPX) removes hydrogen peroxide, and as expected, null gpx mutants are sensitive to peroxides [10]. Another protein in the glutathione system that impacts resistance to nitrosative but not oxidative stress is glutathione reductase [11]. The precise role of Glr in nitrosative stress is puzzling because in other systems, Glr has been shown to destroy alkyl peroxides and remove glutathione from protein thiols [12].
Laccases are important for production of the pigment melanin, which is a free radical scavenger and thus plays a protective role in stress resistance. Laccase also sequesters iron during infection, which interferes with the oxidative burst of phagocytes. Laccase expression decreases as fungal burden increases [13], although the mechanism of regulation of laccase during C. neoformans infection has yet to be fully described. In vivo regulation of laccase proceeds, at least in part, via the co-activator Ssa1, a member of the Hsp70 family [14].
The role of Tsa1 in stress resistance is particularly intriguing. A tsa1Δ strain has shown that Tsa1 is needed for normal resistance to oxidative and nitrosative stress [15]. Reduction is required for recycling of the enzyme to its active form, and in other systems this is catalyzed by thioredoxin (Trx). If Trx alone were responsible for the reduction of CnTsa1, then a Trx deficient strain would be at least as sensitive to stress as is a tsa1Δ strain. In C. neoformans, a strain deleted for both TRX genes is less sensitive to oxidative stress than a tsa1Δ strain. It is possible that components of the glutathione system, such as Glr and perhaps glutathione itself, may be involved in reduction of Tsa1. The existence of such cooperation between oxidative stress systems like the glutathione system and the thioredoxin system has not yet been explored. Conversely, Tsa1 may not be recycled, but may be overoxidized and degraded. Modified forms of Tsa1 are detected on 2D protein gels that may represent overoxidized protein [11].
The tsa1Δ strain is also sensitive to NO stress, yet it is unclear whether Tsa1 is directly involved in NO detoxification. Modified forms of Tsa1 were detected after exposure to NO [11], providing initial support for the direct involvement of Tsa1 in detoxification of NO. The delineation of the amino acids involved in NO resistance, and nature of the modifications will clarify the role of Tsa1 in NO resistance. Alternatively, Tsa1 may serve to propagate a signal that is essential for resistance to nitrosative stress. Tsa1 has already been shown to regulate the laccase genes during nitrosative stress, lending credence to a signaling role [16].
A consumer of nitric oxide is the hemoprotein flavohemoglobin. Deletion of the flavohemoglobin gene (FHB1) in C. neoformans resulted in hypersensitivity to NO and attenuated virulence in a murine model [17]. The reduction in virulence was directly related to NO sensitivity, since the fhb1Δ strain behaved as wild type in an iNOS deficient mouse model.
Does resistance to reactive species produced by cells other than macrophages play a role in pathogenesis?
Most of the research on phagocytosis has focused on macrophages, but C. neoformans can be phagocytosed by other cells, including neutrophils and endothelial cells, which produce additional stressors. A considerable amount of the microbicidal activity of neutrophils depends on the production of hypochlorous acid by the myeloperoxidase system, which produces HOCl from Cl and hydrogen peroxide via myeloperoxidase (MPO). In other organisms, like S. aureus and E. coli, genes that impart resistance to H2O2 are also important for resistance to hypohalous acids and superoxide [18], but the oxidative stress mutants of C. neoformans have not been tested for sensitivity to HOCl. Enticingly, MPO knock out mice are more susceptible to C. neoformans infection compared to wild-type mice [19].
Iron deprivation provokes major responses in C. neoformans
Iron acquisition during growth in a host is known to be a limiting factor for most pathogens. Low iron conditions have been associated with major phenotypic changes in C. neoformans, including induction of the large polysaccharide capsule and repression of laccase activity. Iron deprivation has been shown to induce a siderophore iron transporter (SIT1) and an iron permeases (FTR1), which function to increase intracellular pools of iron available to the fungus [20, 21]. Deletion of the permease or transporter results in very slow growth in low iron conditions.
C. neoformans employs multiple sensing and signaling systems to detect local environmental stresses enabling it to affect a response
Recent work has focused on a histidine kinase (HK), Protein Kinase C (PKC1), and the calmodulin/calcineurin pathways. A C. neoformans HK system activates the Hog1 MAPK signaling pathway [22•], leading to recruitment of downstream effectors that mount the appropriate stress response [23]. Most fungi, with the exception of S. cerevisiae, possess multiple hybrid HK sensors, allowing them to sense a variety of environmental cues including osmotic shock, UV irradiation, oxidative damage, and high temperature. HK sensor systems in the ascomycete fungi have been classified into 11 distinct groups [24]. However, Bahn et al. [22•] identified previously unclassified HK sensor systems in the basidiomycete C. neoformans, suggesting that this sensing system may be even more diversified than previously observed. Furthermore, this system is involved in C. neoformans’ response to low environmental oxygen stress. Both the HK pathway and the SREBP (sterol-response element protein binding) pathway, which activates genes involved in sterol biosynthesis, are involved in C. neoformans ability to adapt to the hypoxic conditions encountered within infected host tissues and are required for virulence [25•]. Both pathways were demonstrated to be required for proliferation of the pathogen within the host tissues, suggesting a link between adaptation to hypoxia and pathogenesis.
Another conserved protein found to be involved in regulating stress responses is calmodulin. The Ca2+ sensor calmodulin is a small protein that upon binding Ca2+, activates the serine threonine-specific protein phosphatase, calcineurin. Calcineurin is critical for growth at 37°C in C. neoformans [26; 27] and calmodulin (CAM1) is essential for viability [28]. Isolation of a CnCAM1-Tsmutant allele led to the delineation of a bifurcated calmodulin/calcineurin signaling system with both a calcium-dependent and -independent mechanism regulating C. neoformans growth at host body temperature [29•]. The downstream pathway activated by the Ca2+ -independent calmodulin remains to be determined.
The PKC1 pathway is a MAP kinase pathway believed to be involved in both sensing stress and maintaining cell wall integrity during stress [30]. This pathway likely includes transmembrane receptors or sensors, and several potential sensor/receptors have been predicted in C. neoformans using a bioinformatic approach and searching for specific stress sensing domains [Gerik et al. abstract 07-GM-A-4356-ASM, ASM General 107th Meeting 2007]. This pathway may be involved in fungal stress resistance [31], and cell wall genes are often regulated during various stresses. The calcineurin pathway can also affect cell wall biosynthesis by intersecting with the PKC1 pathway [32].
Transcription factors have been identified that regulate stress responses
Two putative transcription factors have been found to regulate the thioredoxin system under oxidative and nitrosative stress. Yap4, which is homologous to an AP1 transcription factor, is required for induction of TRX under nitrosative stress, and a yap4Δ mutant is sensitive to NO. An ARF/CREB-like gene, ATF1, is needed for induction of TRX under oxidative stress, and an aft1Δ mutant is sensitive to oxidative stress [33]. Neither of these transcription factors are the major response regulator, since expression of other critical genes such as TSA1 or FHB1 are not regulated by YAP4 or ATF1.
Another transcription factor, Skn7, homologous to a stress response regulator in S. cerevisiae, was upregulated in C. neoformans recovered from endothelial cells. Although its exact role it still unclear, disruption of SKN7 leads to sensitivity to oxidative stress and susceptibility to killing in endothelial cells [34].
Excitingly, the central regulator for the iron response in C. neoformans has been identified as Cir1 [35••]. By microarray analysis, this transcription regulator was shown to impact expression of the iron regulon, as well as the virulence factors laccase, phopholipase, and capsule. Cir1 also regulates cell wall biosynthesis genes possibly by influencing expression of the Ca2+/calmodulin and PKC1 signaling pathways.
Microarrays and proteomic analysis have recently been employed to acquire snapshots of genome and proteome wide changes under a variety of stresses
Microarray analysis of changes in transcript levels have been done using cells with and without exposure to low oxygen, high temperature, reactive species, and phagocytosis.
The transcriptional profile of C. neoformans cells grown under normal oxygen conditions was compared to those grown under low oxygen conditions. Changes were observed in the levels of 347 transcripts with up-regulation of genes involved in stress regulation, carbohydrate metabolism and respiration and down-regulation of those involved in translation, vesicle trafficking, and cell wall and capsule synthesis [25].
Kraus et al [28] conducted microarray analysis comparing transcripts of cells exposed to 25 degrees to those exposed to 37 degrees. Temperature regulated genes included SOD, trehalose synthase, and a putative cell wall stress sensor. Interestingly, a transcription factor ortholog, Mga2, was induced at 37°C found to be important for high temperature growth.
A recent study used microarrays to investigate changes in gene expression during murine macrophage infection [36]. Results showed that while several hundred genes were upregulated, many fewer were downregulated after phagocytosis. Upregulated genes included those involved in membrane transport, oxidative stress response, signaling pathways, metabolism, autophagy, and mating type. Most of the downregulated genes were involved in translation machinery, suggesting that translation is likely repressed as a general stress response which allows the cell to adapt to the environment while conserving energy by reducing protein synthesis. Interestingly, the number of repressed translation genes increased substantially between 2 and 24hr, but this seems paradoxical since C. neoformans efficiently replicates eight hours after being ingested [37]. It is not clear these changes result in non-specific or regulated repression of translation. Proteomic analysis suggests that there is very little proteomic change following peroxide treatment [Brown and Lodge, unpublished], but there is clearly a transcriptional response to oxidative stress as evidenced by analysis of several oxidative stress genes. This suggests that there may be a global repression of translation during oxidative stress. However, serial expression of gene analysis (SAGE) of the transcriptome of C. neoformans in a model of rabbit meningitis, suggested that the translational machinery was induced compared to in vitro growth [38].
The transcriptional and translational response to nitrosative stress was described together showing how these two analyses could complement each other [11]. Upregulated proteins included those involved in stress pathways, signaling, and metabolism. These results were compared to and supported by the microarray analysis of mRNA from the same cells. Modified forms of at least three proteins, including Tsa1, were found by 2D gel analysis of the protein.
How well C. neoformans is able to adapt to external stress likely depends on how well it is able to maintain metabolic homeostasis under stress
The importance of SOD for high temperature growth suggests that at least one stressor, temperature, results in an increased rate of mitochondrial respiration. Since many metabolic pathways, such as the pentose phosphate pathway, are regulated by ATP, these pathways are likely upregulated under stress as well. At the same time, upregulation of genes involved in carbohydrate and lipid transport and autophagy [11, 25, 28, 36], indicate that there is a significant need for uptake of nutrients and energy production after host infection and ingestion by phagocytes.
Since metabolism involves the production of molecules that are important for fueling stress pathways, metabolic flux would directly influence the ability to maintain the redox state of the cell under stressful conditions. For example, the reducing equivalent NADPH produced by the pentose phosphate pathway is essential for maintenance of functional glutathione and thioredoxin systems and is also bound to proteins like Fhb1. Missall [11] reported that transaldolase, a key enzyme in the pentose phosphate pathway, is upregulated and modified during nitrosative stress, and several members of the pentose phosphate pathway were highly expressed in the rabbit model of meningitis [38]. This indicates the pathway may be induced by stress to increase the production of NADPH, as shown in other systems [39].
Conclusions
The stress response in C. neoformans is an intricate network of proteins and pathways that involve sensing stress, reacting to stress, and maintaining homeostasis in a stressful environment. Studies of individual genes and pathways have revealed unique features of stress resistance in C. neoformans. It was anticipated that the catalases would play major roles in stress resistance, and yet they are dispensable. Peroxidases, especially the peroxiredoxin Tsa1, have surprising roles in resistance to nitrosative stress. Two signaling pathways have been implicated in transducing the stress response, but the mechanisms of how the stress is sensed, and how the pathways propagate the signal is still unknown
The application of systems biology approaches, that incorporates microarray, proteomic and metabolomic data, will generate hypotheses about the common and specific stress responses. Similar themes have already emerged from genome-wide analyses of the stress responses, one of which is repression of translation. Whether this repression is specific to a subset of genes is undetermined. The induction of metabolism and oxidative stress genes in several stresses implies that respiration and thus the demand for reductive metabolic intermediates is increased as a common response to stress. These hypotheses are ready to be tested.
Acknowledgments
The authors wish to thank Dr. John Corbett for helpful discussions. Work that contributed this manuscript was supported by National Institute of Health grants RO1AI051209 and R01HL088905 to JKL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah M. Brown, Email: brownsm2@slu.edu.
Leona T. Campbell, Email: lcampb14@slu.edu.
References
- 1.Casadevall A, Perfect J, editors. Cryptococcus neoformans. ASM Press; 1998. [Google Scholar]
- •2.Loftus BJ, Fung E, Roncaglia P, Rowley D, Amedeo P, Bruno D, Vamathevan J, Miranda M, Anderson IJ, Fraser JA, et al. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science. 2005;307:1321–4. doi: 10.1126/science.1103773. In this study, the authors sequenced two strains of Cryptococcus neoformans, which has formed the basis of the genome wide transcriptome and proteome studies described in this manuscript. They also provided an outstanding annotation of the genes of Cryptococcus and demonstrated the presence of alternative splicing and antisense transcripts.
- 3.Crowe JH. Trehalose as a “chemical chaperone”: fact and fantasy. Adv Exp Med Biol. 2007;594:143–158. doi: 10.1007/978-0-387-39975-1_13. [DOI] [PubMed] [Google Scholar]
- 4.Petzold E, Himmelreich U, Mylonakis E, Rude T, Toffaletti D, Cox G, Miller J, Perfect J. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infection and Immunity. 2006;74:5877–5887. doi: 10.1128/IAI.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giles S, Batinic-Haberle I, Perfect J, Cox G. Cryptococcus neoformans mitochondrial superoxide dismutase: and essential link between antioxidant function and high-temperature growth. Eukaryotic Cell. 2005;4:46–54. doi: 10.1128/EC.4.1.46-54.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross NT, Nessa K, Camner P, Jarstrand C. Production of nitric oxide by rat alveolar macrophages stimulated by Cryptococcus neoformans or Aspergillus fumigatus. Medical Mycology. 1999;37:151–157. [PubMed] [Google Scholar]
- 7.Kozel TR, Tabuni A, Young BJ, Levitz S. Influence of opsonization conditions on C3 deposition and phagocyte binding of large and small-capsule Cryptococcus neoformans cells. Infection and Immunity. 1996;64:2336–2338. doi: 10.1128/iai.64.6.2336-2338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox GM, Harrison TS, McDade HC, Taborda CP, Heinrich G, Casadevall A, Perfect JR. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infection and Immunity. 2003;71:173–180. doi: 10.1128/IAI.71.1.173-180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••9.Giles S, Stajich J, Nichols C, Gerrald Q, Alspaugh JA, Dietrich F, Perfect JR. The Cryptococcus neoformans catalase gene family and its role in antioxidant function. Eukaryotic Cell. 2006;5:1447–1459. doi: 10.1128/EC.00098-06. In this study, the authors clearly demonstrated the surprising observation that the catalase gene family is not required for resistance to external oxidative stress. They did this by deleting all four catalase genes and showing that this quadruple deletion strain is resistant to peroxide and has no virulence defect.
- 10.Missall T, Cherry-Harris J, Lodge J. Two glutathione peroxidases in the fungal pathogen Cryptococcus neoformans are expressed in the presence of specific substrates. Microbiology. 2005;151:2573–2581. doi: 10.1099/mic.0.28132-0. [DOI] [PubMed] [Google Scholar]
- 11.Missall T, Pusateri M, Donlin M, Chambers K, Corbett J, Lodge J. Posttranslational, translational, and transcriptional responses to nitric oxide stress in Cryptococcus neoformans: implications for virulence. Eukaryotic Cell. 2006;5:518–529. doi: 10.1128/EC.5.3.518-529.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beer S, Taylor S, Brown S, Dahm C, Costa N, Runswick M, Murphy M. Glutaredoxin 2 catalyzes the reversible glutathionylation of mitochondrial membrane thiol proteins. Journal of Biological Chemistry. 2004;297:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Rivera J, Tucker S, Feldmesser M, Williamson P, Casadevall A. Laccase expression in murine pulmonary Cryptococcus neoformans infection. Infection and Immunity. 2005;73:3124–3127. doi: 10.1128/IAI.73.5.3124-3127.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Hacham M, Panepinto J, Hu G, Shin S, Zhu X, Williamson PR. The Hsp70 member, Ssa1, acts as a DNA-binding transcriptional co-activator of laccase in Cryptococcus neoformans. Molecular Microbiology. 2006;53:1090–101. doi: 10.1111/j.1365-2958.2006.05422.x. [DOI] [PubMed] [Google Scholar]
- 15.Missall T, Pusateri M, Lodge J. Thiol peroxidase is critical for virulence and resistance to nitric oxide and peroxide in the fungal pathogen, Cryptococcus neoformans. Molecular Microbiology. 2004;51:1447–1458. doi: 10.1111/j.1365-2958.2004.03921.x. [DOI] [PubMed] [Google Scholar]
- 16.Missall TA, Moran JM, Corbett JA, Lodge JK. Distinct stress responses of two functional laccases in Cryptococcus neoformans are revealed in the absence of the thiol-specific antioxidant Tsa1. Eukaryotic Cell. 2005;4:202–208. doi: 10.1128/EC.4.1.202-208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jesus-Berrios M, Liu L, Nussbaum JC, Cox GM, Stamler JS, Heitman J. Enzymes that counteract nitrosative stress promote fungal virulence. Current Biology. 2003;13:1983–1988. doi: 10.1016/j.cub.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Dukan S, Touati D. Hypochlorous acid stress in Escherichia coli: resistance, DNA damage, and comparison with hydrogen peroxide stress. Journal of Bacteriology. 1996;178:6145–6150. doi: 10.1128/jb.178.21.6145-6150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Ishida-Okawara A, Suzuki K, Maeda N, Koyama H. Contribution of the myeloperoxidase-dependent oxidative system to host defense against Cryptococcus neoformans. Journal of Medical Microbiology. 2006;55:1291–1299. doi: 10.1099/jmm.0.46620-0. [DOI] [PubMed] [Google Scholar]
- 20.Tangen KL, Jung WH, Sham AP, Lian T, Kronstad JW. The iron- and cAMP-regulated gene SIT1 influences ferrioxamine B utilization, melanization and cell wall structure in Cryptococcus neoformans. Microbiology. 2007;153:29–41. doi: 10.1099/mic.0.2006/000927-0. [DOI] [PubMed] [Google Scholar]
- 21.Lian T, Simmer MI, D’Souza CA, Steen BR, Zuyderduyn SD, Jones SJ, Marra MA, Kronstad JW. Iron-regulated transcription and capsule formation in the fungal pathogen Cryptococcus neoformans. Mol Microbiol. 2005;55:1452–72. doi: 10.1111/j.1365-2958.2004.04474.x. [DOI] [PubMed] [Google Scholar]
- •22.Bahn YS, Kojima K, Cox G, Heitman J. A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Molecular Biology of the Cell. 2006;17:3122–3135. doi: 10.1091/mbc.E06-02-0113. In this extensive study, the authors characterized two sensor kinases and the downstream regulators that signal through the Hog1 MAPK pathway. Each kinase had distinct but overlapping roles in signaling for virulence, mating, differentiation and stress response.
- 23.Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev. 2002;66:300–372. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catlett NL, Yoder OC, Turgeon BG. Whole-genome analysis of two-component signal transduction genes in fungal pathogens. Eukaryotic Cell. 2003;2:1151–1161. doi: 10.1128/EC.2.6.1151-1161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •25.Chun C, Liu O, Madhani H. A link between virulence and homeostatic responses to hypoxia during infection by the human fungal pathogen Cryptococcus neoformans. PLOS Pathogens. 2007;3:225–238. doi: 10.1371/journal.ppat.0030022. The authors explored the transcriptional response to hypoxia in C. neoformans since the organism is exposed to low oxygen conditions in the mammalian host tissues. This study is noteworthy because they have pioneered this area of research in pathogenic fungi.
- 26.Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox DS, Cruz MC, Sia RA, Ke H, Cox GM, Perfect JR, Heitman J. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Molecular Microbiology. 2001;39:835–849. doi: 10.1046/j.1365-2958.2001.02295.x. [DOI] [PubMed] [Google Scholar]
- 28.Kraus P, Boily MJ, Giles S, Stajich JE, Allen A, Cox G, Dietrich F, Perfect J, Heitman J. Identification of Cryptococcus neoformans temperature-related genes with a genomic-DNA microarray. Eukaryotic Cell. 2004;3:1249–1260. doi: 10.1128/EC.3.5.1249-1260.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •29.Kraus PR, Nichols CB, Heitman J. Calcium- and calcineurin-independent roles for calmodulin in Cryptococcus neoformans morphogenesis and high-temperature growth. Eukaryotic Cell. 2005;4:1079–1087. doi: 10.1128/EC.4.6.1079-1087.2005. It has been known that calcineurin was needed for growth and differentiation at mammalian body temperature, and this study extensively explored the signaling mechanisms that regulate growth at 37°C. Their interesting observation was that there is a calcium- and calcineurin independent aspect to the calmodulin pathway.
- 30.Gerik KJ, Donlin MJ, Soto CE, Banks AM, Banks IR, Maligie MA, Selitrennikoff CP, Lodge JK. Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Molecular Microbiology. 2005;58:393–408. doi: 10.1111/j.1365-2958.2005.04843.x. [DOI] [PubMed] [Google Scholar]
- 31.Vilella F, Herrero E, Torres J, de la Torre-Ruiz MA. Pkc1 and the upstream elements of the cell integrity pathway in Saccharomyces cerevisiae, Rom2 and Mtl1, are required for cellular responses to oxidative stress. Journal of Biological Chemistry. 2005;280:9149–9159. doi: 10.1074/jbc.M411062200. [DOI] [PubMed] [Google Scholar]
- 32.Kraus PR, Fox DS, Cox GM, Heitman J. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol Microbiol. 2003;48:1377–87. doi: 10.1046/j.1365-2958.2003.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Missall T, Lodge J. Function of the thioredoxin proteins in Cryptococcus neoformans during stress or virulence and regulation by putative transcriptional modulators. Molecular Microbiology. 2005;57:847–858. doi: 10.1111/j.1365-2958.2005.04735.x. [DOI] [PubMed] [Google Scholar]
- 34.Coenjaerts FE, Hoepelman AI, Scharringa J, Aarts M, Ellerbroek PM, Bevaart L, Van Strijp JA, Janbon G. The Skn7 response regulator of Cryptococcus neoformans is involved in oxidative stress signaling and augments intracellular survival in endothelium. FEMS Yeast Research. 2006;6:652–661. doi: 10.1111/j.1567-1364.2006.00065.x. [DOI] [PubMed] [Google Scholar]
- ••35.Jung WH, Sham A, White R, Kronstad JW. Iron regulation of the major virulence factors in the AIDS-associated pathogen Cryptococcus neoformans. PLoS Biol. 2006;4:e410. doi: 10.1371/journal.pbio.0040410. In this study, the authors identified the master transcription regulator of the iron regulon in Cryptococcus neoformans. Deletion of this putative transcription factor resulted in a complete absence of transcription regulation by iron. This transcription factor also regulates expression of the major virulence factors, capsule, melanin and the ability to grow at 37°C.
- 36.Fan W, Kraus P, Boily MJ, Heitman J. Cryptococcus neoformans gene expression during murine macrophage expression. Eukaryotic Cell. 2005;4:1420–1433. doi: 10.1128/EC.4.8.1420-1433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tucker SC, Casadevall A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci USA. 2002;99:3165–3170. doi: 10.1073/pnas.052702799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larochelle M, Drouin S, Robert F, Turcott B. Oxidative-stress activated zinc cluster protein stb5 has dual activator/repressor functions required for the pentose phosphate pathway regulation and NADPH production. Molecular and Cellular Biology. 2006;26:6690–6701. doi: 10.1128/MCB.02450-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steen BR, Zuyderduyn S, Toffaletti DL, Marra M, Jones SJ, Perfect JR, Kronstad J. Cryptococcus neoformans gene expression during experimental cryptococcal meningitis. Eukaryot Cell. 2003;2:1336–49. doi: 10.1128/EC.2.6.1336-1349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


