Abstract
Periodontal disease is a bacteria-induced chronic inflammatory disease affecting the soft and hard supporting structures encompassing the teeth. When left untreated, the ultimate outcome is alveolar bone loss and exfoliation of the involved teeth. Traditional periodontal diagnostic methods include assessment of clinical parameters and radiographs. Though efficient, these conventional techniques are inherently limited in that only a historical perspective, not current appraisal, of disease status can be determined. Advances in the use of oral fluids as possible biological samples for objective measures of current disease state, treatment monitoring, and prognostic indicators have boosted saliva and other oral-based fluids to the forefront of technology. Oral fluids contain locally and systemically derived mediators of periodontal disease, including microbial, host-response, and bone-specific resorptive markers. Although most biomarkers in oral fluids represent inflammatory mediators, several specific collagen degradation and bone turnover-related molecules have emerged as possible measures of periodontal disease activity. Pyridinoline cross-linked carboxyterminal telopeptide (ICTP), for example, has been highly correlated with clinical features of the disease and decreases in response to intervention therapies, and has been shown to possess predictive properties for possible future disease activity. One foreseeable benefit of an oral fluid-based periodontal diagnostic would be identification of highly susceptible individuals prior to overt disease. Timely detection and diagnosis of disease may significantly affect the clinical management of periodontal patients by offering earlier, less invasive, and more cost-effective treatment therapies.
Keywords: periodontal disease, oral fluids, saliva, disease progression, diagnosis, bone resorption
PERIODONTAL DISEASES: BACKGROUND
Chronic infectious diseases of the oral cavity include dental caries and periodontal disease, the former causing destruction of the teeth, while the latter is, a group of inflammatory conditions, affects the supporting structures of the dentition.1 The unequivocal role of the microbial challenge in the etiology of periodontal disease has been well studied. However, it is the paradoxical impact of the susceptible host’s inflammatory response to the microbial challenge that ultimately leads to the destruction of the periodontal structures and subsequent tooth loss.2-4
Periodontal diseases are further divided into reversible and nonreversible categories. Gingivitis is a reversible inflammatory reaction of the marginal gingiva to dental plaque biofilms. Gingivitis is characterized by an initial increase in blood flow, enhanced vascular permeability, and influx of cells (polymorphonuclear leukocytes [PMNs] and monocyte-macrophages) from the peripheral blood into the periodontal connective tissue. Overt soft tissue alterations during the state of gingivitis include redness, edema, bleeding, and tenderness. The feature distinguishing gingivitis from the destructive form of periodontal disease is the intact anatomical location of the junctional epithelium on the root surface.
Periodontitis, the destructive category of periodontal disease, is a nonreversible inflammatory state of the supporting structures. After its initiation, the disease progresses with the loss of collagen fibers and attachment to the cemental surface, apical migration of the pocket epithelium, formation of deepened periodontal pockets, and the resorption of alveolar bone. If left untreated, the disease continues to progressive bone destruction, leading to tooth mobility and subsequent tooth loss.5
Chronic periodontitis is the most prevalent form of destructive periodontal disease and typically progresses at a slow, steady pace with bouts of extensive disease destruction separated by quiescent periods of bone loss.6-8 Albandar et al. examined the prevalence and severity of chronic periodontitis in the United States adult population (30 years and older) and found that from 1988 to 1994 approximately 35% presented with chronic periodontitis. After adjusting for measurement error due to the partial readings, it was determined that approximately half of U.S. adults had chronic periodontitis. Further breakdown of these findings indicate that 31% of the U.S. population exhibit mild forms of the disease, 13% display moderate severity, and 4% suffer advanced disease.9,10
More than 600 different bacteria are capable of colonizing the human mouth with any individual typically harboring 150 to 200 varying species. Of these many bacteria, it is estimated that approximately 10% play a causal role in the initiation of periodontal disease.2,11-13 Three organisms in particular, Tanerella forsythensis, Porphyromonas gingivalis, and Treponema denticola have been directly associated with chronic periodontitis.13-15 Actinobacillus actinomycetemcomitans, another virulent gram-negative bacterium, has been observed in early-onset forms of periodontal disease and aggressive periodontitis.12 Inherent virulence factors of these pathogenic species enable the bacteria to colonize on the tooth and in the gingival sulcus, defend itself from the host’s antibacterial defense mechanisms, and cause tissue damage by producing potent substances that subsequently trigger the host’s innate inflammatory response.16 Although pathogen-based diagnostic tests are imperative for the initiation of periodontal disease, their utility has not been successful for prediction of periodontal disease.17 At best, pathogen-based tests serve as adjuncts to traditional diagnostic methods by assessing vulnerability of patient and site, classifying disease category, and assisting in treatment modality.
NEED FOR A PERIODONTAL DIAGNOSTIC INDICATOR
A periodontal diagnostic tool provides pertinent information for differential diagnosis, localization of disease, and severity of infection. These diagnostics, in turn, serve as a basis for planning treatment and provide a means for assessing the effectiveness of periodontal therapy.18 Current clinical diagnostic parameters that were introduced more than 50 years ago continue to function as the basic model for periodontal diagnosis in clinical practice today. They include probing pocket depths, bleeding on probing, clinical attachment levels, plaque index, and radiographs that quantify alveolar bone levels.19,20 Albeit easy to use, cost-effective, and relatively noninvasive, clinical attachment loss evaluation by the periodontal probe measures damage from past episodes of destruction and requires a 2- to 3-mm threshold change before a site can be deemed as having experienced significant breakdown. Recent revisions in the design of automated periodontal probes have improved the accuracy and long-term tracking of disease progression. Furthermore, the use of subtraction radiography also offers a method to detect minute changes in the height of alveolar bone. However, both of the above-mentioned techniques are most often seen in the research setting and seldom in clinical practice. In addition to these limitations, conventional disease diagnosis techniques lack the capacity to identify highly susceptible patients who are at risk for future breakdown.21-23 Researchers are confronted then with the need for an innovative diagnostic test that focuses on the early recognition of the microbial challenge to the host. Optimal innovative approaches would correctly determine the presence of current disease activity, predict sites vulnerable for future breakdown, and assess the response to periodontal interventions. A new paradigm for periodontal diagnosis would ultimately affect improved clinical management of periodontal patients.
ORAL FLUID BIOMARKERS OF PERIODONTAL DISEASE
In response to requests from the Office of the Surgeon General and the National Institute of Dental and Craniofacial Research (NIDCR) for concentrated research in salivary diagnostics, significant advancements have been achieved within the past 10 years using saliva, gingival crevicular fluid (GCF), and mucosal transudate as biological samples for the detection of oral and systemic illnesses.24 Easily collected and containing local- and systemic-derived biomarkers of periodontal disease, oral fluids may offer the basis for a patient-specific diagnostic test for periodontal disease.25-28 A biomarker is an objective measure that has been evaluated and confirmed either as an indicator of physiologic health, a pathogenic process, or a pharmacologic response to a therapeutic intervention.29 Oral fluid biomarkers that have been studied for periodontal diagnosis include proteins of host origin (e.g., enzymes and immunoglobulins), phenotypic markers, host cells (e.g., PMNs), hormones, bacteria and bacterial products, ions, and volatile compounds.20,25,30-32 Because of the complex, multifaceted nature of periodontal disease, it is highly unlikely that a single biomarker will prove to be a stand-alone measure for periodontal disease diagnosis. More probable may be the development of an oral fluid-based diagnostic using a combination of host- and site-specific markers that accurately assess periodontal disease status.31,33-36
During the initiation of an inflammatory response in the periodontal connective tissue (Fig. 1), numerous cytokines, such as prostaglandin E2 (PGE2), interleukin (IL)-1β, IL-6, or tumor necrosis factor (TNF)-α are released from cells of the junctional epithelia, connective tissue fibroblasts, macrophages, and PMNs. Subsequently, T and B cells emerge at the infection sites and secrete immunoglobulins as an antigen-specific response.37 Additionally, a number of enzymes, such as matrix metalloproteinase (MMP)-8, MMP-9, or MMP-13 are produced by PMNs and osteoclasts, leading to the degradation of connective tissue collagen and alveolar bone.38 As a consequence of bone resorption, pyridinoline cross-linked carboxyterminal telopeptide (ICTP) and osteocalcin are released into the periodontal tissues. During the inflammatory process intercellular products are created and migrate toward the gingival sulcus or periodontal pocket (Fig.1). These mediators of disease activity have been identified and sampled from various biological fluids, such as saliva and GCF.39
FIGURE 1.
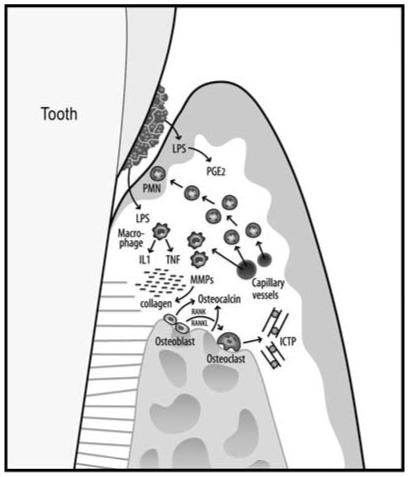
Schematic overview of the pathogenic processes in periodontal disease. Initial events are triggered by LPS from gram-negative plaque biofilms on the tooth root surfaces. As a first line of defense, PMNs are recruited to the site. Monocytes and activated macrophages respond to endotoxin by releasing cytokines TNF and IL-1, which direct further destructive processes. MMP, powerful collagen-destroying enzymes, are produced by fibroblasts and PMNs. TNF, IL-1, and receptor activator of NF-κB ligand (RANKL) are elevated in active sites and mediate osteoclastogenesis and bone breakdown. Bone-specific markers, such as pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP), are released into the surrounding area and transported by way of GCF into the sulcus or pocket and serve as potential biomarkers for periodontal disease detection.
GCF is an inflammatory exudate originating from the gingival plexus of blood vessels in the gingival corium, subjacent to the epithelium lining of the dentogingival space. As GCF traverses through inflamed periodontal tissues en route to the sulcus, biological molecular markers are gathered from the surrounding site.40 GCF sampling methods have been shown to accurately capture inflammatory and connective tissue breakdown mediators.31,33 As recently reviewed by Loos and Tjoa, more than 90 different components in GCF have been evaluated to date for periodontal diagnosis.41 Of the numerous constituents in GCF, however, the vast majority constitute soft tissue inflammatory events, while only a few are regarded as specific biomarkers of alveolar bone destruction (Table 1).
TABLE 1. Bone-related biomarkers from oral fluids associated with periodontal diseases.
| Name | Association | Reference |
|---|---|---|
| ALP | T | 32,34,62,64-66 |
| Cathepsin B | S, T | 68-72 |
| Collagenase-2 (MMP-8) | S, T | 79,81,83-89 |
| Gelatinase (MMP-9) | T | 90 |
| Collagenase-3 (MMP-13) | T | 79,87 |
| Calprotectin | S | 98 |
| Osteocalcin | S | 34,53,79,102-104 |
| Pyridinoline cross-links (ICTP) | S, T | 79,102,109-116 |
| Osteonectin | S | 119 |
| Osteopontin | S | 128,129 |
Note: Association with severity of periodontal disease (S) and treatment planning and outcome (T).
In the early 1970s several researchers demonstrated a correlation between collagenolytic activity of GCF and severity of periodontal disease.42-45 Golub et al. and co-workers discovered that collagenase activity was more highly correlated with pocket depth than with inflammation and therefore “may reflect the degradative activity of the gingival tissues lining the pocket and could, therefore, be of diagnostic value.”42 Villela and Birkedal-Hansen also observed a connection between collagenolytic activity and active disease.46 As a result from these early observations, thought was given to the possible use of collagenases as plausible biochemical markers for disease progression.
Prostaglandins are arachidonic acid metabolites composed of 10 classes, of which D, E, F, G, H, and I are of main importance. Of this group, PGE2 is one of the most extensively studied mediators of periodontal disease activity.47-52 During the host’s innate defense response to bacterial lipopolysaccharide (LPS), monocytes, PMNs, macrophages, and other cells release IL-1, TNF, and PGE2. PGE2 acts as a potent vasodilator and increases capillary permeability, which elicits clinical signs of redness and edema. PGE2 also stimulates fibroblasts and osteoclasts to increase production of MMPs. Ultimately, MMPs affect the remodeling and degradation of the periodontium.33 Offenbacher and co-workers demonstrated that patients with periodontitis had higher levels of GCF-PGE2 than patients with gingivitis.53,54 This group subsequently performed a retrospective analysis of GCF-PGE2 by examining the longitudinal relationship of PGE2 concentrations in GCF to attachment loss in adult patients with periodontitis. Results showed that elevated PGE2 was detectable in GCF 6 months before the identification of periodontal disease activity and significantly decreased 1 month after scaling and root planing was provided.55 Although PGE2 has shown much promise as a biomarker of periodontitis, PGE2-based diagnostics have not entered the clinical arena.
Another oral fluid that has recently gained significant recognition is saliva. Technologies are emerging that use minute amounts of saliva as reliable diagnostic fluids for identification, monitoring, and prediction of various diseases. Saliva-based diagnostic tests are currently being used in a broad range of applications, such as autoimmune disorders, cardiovascular disease, infectious diseases, and in monitoring drugs of abuse.24 Much work is currently under way in the field of salivary diagnostics for periodontal disease. Pederson et al. measured quantities of host-response indicators—cathepsin G, elastase, elastase inhibitors, and C-reactive protein (CRPs)—to determine whether their levels were directly related to the individual’s periodontal status. Forty-five participants were categorized according to periodontal status (healthy, gingivitis, mild-to-moderate periodontitis, or moderate-to-severe periodontitis) and whole-saliva samples were collected. With the exception of α1-antitrypsin, an increase in salivary levels for all of the targeted host-response markers correlated with increasing severity of disease.56 Other early saliva-based investigations detected significantly increased levels of collagenase 2 (MMP-8) in periodontally diseased patients.57 Subsequent explorations of immunoglobulins found in whole saliva directed against periodontal pathogens have indicated some correlations with the status of periodontal disease.58-61 Although saliva-based clinical testing shows much potential, more-extensive research is required to identify the best candidate markers that can be simultaneously evaluated to obtain a profile for diagnosis, monitoring, and prediction of oral diseases.
BIOMARKERS OF BONE RESORPTION OR TURNOVER
Several biomarkers have been studied as applicable to the diagnostics of periodontal bone loss (Table 1). These components, which are evaluated next, are the potential candidates for oral fluid-based diagnostics of periodontal disease.
Alkaline Phosphatase (ALP)
Enzymes found in whole saliva originate from three main sources: (1) the actual salivary secretions per se; (2) the GCF, stemming from PMNs and tissue degradation; and (3) disposed bacterial cells from dental biofilms and mucosal surfaces. ALP is a catalyzing enzyme that accelerates the removal of phosphate groups in the 5 and 3 positions from a variety of molecules, including nucleotides, proteins, and alkaloids. Although present in all tissues, ALP is particularly concentrated in the bone, liver, bile duct, kidney, and placenta. Of interest in oral health, of course, is the association between ALP and periodontal disease.
Early investigations of ALP and periodontal disease in an experimental gingivitis model showed a significant correlation between ALP and pocket depth and between ALP and inflammation.62 Nakamura and Slots studied a total of 76 enzyme activities in mixed whole saliva and noted higher enzyme activity in individuals with periodontal disease than nondiseased individuals.32 Gibert et al. analyzed serum levels of ALP from patients with chronic periodontal disease and compared the findings with those of control patients.63 Results showed a relationship between attachment loss in the periodontal group and a drop in ALP activity in serum. Contrary to these results, Totan et al. investigated the influence of periodontal disease on ALP, aminotransferase (AST), aminopeptidase, and glucuronidase.64 Salivary samples from patients with confirmed periodontal disease were analyzed and revealed that periodontal destruction by measurement of probing depth, gingival bleeding, and suppuration were related to higher ALP levels in saliva. Supporting these results are the findings by Todorovic et al. that increased activity of salivary ALP is seen in patients with periodontal disease in relation to a nondisease control group.65 This group further showed a positive correlation between the salivary enzyme activity and gingival index values. As a predictive indicator for future periodontal breakdown, ALP has not been supported by research findings and therefore may best serve as a marker in periodontal treatment planning and monitoring.34,66
Cathepsin B
As an enzyme belonging to the class of cysteine proteinases, cathepsin B functions in proteolysis. In GCF, macrophages are the main producers of cathepsin B.67 GCF concentrations of cathepsin B were found to be elevated in patients with periodontal disease, but lower in patients with gingivitis.68 Ichimaru et al. concluded that cathepsin B may be correlated with the severity of periodontitis.69 Further investigators have shown positive correlations between cathepin B levels and the severity of periodontal disease, while noting a reduction of cathepsin B levels after periodontal intervention therapies were provided.70,71 Eley and Cox studied cathepsin B and evaluated its use as a predictor of attachment loss.72 Forty-nine patients were monitored after initial periodontal therapy for 2 years. A total of 121 sites were found with attachment loss (90 with rapid loss and 31 with gradual loss). Cathepsin B levels were higher in the sites with rapid loss than in the paired control sites. Moreover, in the sites with gradual attachment loss, cathepsin B levels were elevated when compared with the paired control sites. With a cut-off value of 7.5 μU/30 sec GCF sample for the total cathepsin B activity and 30 μU/uL for enzyme concentration, remarkable results of 100% sensitivity and 99.8% specificity for both cathepsin B parameters were reported. Cathepsin B may have a potential use in distinguishing periodontitis from gingivitis and in planning treatment and monitoring treatment outcomes.41
Collagenase-2 (MMP-8)
MMPs are host proteinases responsible for both tissue degradation and remodeling.73-77 During progressive periodontal breakdown, gingival and periodontal ligament collagens are cleaved by host cell-derived interstitial collagenases. One vital interstitial collagenase capable of degrading the triple helical structures of native types I, II, and III collagens found in alveolar bone matrix is collagenase-2. Collagenase-2, also referred to as MMP-8, is released during the maturation of PMNs in the bone marrow. Once produced, it becomes glycosylated and is prestored in the sub-cellular-specific granules, where it is subsequently released in large quantities as the PMNs are recruited to a site of inflammation. Chubinskaya et al. demonstrated the ability of non-neutrophil-lineage mesenchymal cells, such as human gingival and periodontal ligament fibroblasts and chondrocytes, to also be able to produce MMP-8.78
MMP-8 is the most prevalent MMP found in diseased periodontal tissue and GCF.79-81 Nomura et al. found no difference in MMP-8 levels from patients with periodontal disease when compared to patients with gingivitis.82 From this early investigation, it was believed that MMP-8 may serve as a proinflammatory marker, but not as a discriminating marker for chronic periodontitis and gingivitis. However, Mancini and co-workers found an 18-fold increase of MMP-8 in patients experiencing active periodontal tissue breakdown as compared with patients under stable conditions. Conclusions from this investigation indicated the potential use of MMP-8 as a screening test for detection of active disease progression.83 Elevated MMP-8 levels in active disease progression were observed by Lee et al. in a longitudinal study using patients with gingivitis, nonprogressive, and progressive periodontitis. The total collagenase activity was observed to be 50% higher in the disease progression group.81
Golub et al. introduced a 20-mg low-dose doxycycline (LDD) capsule, which preserved its proteinase-inhibitory ability to suppress connective tissue breakdown, but without antibiotic/antimicrobial capabilities. The group went on to conduct several studies demonstrating that LDD can function as an MMP by way of suppressing the collagenase activity in GCF and gingival tissues of patients with adult periodontitis.84,85 To test the hypothesis that LDD could lower GCF levels of bone-type collagen fragments, clinical parameters (gingival inflammation, pocket depth, and radiographic evidence of bone loss) that predicted excessive MMP activity in periodontal pockets of 18 adult patients were evaluated. All patients received supragingival scaling 1 month before the baseline appointment. At the baseline visit and at the subsequent 1- and 2-month visits, GCF samples were collected. Conventional clinical measures (gingival index, plaque index, probing depth, and attachment level) were taken at each time point in the study. Western blots analyses determined that neutrophil-type collagenase (MMP-8) was increased in disease and substantially reduced by approximately 60% during the 2-month protocol of LDD.79 MMP-8 may have some future value as a diagnostic marker for periodontal disease, an indicator for disease progression, and as a signal to determine the efficacy of treatment.41
MMP-8 has also been detected in elevated amounts in peri-implant sulcular fluid (PISF) from peri-implantitis lesions. Teronen et al. identified higher collagenase-2 levels in failing dental implants compared to nonmobile implants.86 Ma et al. went on to explore for the presence of MMP-8 and collagenase-3, MMP-13, in peri-implant sulcus fluid. Forty-nine randomly selected dental implant sites in 13 patients were studied. Implants were categorized into three groups according to the amount of bone loss in the vertical dimension: <1 mm, from 1 to 3 mm, or >3 mm. Results from this investigation showed that both MMP-8 and MMP-13 levels were significantly higher in the >3 mm bone loss group when compared to the groups that had less bone loss.87 Additional studies were conducted by Kivela-Rajamaki et al., looking at MMP-8 levels in combination with laminin-5 and, during a separate study, with MMP-7. Conclusions drawn from both investigations indicate that elevated levels of MMP-8 can be seen in diseased PISF as compared to healthy PISF.88,89 Collectively, these findings offer hope for the use of MMP-8 as a marker for active phase of peri-implant disease. Longitudinal studies are required to evaluate MMP-8 either alone or in conjunction with other molecular biomarkers to predict the risk of future disease occurrence and to monitor treatment interventions.
Gelatinase (MMP-9)
Gelatinase (MMP-9), another member of the collagenase family, is produced by neutrophils and degrades collagen extracellular ground substance. In a longitudinal study conducted by Teng et al., patients were asked to rinse and expectorate, providing subject-based instead of individual site-based GCF rinse samples.90 When analyzed, a twofold increase in mean MMP-9 levels was reported in patients with recurrent attachment loss. Once given systemic metronidazole, mouthrinse samples from patients with initial elevated MMP-9 concentrations markedly dropped. Given these results, future use of MMP-9 in oral diagnostics may best serve as a guide in periodontal treatment monitoring.
Collagenase-3 (MMP-13)
Collagenase-3, referred to as MMP-13, is another collagenolytic MMP with an exceptionally wide substrate specificity.91,92 MMP-13 is expressed during bone formation and gingival wound healing and at heightened quantities during pathological tissue destructive states, such as arthritis, chronic ulcers, atherosclerosis, and several types of malignant tumors.93,94 Uitto et al. examined MMP-13 in chronically inflamed oral mucosa and found that during the course of prolonged inflammation undifferentiated epithelial cells produce significant concentrations of MMP-13.95 Tervaharitiala et al. examined diseased gingival sulcular epithelium of patients with adult periodontitis (AD) and patients with localized juvenile periodontitis (LJP) for evidence of MMP and found sulcular epithelium expressing detectible levels of MMP-13, MMP-8, and MMP-2 in vivo.93 Golub et al. were the first to discover MMP-13 in GCF of periodontal patients, albeit in only a small proportion (3-4%) of the total amount of GCF collagenase. Golub further investigated the effects of LDD as a MMP inhibitor and found that LDD reduced GCF concentrations of MMP-13 faster and more efficiently than MMP-8 levels.79 MMP-13 has also been implicated in peri-implantitis. Ma et al. concluded that elevated levels of both MMP-13 and MMP-8 correlated with irreversible peri-implant vertical bone loss around loosening dental implants.87 In the future, MMP-13 may be useful for diagnosing and monitoring the course of periodontal disease as well as tracking the efficacy of therapy.
Calprotectin
Calprotectin is a 36-kDa protein composed of a dimeric complex of 8- and 14-kDa subunits. Neutrophils are the primary source of calprotectin although other cells, such as activated monocytes and macrophages and specific epithelial cells, are also capable of manufacturing the protein. Calprotectin acts as a calcium- and zinc-binding protein with both antimicrobial and antifungal activities. Furthermore, calprotectin plays a role in immune regulation through its ability to inhibit immunoglobulin production and, of particular interest, its role as a proinflammatory protein for neutrophil recruitment and activation.
Current research is using calprotectin as a marker for medical conditions such as ulcerative colitis and Crohn’s disease.96,97 In periodontology, Kido et al. identified calprotectin in GCF and found that GCF concentration levels in patients with periodontal disease were higher than those in GCF from healthy subjects.98 The expression of calprotectin from inflammatory cells appears to offer protection of the epithelial cells against binding and invasion by P. gingivalis. In periodontal disease, calprotectin appears to improve resistance to P. gingivalis by boosting the barrier protection and innate immune functions of the gingival epithelium.99
Osteocalcin
Elevated serum osteocalcin levels have been found during periods of rapid bone turnover, such as osteoporosis, multiple myeloma, and fracture repair.100,101 Therefore, studies have investigated the relationship between GCF osteocalcin levels and periodontal disease.34,53,79,102-104 Kunimatsu et al. reported a positive correlation between GCF osteocalcin N-terminal peptide levels and clinical parameters in a cross-sectional study of patients with periodontitis and gingivitis.103 The authors also reported that osteocalcin could not be detected in patients with gingivitis. In contrast, Nakashima et al. reported significant GCF osteocalcin levels from both periodontitis and gingivitis patients.53 Osteocalcin levels were also significantly correlated with pocket depth and gingival index scores, as well as GCF levels of ALP and PGE2. In a longitudinal study of untreated periodontitis patients with ≥1.5 mm attachment loss during the monitoring period, GCF osteocalcin levels alone were unable to discriminate between active and inactive sites.34 However, when a combination of the biochemical markers osteocalcin, collagenase, PGE2, α-2 macroglobulin, elastase, and ALP was evaluated, increased diagnostic sensitivity and specificity values of 80% and 91%, respectively, were reported.34
A longitudinal study using an experimental periodontitis model in beagle dogs reported a strong correlation between GCF osteocalcin levels and active bone turnover as assessed by bone-seeking radiopharmaceutical uptake (BSRU).102 However, osteocalcin was shown to possess only modest predictive value for future bone loss measured by computer-assisted digitizing radiography. Moreover, treatment of chronic periodontitis patients with subanti-microbial doxycycline failed to reduce GCF osteocalcin levels,79 and a cross-sectional study of periodontitis patients reported no differences in GCF osteocalcin levels between deep and shallow sites in the same patients.105 Moreover, osteocalcin levels in the GCF during orthodontic tooth movement were highly variable between subjects and lacked a consistent pattern related to the stages of tooth movement.106 In summary, the results of these studies show a role for intact osteocalcin as a bone-specific marker of bone turnover.
Pyridinoline Cross-Linked Carboxyterminal Telopeptide of Type I Collagen (ICTP)
Given the specificity and sensitivity for bone resorption, pyridinoline crosslinks represent a potentially valuable diagnostic aid for periodontal disease, since biomarkers specific for bone degradation may be useful in differentiating between the presence of gingival inflammation and active periodontal or periimplant bone destruction.107 Several investigations have explored the ability of pyridinoline cross-links to detect bone resorption in periodontitis and periimplantitis as well as in response to periodontal therapy.79,102,105,108-116
Palys et al. related ICTP levels to the subgingival microflora of various disease states on GCF.110 Subjects were divided into groups representing health, gingivitis, and chronic periodontitis, and GCF and plaque samples were collected from each subject. The samples were analyzed for ICTP levels and the presence of 40 subgingival species by using checkerboard DNA-DNA hybridization techniques. ICTP levels differed significantly between health, gingivitis, and periodontitis subjects, and related modestly to several clinical disease parameters. ICTP levels were also strongly correlated with whole subject levels of several periodontal pathogens including T. forsythensis, P. gingivalis, P. intermedia, and T. denticola. In a subsequent study, Oringer et al. examined the relationship between ICTP levels and subgingival species around implants and teeth in 20 partially edentulous and two fully edentulous patients. No significant differences were found among ICTP levels and subgingival plaque composition between implants and teeth. Strong correlations between elevated ICTP levels at implant sites and colonization with organisms associated with failing implants, such as P. intermedia, F. nucleatum ss vincentii, and S. gordonii were found.109
Golub et al. found that treatment of chronic periodontitis patients with nonsurgical periodontal therapy and LDD resulted in a 70% reduction in GCF ICTP levels after 1 month, concomitant with a 30% reduction in collagenase levels.79 An investigation of periodontitis patients treated with scaling and root planing also demonstrated significant correlations between GCF ICTP levels and clinical periodontal disease parameters, including attachment loss, pocket depth, and bleeding on probing.107 In addition, elevated GCF ICTP levels at baseline, especially at shallow sites, were found to be predictive for future attachment loss as early as 1 month after sampling. Furthermore, treatment of a group of periodontitis subjects by SRP and locally delivered minocycline led to rapid reductions in GCF ICTP levels.79
In summary, studies assessing the role of GCF ICTP levels as a diagnostic marker of periodontal disease activity have produced promising results to date. ICTP has been shown to be a promising predictor of both future alveolar bone and attachment loss. Furthermore, ICTP was strongly correlated with clinical parameters and putative periodontal pathogens, and demonstrated significant reductions after periodontal therapy. Controlled human longitudinal trials are needed to fully establish the role of ICTP as a predictor of periodontal tissue destruction, disease activity, and response to therapy in periodontal patients.
Therefore, the measurement of connective tissue-derived molecules, such as ICTP or osteocalcin, may lead to a more accurate assessment of tissue breakdown.117
Osteonectin
Also referred to as secreted protein acidic and rich in cysteine and basement membrane protein (BM-40), osteonectin is a single-chain polypeptide that binds strongly to hydroxyapatite and other extracellular matrix proteins including collagens. Because of its affinity for collagen and hydroxylapatite, osteonectin has been implicated in the early phases of tissue mineralization.118 In a cross-sectional study by Bowers et al., GCF samples were analyzed from patients with gingivitis, at moderate or severe periodontal disease states. Using a dot blot assay, both osteonectin and N-propeptide alpha I type I collagen were significantly increased in patients with periodontal disease. Furthermore, the protein concentrations found in GCF were elevated as probe depth measures increased in the sites evaluated.119 At the final analysis of this study, osteonectinappeared to be the more sensitive marker for detection of periodontal disease status, when compared with N-propeptide alpha I type I collagen.
Osteopontin (OPN)
OPN is a single-chain polypeptide having a molecular weight of approximately 32,600.120 It is found in the kidney, blood, mammary gland, salivary glands, and bone. In bone matrix, OPN is highly concentrated at sites where osteoclasts are attached to the underlying mineral surface, that is, the clear zone attachment areas of the plasma membrane.121,122 However, since OPN is produced by both osteoblasts and osteoclasts, it holds a dual function in bone maturation and mineralization as well as bone resorption.123-127 Kido et al. investigated the presence of OPN in GCF and the correlation between these levels and probing depth measures of periodontally healthy and diseased patients. Results from this study revealed that OPN could be detected in GCF, and increased OPN levels coincided with increased probing depth measures.128 Sharma et al. recently published findings from an investigation of GCF OPN. A total of 45 subjects were divided into three groups (healthy, gingivitis, and chronic periodontitis) based on clinical examination, modified gingival index, Ramfjord periodontal disease index scores, and radiographic evidence of bone loss. The chronic periodontitis group subsequently received nonsurgical therapy and GCF samples were collected again 6 to 8 weeks after treatment. Results indicated that GCF OPN concentrations increased proportionally with the progression of disease and when nonsurgical periodontal treatment was provided, GCF OPN levels were significantly reduced. Although additional long-term prospective studies are needed, at this point OPN appears to hold promise as a possible biomarker of periodontal disease progression.129
FUTURE DIRECTIONS FOR PERIODONTAL ORAL FLUID-BASED DIAGNOSTICS
Researchers involved in delivery of periodontal therapy are currently investigating the possible use of oral fluids in the diagnosis of oral diseases and drug development. The movement of the pharmaceutical industry toward pharmacogenomics will require the tailored design of specific diagnostic profiles of patients for individualized dental therapy. Professionals in seemingly unrelated arenas, such as the insurance industry, Environment Protection Agency, and Homeland Security, are interested in the possibility of oral fluid use as well for rapid screening of oral and systemic health status.
As it relates to periodontology, the great need for periodontal diagnostics has become increasingly evident. From physical measurements by periodontal probing to sophisticated genetic susceptibility analysis and molecular arrays for the detection of biomarkers on the different stages of the disease, substantial improvements have been made in the understanding of the mediators implicated on the initiation, pathogenesis, and progression of periodontitis. Through the biomarker discovery process, new therapeutics have been designed linking therapeutic and diagnostic approaches together, especially in the area of host modulatory drugs for periodontal disease treatment. Moreover, new diagnostic technologies, such as microarray and microfluidics, are now currently available for risk assessment and comprehensive screening of biomarkers. The future is bright for the use of rapid, easy-to-use diagnostics that will provide an enhanced patient assessment that can guide and transform customized therapies for dental patients, leading to more individualized, targeted treatments for oral health.
ACKNOWLEDGMENTS
This work was supported by NIH/NIDCR grant U01-DE14961 and NCRR grant M01-RR000042. The authors acknowledge Dr. Thiago Morelli and Noah Smith for their assistance in the preparations of the manuscript and Mr. Chris Jung for design of the figure.
REFERENCES
- 1.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J. Periodontol. 1992;63(4 Suppl):322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 3.Genco RJ. Host responses in periodontal diseases: current concepts. J. Periodontol. 1992;63(4 Suppl):338–355. doi: 10.1902/jop.1992.63.4s.338. [DOI] [PubMed] [Google Scholar]
- 4.Page RC, Kornman KS. The pathogenesis of human periodontitis: an introduction. Periodontol. 2000;14:9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. 1997. [DOI] [PubMed] [Google Scholar]
- 5.Offenbacher S. Periodontal diseases: pathogenesis. Ann. Periodontol. 1996;1:821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 6.Albandar JM. A 6-year study on the pattern of periodontal disease progression. J. Clin. Periodontol. 1990;17(7 Pt 1):467–471. doi: 10.1111/j.1600-051x.1990.tb02346.x. [DOI] [PubMed] [Google Scholar]
- 7.Albandar JM, Abbas DK. Radiographic quantification of alveolar bone level changes. Comparison of 3 currently used methods. J. Clin. Periodontol. 1986;13:810–813. doi: 10.1111/j.1600-051x.1986.tb02235.x. [DOI] [PubMed] [Google Scholar]
- 8.Socransky SS, Haffajee AD, Goodson JM, Lindhe J. New concepts of destructive periodontal disease. J. Clin. Periodontol. 1984;11:21–32. doi: 10.1111/j.1600-051x.1984.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 9.Albandar JM. Periodontal diseases in North America. Periodontol. 2002;29:31–69. doi: 10.1034/j.1600-0757.2002.290103.x. 2000. [DOI] [PubMed] [Google Scholar]
- 10.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J. Periodontol. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 11.Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. 2000. [DOI] [PubMed] [Google Scholar]
- 12.Zambon JJ. Periodontal diseases: microbial factors. Ann. Periodontol. 1996;1:879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- 13.Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 14.Loesche WJ, Lopatin DE, Giordano J, et al. Comparison of the benzoyl-DL-arginine-naphthylamide (BANA) test, DNA probes, and immunological reagents for ability to detect anaerobic periodontal infections due to Porphyromonas gingivalis, Treponema denticola, and Bacteroides forsythus. J. Clin. Microbiol. 1992;30:427–433. doi: 10.1128/jcm.30.2.427-433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loesche WJ, Kazor CE, Taylor GW. The optimization of the BANA test as a screening instrument for gingivitis among subjects seeking dental treatment. J. Clin. Periodontol. 1997;24:718–726. doi: 10.1111/j.1600-051x.1997.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 16.Listgarten MA. Structure of surface coatings on teeth. A review. J. Periodontol. 1976;47:139–147. doi: 10.1902/jop.1976.47.3.139. [DOI] [PubMed] [Google Scholar]
- 17.Listgarten MA, Loomer PM. Microbial identification in the management of periodontal diseases. A systematic review. Ann. Periodontol. 2003;8:182–192. doi: 10.1902/annals.2003.8.1.182. [DOI] [PubMed] [Google Scholar]
- 18.Armitage GC. Periodontal diseases: diagnosis. Ann. Periodontol. 1996;1:37–215. doi: 10.1902/annals.1996.1.1.37. [DOI] [PubMed] [Google Scholar]
- 19.Armitage GC. The complete periodontal examination. Periodontol. 2004;34:22–33. doi: 10.1046/j.0906-6713.2002.003422.x. 2000. [DOI] [PubMed] [Google Scholar]
- 20.Lamster IB, Grbic JT. Diagnosis of periodontal disease based on analysis of the host response. Periodontol. 2000;7:83–99. doi: 10.1111/j.1600-0757.1995.tb00038.x. 1995. [DOI] [PubMed] [Google Scholar]
- 21.Haffajee AD, Socransky SS, Goodson JM. Clinical parameters as predictors of destructive periodontal disease activity. J. Clin. Periodontol. 1983;10:257–265. doi: 10.1111/j.1600-051x.1983.tb01274.x. [DOI] [PubMed] [Google Scholar]
- 22.Goodson JM. Diagnosis of periodontitis by physical measurement: interpretation from episodic disease hypothesis. J. Periodontol. 1992;63(4 Suppl):373–382. doi: 10.1902/jop.1992.63.4s.373. [DOI] [PubMed] [Google Scholar]
- 23.Haffajee AD, Socransky SS, Lindhe J, et al. Clinical risk indicators for periodontal attachment loss. J. Clin. Periodontol. 18:117–125. doi: 10.1111/j.1600-051x.1991.tb01700.x. [DOI] [PubMed] [Google Scholar]
- 24.Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis. 2002;8:69–76. doi: 10.1034/j.1601-0825.2002.1o834.x. [DOI] [PubMed] [Google Scholar]
- 25.Mandel ID. The diagnostic uses of saliva. J. Oral Pathol. Med. 1990;19:119–125. doi: 10.1111/j.1600-0714.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 26.Mandel ID. Salivary diagnosis: promises, promises. Ann. N. Y. Acad. Sci. 1993;694:1–10. doi: 10.1111/j.1749-6632.1993.tb18336.x. [DOI] [PubMed] [Google Scholar]
- 27.Mandel ID. A contemporary view of salivary research. Crit. Rev. Oral Biol. Med. 1993;4:599–604. doi: 10.1177/10454411930040034701. [DOI] [PubMed] [Google Scholar]
- 28.Mandel ID. Salivary diagnosis: more than a lick and a promise. J. Am. Dent. Assoc. 1993;124:85–87. doi: 10.14219/jada.archive.1993.0007. [DOI] [PubMed] [Google Scholar]
- 29.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 30.Ferguson DB. Current diagnostic uses of saliva. J. Dent. Res. 1987;66:420–424. doi: 10.1177/00220345870660020601. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis a review. J. Clin. Periodontol. 2000;27:453–465. doi: 10.1034/j.1600-051x.2000.027007453.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura M, Slots J. Salivary enzymes. Origin and relationship to periodontal disease. J. Periodontal Res. 1983;18:559–569. doi: 10.1111/j.1600-0765.1983.tb00393.x. [DOI] [PubMed] [Google Scholar]
- 33.Ozmeric N. Advances in periodontal disease markers. Clin. Chim. Acta. 2004;343:1–16. doi: 10.1016/j.cccn.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 34.Nakashima K, Giannopoulou C, Andersen E, et al. A longitudinal study of various crevicular fluid components as markers of periodontal disease activity. J. Clin. Periodontol. 1996;23:832–838. doi: 10.1111/j.1600-051x.1996.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 35.Singh AK, Herr AE, Hatch AV, et al. Integrated microfluidic platform for oral diagnostics (IMPOD) [abstract]; New York Academy of Sciences: Oral-Based Diagnostics—A New York Academy of Sciences Meeting; 2006; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramseier CA, Kinney JS, Herr AE, et al. Salivary diagnostics for inflammatory periodontal diseases [abstract]; New York Academy of Sciences: Oral-Based Diagnostics—A New York Academy of Sciences Meeting; 2006. [Google Scholar]
- 37.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol. 2000;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. 1997. [DOI] [PubMed] [Google Scholar]
- 38.Mantyla P, Stenman M, Kinane DF, et al. Gingival crevicular fluid collagenase-2 (MMP-8) test stick for chair-side monitoring of periodontitis. J. Periodontal Res. 2003;38:436–439. doi: 10.1034/j.1600-0765.2003.00677.x. [DOI] [PubMed] [Google Scholar]
- 39.Taba M, Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent. Clin. North Am. 2005;49:551–571. vi. doi: 10.1016/j.cden.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cimasoni G. Crevicular fluid updated. Monogr. Oral Sci. 1983;12:III–VII. 1–152. [PubMed] [Google Scholar]
- 41.Loos BG, Tjoa S. Host-derived diagnostic markers for periodontitis: do they exist in gingival crevice fluid? Periodontol. 2005;39:53–72. doi: 10.1111/j.1600-0757.2005.00129.x. 2000. [DOI] [PubMed] [Google Scholar]
- 42.Golub LM, Siegel K, Ramamurthy NS, Mandel ID. Some characteristics of collagenase activity in gingival crevicular fluid and its relationship to gingival diseases in humans. J. Dent. Res. 1976;55:1049–1057. doi: 10.1177/00220345760550060701. [DOI] [PubMed] [Google Scholar]
- 43.Golub LM, Kleinberg I. Gingival crevicular fluid: a new diagnostic aid in managing the periodontal patient. Oral Sci. Rev. 1976;8:49–61. [PMC free article] [PubMed] [Google Scholar]
- 44.Robertson PB, Grupe HE, Jr, Taylor RE, et al. The effect of collagenase-inhibitor complexes on collagenolytic activity of normal and inflamed gingival tissue. J. Oral Pathol. 1973;2:28–32. doi: 10.1111/j.1600-0714.1973.tb01671.x. [DOI] [PubMed] [Google Scholar]
- 45.Kowashi Y, Jaccard F, Cimasoni G. Increase of free collagenase and neutral protease activities in the gingival crevice during experimental gingivitis in man. Arch. Oral Biol. 1979;24:645–650. doi: 10.1016/0003-9969(79)90112-2. [DOI] [PubMed] [Google Scholar]
- 46.Villela B, Cogen RB, Bartolucci AA, Birkedal-Hansen H. Collagenolytic activity in crevicular fluid from patients with chronic adult periodontitis, localized juvenile periodontitis and gingivitis, and from healthy control subjects. J. Periodontal. Res. 1987;22:381–389. doi: 10.1111/j.1600-0765.1987.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 47.Airila-Mansson S, Soder B, Kari K, Meurman JH. Influence of combinations of bacteria on the levels of prostaglandin E2, interleukin-1beta, and granulocyte elastase in gingival crevicular fluid and on the severity of periodontal disease. J. Periodontol. 2006;77:1025–1031. doi: 10.1902/jop.2006.050208. [DOI] [PubMed] [Google Scholar]
- 48.Ikarashi F, Yamazaki K, Hara K, Nohara H. Production of prostaglandin E2 by polymorphonuclear neutrophils isolated from gingival crevicular fluid and peripheral blood of dogs in periodontal health and disease. Nippon Shishubyo Gakkai Kaishi. 1990;32:121–128. doi: 10.2329/perio.32.121. [DOI] [PubMed] [Google Scholar]
- 49.Weyna E. Comparative evaluation of histological studies of the gingiva and levels of prostaglandin-like substances in cases of deep inflammatory periodontal disease. Czas. Stomatol. 1985;38:53–56. [PubMed] [Google Scholar]
- 50.Weyna E. Levels of prostaglandin-like substances in the gingiva of subjects with healthy periodontium or in patients with inflammatory periodontal disease as determined by biological methods. Czas. Stomatol. 1983;36:289–293. [PubMed] [Google Scholar]
- 51.Loning T, Albers HK, Lisboa BP, et al. Prostaglandin E and the local immune response in chronic periodontal disease. Immunohistochemical and radioimmunological observations. J. Periodontal. Res. 1980;15:525–535. doi: 10.1111/j.1600-0765.1980.tb00310.x. [DOI] [PubMed] [Google Scholar]
- 52.Goodson JM, Dewhirst FE, Brunetti A. Prostaglandin E2 levels and human periodontal disease. Prostaglandins. 1974;6:81–85. doi: 10.1016/s0090-6980(74)80043-2. [DOI] [PubMed] [Google Scholar]
- 53.Nakashima K, Roehrich N, Cimasoni G. Osteocalcin, prostaglandin E2 and alkaline phosphatase in gingival crevicular fluid: their relations to periodontal status. J. Clin. Periodontol. 1994;21:327–333. doi: 10.1111/j.1600-051x.1994.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 54.Offenbacher S, Odle BM, Gray RC, Van Dyke TE. Crevicular fluid prostaglandin E levels as a measure of the periodontal disease status of adult and juvenile periodontitis patients. J. Periodontal. Res. 1984;19:1–13. doi: 10.1111/j.1600-0765.1984.tb01190.x. [DOI] [PubMed] [Google Scholar]
- 55.Offenbacher S, Odle BM, Van Dyke TE. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J. Periodontal. Res. 1986;21:101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 56.Pederson ED, Stanke SR, Whitener SJ, et al. Salivary levels of alpha 2-macroglobulin, alpha 1-antitrypsin, C-reactive protein, cathepsin G and elastase in humans with or without destructive periodontal disease. Arch. Oral Biol. 1995;40:1151–1155. doi: 10.1016/0003-9969(95)00089-5. [DOI] [PubMed] [Google Scholar]
- 57.Iijima K, Ando K, Kishi M, et al. Collagenase activity in human saliva. J. Dent. Res. 1983;62:709–712. doi: 10.1177/00220345830620060301. [DOI] [PubMed] [Google Scholar]
- 58.Basu MK, Fox EC, Becker JF. Salivary IgG and IgA before and after periodontal therapy. A preliminary report. J. Periodontal. Res. 1976;11:226–229. doi: 10.1111/j.1600-0765.1976.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 59.Sandholm L, Gronblad E. Salivary immunoglobulins in patients with juvenile periodontitis and their healthy siblings. J. Periodontol. 1984;55:9–12. doi: 10.1902/jop.1984.55.1.9. [DOI] [PubMed] [Google Scholar]
- 60.Sandholm L, Tolo K, Olsen I. Salivary IgG, a parameter of periodontal disease activity? High responders to Actinobacillus actinomycetemcomitans Y4 in juvenile and adult periodontitis. J. Clin. Periodontol. 1987;14:289–294. doi: 10.1111/j.1600-051x.1987.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 61.Schenck K, Poppelsdorf D, Denis C, Tollefsen T. Levels of salivary IgA antibodies reactive with bacteria from dental plaque are associated with susceptibility to experimental gingivitis. J. Clin. Periodontol. 1993;20:411–417. doi: 10.1111/j.1600-051x.1993.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 62.Ishikawa I, Cimasoni G. Alkaline phosphatase in human gingival fluid and its relation to periodontitis. Arch. Oral Biol. 1970;15:1401–1404. doi: 10.1016/0003-9969(70)90032-4. [DOI] [PubMed] [Google Scholar]
- 63.Gibert P, Tramini P, Sieso V, Piva MT. Alkaline phosphatase isozyme activity in serum from patients with chronic periodontitis. J. Periodontal. Res. 2003;38:362–365. doi: 10.1034/j.1600-0765.2003.00388.x. [DOI] [PubMed] [Google Scholar]
- 64.Totan A, Greabu M, Totan C, Spinu T. Salivary aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase: possible markers in periodontal diseases? Clin. Chem. Lab. Med. 2006;44:612–615. doi: 10.1515/CCLM.2006.096. [DOI] [PubMed] [Google Scholar]
- 65.Todorovic T, Dozic I, Vicente-Barrero M, et al. Salivary enzymes and periodontal disease. Med. Oral Patol. Oral Cir. Bucal. 2006;11:E115–E119. [PubMed] [Google Scholar]
- 66.McCauley LK, Nohutcu RM. Mediators of periodontal osseous destruction and remodeling: principles and implications for diagnosis and therapy. J. Periodontol. 2002;73:1377–1391. doi: 10.1902/jop.2002.73.11.1377. [DOI] [PubMed] [Google Scholar]
- 67.Kennett CN, Cox SW, Eley BM. Investigations into the cellular contribution to host tissue proteases and inhibitors in gingival crevicular fluid. J. Clin. Periodontol. 1997;24:424–431. doi: 10.1111/j.1600-051x.1997.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 68.Kunimatsu K, Yamamoto K, Ichimaru E, et al. Cathepsins B, H and L activities in gingival crevicular fluid from chronic adult periodontitis patients and experimental gingivitis subjects. J. Periodontal. Res. 1990;25:69–73. doi: 10.1111/j.1600-0765.1990.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 69.Ichimaru E, Tanoue M, Tani M, et al. Cathepsin B in gingival crevicular fluid of adult periodontitis patients: identification by immunological and enzymological methods. Inflamm. Res. 1996;45:277–282. doi: 10.1007/BF02280991. [DOI] [PubMed] [Google Scholar]
- 70.Chen HY, Cox SW, Eley BM. Cathepsin B, alpha2-macroglobulin and cystatin levels in gingival crevicular fluid from chronic periodontitis patients. J. Clin. Periodontol. 1998;25:34–41. doi: 10.1111/j.1600-051x.1998.tb02361.x. [DOI] [PubMed] [Google Scholar]
- 71.Cox SW, Eley BM. Cathepsin B/L-, elastase-, tryptase-, trypsin- and dipeptidyl peptidase IV-like activities in gingival crevicular fluid. A comparison of levels before and after basic periodontal treatment of chronic periodontitis patients. J. Clin. Periodontol. 1992;19:333–339. doi: 10.1111/j.1600-051x.1992.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 72.Eley BM, Cox SW. The relationship between gingival crevicular fluid cathepsin B activity and periodontal attachment loss in chronic periodontitis patients: a 2-year longitudinal study. J. Periodontal. Res. 1996;31:381–392. doi: 10.1111/j.1600-0765.1996.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 73.Woessner JF., Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 74.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J. Periodontol. 1993;64(5 Suppl):474–484. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 75.Salo T, Makela M, Kylmaniemi M, et al. Expression of matrix metalloproteinase-2 and -9 during early human wound healing. Lab. Invest. 1994;70:176–182. [PubMed] [Google Scholar]
- 76.Llano E, Pendas AM, Knauper V, et al. Identification and structural and functional characterization of human enamelysin (MMP-20) Biochemistry. 1997;36:15101–15108. doi: 10.1021/bi972120y. [DOI] [PubMed] [Google Scholar]
- 77.Pirila E, Ramamurthy N, Maisi P, et al. Wound healing in ovariectomized rats: effects of chemically modified tetracycline (CMT-8) and estrogen on matrix metalloproteinases -8, -13 and type I collagen expression. Curr. Med. Chem. 2001;8:281–294. doi: 10.2174/0929867013373552. [DOI] [PubMed] [Google Scholar]
- 78.Chubinskaya S, Huch K, Mikecz K, et al. Chondrocyte matrix metalloproteinase-8: up-regulation of neutrophil collagenase by interleukin-1 beta in human cartilage from knee and ankle joints. Lab. Invest. 1996;74:232–240. [PubMed] [Google Scholar]
- 79.Golub LM, Lee HM, Greenwald RA, et al. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm. Res. 1997;46:310–319. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- 80.Lee W, Aitken S, Kulkarni G, et al. Collagenase activity in recurrent periodontitis: relationship to disease progression and doxycycline therapy. J. Periodontal. Res. 1991;26:479–485. doi: 10.1111/j.1600-0765.1991.tb01798.x. [DOI] [PubMed] [Google Scholar]
- 81.Lee W, Aitken S, Sodek J, McCulloch CA. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. J. Periodontal. Res. 1995;30:23–33. doi: 10.1111/j.1600-0765.1995.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 82.Nomura T, Ishii A, Oishi Y, et al. Tissue inhibitors of metalloproteinases level and collagenase activity in gingival crevicular fluid: the relevance to periodontal diseases. Oral Dis. 1998;4:231–240. doi: 10.1111/j.1601-0825.1998.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 83.Mancini S, Romanelli R, Laschinger CA, et al. Assessment of a novel screening test for neutrophil collagenase activity in the diagnosis of periodontal diseases. J. Periodontol. 1999;70:1292–1302. doi: 10.1902/jop.1999.70.11.1292. [DOI] [PubMed] [Google Scholar]
- 84.Golub LM, Ciancio S, Ramamamurthy NS, et al. Low-dose doxycycline therapy: effect on gingival and crevicular fluid collagenase activity in humans. J. Periodontal. Res. 1990;25:321–330. doi: 10.1111/j.1600-0765.1990.tb00923.x. [DOI] [PubMed] [Google Scholar]
- 85.Golub LM, Wolff M, Roberts S, et al. Treating periodontal diseases by blocking tissue-destructive enzymes. J. Am. Dent. Assoc. 1994;125:163–169. doi: 10.14219/jada.archive.1994.0261. discussion 169-171. [DOI] [PubMed] [Google Scholar]
- 86.Teronen O, Konttinen YT, Lindqvist C, et al. Human neutrophil collagenase MMP-8 in peri-implant sulcus fluid and its inhibition by clodronate. J. Dent. Res. 1997;76:1529–1537. doi: 10.1177/00220345970760090401. [DOI] [PubMed] [Google Scholar]
- 87.Ma J, Kitti U, Teronen O, et al. Collagenases in different categories of peri-implant vertical bone loss. J. Dent. Res. 2000;79:1870–1873. doi: 10.1177/00220345000790110901. [DOI] [PubMed] [Google Scholar]
- 88.Kivela-Rajamaki MJ, Teronen OP, Maisi P, et al. Laminin-5 gamma2-chain and collagenase-2 (MMP-8) in human peri-implant sulcular fluid. Clin. Oral Implants. Res. 2003;14:158–165. doi: 10.1034/j.1600-0501.2003.140204.x. [DOI] [PubMed] [Google Scholar]
- 89.Kivela-Rajamaki M, Maisi P, Srinivas R, et al. Levels and molecular forms of MMP-7 (matrilysin-1) and MMP-8 (collagenase-2) in diseased human peri-implant sulcular fluid. J. Periodontal. Res. 2003;38:583–590. doi: 10.1034/j.1600-0765.2003.00688.x. [DOI] [PubMed] [Google Scholar]
- 90.Teng YT, Sodek J, McCulloch CA. Gingival crevicular fluid gelatinase and its relationship to periodontal disease in human subjects. J. Periodontal. Res. 1992;27:544–552. doi: 10.1111/j.1600-0765.1992.tb01830.x. [DOI] [PubMed] [Google Scholar]
- 91.Johansson N, Kahari VM. Matrix metalloproteinases in squamous cell carcinoma. Histol. Histopathol. 2000;15:225–237. doi: 10.14670/HH-15.225. [DOI] [PubMed] [Google Scholar]
- 92.Konttinen YT, Salo T, Hanemaaijer R, et al. Collagenase-3 (MMP-13) and its activators in rheumatoid arthritis: localization in the pannus-hard tissue junction and inhibition by alendronate. Matrix Biol. 1999;18:401–412. doi: 10.1016/s0945-053x(99)00030-x. [DOI] [PubMed] [Google Scholar]
- 93.Tervahartiala T, Pirila E, Ceponis A, et al. The in vivo expression of the collagenolytic matrix metalloproteinases (MMP-2, -8, -13, and -14) and matrilysin (MMP-7) in adult and localized juvenile periodontitis. J. Dent. Res. 2000;79:1969–1977. doi: 10.1177/00220345000790120801. [DOI] [PubMed] [Google Scholar]
- 94.Kiili M, Cox SW, Chen HY, et al. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: molecular forms and levels in gingival crevicular fluid and immunolocalisation in gingival tissue. J. Clin. Periodontol. 2002;29:224–232. doi: 10.1034/j.1600-051x.2002.290308.x. [DOI] [PubMed] [Google Scholar]
- 95.Uitto VJ, Airola K, Vaalamo M, et al. Collagenase-3 (matrix metalloproteinase-13) expression is induced in oral mucosal epithelium during chronic inflammation. Am. J. Pathol. 1998;152:1489–1499. [PMC free article] [PubMed] [Google Scholar]
- 96.Fagerhol MK. Calprotectin, a faecal marker of organic gastrointestinal abnormality. Lancet. 2000;356:1783–1784. doi: 10.1016/S0140-6736(00)03224-4. [DOI] [PubMed] [Google Scholar]
- 97.Poullis A, Foster R, Mendall MA, Fagerhol MK. Emerging role of calprotectin in gastroenterology. J. Gastroenterol. Hepatol. 2003;18:756–762. doi: 10.1046/j.1440-1746.2003.03014.x. [DOI] [PubMed] [Google Scholar]
- 98.Kido J, Nakamura T, Kido R, et al. Calprotectin in gingival crevicular fluid correlates with clinical and biochemical markers of periodontal disease. J. Clin. Periodontol. 1999;26:653–657. doi: 10.1034/j.1600-051x.1999.261004.x. [DOI] [PubMed] [Google Scholar]
- 99.Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression in vitro by oral epithelial cells confers resistance to infection by Porphyromonas gingivalis. Infect. Immun. 2001;69:4242–4247. doi: 10.1128/IAI.69.7.4242-4247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bataille R, Delmas P, Sany J. Serum bone gla-protein in multiple myeloma. Cancer. 1987;59:329–334. doi: 10.1002/1097-0142(19870115)59:2<329::aid-cncr2820590227>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 101.Slovik DM, Gundberg CM, Neer RM, Lian JB. Clinical evaluation of bone turnover by serum osteocalcin measurements in a hospital setting. J. Clin. Endocrinol. Metab. 1984;59:228–230. doi: 10.1210/jcem-59-2-228. [DOI] [PubMed] [Google Scholar]
- 102.Giannobile WV, Lynch SE, Denmark RG, et al. Crevicular fluid osteocalcin and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) as markers of rapid bone turnover in periodontitis. A pilot study in beagle dogs. J. Clin. Periodontol. 1995;22:903–910. doi: 10.1111/j.1600-051x.1995.tb01793.x. [DOI] [PubMed] [Google Scholar]
- 103.Kunimatsu K, Mataki S, Tanaka H, et al. A cross-sectional study on osteocalcin levels in gingival crevicular fluid from periodontal patients. J. Periodontol. 1993;64:865–869. doi: 10.1902/jop.1993.64.9.865. [DOI] [PubMed] [Google Scholar]
- 104.Lee AJ, Walsh TF, Hodges SJ, Rawlinson A. Gingival crevicular fluid osteocalcin in adult periodontitis. J. Clin. Periodontol. 1999;26:252–256. doi: 10.1034/j.1600-051x.1999.260409.x. [DOI] [PubMed] [Google Scholar]
- 105.Williams RC, Paquette DW, Offenbacher S, et al. Treatment of periodontitis by local administration of minocycline microspheres: a controlled trial. J. Periodontol. 2001;72:1535–1544. doi: 10.1902/jop.2001.72.11.1535. [DOI] [PubMed] [Google Scholar]
- 106.Griffiths GS, Moulson AM, Petrie A, James IT. Evaluation of osteocalcin and pyridinium crosslinks of bone collagen as markers of bone turnover in gingival crevicular fluid during different stages of orthodontic treatment. J. Clin. Periodontol. 1998;25:492–498. doi: 10.1111/j.1600-051x.1998.tb02478.x. [DOI] [PubMed] [Google Scholar]
- 107.Giannobile WV, Al-Shammari KF, Sarment DP. Matrix molecules and growth factors as indicators of periodontal disease activity. Periodontol. 2003;31:125–134. doi: 10.1034/j.1600-0757.2003.03108.x. 2000. [DOI] [PubMed] [Google Scholar]
- 108.Armitage GC, Jeffcoat MK, Chadwick DE, et al. Longitudinal evaluation of elastase as a marker for the progression of periodontitis. J. Periodontol. 1994;65:120–128. doi: 10.1902/jop.1994.65.2.120. [DOI] [PubMed] [Google Scholar]
- 109.Oringer RJ, Palys MD, Iranmanesh A, et al. C-telopeptide pyridinoline cross-links (ICTP) and periodontal pathogens associated with endosseous oral implants. Clin. Oral Implants. Res. 1998;9:365–373. doi: 10.1034/j.1600-0501.1996.090602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Palys MD, Haffajee AD, Socransky SS, Giannobile WV. Relationship between C-telopeptide pyridinoline cross-links (ICTP) and putative periodontal pathogens in periodontitis. J. Clin. Periodontol. 1998;25(11 Pt 1):865–871. doi: 10.1111/j.1600-051x.1998.tb02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shibutani T, Murahashi Y, Tsukada E, et al. Experimentally induced periodontitis in beagle dogs causes rapid increases in osteoclastic resorption of alveolar bone. J. Periodontol. 1997;68:385–391. doi: 10.1902/jop.1997.68.4.385. [DOI] [PubMed] [Google Scholar]
- 112.Talonpoika JT, Hamalainen MM. Type I collagen carboxyterminal telopeptide in human gingival crevicular fluid in different clinical conditions and after periodontal treatment. J. Clin. Periodontol. 1994;21:320–326. doi: 10.1111/j.1600-051x.1994.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 113.Gapski R, Barr JL, Sarment DP, et al. Effect of systemic matrix metalloproteinase inhibition on periodontal wound repair: a proof of concept trial. J. Periodontol. 2004;75:441–452. doi: 10.1902/jop.2004.75.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perez LA, Al-Shammari KF, Giannobile WV, Wang HL. Treatment of periodontal disease in a patient with Ehlers-Danlos syndrome. A case report and literature review. J. Periodontol. 2002;73:564–570. doi: 10.1902/jop.2002.73.5.564. [DOI] [PubMed] [Google Scholar]
- 115.Oringer RJ, Al-Shammari KF, Aldredge WA, et al. Effect of locally delivered minocycline microspheres on markers of bone resorption. J. Periodontol. 2002;73:835–842. doi: 10.1902/jop.2002.73.8.835. [DOI] [PubMed] [Google Scholar]
- 116.Al-Shammari KF, Giannobile WV, Aldredge WA, et al. Effect of non-surgical periodontal therapy on C-telopeptide pyridinoline cross-links (ICTP) and interleukin-1 levels. J. Periodontol. 2001;72:1045–1051. doi: 10.1902/jop.2001.72.8.1045. [DOI] [PubMed] [Google Scholar]
- 117.Giannobile WV. C-telopeptide pyridinoline cross-links. Sensitive indicators of periodontal tissue destruction. Ann. N. Y. Acad. Sci. 1999;878:404–412. doi: 10.1111/j.1749-6632.1999.tb07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Termine JD, Kleinman HK, Whitson SW, et al. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 119.Bowers MR, Fisher LW, Termine JD, Somerman MJ. Connective tissue-associated proteins in crevicular fluid: potential markers for periodontal diseases. J. Periodontol. 1989;60:448–451. doi: 10.1902/jop.1989.60.8.448. [DOI] [PubMed] [Google Scholar]
- 120.Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cellbinding sequence. Proc Natl. Acad. Sci. USA. 1986;83:8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rodan GA. Osteopontin overview. Ann. N. Y. Acad. Sci. 1995;760:1–5. doi: 10.1111/j.1749-6632.1995.tb44614.x. [DOI] [PubMed] [Google Scholar]
- 122.Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Osteopontin—a possible anchor of osteoclasts to bone. Proc. Natl. Acad. Sci. USA. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kadono H, Kido J, Kataoka M, et al. Inhibition of osteoblastic cell differentiation by lipopolysaccharide extract from Porphyromonas gingivalis. Infect. Immun. 1999;67:2841–2846. doi: 10.1128/iai.67.6.2841-2846.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heinegard D, Andersson G, Reinholt FP. Roles of osteopontin in bone remodeling. Ann. N. Y. Acad. Sci. 1995;760:213–222. doi: 10.1111/j.1749-6632.1995.tb44632.x. [DOI] [PubMed] [Google Scholar]
- 125.Owen TA, Aronow M, Shalhoub V, et al. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J. Cell. Physiol. 1990;143:420–430. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 126.Ikeda T, Nomura S, Yamaguchi A, et al. In situ hybridization of bone matrix proteins in undecalcified adult rat bone sections. J. Histochem. Cytochem. 1992;40:1079–1088. doi: 10.1177/40.8.1619274. [DOI] [PubMed] [Google Scholar]
- 127.Horton MA, Nesbit MA, Helfrich MH. Interaction of osteopontin with osteoclast integrins. Ann. N. Y. Acad. Sci. 1995;760:190–200. doi: 10.1111/j.1749-6632.1995.tb44630.x. [DOI] [PubMed] [Google Scholar]
- 128.Kido J, Nakamura T, Asahara Y, et al. Osteopontin in gingival crevicular fluid. J. Periodontal. Res. 2001;36:328–333. doi: 10.1034/j.1600-0765.2001.360509.x. [DOI] [PubMed] [Google Scholar]
- 129.Sharma CG, Pradeep AR. Gingival crevicular fluid osteopontin levels in periodontal health and disease. J. Periodontol. 2006;77:1674–1680. doi: 10.1902/jop.2006.060016. [DOI] [PubMed] [Google Scholar]


