Abstract
Objectives
To describe the prevalence of epilepsy after 6 months-of-age in children with perinatal stroke and examine whether perinatal data predict epilepsy onset and resolution.
Study design
A retrospective review of 64 children with perinatal stroke. In children with at least 6 months of follow-up data, Kaplan-Meier curves, univariate log-rank tests, and Cox proportional hazards models were used to examine predictors of time to development of seizures, and time to resolution of seizures in children with epilepsy. The association of risk factors with the presence of epilepsy at any time after 6 months-of-age was examined using Fisher’s exact test.
Results
Forty-one of the 61 children with at least 6 months of follow-up data (67%) had epilepsy between 6 months-of-age and last follow-up, but in 13 of 41 seizures eventually resolved and anticonvulsants were discontinued. Infarct on prenatal ultrasound (p=0.0065) and family history of epilepsy (p=0.0093) were significantly associated with time to development of seizures after 6 months-of-age in the univariate analysis. No assessed variables were associated with time to resolution of epilepsy or with the presence of epilepsy after 6 months-of-age.
Conclusions
Childhood epilepsy is frequent after perinatal stroke. Evidence of infarction on prenatal ultrasound and a family history of epilepsy predict earlier onset of active seizures.
Epilepsy affects approximately 5 out of 1000 children; however, children with central nervous system injury are at increased risk1. Children with perinatal arterial ischemic stroke appear to be at increased risk for childhood epilepsy, but estimates of epilepsy after perinatal stroke vary widely. Epilepsy rates ranging from 0–50%2–9 were reported for studies of 8–46 children with a median of approximately 35 months of follow-up. There are few studies examining clinical factors that predict the development of childhood epilepsy in children with perinatal stroke, and little data on when children with perinatal stroke develop epilepsy. When parents are told that their child had a perinatal stroke and the possible outcomes are described, physicians are often faced with the following three questions: will my child have epilepsy when he is older? If my child does have epilepsy, will the medication stop all his seizures? When would he develop seizures? The purpose of this study is to answer these questions by describing the prevalence and severity of epilepsy after 6 months-of-age in children with perinatal stroke, examine whether data present in the perinatal period predict the presence of childhood epilepsy, describe when these children develop active seizures and examine predictors of time to onset of seizure activity.
Methods
Population
Sixty-four children with perinatal arterial ischemic stroke were initially identified using a combination of the following techniques: review of neurology clinic records from January 1990-January 2007; patient referral; and International Classification of Disease, 9th edition code searches from May 1 1999-May 1 2004 using codes 767 (neonatal stroke), 433 (occlusion and stenosis/precerebral ischemia), 434 (occlusion of cerebral arteries), 435 (transient cerebral ischemia), 436 (acute but ill-defined cerebrovascular disease), 437 (other and ill-defined cerebrovascular disease), 438 (late effects of cerebrovascular disease) and 342 (acute hemiplegia)10. ICD-9 codes searches of additional years were not performed due to the limitations of these searches.11 Birth years of patients ranged from 1990–2005. The perinatal period was defined as the time from the end of the 20th week of gestation to day 28 after birth12. All children had a gestational age of at least 36 weeks at birth, presented with neurological symptoms during the neonatal period (first 28 days of life) and were diagnosed with arterial ischemic stroke before discharge home from the neonatal intensive care unit (NICU). Children with radiographic evidence of generalized hypoxic ischemic injury or dysmorphic features suggestive of a genetic syndrome were excluded.
Data Collection
Data were collected from the medical record on prenatal ultrasound imaging abnormalities, clinical presentation, perinatal comorbidities, initial cranial computed tomography (CT) and/or magnetic resonance imaging (MRI), initial and follow-up electroencephalograms (EEG), family history of epilepsy in first- or second-degree relatives, and epilepsy status during all available follow-up. Based on radiographic findings, stroke locations were identified, described by arterial territory, and classified as unilateral or bilateral. Unilateral middle cerebral artery (MCA) infarctions were classified as large branch (involving the M1 territory) or small branch. EEG reports were reviewed and classified as normal or abnormal. Abnormalities were classified as abnormal background consistent with encephalopathy, slowing or voltage attenuation, epileptiform discharges, or electrographic seizure activity. Epilepsy outcomes were assessed in children at least 6 months-of-age at the last clinical follow-up. All subjects had clinic notes that included the clinician’s record of last seizure and estimated frequency of seizures. Children were classified as having or not having epilepsy, and were classified according to a modified form of the Engel classification13, 14 adjusted by our group for a young population with limited follow-up and no surgical intervention: Class 0= seizure-free and off anticonvulsants for at least 6 months, Class 1= seizure-free for at least 6 months while on medication or seizure-free off medication for fewer than 6 months, Class 2= less than one seizure a month, Class 3= 1–4 seizures a month, Class 4= 5–30 seizures a month, Class 5= 30 or more seizures a month. Epilepsy was defined as modified Engel (ME) class 1 or higher, active seizures were defined as ME class 2 or higher, and severe epilepsy was defined as ME class 3 or higher. All children with epilepsy had at least 2 nonfebrile clinical seizures or 1 nonfebrile clinical seizure with an EEG demonstrating epileptiform discharges.
Statistical Analysis
Totals and proportions were used to describe the clinical characteristics of the population. Time to development of first seizure after six months-of-age was the primary outcome examined. Six months-of-age was chosen as a starting point because in our clinical experience children with perinatal stroke are often maintained on phenobarbital for up to 6 months after NICU discharge; we wanted to examine what happens after that point. For children who presented with seizures at six months-of-age, the time to first seizure was zero. For children who never developed seizures, the time to first seizure was length of follow-up, and the observation was censored.
Time to resolution of seizures was also examined; in some children with epilepsy, seizures resolve and anticonvulsants can be discontinued. This analysis only included children who had seizures after six months-of-age. For children whose seizures resolved, defined as both being seizure-free for at least 6 months and being taken off anticovulsants, the time to seizure resolution was months from start of seizures to date taken off anticonvulsants. For children whose seizures did not resolve, the time to seizure resolution was months from start of seizures to date of last follow-up, and the observation was censored.
Six variables were examined as predictors of outcome: evidence of infarction on prenatal ultrasound; initial presentation with seizures; abnormality on initial NICU EEG; presence of bilateral infarcts on radiographic imaging; in children with MCA infarcts, the presence of large-versus-small-branch infarction; and family history of seizures. Univariate log-rank tests were done to test the association of each of these factors with time to development of seizures. Last, a multivariable Cox proportional hazards model was fit to examine the association of all factors except abnormality on initial NICU EEG and the presence of large-versus-small-branch MCA infarction. Abnormality on initial NICU EEG was omitted because no children with evidence of stroke on prenatal ultrasound had a neonatal EEG. The presence of large-versus-small-branch infarction was omitted because it did not apply to children who did not have an MCA infarction. The proportional hazards assumption was tested with the Kolmogorov-type supremum test for all variables and was met in all cases.
As a secondary analysis, Fisher’s exact tests were done to examine the association of the six factors with development of epilepsy at any time after six months-of-age (yes/no).
Ethics
This study was approved by the Institutional Review Board of the Indiana University School of Medicine (Study # 0207-55)
Results
Patient population
Sixty-four children with perinatal arterial ischemic stroke were identified. Thirty-six (56%) were male. Forty-eight (75%) presented in the NICU with seizures. All children with seizures were treated with phenobarbital. Additional medications used included lorazepam (8), phenytoin (8), diazepam (1) and fosphenytoin (1). Additional presentations which prompted neurological evaluation included apnea (19), respiratory distress (12), abnormal tone (11), poor feeding (9), poor respiratory effort (6), and irritability (5). Cormorbidities included infection in 11 (17%); cardiac abnormalities in 11 (17%); extracorporeal membrane oxygenation in 4 (6%), and renal failure in 3 (5%). Seven of the 64 children with perinatal arterial ischemic stroke (11%) had a family history of seizures.
Radiographic Findings
Evidence of stroke was present on prenatal ultrasound for 4 patients (6%). All patients had confirmation of infarction by cranial CT (20), MRI (20), or both (24). Forty-six children (72%) had unilateral hemispheric infarction in isolated arterial territories: 1 in the right internal carotid artery territory, 1 in the right anterior cerebral artery (ACA) territory, 4 in the posterior cerebral artery (PCA) territory (2 left, 2 right) and 40 in the MCA territory (27 left, 13 right). Of the unilateral MCA infarctions, 18 were large branch and 19 were small branch; original films could not be accessed for 3 and infarction size could not be judged from radiographic reports. Four children had multiple unilateral infarctions in multiple territories. Fourteen children had bilateral infarctions involving the following regions: the basal ganglia (1 right), brainstem (1), cerebellar (1 left, 2 right), anterior cerebral artery (2 left, 2 right), PCA (7 left, 7 right), and MCA (12 left, 11 right) territories. Four had watershed infarctions. Nineteen had infarction with hemorrhagic transformation (9 left, 3 right, 7 bilateral).
Initial EEG findings on reports
Initial EEG reports were available for 44 of the 48 newborns that presented with seizures; 40 (91%) had at least one abnormality on initial EEG. (Table I; available at www.jpeds.com)
Table I.
EEG findings on initial EEG (n= 44*)
| EEG finding | # (%) |
|---|---|
| Abnormal background | 26 (59.1%) |
| Slowing/voltage attenuation | 26 (59.1%) |
| Epileptiform discharges | 35 (79.5%) |
| Electrographic seizures | 17 (38.6%) |
| Normal | 4 (9.1%) |
Not all children had initial EEG performed
Epilepsy status after 6 months-of-age
At least 6 months of follow-up data were available on 61 patients (95% of 64). Median age of these 61 patients at last follow-up was 43 months (range 9–179). Forty-one children (67 %) had epilepsy (ME class 1 or higher) between 6 months-of-age and last follow-up. Eleven of those children were maintained on phenobarbital after NICU discharge for more than 6 months, but were seizure-free (ME class 1); children were maintained on phenobarbital for that length of time usually due to abnormal EEG or variations in physician practice. Eleven children had seizure activity after leaving the NICU which continued or started in the first 6 months of life (ME class 2 or higher). Nineteen children had seizure onset after 6 months of age; 6 of those children had not presented with seizures in the NICU. Median age at onset of seizures for those 19 children was 16 months (range 7–124 months). Seizures resolved in 13 (32% of 41 children with epilepsy); these children were seizure-free and off medications for at least 6 months at the last follow-up visit.
A total of 28 children (46% of the 61 included patients) were classified as having epilepsy at the time of last follow-up (Table II). Five children (8% of the 61 children) developed infantile spasms; these children have been previously described15. Most of the children with epilepsy (64%; 18 of 28) were seizure-free on medication or while being weaned off.
Table II.
Epilepsy outcomes at last follow-up (N=61)
| Outcome | # (%) |
|---|---|
| ME Class 0 | 33 (54%) |
| ME Class 1 | 18 (30%) |
| ME Class 2 | 3 (5%) |
| ME Class 3 | 4 (7%) |
| ME-Class 4 | 2 (3%) |
| ME-Class 5 | 1 (2%) |
| Infantile spasms | 5 (8%) |
ME= Modified Engel
Class 0= seizure-free and off anticonvulsants for at least 6 months
Class 1= seizure-free on medication for at least 6 months or seizure-free off medication for fewer than 6 months
Class 2= less than one seizure a month
Class 3= 1–4 seizures a month
Class 4= 5–30 seizures a month
Class 5= 30 or more seizures a month.
Twenty-five patients were on seizure medications at last follow-up; 3 had been weaned off but had been seizure-free off medication for fewer than 6 months. The most commonly used medications used at last follow up were: phenobarbital (10), lamotrigine(5), oxcarbazepine (7), valproic acid (4), and topiramate(4). Six (24% of the 25 patients with medication) patients required multiple medications to control their seizures at last follow-up.
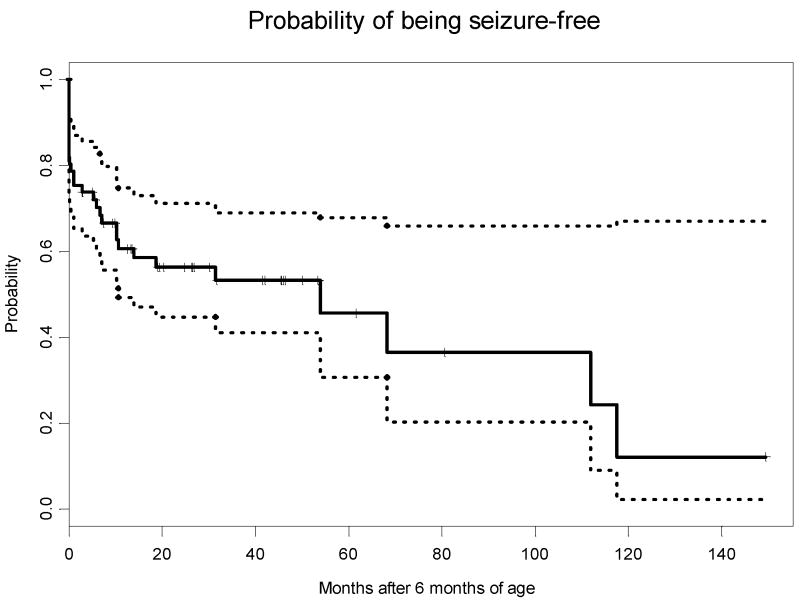
Follow-up EEGs performed at 6 months-of-age or later were available for 42 of the children: 17 were normal; 19 had slowing and/or voltage attenuation, which was diffuse in 9 and focal in 10; 19 had epileptiform discharges. Five children had infantile spasms with onset at a median age of 7 months; three of these children later developed severe seizures (ME class 3 or higher). Several of these children were previously described.15 The Kaplan-Meier curve demonstrating the probability of being seizure-free (ME class 0 or 1) over time is in Figure 1.
Figure 1.
The light dotted lines represent 95% confidence intervals.
On univariate analysis, only presence of infarct on prenatal ultrasound (p=0.0065) (Figure 2; available at www.jpeds.com) and family history of seizures (p=0.0093) (Figure 3; available at www.jpeds.com) were significantly associated with time to development of seizures after six months of age. The median time to development of seizures after 6 months-of-age was shorter in children with evidence of infarct on prenatal ultrasound (3.8 months with evidence vs 53.9 without evidence) and in children with a family history of seizures (1.1 months with a family history vs 53.9 months without). Results of the log-rank tests are shown in Table III. On multivariable analysis, none of the variables were associated with time until development of seizures, though there was a trend towards significance for presence of infarct on prenatal ultrasound and family history of seizures: presence of infarct on prenatal ultrasound (p=0.053), NICU presentation with seizures (p=0.397), bilateral infarcts (p=0.826), family history (p=0.087).
Figure 2.

Comparison of children with and without evidence of infarction prenatal ultrasound, difference is significant, p= 0.0065
Figure 3.

Comparison of children with and without family history of epilepsy, difference is significant, p=0.0093.
Table III.
Log-rank tests- Prediction of time to seizure onset after 6 months
| N | Median months | p-value | |
|---|---|---|---|
| Evidence of infarct on ultrasound | 4 | 3.8 | 0.007 |
| No evidence of infarct on ultrasound | 57 | 53.9 | |
| Initial presentation with seizures | 45 | 53.9 | 0.890 |
| No initial presentation with seizures | 16 | 111.8 | |
| Abnormal NICU EEG | 40 | 31.4 | 0.701 |
| Normal NICU EEG | 5 | >53.5 | |
| Bilateral infarcts | 13 | 31.4 | 0.772 |
| No bilateral infarcts | 48 | 53.9 | |
| MCA infarct- Large branch | 18 | 111.8 | 0.718 |
| MCA infarct- Small branch | 18 | 53.9 | |
| Family history | 7 | 1.1 | 0.009 |
| No family history | 54 | 53.9 |
NICU= neonatal intensive care unit; EEG= electroencephalogram;
MCA= middle cerebral artery
On univariate analysis, no variables were significantly associated with time to resolution of seizures after six months-of-age. Results of the log-rank tests are shown in Table IV. On multivariable analysis, none of the variables with significantly associated with time to resolution of seizures: presence of infarct on prenatal ultrasound (p=0.998), NICU presentation with seizures (p=0.874), bilateral infarcts (p=0.998), or family history (p=0.874).
Table IV.
Log-rank tests- Predictors of resolution of epilepsy
| N | Median months | p-value | |
|---|---|---|---|
| Presence of infarct on prenatal ultrasonography | 4 | >178.8 | 0.2535 |
| No presence of infarct on prenatal ultrasonography | 26 | >152.9 | |
| Initial presentation with seizures | 21 | >88.0 | 0.2609 |
| No initial presentation with seizures | 9 | >178.8 | |
| Abnormal NICU EEG | 20 | >150.5 | 0.3830 |
| Normal NICU EEG | 2 | >78.4 | |
| Bilateral infarcts | 7 | >152.9 | 0.2619 |
| No bilateral infarcts | 23 | >178.8 | |
| Family history of epilepsy | 6 | >178.8 | 0.7023 |
| No family history of epilepsy | 24 | >152.9 | |
| Large branch | 9 | >178.8 | 0.0984 |
| Small branch | 8 | 65.4 |
None of the six factors were significantly associated with development of seizures at any time after six months of age on Fisher’s exact tests. These results are shown in Table V (available at www.jpeds.com).
Table V.
Fisher’s exact test- predictors of epilepsy
| p-value | |
|---|---|
| Evidence of infarct on ultrasound | 0.293 |
| Initial presentation with seizures | 0.356 |
| Abnormal NICU EEG | 0.136 |
| Bilateral infarcts | 0.742 |
| MCA infarcts: Large vs. small branch | 0.443 |
| Family history | 0.409 |
NICU= neonatal intensive care unit; EEG= electroencephalogram;
MCA= middle cerebral artery
Discussion
Seizures are a frequent presenting sign of perinatal arterial ischemic stroke, and childhood epilepsy is a frequent resulting morbidity. In our cohort, 75% (48 of 64) of children with perinatal stroke presented with seizures, and 67 % (41 of 61 with >= 6 months of follow-up) had or developed epilepsy after 6 months-of-age. Epilepsy resolved in 13 children, so 46% (28 of 61) had epilepsy as of last follow-up. Sixty-four percent (18 of 28) of the children with epilepsy were eventually seizure-free with treatment and 25 % (7 of 28) of the children with epilepsy had severe epilepsy at last follow-up with 1 or more seizures per month (ME class 3 or higher). Seizures that started or re-started after 6 months-of-age began at a median age of 16 months, but one child had seizures start at 10.3 years of age. Evidence of infarct on prenatal ultrasound and family history of epilepsy were both associated with earlier onset of seizures. We were unable to identify predictors of what caused epilepsy to resolve in some children, or whether or not children would develop epilepsy.
This prevalence of childhood epilepsy after perinatal stroke was higher than what has been reported in previous studies2–9, but we did note that seizures resolved in some children; our incidence of epilepsy at last follow-up falls within the previously described range. There are larger studies of epilepsy in the hemiplegic cerebral palsy literature, where it is reported approximately 1/3 of children have epilepsy16, 17; in this population, neonatal seizures are a predictor for later seizures18. In our cohort, neonatal seizures did not predict later epilepsy. Stroke is responsible for at least 20% of hemiplegic cerebral palsy19, 20, but the wide range of other etiologies included in this population makes it difficult to draw conclusions on the outcomes after perinatal stroke from published cerebral palsy data.
The median age at time of seizure development for children over 6 months-of-age in our cohort was 16 months (range 7 months to 10 years and 4 months). Sran et al2 described children with perinatal stroke who were seizure-free for 1–8 years, then developed epilepsy. It is possible that the incidence of epilepsy after perinatal stroke in our cohort will rise as we continue to follow these children over the next several years.
Evidence of infarction on prenatal ultrasound predicted the earlier onset of epilepsy in our cohort. There are few large studies of fetal stroke, but the data that have been published suggest that these children may be at increased risk for neurological impairment. Ozduman et al21 described epilepsy in 2 of 3 children with fetal stroke who had at least 18 months of follow-up; they reviewed the previous literature but few details on the long-term neurological outcomes were available.
Family history was also a predictor of the development of seizures over time; children developed epilepsy earlier if they had a family history of epilepsy. This suggests that genetic background may influence outcome, but may also reflect increased awareness and detection of seizures in families with prior experience with epilepsy.
Although we found predictors of time to onset of epilepsy, none of the risk factors we evaluated predicted time to resolution of seizures or whether children would develop epilepsy. Lee and colleagues also found it difficult to identify predictors of epilepsy6. Our sample size may have limited our ability to identify strong predictors. We may be able to revisit this in future, larger studies.
There are several limitations in this study. Most of the data are retrospective. We started to collect prospective data on some of these children two years ago, but the rarity of perinatal stroke makes it difficult to collect information on large numbers of children at a single center. Our cohort may have been too small to detect variables predictive of later epilepsy due to type II error. We have limited follow-up, with a median age at last follow-up of 43 months; some of the children developed epilepsy as late as 10 years of age, so we may have underestimated the rate of childhood epilepsy. It is also possible that some of the children whose epilepsy “resolved” will have their epilepsy restart later in life. This cohort may be biased towards sicker patients who have higher rates of epilepsy. Some children with perinatal stroke have normal neurological outcomes22; their parents might be less likely to return for follow-up. Some of the children who were “well-controlled on one medication” may have had resolution of their epilepsy that was not detected because follow-up EEG was not performed. We had 11 children who were maintained on phenobarbital for more than 6 months after NICU discharge, in part because there are no well-established guidelines on how long to maintain anticonvulsant therapy in children with neonatal stroke. However, for a parent, a child who has epilepsy and is “well-controlled on medication” and a child who is actually epilepsy-free but still on medication require the same amount of work. For parents, the experienced outcome is the same, so we felt it was reasonable to group them together. For the Kaplan-Meier curves and Cox proportional hazards models, we chose to examine time to development of active seizures after 6 months-of-age becasue it was not always clear when epilepsy began and ended for children maintained on phenobarbital while seizure-free for prolonged periods. The object of this paper was to provide information to counsel parents about future care their children would require. In the future, when we have more follow-up data on these children, we may find it easier to identify predictors of chronic epilepsy.
Previous work has suggested that abnormal background on neonatal EEG or early seizures may be predictive of later poor neuromotor outcome3, 7. The next step in our research will be to examine other outcome measures and see if this holds true in our cohort. We will also assess the association of epilepsy with other disability.
Acknowledgments
The authors would like to thank Ms. Nina Talib M.Sc. for technical assistance.
Dr. Golomb is supported by the National Institutes of Health NINDS grant K23 NS048024 and the Clarian Values Fund grant VFR-171. Dr. Williams is supported by the National Institutes of Health NINDS R01 NS 39571 and a VA Health Services Research and Development Career Award.
Abbreviations
- NICU
neonatal intensive care unit
- CT
computed tomography
- MRI
magnetic resonance imaging
- EEG
electroencephalogram
- MCA
middle cerebral artery
- ME
modified Engel
- PCA
posterior cerebral artery
Footnotes
This work was done at the Riley Hospital for Children, Indianapolis, Indiana
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cowan LD. The epidemiology of the epilepsies in children. Ment Retard Dev Disabil Res Rev. 2002;8:171–81. doi: 10.1002/mrdd.10035. [DOI] [PubMed] [Google Scholar]
- 2.Sran SK, Baumann RJ. Outcome of neonatal strokes. American Journal of Diseases of Children. 1988;142:1086–8. doi: 10.1001/archpedi.1988.02150100080031. [DOI] [PubMed] [Google Scholar]
- 3.Sreenan C, Bhargava R, Robertson CM. Cerebral infarction in the term newborn: clinical presentation and long-term outcome. Journal of Pediatrics. 2000;137:351–5. doi: 10.1067/mpd.2000.107845. [DOI] [PubMed] [Google Scholar]
- 4.Estan J, Hope P. Unilateral neonatal cerebral infarction in full term infants. Arch Dis Child Fetal Neonatal Ed. 1997;76:F88–93. doi: 10.1136/fn.76.2.f88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koelfen W, Freund M, Varnholt V. Neonatal stroke involving the middle cerebral artery in term infants: clinical presentation, EEG and imaging studies, and outcome. Developmental Medicine & Child Neurology. 1995;37:204–12. doi: 10.1111/j.1469-8749.1995.tb11993.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Croen LA, Lindan C, Nash KB, Yoshida CK, Ferriero DM, et al. Predictors of outcome in perinatal arterial stroke: a population-based study. Ann Neurol. 2005;58:303–8. doi: 10.1002/ana.20557. [DOI] [PubMed] [Google Scholar]
- 7.Mercuri E, Rutherford M, Cowan F, Pennock J, Counsell S, Papadimitriou M, et al. Early prognostic indicators of outcome in infants with neonatal cerebral infarction: a clinical, electroencephalogram, and magnetic resonance imaging study. Pediatrics. 1999;103:39–46. doi: 10.1542/peds.103.1.39. [DOI] [PubMed] [Google Scholar]
- 8.Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–80. doi: 10.1542/peds.2005-1178. [DOI] [PubMed] [Google Scholar]
- 9.Koelfen W, Freund M, Konig S, Varnholt V, Rohr H, Schultze C. Results of parenchymal and angiographic magnetic resonance imaging and neuropsychological testing of children after stroke as neonates. European Journal of Pediatrics. 1993;152:1030–5. doi: 10.1007/BF01957231. [DOI] [PubMed] [Google Scholar]
- 10.International Classification of Diseases. 9th rev, clinical modification: ICD-9:CM. Denver, DE: American Medical Association; 1999. [Google Scholar]
- 11.Golomb MR, Garg BP, Saha C, Williams LS. Accuracy and yield of ICD-9 codes for identifying children with ischemic stroke. Neurology. 2006;67:2053–5. doi: 10.1212/01.wnl.0000247281.98094.e2. [DOI] [PubMed] [Google Scholar]
- 12.Anderson KN, Anderson LE. Mosby’s pocket dictionary of medicine, nursing, and allied health. The C.V. Mosby Company; 1990. [Google Scholar]
- 13.Engel J. Surgical treatment of the epilepsies. New York: Raven Press; 1987. [Google Scholar]
- 14.Koh S, Nguyen S, Asarnow RF, LoPresti C, Yudovin S, Shields WD, et al. Five or more acute postoperative seizures predict hospital course and long-term seizure control after hemispherectomy. Epilepsia. 2004;45:527–33. doi: 10.1111/j.0013-9580.2004.50203.x. [DOI] [PubMed] [Google Scholar]
- 15.Golomb MR, Garg BP, Williams LS. Outcomes of children with infantile spasms after perinatal stroke. Pediatr Neurol. 2006;34:291–5. doi: 10.1016/j.pediatrneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson M, Hagberg G, Olsson I. Clinical and aetiological aspects of epilepsy in children with cerebral palsy. Dev Med Child Neurol. 2003;45:371–6. doi: 10.1017/s0012162203000719. [DOI] [PubMed] [Google Scholar]
- 17.Oskoui M, Shevell MI. Profile of pediatric hemiparesis. J Child Neurol. 2005;20:471–6. doi: 10.1177/088307380502000601. [DOI] [PubMed] [Google Scholar]
- 18.Bruck I, Antoniuk SA, Spessatto A, Bem RS, Hausberger R, Pacheco CG. Epilepsy in children with cerebral palsy. Arq Neuropsiquiatr. 2001;59:35–9. doi: 10.1590/s0004-282x2001000100008. [DOI] [PubMed] [Google Scholar]
- 19.Uvebrant P. Hemiplegic cerebral palsy. Aetiology and outcome Acta Paediatrica Scandinavica - Supplement. 1988;345:1–100. doi: 10.1111/j.1651-2227.1988.tb14939.x. [DOI] [PubMed] [Google Scholar]
- 20.Humphreys P, Whiting S, Pham B. Hemiparetic cerebral palsy: clinical pattern and imaging in prediction of outcome. Canadian Journal of Neurological Science. 2000;27:210–9. [PubMed] [Google Scholar]
- 21.Ozduman K, Pober BR, Barnes P, Copel JA, Ogle EA, Duncan CC, et al. Fetal stroke. Pediatr Neurol. 2004;30:151–62. doi: 10.1016/j.pediatrneurol.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 22.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. Journal of Child Neurology. 2000;15:316–24. doi: 10.1177/088307380001500508. [DOI] [PubMed] [Google Scholar]