Abstract
Isoflavones may influence insulin action by means of their well-known receptor-mediated estrogenic activity. However, isoflavones also bind to PPAR’s which are strongly associated with insulin action. Soy protein with its isoflavones has previously been shown to improve glycemic control in diabetic postmenopausal women and to improve insulin sensitivity in ovariectomized monkeys. The purpose of the current report was to extend our studies of dietary soy protein to male monkeys and determine effects of the soy isoflavones on insulin resistance. Two studies are reported here. Study one involved 91 male monkeys consuming three diets differing only by the source of protein (casein-lactalbumin, soy protein with a low isoflavone concentration or soy protein with a high isoflavone concentration). Intravenous glucose tolerance tests (IVGTTs) were done and plasma adiponectin and lipoprotein concentrations were determined after 25 months of study. Samples of visceral fat were obtained at 31 months for assessment adiponectin and PPARγ expression. The second study involved 8 monkeys in a Latin square design that compared the effects of diets with either casein/lactalbumin, soy protein with a high isoflavone concentration, or soy protein that was alcohol-washed to deplete the isoflavones. After eight weeks of treatment, insulin sensitivity and plasma lipoproteins were assessed. At ten weeks, skeletal muscle was biopsied for determination of insulin receptor, PPARα and PPARγ content. The major findings were that consumption of isoflavone-containing soy protein dose-dependently increased insulin responses to the glucose challenge and decreased plasma adiponectin while isoflavone-depleted soy protein decreased body weight and had no effect on plasma adiponectin concentrations. Muscle PPARα and γ expression was also increased with the isoflavone-depleted soy relative to either casein or soy protein containing the isoflavones. Further studies are needed to determine the mechanisms involved in these effects of a high soy isoflavone diet and to optimize dietary isoflavone content for maximal health benefits in males.
Keywords: soy, isoflavones, insulin, PPAR, adiponectin, nonhuman primates
INTRODUCTION
Current estimates are that 50% of the adult population of the United States is obese (1). About half of the obese population also suffers from pre-diabetes or the metabolic syndrome (2). Insulin resistance and obesity are key features of the metabolic syndrome and Type 2 diabetes mellitus (T2DM) (2,3). One potential mechanism involves the production of hormones or adipokines by adipose tissue. Plasma concentrations of many adipokines, such as leptin, TNFα, and PAI-1, have been associated positively with insulin resistance, whereas adiponectin is associated negatively (4,5). Further, lower plasma concentrations of adiponectin are associated with increased incidence of metabolic syndrome, diabetes, and vascular disease (5–7).
Pharmacologic agents, such as thiazolidinediones, are potent agonists of PPARγ and have become useful clinical tools to improve insulin resistance and raise adiponectin concentrations (5,7). Interestingly, the isoflavones genistein and daidzein, plant estrogens found in soy beans and processed soy protein, have also been shown to bind to PPARγ as well as PPAR α and δ(8–10), suggesting the potential value of isoflavones as a nutritional approach to modulating insulin action. Soy is the most commonly used botanical in the US, and the FDA has approved a health claim for soy protein and soy-based food products, based largely on the evidence that soy consumption improves plasma lipid and lipoprotein concentrations and might reduce risk of CHD, yet does not appear to increase cancer risk (11).
The earliest report of soy beans having beneficial effects on glycemic control was in 1910 (12) when the consumption of soy beans was found to decrease glycosuria in diabetics. Until relatively recently, however, there have been few studies of the effects of soy or its isoflavones on glycemic indices. Among the somewhat limited data is the finding that consumption of soy protein containing isoflavones was associated with improved lipoprotein and glycemic control in T2DM postmenopausal women (13). In nondiabetic postmenopausal women soy consumption was associated with decreased fasting insulin concentrations and isoflavone intake was inversely associated with postchallenge insulin concentrations (14). Similarly, we have found improved plasma lipoprotein profiles and insulin sensitivity in premenopausal monkeys fed soy rich diets (15). However, in smaller studies of predominantly male T2DM subjects (14 men and 6 women) the improvement in lipoprotein profiles has been seen, but not the improved glycemic control (16). Similarly, in a small study of diabetic and nondiabetic male monkeys, soy protein improved plasma lipoproteins and atherosclerosis but did not affect glycemic control (17).
These few studies suggest that the benefits of soy on carbohydrate metabolism are more apparent in females than males. The studies did not assess potential mechanisms involved with changes in insulin action or whether the effect is due to the soy protein, its isoflavones, or both. In addition to the isoflavones binding to PPAR, they have estrogenic activity, binding to both ERα and ERβ, but with greater affinity to ERβ (11). Genistein is also a tyrosine kinase inhibitor (18), so high concentrations may inhibit insulin signaling pathways.
The purpose of the current studies was to extend our studies of dietary soy protein and isoflavones to address their effects on insulin resistance in males. Further, since adiponectin is strongly associated with insulin action and is also regulated by PPARγ, we explored whether changes in insulin resistance were related to changes in plasma concentrations of this adipokine.
Methods
Animal Studies
Study 1
Ninety-one adult male cynomolgus monkeys (Macaca fascicularis) were imported from Indonesia (Institut Pertanian Bogor). Effects on plasma lipoprotein and isoflavone concentrations and the cardiovascular system have been reported previously (19). All monkeys consumed a Western-type diet differing only by the source of dietary protein (19). The major source of protein for Group 1 was casein-lactalbumin (Casein, n=30), for Group 2 was a mixture of unmodified soy protein isolate and alcohol-washed (isoflavone-depleted) soy protein isolate approximating human intake of 75 mg isoflavones/day (low-ISO, n=30), and for Group 3 was unmodified soy protein isolate containing an amount approximating human intake of 150 mg isoflavones/day (high-ISO, n=31). Other than protein source, diets were equal in macronutrients with 19% of calories from protein, 35% from lipid (0.28 mg cholesterol/Cal), and 46% from carbohydrates. Detailed descriptions of the diet compositions have been published previously (19). Glucose and insulin responses to an IVGTT and plasma adiponectin concentrations were determined after 25 months of study as described previously (20). Visceral fat samples were collected after 31 months of treatment.
Study 2
Eight old, obese, hyperinsulinemic male monkeys were used in this study (note increased body weight and fasting insulin of monkeys in Table 2 vs. Table 1). The study was a 3-phase Latin square design, such that each monkey received each diet after a baseline period during which animals consumed the control diet. Animals were randomized to one of 3 diet groups containing either 1- casein, 2-alcohol-washed (isoflavone-depleted) soy (SOY−, 8 mg isoflavones/d human equivalent), or 3-intact soy protein (SOY+, 132 mg isoflavones/d human equivalent). Diets were equal in macronutrients with the exception of the protein source and composed of 19% of calories from protein, 20% from lipid (0.19 mg cholesterol/Cal), and 61% from carbohydrates. Each phase lasted 10 weeks.
The first phase of diet interventions began the week after baseline measures were completed. After 4 and 8 weeks of treatment, measurement of body weight and blood collection for analysis of TC, HDLC, and TG concentrations were done. At 8 weeks, minimal model analysis (frequently sampled intravenous glucose tolerance test) was performed (21). At 10 weeks treatment, animals were sedated for measurement of body weight, plasma lipids and lipoproteins, and muscle biopsy of the vastus lateralis muscle.
All procedures involving animals were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by the Institutional Animal Care and Use Committee.
Clinical chemistry measures
Animals were fasted overnight and sedated with ketamine hydrochloride (15 mg/kg intramuscularly) (Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) prior to blood collection. TC, HDLC and TG were determined by enzymatic techniques (19). Lipoprotein fractions were separated by ultracentrifugation and HPLC and the cholesterol content of each fraction determined enzymatically (19) Plasma glucose, fructosamine, insulin, C-peptide, leptin, and adiponectin were determined as described (20–22).
Measurements for the IVGTT included the glucose area under the curve (AUC), calculated as the total area including all time points, the disappearance rates (K value) for glucose and insulin were calculated from the linear portion of the curve (20). Measurements for the FSIVGTT included the insulin sensitivity index (SI) as described previously (15,21).
Western Blot Analyses
Biopsies of visceral fat (Study 1) and skeletal muscle (Study 2) were snap frozen in liquid nitrogen and stored at −70C until processed as described previously (23). Fat samples were assessed for adiponectin (mouse monoclonal anti-adiponectin, BioVision Research Products, Mountain View, CA), and PPARγ (rabbit polyclonal anti-PPARγ, Santa Cruz Biotechnology Inc, Santa Cruz, CA). Skeletal muscle homogenates were assessed for insulin receptor (IR) expression (mouse monoclonal anti-lR, Research Diagnostics, Inc, Flanders, NJ). Basal IR activity was determined using a phosphorylation state specific antibody generated against the phosphorylated tyrosine residue 1158 of the human IR (anti-IRpY1158, Biosource International; Camarillo, CA). The blots were also probed with anti-PPARα (rabbit polyclonal anti-PPARα; Santa Cruz Biotechnology Inc, Santa Cruz, CA) or anti-PPARγ antibody (rabbit polyclonal anti-PPAR γ; Biomol Intl, Plymouth Meeting, PA). To account for equal protein loading, blots were stripped (Re-Blot Plus; Chemicon International; Temecula, CA) and reprobed for actin in muscle (monoclonal actin Ab-1; Oncogene Research Products; Boston, MA), or guanidine disassociation inhibitor (GDI) in fat (Rabbit anti-Rho GDI polyclonal antibody, Santa Cruz Biotechnology Inc, Santa Cruz, CA)(24). As described previously (23), signals were detected using a Storm Phosphorimager 860 (Molecular Dynamics; Sunnyvale, CA) and densitometry quantified using ImageQuant Software (Version 5.2; Amersham Biosciences; Sunnyvale, CA). Densitometry results are presented as arbitrary scanning units after correcting for loading; however, results were similar regardless of this correction.
Statistics
All data are reported as mean ± SEM. Study 1: The glucose tolerance test outcomes (glucose response curve and insulin response curve) were analyzed using linear mixed effects models (LMM). Other outcomes were analyzed using one-way analysis of variance, adjusting for baseline measure when available. Study 2: As the study design was a Latin-square design, treatment means were assessed for time trends, treatment by phase interactions, and homogeneity of variance (Levene’s). Data were analyzed for the difference between treatments by paired t-test, since there were no treatment by period interactions. Analyses were performed by SAS 9.1 (Cary, NC).
Results
Study 1
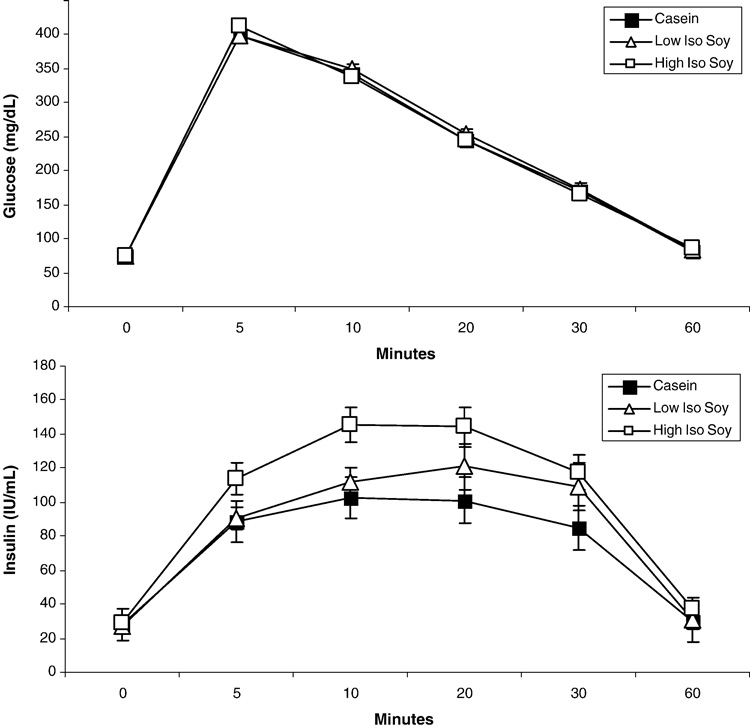
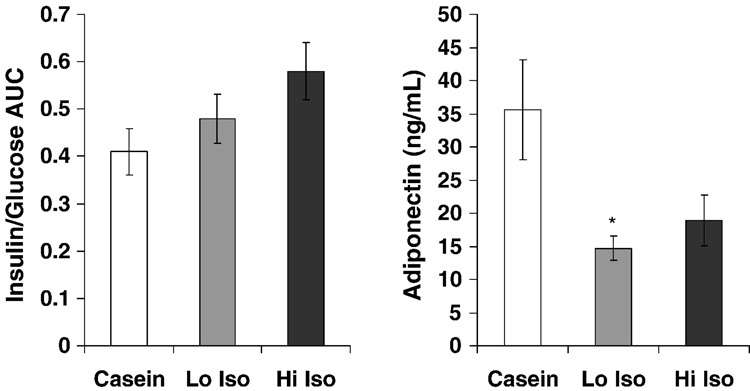
There were no treatment effects on fasting glucose, insulin or overall glycemic control as assessed by fructosamine concentrations (Table 1). As reported previously (19), plasma LDLC was decreased by 21% and 17% and HDLC was increased 36% and 18% in the groups fed low ISO and hi ISO respectively (all p’s < 0.05). Plasma TG was unaffected (19). Figure 1 depicts the glucose and insulin responses to the IVGTT. There was no treatment effect on glucose responses (Fig 1 and Table 1). However, insulin responses were significantly increased (p<0.05) with treatment (Fig 1) with a dose-dependent increase in the maximal insulin response (p=0.01). The ratio of insulin:glucose AUC, an index of insulin resistance, was 17% and 41% greater with increasing dietary isoflavone content (Fig 2) but this did not reach statistical significance (p>0.05). Males fed hi ISO also tended to gain more weight (Fig 2). Consistent with an insulin resistant condition, plasma adiponectin concentrations (Fig 2) were significantly decreased with isoflavone consumption (p=0.02).
Table 1.
Study 1: Measures (Mean ± SEM) for male monkeys consuming protein from either casein-lactalbumin (Casein), soy with low isoflavones (Low Iso Soy), or soy with high isoflavones (High Iso Soy)
| Outcome | Casein N=30 | Low Iso Soy n=30 | High Iso Soy n=31 |
|---|---|---|---|
| Glucose (mg/dL), Fasting | 74.5 ± 1.68 | 73.6 ± 1.94 | 74.2 ± 1.91 |
| Glucose, IVGTT, Max | 398.4 ± 6.59 | 399.5 ± 7.82 | 416.1 ± 6.80 |
| Glucose, IVGTT, Kval | 3.63 ± 0.19 | 3.55 ± 0.21 | 3.87 ± 0.23 |
| Insulin (IU/mL), Fasting | 28.00 ± 3.73 | 27.30 ± 3.55 | 28.92 ± 9.00 |
| Insulin, IVGTT, Max | 115.6 ± 10.36 | 134.7 ± 12.49 | 172.4 ± 14.96* |
| Fructosamine, Baseline | 263.9 ± 6.12 | 272.9 ± 6.28 | 271.2 ± 6.05 |
| Fructosamine, Treatment | 260.1 ± 6.53 | 252.7 ± 4.61 | 253.1 ± 6.59 |
| Body Weight (kg), Baseline | 5.51 ± 0.12 | 5.70 ± 0.13 | 5.68 ± 0.13 |
| Body Weight (kg), Treatment | 5.95 ± 0.14 | 6.05 ± 0.16 | 6.22 ± 0.17 |
| Fat Adiponectin (ASU) | 0.58 ± 0.07 | 0.58 ± 0.08 | 0.58 ± 0.08 |
| Fat PPARγ (ASU) | 10.80 ± 0.52 | 9.70 ± 0.78 | 11.20 ± 1.05 |
p< 0.05 vs. Casein
Figure 1.
Glucose (top) and insulin (bottom) responses to an intravenous glucose tolerance test for monkeys consuming casein, soy protein with low isoflavone dose (Low Iso Soy) and high isoflavone dose (High Iso Soy). There were no changes in glucose response but a dose-dependent increase in insulin responses was found with isoflavone intake (ANOVA, p < 0.05). The treatment differences was due to High Iso Soy compared to Casein (P=0.03) and an intermediate response with Low Iso Soy compared to Casein (p=0.11).
Figure 2.
Changes in insulin resistance (as determined by insulin/glucose areas under the curve (AUC) following a glucose challenge (as depicted in Fig1) and plasma adiponectin concentrations with monkeys consuming casein, soy protein with low isoflavone dose (Low Iso Soy) and high isoflavone dose (High Iso Soy). Adiponectin was significantly less in Low Iso compared to Casein (p = 0.02) with a similar trend for Hi Iso compared to Casein (p=0.08).
Despite the lower plasma adiponectin concentrations with soy isoflavones, there was no difference in the abundance of adiponectin in fat, the primary source of circulating adiponectin. Adiponectin levels are controlled in part by PPARγ, the expression of which was also not affected by soy isoflavones (Table 1).
Study 2
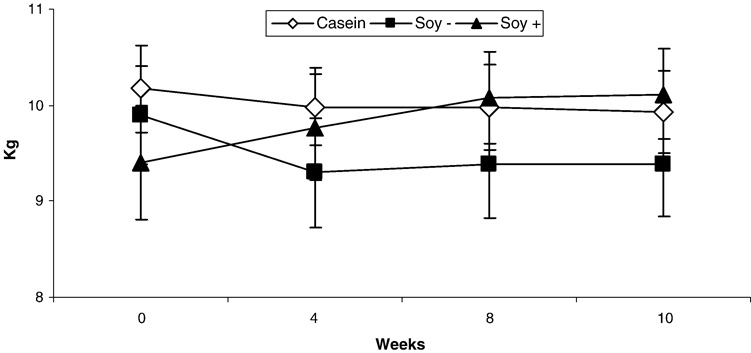
Treatment with SOY− decreased body weight compared to SOY+ (p = 0.02) but not Casein (p = 0.17) (Fig 3) despite no effect on leptin concentrations (Table 2). Compared to Casein, SOY− treatment resulted in lower TC (p = 0.02) and LDL-C (p = 0.02) but had no effect on TG or HDLC. There was no significant effect of SOY+ treatment on TC, LDL-C, HDLC, or TG compared to Casein. There was also no significant effect of SOY− or SOY+ treatment on insulin sensitivity, glucose effectiveness, fasting blood glucose, insulin or C-peptide concentrations compared to Casein (all p’s > 0.05).
Figure 3.
Change in body weight in monkeys with consumption of casein, soy protein alcohol washed to deplete isoflavones (SOY −), and soy protein with its isoflavones intact (SOY+) over a 10 week period using a Latin-square design. Body weights decreased (p<0.05) with consumption of SOY − compared to SOY+.
Table 2.
Study 2: Measures (Mean ± SEM) for male monkeys consuming protein from either casein-lactalbumin (Casein), soy washed to remove isoflavones (Soy −), or soy with high isoflavones (Soy +)
| Outcome | Casein n=8 | Soy − n=8 | Soy + n=8 |
|---|---|---|---|
| Glucose (mg/dL), Fasting | 67.8 ± 4.8 | 81.9 ± 6.0 | 69.9 ± 5.2 |
| Insulin (IU/mL), Fasting | 54.3 ± 8.6 | 53.0 ± 16.5 | 42.5 ± 8.8 |
| C-Peptide (ng/mL) | 9.87 ± 2.7 | 7.31 ± 3.1 | 6.83 ± 1.7 |
| SI (10-4.min-1.mU-1.mL) | 3.10 ± 1.15 | 3.19 ± 0.85 | 2.85 ± 1.11 |
| Glucose Effectiveness (min-1) | 0.037 ± 0.010 | 0.036 ± 0.014 | 0.020 ± 0.006 |
| HDL-C (mg/dL) | 71.8 ± 11.6 | 71.3 ± 12.1 | 71.5 ±16.5 |
| LDL-C (mg/dL) | 106.1 ± 18.5 | 78.9 ±15.4* | 96.3 ±16.1 |
| Adiponectin (ng/mL) | 4.26 ± 1.39 | 5.42 ± 2.08 | 3.96 ± 1.43 |
| Fasting Leptin (ng/mL) | 53.5 ± 11.9 | 40.6 ± 5.2 | 40.3 ± 6.4 |
| Body Weight (kg) | 9.99 ± 0.43 | 9.53 ± 0.55† | 10.22 ± 0.47 |
| Muscle IR Activity (ASU) | 16794 ± 645 | 31387 ± 3350 | 29454 ± 5317 |
| Muscle IR Expression (ASU) | 4726 ± 820 | 2879 ± 268 | 2721 ± 596* |
p< 0.05 vs. Casein
p < 0.05 vs Soy +
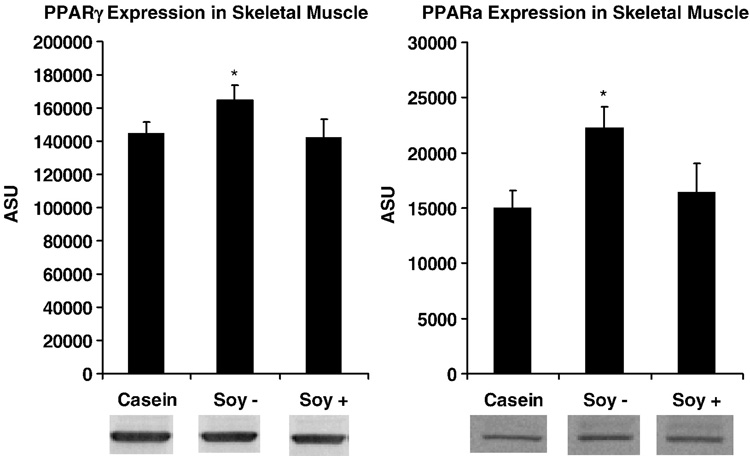
SOY− treatment increased PPARα and PPARγ expression in skeletal muscle compared to Casein (p=0.009, p=0.03), but SOY+ treatment had no effect (p=0.61, p=0.86), suggesting that isoflavones attenuated the effect of soy protein (Fig 4). There were no significant correlations between either PPARα and PPARγ expression and insulin sensitivity, or fasting glucose and insulin concentrations, or body weight.
Figure 4.
PPARγ and PPARα expression in skeletal muscle of monkeys with consumption of casein, soy protein alcohol-washed to deplete isoflavones (SOY−), and soy protein with its isoflavones intact (SOY+) after a 10 week period using a Latin-square design. Expression is significantly greater (p<0.05) for SOY− compared to casein.
SOY+ treatment resulted in less skeletal muscle IR expression compared to Casein treatment (p = 0.01) with a similar tendency for SOY− treatment (p > 0.05). However, there was no difference in basal IR activity (tyrosine phosphorylation) with either of the Soy treatments (Table 2).
Discussion
The major findings from these studies are that in male monkeys, consumption of soy protein with its isoflavones increases insulin secretion following a glucose challenge (Fig 1). Despite the increased insulin secretion there no were no changes in glucose disposal and a dose-dependent increase in the ratio of insulin:glucose AUCs determined from the IVGTTs (Fig 2) indicating an increase in peripheral insulin resistance due to the isoflavones. Further, consistent with an insulin resistance state there is a significant decrease in plasma adiponectin concentrations with soy isoflavones (Fig 2). In contrast, consumption of isoflavone-depleted soy protein resulted in loss of body weight and no effect on plasma adiponectin concentrations. Muscle PPARα and γ expression was also increased in this group compared to either casein or soy protein containing the isoflavones.
The increase in insulin secretion following the glucose challenge was not unexpected. As earlier studies have shown with estradiol (25), studies of genistein and (26, 27) and to a lesser extent daidzein (27) found increased insulin secretion from islet preparations. A more recent study by Liu et al (28) found that genistein increases glucose-stimulated insulin secretion in cell lines and mouse pancreatic islets at micromolar concentrations via a cAMP-dependent protein kinase mechanism. This action could be beneficial and may be the basis for the early report suggesting clinically-relevant decreases in glucosuria in diabetics (12), consistent with a secretagogue-like effect. However, in the current studies, the lack of increased glucose removal in spite of the increased insulin secretion, indicates peripheral insulin resistance.
Potential mechanisms for increased insulin resistance could relate to the fact that genistein is a potent tyrosine kinase inhibitor for both platelet-derived growth factor and epidermal growth factor (18, 29). Our data (Table 2) and others (30) suggest basal IR activity is not affected by soy isoflavones. However, high concentrations of genistein could be inhibitory, while daidzein, which is not a tyrosine kinase inhibitor, likely would not be inhibitory.
Other postulated mechanisms that could increase peripheral insulin resistance include changes in insulin receptor number, affinity, intracellular phosphorylation, and alterations in the glucose transport apparatus (31–36). Insulin receptor number was found to be decreased in rat livers perfused with genistein (31). This result is consistent with the in vivo finding reported here for skeletal muscle (Table 2). Other effects that could be detrimental to insulin action include genistein-induced inhibition of Glut4 translocation in rat adipocytes (32) and effects on glucose oxidation (30). These inhibitory effects on hormone signal transduction could be due to inhibition of other protein kinases, such as those with ATP binding at the catalytic sites (30). In support of this, genistein has been shown to inhibit Akt kinase activity (33) and effects of soy diet on Akt activity has also been shown to result in worsening of heart disease in male mice, but not females (34).
In vitro studies have shown that soy isoflavones increase expression of PPARs (8–10). In murine macrophage-like RAW 264.7 cells expressing a peroxisome-proliferator response element (PPRE)-containing reporter and either PPARα or PPARγ plasmids, unconjugated genistein and daidzein increased both PPARα and γ-directed gene expression (10). Consistent with a PPARγ effect, when obese Zucker rats were fed diets containing soy protein with isoflavones the animals had improved lipid metabolism and glucose tolerance but they gained weight consistent with PPARγ agonist treatment (10).
Genistein (>1 µm) was also shown to act as a ligand for PPARγ in mesenchymal progenitor cells (precursor cells for osteoblasts and adipocytes), resulting in up-regulation of adipogenesis and down-regulation of osteogenesis. Transfection experiments showed that activation of PPARγ by genistein at micromolar concentrations down-regulates its estrogenic transcriptional activity while activation of ERα and ERβ by genistein down-regulates PPARγ transcriptional activity (8). These same investigators reported similar effects with daidzein (9). In addition, there were concentration-dependent biphasic effects of daidzein on osteogenesis and adipogenesis that were not apparent when ERs were blocked. In addition to transactivating PPARγ, daidzein also transactiviated PPARα and δ. These studies suggest cross-talk between ER and PPAR with outcomes dependent on the balance between activated ERs and PPARγ.
PPAR action is modified by cofactors such as PPARγ coactivator-1 (PGC-1). PGC-1 is also estrogen responsive and may mediate some of the ER transcriptional effects (37). As isoflavones also have estrogenic activity, some effect on glycemic control may be mediated through PGC-1 (38). Taken together, these studies suggest an intriguing mechanism pathway whereby soy isoflavones and endogenous hormones may interact to affect PPAR action resulting in different action in males and females.
Since genistein and daidzein have both been shown to bind to and activate PPARγ (8–10), it is likely that changes in insulin sensitivity could then be modified by adiponectin, which is increased in response to PPARγ agonists (5–7). Studies in mice have shown that soy protein isolate containing isoflavones increased both plasma concentrations and adipose tissue mRNA abundance of adiponectin (39,40). This is opposite of our finding in monkeys of lower plasma adiponectin concentrations with no difference in adipose tissue expression (Fig 2, Table 1). The only data we are aware of in humans suggests that soy isoflavones do not affect plasma adiponectin concentrations (41). Interestingly, low adiponectin levels have been shown to be associated with impaired vasodilation in people (6). In this study, soy isoflavones did not improve arterial vasodilation in male monkeys (19) but plasma lipids and atherosclerosis were improved with soy isoflavones in this study (19) and others (11).
There are also sex specific differences in metabolism of soy isoflavones. Stroud et al (42) found that male monkeys had higher plasma genistein, daidzein, and total isoflavones concentrations compared to premenopausal females fed the same soy isoflavone containing diet. It is not known how these varying plasma isoflavone concentrations relate to different tissue levels but it is likely that tissue differences occur.
While soy isoflavones have often been thought to be the active, beneficial ingredient of soy beans, others have proposed components of soy protein to be the health-beneficial component. For example, Moriyama et al. (43), suggest that soy protein, in particular the 7S component, has therapeutic benefits for treatment of obesity and metabolic syndrome. Likewise, Sirtori’s group (44) has shown beneficial effects with the 7S component on lipoprotein metabolism. Thus, it is likely that while soy protein is both heart-healthy and improves a number of aspects of the metabolic syndrome, these effects are attenuated by the soy isoflavones in males, but not necessarily in females. We show here that the isoflavones resulted in a dose-dependent increase in insulin resistance in male monkeys (Fig 1) and lower plasma adiponectin concentrations (Fig 2). However, the smaller study with isoflavone-depleted soy (Study 2) resulted in lower body weight (Fig 3) and greater muscle PPARα and γ expression (Fig 4). Future studies with a comparison of soy protein, in particular the 7S component, and soy protein containing lower isoflavone concentrations would be warranted, especially in males where the plasma isoflavone concentrations are higher than in females. The adverse effects of a high soy isoflavones diet on insulin and glucose metabolism in male monkeys are of potential public health relevance and studies are needed to determine the mechanisms involved and to optimize dietary isoflavone content for maximal health benefits.
Acknowledgements
These studies were supported in part by grants HL45666 and HL79421, from the National Heart, Lung and Blood Institute, and P40RR021380 from the National Center for Research Resources, all from the National Institutes of Health. The authors thank Ms. Mary Jo Busa for editorial assistance.
Footnotes
This work was previously presented in abstract form at the American Diabetes Association, 2004
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163(4):427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Reviews. 1997;5(3):177–269. [Google Scholar]
- 4.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan KCB, Xu A, Chow WS, et al. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab. 2004;84:765–769. doi: 10.1210/jc.2003-031012. [DOI] [PubMed] [Google Scholar]
- 7.Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obesity Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 8.Dang ZC, Audinot V, Papapoulos SE, et al. Peroxisome proliferator-activated receptor gamma (PPARgamma ) as a molecular target for the soy phytoestrogen genistein. J Biol Chem. 2003;278(2):962–967. doi: 10.1074/jbc.M209483200. [DOI] [PubMed] [Google Scholar]
- 9.Dang ZC, Löwik CWGM. The balance between concurrent activation of ERs and PPARs determine daidzein-induced osteogenesis and adipogenesis. J Bone &Min Res. 2004;19:853–861. doi: 10.1359/JBMR.040120. [DOI] [PubMed] [Google Scholar]
- 10.Mezei O, Banz WJ, Steger RW, et al. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr. 2003;133(5):1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- 11.Wagner JD, Anthony MS, Cline JM. Soy phytoestrogens: research on benefits and risks. Clin Obstet Gynecol. 2001;44(4):843–852. doi: 10.1097/00003081-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Friedenwald J, Ruhrah J. The use of the soy bean as a food in diabetes. American Journal of Medical Science. 1910;140:793–803. [Google Scholar]
- 13.Jayagopal V, Albertazzi P, Kilpatrick ES, et al. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25(10):1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- 14.Goodman-Gruen D, Kritz-Silverstein D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J Nutr. 2001;131(4):1202–1206. doi: 10.1093/jn/131.4.1202. [DOI] [PubMed] [Google Scholar]
- 15.Wagner JD, Cefalu WT, Anthony MS, et al. Dietary soy protein and estrogen replacement therapy improve cardiovascular risk factors and decrease aortic cholesteryl ester content in ovariectomized cynomolgus monkeys. Metabolism. 1997;46(6):698–705. doi: 10.1016/s0026-0495(97)90016-0. [DOI] [PubMed] [Google Scholar]
- 16.Hermansen K, Sondergaard M, Hoie L, et al. Beneficial effects of a soy-based dietary supplement on lipid levels and cardiovascular risk markers in type 2 diabetic subjects. Diabetes Care. 2001;24(2):228–233. doi: 10.2337/diacare.24.2.228. [DOI] [PubMed] [Google Scholar]
- 17.Wagner JD, Zhang L, Greaves KA, et al. Soy protein reduces the arterial low-density lipoprotein (LDL) concentration and delivery of LDL cholesterol to the arteries of diabetic and nondiabetic male cynomolgus monkeys. Metabolism-Clinical and Experimental. 2000;49(9):1188–1196. doi: 10.1053/meta.2000.8620. [DOI] [PubMed] [Google Scholar]
- 18.Akiyama T, Ishida J, Nakagawa S, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262(12):5592–5595. [PubMed] [Google Scholar]
- 19.Adams MR, Golden DL, Williams JK, et al. Soy protein containing isoflavones reduces the size of atherosclerotic plaques without affecting coronary artery reactivity in adult male monkeys. J Nutr. 2005;135:2852–2856. doi: 10.1093/jn/135.12.2852. [DOI] [PubMed] [Google Scholar]
- 20.Shadoan MK, Zhang L, Kavanagh K, et al. Addition of Medroxyprogesterone Acetate to Conjugated Equine Estrogens Results in Insulin Resistance in Adipose Tissue. Metabolism. 2007;56:830–837. doi: 10.1016/j.metabol.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Wagner JD, Thomas MJ, Williams JK, et al. Insulin sensitivity and cardiovascular risk factors in ovariectomized monkeys with estradiol alone or combined with nomegestrol acetate. J Clin Endocrinol Metab. 1998;83:896–901. doi: 10.1210/jcem.83.3.4628. [DOI] [PubMed] [Google Scholar]
- 22.Wagner JD, Kavanagh K, Ward G, Kaplan J. Old world primate models of type 2 diabetes mellitus. ILAR Journal. 2006;47:259–271. doi: 10.1093/ilar.47.3.259. [DOI] [PubMed] [Google Scholar]
- 23.Berg AH, Lin Y, Lisanti MP, et al. Adipocyte differentiation induces dynamic changes in NF-kB expression and activity. Am J Physiol Endcrinol Metab. 2004;287:E1178–E1188. doi: 10.1152/ajpendo.00002.2004. [DOI] [PubMed] [Google Scholar]
- 24.Shadoan MK, Zhang L, Wagner JD. Effects of hormone replacement therapy on insulin signaling proteins in skeletal muscle of cynomolgus monkeys. Steroids. 2004;69:313–318. doi: 10.1016/j.steroids.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Costrini NV, Kalkhoff RK. Relative effects of pregnancy, estradiol, and progesterone on plasma insulin and pancreatic islet insulin secretion. J Clin Invest. 1971;50:992–999. doi: 10.1172/JCI106593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorenson RL, Brelje TC, Roth C. Effect of tyrosine kinase inhibitors on islets of Langerhans: evidence for tyrosine kinases in the regulation of insulin secretion. Endocrinology. 1994;134(4):1975–1978. doi: 10.1210/endo.134.4.8137766. [DOI] [PubMed] [Google Scholar]
- 27.Jonas JC, Plant TD, Gilon P, et al. Multiple effects and stimulation of insulin secretion by the tyrosine kinase inhibitor genistein in normal mouse islets. Br J Pharmacol. 1995;114:872–880. doi: 10.1111/j.1476-5381.1995.tb13285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D, Zhen W, Yang Z, et al. Genistein acutely stimulates insulin secretion in pancreatic β-cells through cAMP-dependent protein kinase pathway. Daibetes. 2006;55:1043–1050. doi: 10.2337/diabetes.55.04.06.db05-1089. [DOI] [PubMed] [Google Scholar]
- 29.Hill TD, Dean NM, Mordan LJ, et al. PDGF-induced activation of phospholipase C is not required for induction of DNA synthesis. Science. 1990;248(4963):1660–1663. doi: 10.1126/science.2163545. [DOI] [PubMed] [Google Scholar]
- 30.Abler A, Smith JA, Randazzo PA, et al. Genistein differentially inhibits postreceptor effects of insulin in rat adipocytes without inhibiting the insulin receptor kinase. J Biol Chem. 1992;267(6):3946–3951. [PubMed] [Google Scholar]
- 31.Mackowiak P, Nogowski L, Nowak KW. Effect of isoflavone genistein on insulin receptors in perfused liver of ovariectomized rats. J Recept Signal Transduct Res. 1999;19(1–4):283–292. doi: 10.3109/10799899909036651. [DOI] [PubMed] [Google Scholar]
- 32.Smith RM, Tiesinga JJ, Shah N, et al. Genistein inhibits insulin-stimulated glucose transport and decreases immunocytochemical labeling of GLUT4 carboxyl-terminus without affecting translocation of GLUT4 in isolated rat adipocytes: additional evidence of GLUT4 activation by insulin. Arch Biochem Biophys. 1993;300(1):238–246. doi: 10.1006/abbi.1993.1033. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Sarkar FH. Inhibition of nuclear factor κB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002;8:2369–2377. [PubMed] [Google Scholar]
- 34.Stauffer BL, Konhilas JP, Luczak ED, et al. Soy diet worsens heart disease in mice. J Clin Invest. 2006;116:209–216. doi: 10.1172/JCI24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harmon AW, Harp JB. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am J Physiol Cell Physiol. 2001;280(4):C807–C813. doi: 10.1152/ajpcell.2001.280.4.C807. [DOI] [PubMed] [Google Scholar]
- 36.Harmon AW, Patel YM, Harp JB. Genistein inhibits CCAAT/enhancer-binding protein beta (C/EBPbeta) activity and 3T3-L1 adipogenesis by increasing C/EBP homologous protein expression. Biochem J. 2002;367(Pt 1):203–208. doi: 10.1042/BJ20020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tcherepanova I, Puigserver P, Norris JD, et al. Modulation of estrogen receptor-alpha transcriptional activity by the coactivator PGC-1. J Biol Chem. 2000;275(21):16302–16308. doi: 10.1074/jbc.M001364200. [DOI] [PubMed] [Google Scholar]
- 38.Morifuji M, Sanbongi C, Sugiura K. Dietary soya protein intake and exercise training have an additive effect on skeletal muscle fatty acid oxidation enzyme activities and mRNA levels in rats. Br J Nutrition. 2006;96:469–475. [PubMed] [Google Scholar]
- 39.Nagasawa A, Fukui K, Funahashi T, et al. Effects of soy protein diet on the expression of adipose genes and plasma adiponectin. Horm Metab Res. 2002;34:635–639. doi: 10.1055/s-2002-38254. [DOI] [PubMed] [Google Scholar]
- 40.Nagasawa A, Fukui K, Kojima M, et al. Divergent effects of soy protein diet on the expression of adipocytokines. Biochem Biophys Res Commun. 2003;311:909–914. doi: 10.1016/j.bbrc.2003.10.087. [DOI] [PubMed] [Google Scholar]
- 41.Christie DR, Cooper BC, Lyon D, et al. Effect of soy phytoestrogens on systemic markers of inflammation and adiposity in postmenopausal women. Fertil Steril. 2006;86 Suppl 2:S90. [Google Scholar]
- 42.Stroud FC, Appt SE, Wilson ME, et al. Concentrations of isoflavones in macaques consuming standard laboratory monkey diet. J Am Assoc Lab Anim Sci. 2006;45:20–23. [PubMed] [Google Scholar]
- 43.Moriyama T, Kishimoto K, Nagai K, et al. Soybean beta-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of beta-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci Biotech Biochem. 2004;68:352–359. doi: 10.1271/bbb.68.352. [DOI] [PubMed] [Google Scholar]
- 44.Lovati M, Manzoni C, Gianazza E, et al. Soy protein peptides regulate cholesterol homeostasis in Hep G2 cells. J Nutr. 2000;130:2543–2549. doi: 10.1093/jn/130.10.2543. [DOI] [PubMed] [Google Scholar]