Abstract
Many models have been proposed over the years to explain how motivated feeding behavior is controlled. One of the most compelling is based on the original concepts of Eliot Stellar whereby sets of interosensory and exterosensory inputs converge on a hypothalamic control network that can either stimulate or inhibit feeding. These inputs arise from information originating in the blood, the viscera, and the telencephalon. In this manner the relative strengths of the hypothalamic stimulatory and inhibitory networks at a particular time dictates how an animal feeds. Anorexia occurs when the balance within the networks consistently favors the restraint of feeding. This article discusses experimental evidence supporting a model whereby the increases in plasma osmolality that result from drinking hypertonic saline activate pathways projecting to neurons in the paraventricular nucleus of the hypothalamus (PVH) and lateral hypothalamic area (LHA). These neurons constitute the hypothalamic controller for ingestive behavior, and receive a set of afferent inputs from regions of the brain that process sensory information that is critical for different aspects of feeding. Important sets of inputs arise in the arcuate nucleus, the hindbrain, and in the telencephalon. Anorexia is generated in dehydrated animals by way of osmosensitive projections to the behavior control neurons in the PVH and LHA, rather than by actions on their afferent inputs.
Introduction
A tremendous amount of work currently focuses on clarifying the neural mechanisms of feeding. Much of this effort is directed at the cellular and molecular level, and has generated a wealth of data about the extracellular and intracellular signaling molecules that can affect feeding [1,2]. Moving out from these essentially reductionistic perspectives, a more structuralistic approach attempts to elaborate the functional systems within the brain and beyond that regulate feeding. The rationale behind this systems-level analysis is that identifying the important neurons and the connections they form will allow us to construct models of neural network organization. This will help us to understand the constituents of these networks, how they are organized, how they interact, and how they function to control feeding in normal and anorexic states. Indeed many circuit diagrams for feeding control systems have been developed using sophisticated neuroanatomical tracing techniques and the chemical phenotyping of neurons [3–6].
The emergent models that account for overall function are quite rich in delineating the sensory and motor ends of the control networks, but to elaborate a more complete explanation of different feeding behaviors we still need to identify the critical cell types located deeper within these networks. Thus, an important focus for increasing the scope of our control network is how the telencephalon fits into the overall scheme. The importance of telencephalic regions derives from the complexity and adaptability they can add to behavioral expression; for example, the ability to invoke the types of learned strategies underlying foraging behavior resides in the telencephalon. Furthermore, the more complex features of computing the incentive value and relative rewarding aspects of food very likely requires telencephalic contributions.
In terms of function, a popular and persuasive model is based on the seminal concepts of Stellar [7]. It posits at the absolute simplest level that feeding behavior results from an ongoing interaction between mechanisms engaged by various stimuli to drive feeding on the one hand, and those mechanisms that restrain feeding on the other. Again, at the absolute simplest level, anorexia occurs when a stimulus or disturbance tips the balance in favor of restraint [6,8].
This article will describe experiments directed towards understanding how feeding control networks in the brain are impacted by a simple physiological stimulus—cellular dehydration—that leads to anorexia. We will begin by reviewing current neural models of motivated behavioral control. We will then provide examples that support a model whereby dehydration-anorexia is generated largely by modulating the way behavioral controllers in the paraventricular nucleus of the hypothalamus (PVH) and parts of the lateral hypothalamic area (LHA) respond to important sets of afferent inputs, rather than by affecting the efficacy of the afferents themselves.
Basic Circuits and Their Organization
Investigations about how feeding is centrally regulated have focused for almost 70 years on two parts of the brain: the hypothalamus and hindbrain. The primacy of one region versus the other for controlling feeding has each had its strong advocates, and over the years debate has ebbed and flowed about their relative importance of each. The current consensus view is that the hypothalamus and hindbrain each contribute different aspects to feeding regulation, with the hypothalamus adding ‘motivated’ components to the networks in the hindbrain that control the more reflex aspects of feeding. However, it is now quite clear that focusing only on the hindbrain and hypothalamus cannot explain all aspects of feeding; regions in the telencephalon must also be considered if we are to understand how animals express the full repertoire of behaviors needed to survive.
The contributions from each of the three brain parts to ingestive behavior control can be very broadly summarized as follows [9–12]:
The Hindbrain contains motor pattern generators and some of the motor neurons responsible for the rhythmic movements contributing to chewing and swallowing. These elements comprise the circuitry that allows animals to feed and maintain body weight only if food and water are provided directly to the animal’s mouth. Animals with complete isolation of the caudal hindbrain are unable to initiate spontaneous feeding and die unless they are provided with food directly into the mouth or stomach [9]. All viscerosensory information that enters the brain by way of the vagus nerve or spinal cord is first processed in the hindbrain. This information includes gastrointestinal state along with information from the liver and hepatic portal vein about energy balance and metabolite status. Core networks for accepting or rejecting food items placed in the mouth are located in the hindbrain.
The Hypothalamus contains the neural components that allow animals to feed spontaneously (ie. in a ‘motivated’ manner). Swanson [10] has proposed that these components are part of a behavioral control column in the medial hypothalamus for all motivated behaviors. The PVH contains crucial parts of the behavioral controller for ingestive behaviors. The mechanisms for coordinating ingestive behaviors with other motivated behaviors such as reproduction, as well as with autonomic and neuroendocrine output are also located in the hypothalamus [see 13,14].
The Telencephalon is made up of a layered cortical plate (cerebral cortex), and underlying cerebral nuclei (striatum and pallidum). These regions add what can be called cognitive components to feeding; for example, the egocentric and allocentric cognitive maps that are required for constructing complex foraging behaviors. The telencephalon is also required for the feeding associated with learned cues [eg. 15,16]. In decorticate rats (ie. where only the cerebral cortex is removed leaving the cerebral nuclei intact) feeding continues virtually unimpeded if food is present in the immediate environment, and if it does not require sophisticated oral or dexterous manipulation [17].
The end point of all feeding episodes is generally the same: the placement of food into the mouth followed by oral manipulation and then swallowing. The efficient execution of these actions requires very tight coordination between sets of neck, head, facial, and oropharyngeal muscles. In some mammals the initial part of the consummatory phase may also involve the forelimb musculature for precise manipulation of the food object.
An important point to remember is that even with this convergent set of actions, feeding cannot be regarded as a unitary episode controlled by a single set of neurons. It is a complex set of events that can be activated or inhibited in many circumstances by a highly diverse set of stimuli. In turn, these stimuli engage different sets of neurons. For example, neurons in the arcuate nucleus of the hypothalamus (ARH) are required for feeding in certain circumstances, but are completely dispensable in others. Instead, groups of neurons are organized into networks that span all three brain parts. There must be a degree of parallel processing across these networks because it is possible to compromise one network so that the animal loses the ability to feed in one way, but other types of feeding are unaffected. Compromise another network and a different facet of feeding behavior is lost. Only if the networks directly responsible for motor control are damaged is the ability to perform any form ingestive behaviors totally lost. For example, damage to the motor neurons controlling the muscles of the tongue or jaws, or bilateral electrolytic lesions of the LHA that interrupt dopaminergic pathways essential for motor control (the ‘lateral hypothalamic syndrome’) are two conditions where complete aphagia occurs.
Network Convergence
Given the broad range of circumstances in which feeding is expressed, a fundamental organizing principle of behavioral control is that neural information derived from different feeding stimuli must converge onto a core network that ultimately controls eating—the presumptive final common feeding pathway. Again, this convergence was a feature of Stellar’s original model [7]. Although the notion of a simple final common pathway for a behavior as complex as feeding probably has limited value when attempting a detailed systems analysis, the commonality of the motor actions expressed during the consummatory phase of behavior clearly supports the existence of a core network of some type. The major question then becomes how is this core network structured so that it becomes engaged by the many stimuli that can control feeding?
As a starting point, Swanson has proposed a model whereby all motivated behaviors are controlled by a hierarchically-ordered network of motor neurons, pattern generators, initiators and controllers [10]. Behavioral specificity derives from discrete controllers located in the medial zone of the hypothalamus, each of which is responsible for a particular motivated behavior. As yet unidentified elements in the PVH appear to contribute to the behavioral controller for feeding. A great deal of structural and experimental evidence also strongly implicates parts of the LHA as being important contributors to the control network [see 18 for review]. The overall control functions of the PVH and parts of the LHA on ingestive behaviors are then mediated by their connections to the hindbrain and telencephalon (Fig. 1).
Figure 1.
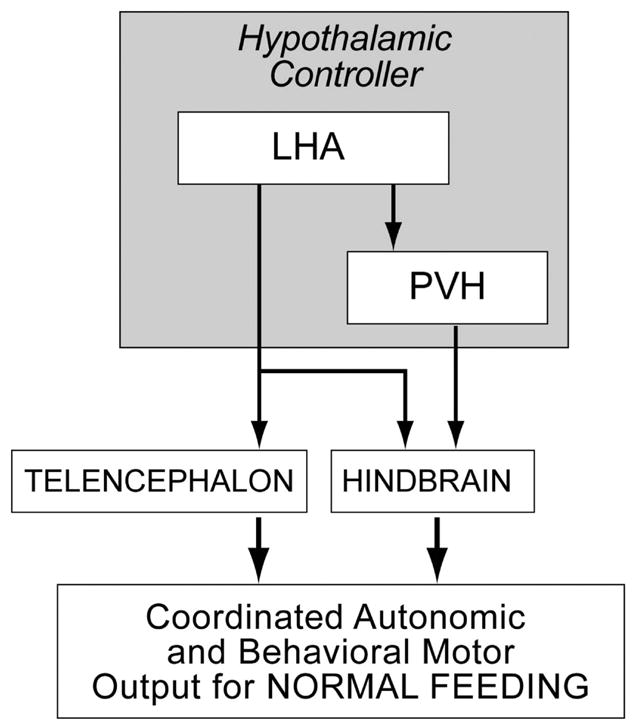
Parts of the paraventricular nucleus of the hypothalamus (PVH) and lateral hypothalamic area (LHA) are critical nodes in the a hypothalamic controller for ingestive behavior [Swanson, 2000]. In this model, feeding, together with its coordinated autonomic motor events, is controlled by their extensive projections to the telencephalon and hindbrain.
If the PVH and parts of the LHA act as nodes in a hierarchical ingestive behavioral control network, then they must receive convergent inputs from other regions known to regulate feeding. When we examine other brain regions that are important for different types of feeding behavior, we see that many of their efferent connections target the PVH and LHA, suggesting that these two regions are indeed key points of convergence. This type of convergence is illustrated by three examples (Fig. 2).
Figure 2.
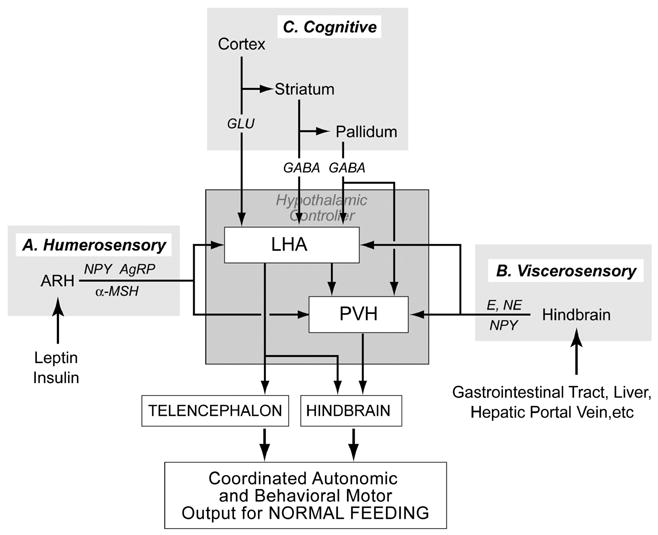
The hypothalamic ingestive behavior controllers in the paraventricular nucleus of the hypothalamus (PVH) and lateral hypothalamic area (LHA) illustrated in Figure 1 receive a variety of inputs from regions that can influence feeding behavior. Three examples are illustrated here. Group A inputs from the arcuate nucleus (ARH) contain neuropeptide Y (NPY), agouti-related peptide (AgRP) and _-MSH. In turn, ARH neurons are regulated by humerosensory information in the form of leptin and insulin. Group B inputs originate in the hindbrain and convey a range of viscerosensory information from the gastrointestinal tract, liver, hepatic portal vein, etc. Nor-epinephrine (NE), epinephrine (E), and NPY are important constituents of these projections. Group C inputs constitute a complex set of projections from the telencephalon that convey cognitive influences on feeding behavior. Swanson [10] has proposed that these projections take the form of a triple descending pathway from the cortex (glutamate), striatum (GABA), and pallidum (GABA).
First, the full effects of leptin and ghrelin on feeding require neurons in the ARH that project to the PVH and LHA [19,20]. In this manner, these projections can be thought of as conveying essential blood-borne (humerosensory) information to the behavioral controllers (Fig. 2 Group A).
Second, the feeding that follows 2-deoxy-D-glucose (2DG) administration requires mechanisms in the hindbrain that engage catecholaminergic projections to the PVH and LHA [21,22]. These ascending projections seem to be an important component of a neural system that conveys interosensory information from the viscera to the hypothalamic ingestive behavioral controllers after it has been processed by integrative networks in the hindbrain (Fig. 2 Group B). In terms of separation of function, it is interesting to note that ARH neurons are not required for 2DG-induced feeding [19].
Third, inputs from the cortex, striatum, and pallidum provide cognitive influences on the feeding behavior controllers in the hypothalamus by way of a triple descending projection network [10,11] (Fig. 2 Group C). An interesting example of telencephalic influences on feeding was described about 10 years ago by Kelley and colleagues who showed that inhibiting the rostral shell of the nucleus accumbens (ACBsh) with muscimol (a GABA-A receptor agonist) rapidly, robustly, and specifically stimulated feeding [23]. Feeding occurs within 2–3 minutes, and with larger doses, the amount eaten is quite substantial, suggesting that efferent projections of the ACBsh closely interact with the primary network that controls feeding. GABAergic projections from the ACBsh are restricted to the substantia innominata (SI) and parts of the LHA [24], but the identity of the network that is directly disinhibited to stimulate feeding following ACBsh mucimol injections remains unknown. However, these injections strongly activate Fos in the LHA and PVH [25–27], suggesting that these two regions are somehow engaged by the ACBsh to initiate feeding.
Experimental Approaches to Anorexia
Although much of the popular press uses the term anorexia synonymously with anorexia nervosa, ‘anorexia’ simply describes any loss of appetite and concomitant reduction in food intake that occurs in the presence of readily accessible food sources. We have previously discussed that anorexia is seen in two broad sets of conditions [18]:
- As a symptom that accompanies two groups of pathologies:
- anorexia that originates psychologically, of which anorexia nervosa is clinically the most important;
- anorexia associated with disease states, of which the disease-associated wasting or cachexia that accompanies AIDS, cancer and other conditions is clinically the most important and widely known.
As an adaptive behavioral response to some homeostatic challenges, which may originate externally, as with the anorexia that accompanies stress; or it may be an adjunct to a physiological challenge such as the cellular dehydration that occurs following the ingestion of hypertonic saline [28].
As described earlier, a simple explanation for anorexia is that over time the balance within the control network is tipped in favor of restraining food intake. The way that the network is impacted to generate anorexia will be very different depending on the stimulus. For example, with clinically-relevant anorexias, and particularly with anorexia nervosa, it will be critical to understand how inputs from the cortex and other parts of the telencephalon interact with feeding networks in the diencephalon and hindbrain. For other types of anorexia, the interactive mechanisms are likely to be very different. But the overall principle is that we need to understand the underlying structure of the control network and how it functions to be able to fully understand how anorexias develop.
Network Function in Dehydration-Anorexia
Having established a working model for the basic control network of ingestive behaviors (Fig. 2), we will now describe how the function of this network is altered during the development of a physiologically-adaptive anorexia: dehydration-anorexia. We will see that the impact of each of the three sets of inputs on feeding are specifically altered as dehydration progresses. The evidence from these experiments supports a model whereby the PVH and parts of the LHA (ie. the hypothetical hypothalamic behavior controllers for feeding) are impacted in a way that favors anorexia rather than continued feeding.
The dehydrated-anorexic rat has some very simple properties that effectively constrain potential effector mechanisms to make it a good model for investigating neural mechanisms of anorexia. Anorexia develops within 2 days of drinking 2.5% saline, and nocturnal food intake continues to fall, until by the fifth night, it may be reduced by as much as 75% [28]. Compensatory feeding develops within minutes when rats are again provided with water, which is a readily identifiable and controllable stimulus [28]. The sensory signals and transduction systems responsible for dehydration-anorexia are restricted to those derived from cellular dehydration and perhaps the taste of salt in the water. Together these factors permit us to investigate with relative ease and precision the interactions at the network level that turn ingestive behaviors on and off. It is important to note that investigating neural mechanisms in dehydrated-anorexic rats is not going to reveal how specific signals initiate particular types of pathological anorexias; clearly these are going to be different in each case—e.g. cytokines for some types of anorexia, complex psychiatric processes for anorexia nervosa.
How Inputs from the Hindbrain Function During Dehydration-Anorexia
Viscerosensory information such as blood glucose, gastrointestinal distension, hepatic function etc. forms an important set of control signals for ingestive behavior. This information is relayed to the hindbrain by vagal and spinal pathways where it is processed to drive reflex loops for behavior and visceromotor functions, as well as being relayed to the forebrain to affect an array of functions including ingestive behavior [29]. Ascending catecholaminergic neurons form a critical part of this system (Fig. 2 group B).
To investigate how these viscerosensory relay systems are impacted during dehydration-anorexia, we determined how animals responded to intravenous injections of 2DG [30]. Ordinarily 2DG will stimulate feeding, ACTH release, and hyperglycemia. We found that these three responses were differentially affected by DE. Food intake was significantly decreased in dehydrated animals following 2DG injections, whereas corticosterone secretion and blood glucose both significantly increased following 2DG regardless of whether animals were anorexic or not (Fig. 3). These results strongly suggest that the overall function of the ascending hindbrain projections that convey information about metabolic state is unaffected by dehydration. Instead, it seems that dehydration specifically targets the neurons that comprise the ingestive behavior controllers in the PVH and parts of the LHA to inhibit feeding in response to glucoprivic challenge.
Figure 3.
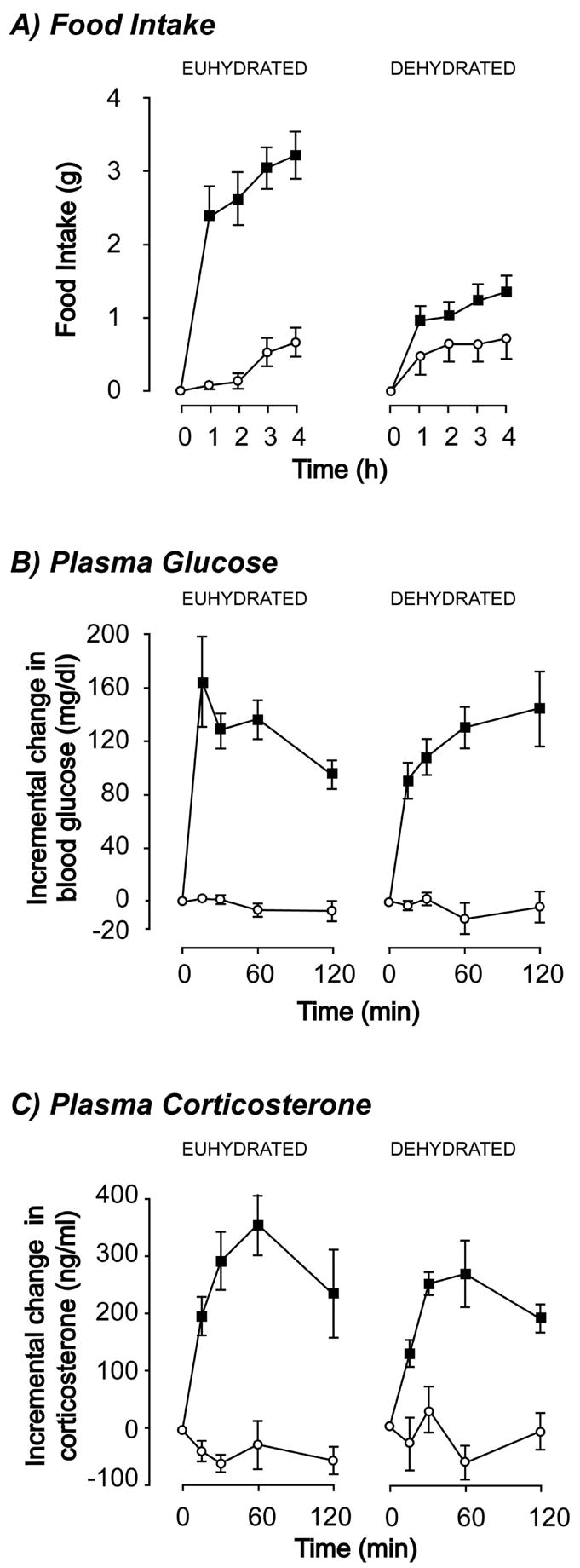
Intravenous injections of 2-deoxy-D-glucose (2DG) in euhydrated control rats leads to increased feeding (A), hyperglycemia (B), and elevated plasma corticosterone concentrations (C). In animals dehydrated by drinking 2.5% hypertonic saline for 5 days only the feeding response to 2DG is suppressed. In these same animals, the hyperglycemic and plasma corticosterone responses remain intact (Data adapted from [30])
How NPY-Containing Inputs Function During Dehydration-Anorexia
Neuropeptide-Y (NPY) was one of the first peptides to be characterized as orexigenic. Although it is very widely expressed in the brain, numerous studies have shown that the critical NPY-containing pathways for feeding are those originating in the ARH and hindbrain, and terminate in the PVH and LHA (Fig. 2 Groups A & B). We have previously shown that dehydrated animals have increased NPY mRNA levels in the ARH, suggesting that arcuate NPY/AgRP neurons synthesize more NPY in the anorexic state [31]. One possible explanation of these data is that the sensitivity of downstream elements to NPY neurons is reduced during dehydration-anorexia.
To test this hypothesis we measured the dose-dependent effects of NPY injections into the PVH or LHA of 3 or 5 day dehydration-animals [32]. We showed that higher doses (1μg) of NPY could reverse anorexia when injected into the LHA, but not the PVH of dehydrated animals, whereas lower doses (0.5μg) were less effective in both regions. Furthermore, there was a very significant negative correlation between the intensity of anorexia and the ability of 1μg NPY in the LHA to reverse anorexia (Fig. 4). However, the latency to eat was not different between the normal euhydrated (EU) and dehydration states (7–8mins). These results show that even with increased NPY synthesis in ARH neurons, dehydration targets downstream elements in a way that renders them less sensitive to NPY. Within this model, our data show that dehydration targets PVH neurons more effectively than LHA neurons to suppress feeding. Combined with the results from our 2DG experiments [30], our NPY data suggest that one reason that 2DG feeding is suppressed is because the sensitivity to the NPY colocalized within adrenergic inputs to the PVH is diminished by DE. Furthermore, a desensitization of NPY mechanisms in the PVH and LHA may explain why dehydrated animals have a reduced feeding response to overnight starvation [30], which is an NPY-dependent mechanism [33,34].
Figure 4.
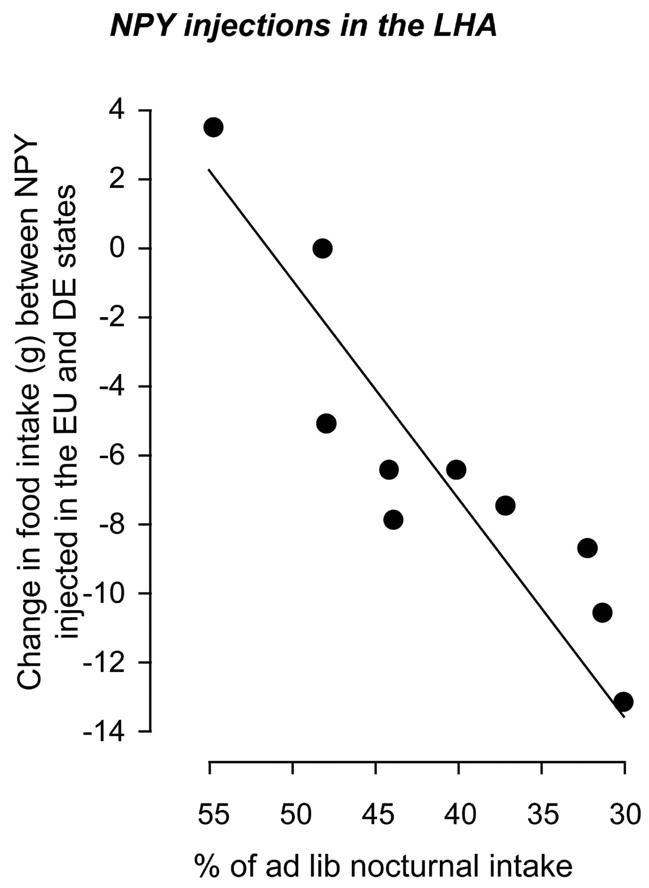
The difference between the 4 hour feeding response of individual animals injected with 1μg of neuropeptide Y in the lateral hypothalamic area in the euhydrated state and then injected again later in the dehydrated state (after drinking 2.5% hypertonic saline). The data show that the suppression of NPY-induced feeding is inversely correlated to the intensity of the anorexia that develops as a consequence of dehydration (Data adapted from [32]).
How Inputs from the Telencephalon function during Dehydration-Anorexia
Muscimol injected into the ACBsh is one of the most powerful ways to stimulate feeding [23,35]. This manipulation is particularly useful from a systems perspective because—in contrast to virtually all other manipulations—feeding is initiated in the telencephalon, not in the periphery, hypothalamus or hindbrain. This makes manipulation of the ACBsh a useful way to investigate how cognitive systems might engage feeding networks [4,10]. The rapidity of feeding onset (less than 2mins, compared to 7–8mins for NPY feeding) suggests that the neural systems engaged by the ACBsh must closely interact with the core network controlling feeding (Fig. 2 Group C).
To test whether dehydration-anorexia is attenuated by manipulating the ACBsh, we used the experimental model of Stratford and colleagues [23,35], and compared the effects of muscimol injections in the ACBsh on feeding in control euhydrated or dehydrated animals [36]. The idea was to determine whether outputs from the telencephalon were sufficient to overcome the anorexia resulting from DE. If they were, this would be consistent with the hypothesis that dehydration only targets a subset of the overall control network for feeding to initiate anorexia, while leaving other parts of the network with the ability to function normally. If ACBsh-muscimol feeding was reduced in DE, it would suggest that dehydration in fact targets the same part of the core network that is engaged when the ACBsh is inhibited, and would support the idea of a central ingestive behavioral controller that processes convergent inputs from a variety of sources to control feeding [10,11].
What we found was that both the amount of food eaten and the cumulative time spent eating were significantly reduced after ACBsh muscimol injections in dehydrated animals compared to EU animals. The amount of food eaten was also negatively correlated to the depth of anorexia (Fig. 5); a finding again similar to dehydrated animals tested with NPY injections in the LHA (Fig. 4). However, as with hypothalamic NPY injections in dehydrated animals, the latency to eat was unchanged from the response in the EU state. These findings strongly support the idea that dehydration targets the downstream elements of the ACBsh in a way that ultimately suppresses feeding (Fig. 2 Group C).
Figure 5.
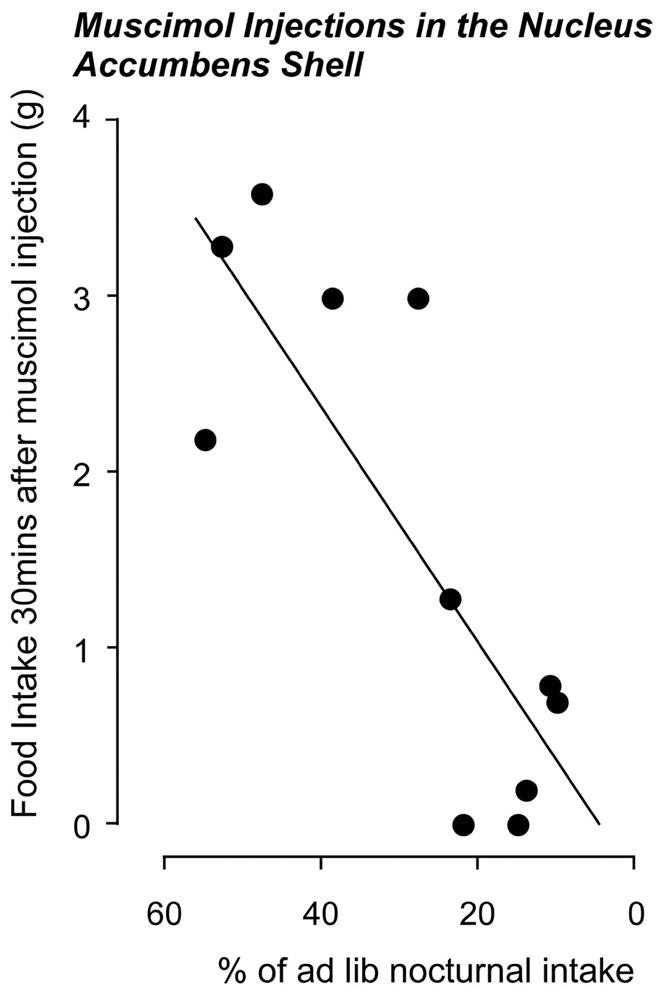
The 30 minute feeding response to 50ng of muscimol injected bilaterally into the shell of the nucleus accumbens of dehydrated by drinking 2.5% hypertonic saline is inversely correlated to the intensity of the resulting anorexia (Data adapted from [36]).
Summary and Conclusion
Our studies collectively support a model whereby dehydration generates anorexia by targeting the hypothalamic behavior control elements located in the PVH and parts of the LHA rather than the input pathways they receive (Fig. 6). In this way, NPY-sensitive targets in the LHA and particularly the PVH, are actively suppressed during dehydration-anorexia. This suppression may also be sufficient to account for the reduced feeding we have previously reported following overnight starvation or 2DG injections [30]. Furthermore, the feeding that follows inhibition of the ACBsh is suppressed by DE, showing that the downstream targets of the ACBsh are also affected. Although the exact nature of these targets is largely unknown, the fact that ACBsh inhibition increases Fos accumulation in the PVH and LHA [26,27], and also requires an NPY component for feeding [37] suggests that ACBsh inhibition also activates the same hypothalamic behavior controller as other feeding stimuli. It must also be noted that the PVH and LHA are not the only regions that show Fos accumulation under these circumstances; the ARH as well as the septohypothalamic, parataenial, lateral habenular nuclei, and medial substantia nigra also express Fos under following this manipulation [26,27]. However, the most parsimonious explanation for our muscimol data is that dehydration interferes with the feeding network engaged by the ACBsh at the level of the PVH and parts of the LHA (Fig. 2 Group C).
Figure 6.
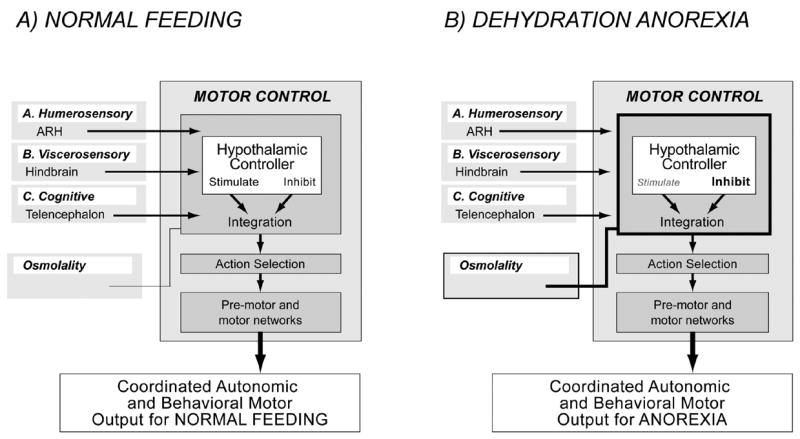
A) Ingestive behavior control is thought to be controlled by a brain-wide network that converges onto a motor control network, which is organized at three levels [adapted from 10,11,12,18]: A hypothalamic behavior controller that contains drive circuits that can either stimulate, or inhibit behaviors; action selection networks that integrate the outputs of the hypothalamic controller with those of other systems; and executive pre-motor and motor neuron networks. The generation of motivated behavioral actions by motor control networks can be initiated by a variety of inputs. The three examples are shown in Figure 2 are reproduced here. They are: A, inputs from the arcuate nucleus (ARH) that are regulated by humerosensory information;. B, inputs originating in the hindbrain that convey a range of viscerosensory information; and C, inputs from the telencephalon that convey cognitive influences on feeding behavior.
B) Evidence discussed in this article suggests that the increased osmolality that results from dehydration acts at the level of the hypothalamic behavior controller to suppress the actions of the inputs A) – C), which in turn generates anorexia.
Dehydration does not appear to generate anorexia by suppressing the functions of more distal humerosensory elements in the ARH or the interoceptive integrators in the hindbrain that provide important information to the PVH and LHA (Fig. 2 Groups 1 & 2). We find no evidence to show that dehydration suppresses the normal output of either the ARH or the catecholaminergic neurons in the hindbrain required for 2DG feeding. Indeed, since NPY and POMC gene expression are both altered during dehydration-anorexia in a manner consistent with the increased hunger rather than a pattern that would be expected to reduce feeding [31], the output of the ARH may actually be tipped in favor of stimulating feeding. Furthermore, the fact that dehydration suppresses feeding but not the glucocorticoid response to 2DG—both of which require ascending catecholaminergic projections to the PVH [22]—clearly demonstrates that ascending catecholaminergic afferents remain functionally intact during dehydration-anorexia. The fact the dehydration differentially targets control elements in the PVH for feeding and neuroendocrine CRH release—suppressing one but not the other [30]—shows that the effects of dehydration within the PVH are exquisitely site specific.
The major unanswered questions for this model are what and where exactly are the control elements within the PVH and parts of the LHA that constitute the feeding controller, and how are they targeted by DE?
The PVH contains three functional compartments, of which only one is seemingly in a position to control behavior. Consequently it appears very unlikely that neuroendocrine neurons in the medial parvicellular parts and magnocellular of the PVH play a significant role in controlling ingestive behaviors, given that their only know connections are to the neurohypophysis. This leaves the large population of parvicellular neurons that have descending connections as the most likely candidates. These neurons are concentrated within in the dorsal, ventral, and lateral parvicellular parts of the PVH and have extensive projections to the midbrain, hindbrain, and spinal cord. They express a variety of neuropeptides including oxytocin, which has been implicated in some forms of dehydration-anorexia [38].
With regard to the LHA, we are really only just beginning to understand how this complex region of the hypothalamus is organized in terms of neuropeptide distribution and connectional architecture [eg. 39,40]. If we are to understand how ingestive behaviors are controlled, a major challenge is now to define the detailed connectional and functional architecture of this highly complex brain region and to determine whether any of these neurons receive convergent information from the different inputs that can control feeding (Fig. 2).
Acknowledgments
The work from the authors laboratory described in this article was supported by MH066168 (AGW). Further support was provided by a NIH National Research Service Award (MH067392) to DSS, and a graduate merit fellowship from USC College to CMN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 2.Munzberg H, Myers MG., Jr Molecular anatomical, determinants of central leptin resistance. Nat Neurosci. 2005;8:566–70. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 3.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 4.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 5.Sawchenko PE. Toward a new neurobiology of energy balance, appetite, and obesity: the anatomists weigh in. J Comp Neurol. 1998;402:435–41. [PubMed] [Google Scholar]
- 6.Watts AG. Understanding the neural control of ingestive behaviors:helping to separate cause from effect with dehydration-associated anorexia. Horm Behav. 2000;37:261–83. doi: 10.1006/hbeh.2000.1581. [DOI] [PubMed] [Google Scholar]
- 7.Stellar E. The physiology of motivation. Psychol Rev. 1954;61:5–22. doi: 10.1037/h0060347. [DOI] [PubMed] [Google Scholar]
- 8.Watts AG. Neuropeptides and the integration of motor responses to dehydration. Ann Rev Neuroscience. 2001;24:357–384. doi: 10.1146/annurev.neuro.24.1.357. [DOI] [PubMed] [Google Scholar]
- 9.Grill HJ, Kaplan JM. Caudal Brainstem Participates In The Distributed Neural Control Of Feeding. In: Edward M Stricker., editor. The Handbook of Behavioral Neurobiology. Food and Fluid Intake. 1. Plenum Press; 1990. pp. 125–149. [Google Scholar]
- 10.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 11.Swanson LW. Anatomy of the soul as reflected in the cerebral hemispheres: neural circuits underlying voluntary control of basic motivated behaviors. J Comp Neurol. 2005;493:122–31. doi: 10.1002/cne.20733. [DOI] [PubMed] [Google Scholar]
- 12.Watts AG, Swanson LW. Learning, Motivation, and Emotion. 3. Vol. 3. John Wiley & Sons; 2002. Anatomy of Motivational Systems. ‘Stevens’ Handbook of Experimental Psychology; p. 632. Hal Pashler Randy Gallistell. [Google Scholar]
- 13.Schneider JE, Watts AG. Energy Homeostasis and Behavior. In: Pfaff DW, editor. Hormones, Brain, and Behavior. Vol. 1. Academic Press; 2002. pp. 435–523. [Google Scholar]
- 14.Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Brain Res Rev. 2003;41:153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- 15.Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav. 2005;86:747–61. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J Neurosci. 2005;25:8295–302. doi: 10.1523/JNEUROSCI.2480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wishaw IQ. The Decorticate Rat. In: Kolb B, Tees RC, editors. The Cerebral Cortex of the Rat. MIT Press; Cambridge MA: 1990. pp. 239–267. [Google Scholar]
- 18.Watts AG, Salter DS. Neural Mechanisms of Anorexia. In: Edward M Stricker, Steven C Woods., editors. The Handbook of Behavioral Neurobiology. Food and Fluid Intake. 2. Plenum Press; 2004. pp. 383–420. [Google Scholar]
- 19.Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology. 2005;146:1179–91. doi: 10.1210/en.2004-1166. [DOI] [PubMed] [Google Scholar]
- 20.Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–61. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 21.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol. 2001;432:197–216. doi: 10.1002/cne.1097. [DOI] [PubMed] [Google Scholar]
- 22.Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology. 2003;144:1357–67. doi: 10.1210/en.2002-221076. [DOI] [PubMed] [Google Scholar]
- 23.Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–40. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- 25.Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19:376–86. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- 26.Stratford TR. Activation of feeding-related neural circuitry after unilateral injections of muscimol into the nucleus accumbens shell. Brain Res. 2005;1048:241–50. doi: 10.1016/j.brainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1436–44. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]
- 28.Watts AG. Dehydration-associated anorexia:development and rapid reversal. Physiol Behav. 1999;65:871–878. doi: 10.1016/s0031-9384(98)00244-3. [DOI] [PubMed] [Google Scholar]
- 29.Berthoud HR. The Caudal Brainstem And The Control Of Food Intake And Energy Balance. In: Edward M Stricker, Steven C Woods., editors. The Handbook of Behavioral Neurobiology. Food and Fluid Intake. 2. Plenum Press; 2004. pp. 195–240. [Google Scholar]
- 30.Salter D, Watts AG. Differential suppression of hyperglycemic, feeding, and neuroendocrine responses in anorexia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R174–82. doi: 10.1152/ajpregu.00275.2002. [DOI] [PubMed] [Google Scholar]
- 31.Watts AG, Sanchez-Watts G, Kelly AB. Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration-induced anorexia. J Neurosci. 1999;19:6111–21. doi: 10.1523/JNEUROSCI.19-14-06111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salter D, Watts AG. Feeding response to hypothalamic injections of neuroptide Y is attenuated in dehydration anorexia. Soc Neurosci Abstr. 2003;28:Abs#615.5. [Google Scholar]
- 33.Ishihara A, Tanaka T, Kanatani A, Fukami T, Ihara M, Fukuroda T. A potent neuropeptide Y antagonist, 1229U91, suppressed spontaneous food intake in Zucker fatty rats. Am J Physiol. 1998;274:R1500–R1504. doi: 10.1152/ajpregu.1998.274.5.R1500. [DOI] [PubMed] [Google Scholar]
- 34.Kanatani A, Ishihara A, Asahi S, Tanaka T, Ozaki S, Ihara M. Potent neuropeptide Y Y1 receptor antagonist, 1229U91:blockade of neuropeptide Y-induced and physiological food intake. Endocrinology. 1996;137:3177–318. doi: 10.1210/endo.137.8.8754736. [DOI] [PubMed] [Google Scholar]
- 35.Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–8. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neuner CM, Watts AG. Effects of GABA in the nucleus accumbens shell on ingestive behavior after dehydration-anorexia. Appetite. 2006;46:372. [Google Scholar]
- 37.Stratford TR, Wirtshafter D. NPY mediates the feeding elicited by muscimol injections into the nucleus accumbens shell. Neuroreport. 2004;15:2673–6. doi: 10.1097/00001756-200412030-00024. [DOI] [PubMed] [Google Scholar]
- 38.Rinaman L, Vollmer RR, Karam J, Phillips D, Li X, Amico JA. Dehydration anorexia is attenuated in oxytocin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1791–9. doi: 10.1152/ajpregu.00860.2004. [DOI] [PubMed] [Google Scholar]
- 39.Goto M, Canteras NS, Burns G, Swanson LW. Projections from the subfornical region of the lateral hypothalamic area. J Comp Neurol. 2005;493:412–38. doi: 10.1002/cne.20764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parcelating scheme of the lateral hypothalamic zone. Neuroscience Letters. 2005;387:80–84. doi: 10.1016/j.neulet.2005.06.066. [DOI] [PubMed] [Google Scholar]


