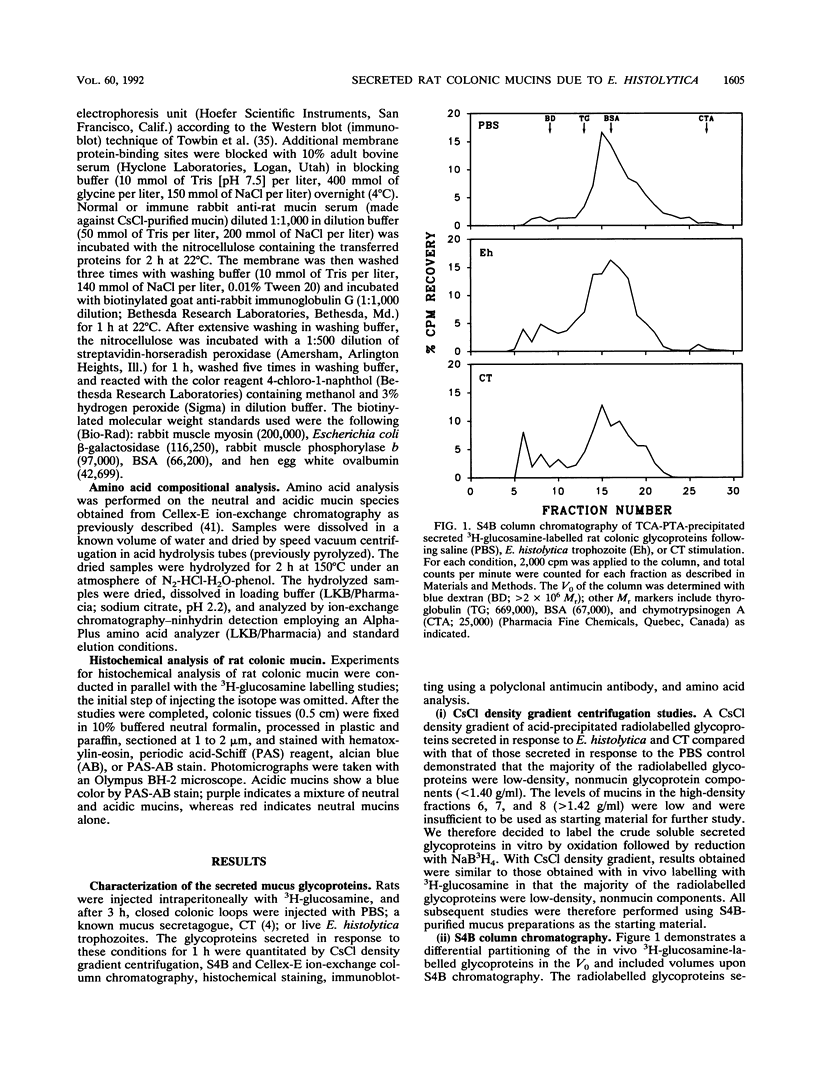
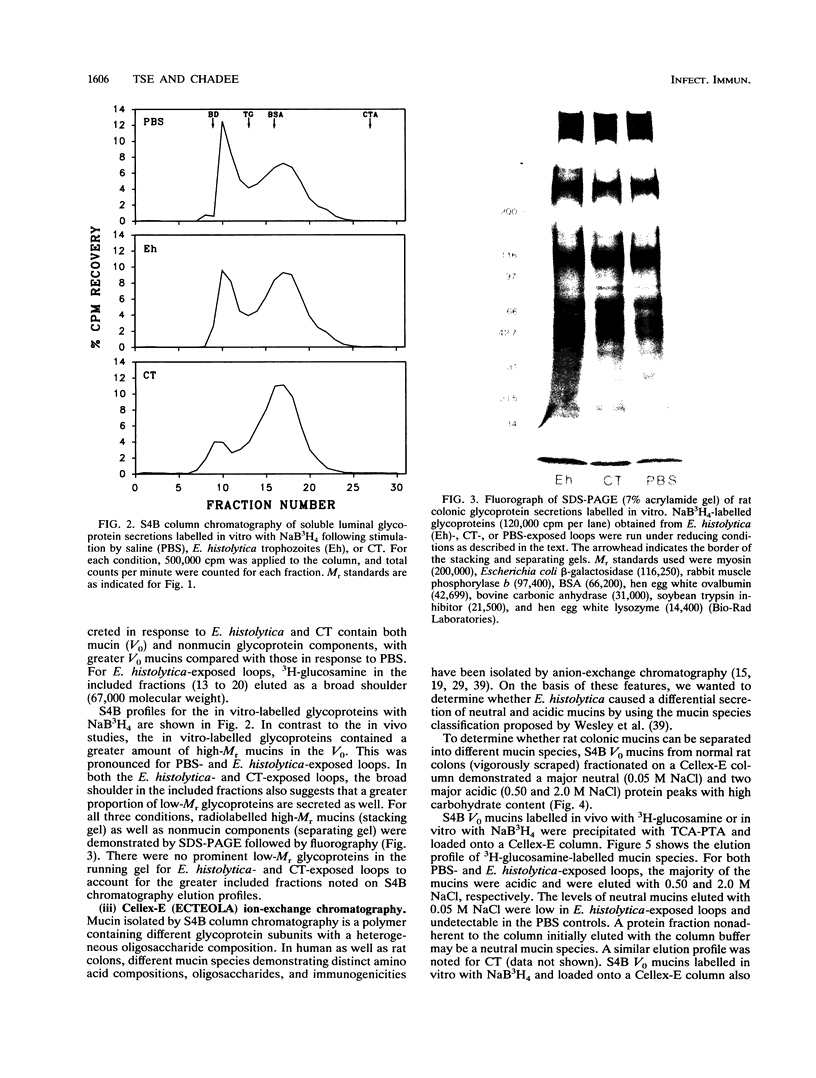
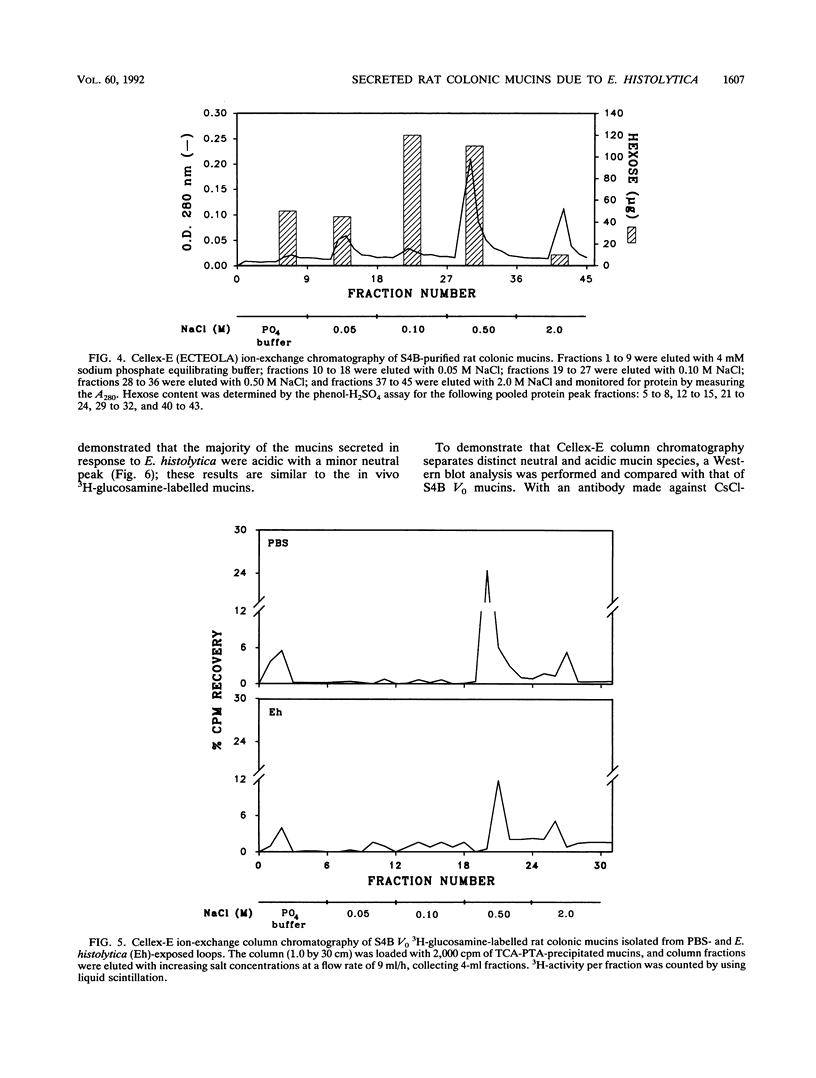
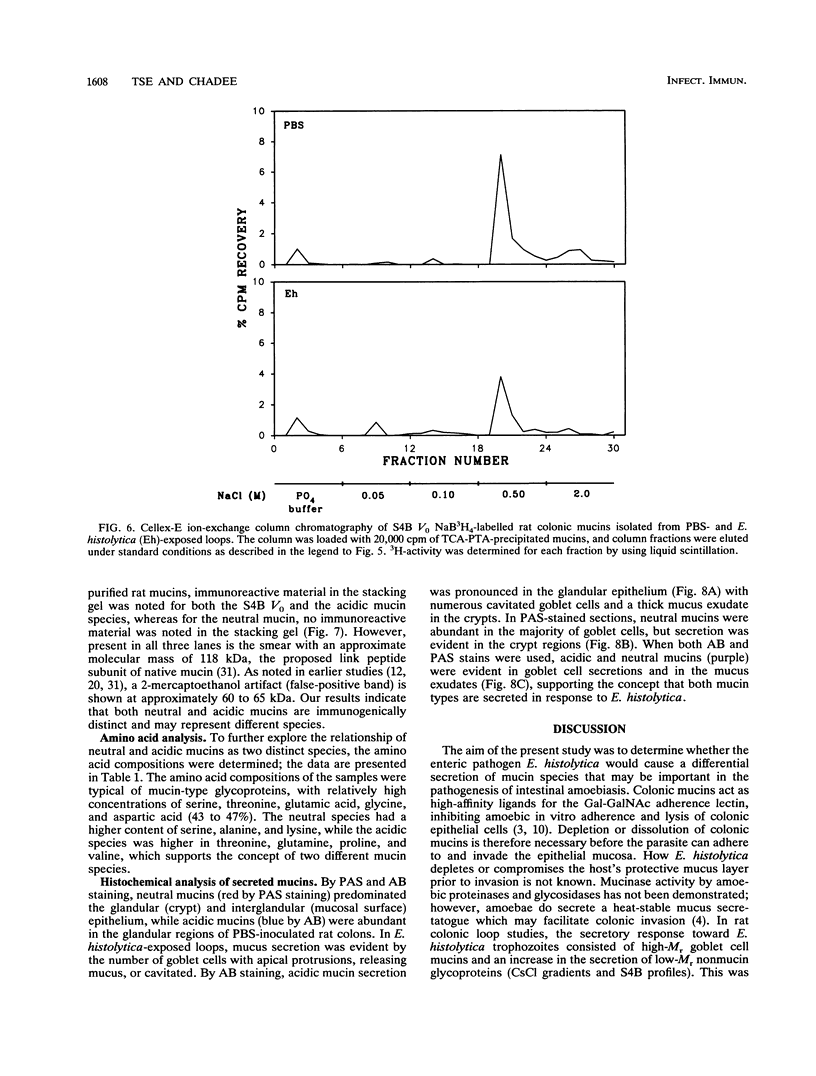
Abstract
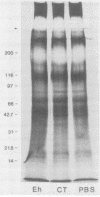
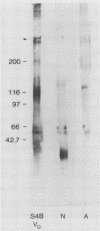
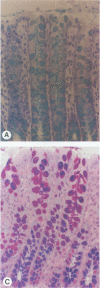
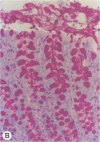
Invasion of the colonic mucosa by Entamoeba histolytica trophozoites is preceded by colonic mucus depletion. The aim of our studies was to determine whether E. histolytica caused a differential secretion of mucin species in a rat colonic loop model. Mucus secretion in response to amoebae was followed by release of acid-precipitable 3H-glucosamine metabolically labelled glycoproteins and in vitro labelling of glycoprotein secretion with NaB3H4. The secretory response consisted of high-Mr goblet cell mucins and an increase in the secretion of low-Mr nonmucin glycoproteins as determined by Sepharose 4B column chromatography. High-Mr mucins subfractionated by Cellex-E (ECTEOLA) ion-exchange chromatography demonstrated a minor neutral and a major acidic mucin (greater than 98%) species. Marked differences between the neutral and acidic mucin species were indicated by immunogenicity and amino acid compositions. Thin-section histochemistry of rat colons confirmed secretion of neutral and acidic mucins in response to E. histolytica and demonstrated secretory activity from goblet cells from both the crypts and interglandular epithelium. E. histolytica mucus secretagogue activity was generalized and may function to deplete the host's protective mucus layer, facilitating invasion by the parasites.
Full text
PDF

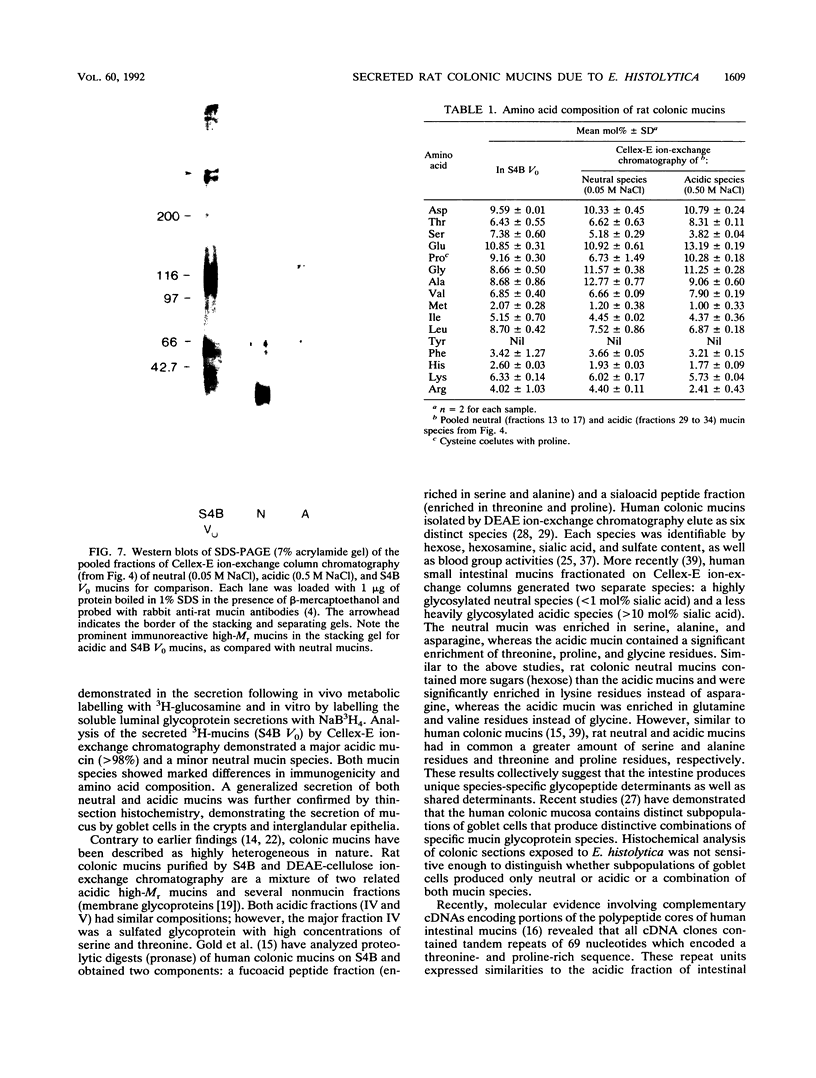



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Bell A., Mantle M., Pearson J. P. The structure and physiology of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:115–133. doi: 10.1007/978-1-4615-9254-9_15. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chadee K., Johnson M. L., Orozco E., Petri W. A., Jr, Ravdin J. I. Binding and internalization of rat colonic mucins by the galactose/N-acetyl-D-galactosamine adherence lectin of Entamoeba histolytica. J Infect Dis. 1988 Aug;158(2):398–406. doi: 10.1093/infdis/158.2.398. [DOI] [PubMed] [Google Scholar]
- Chadee K., Keller K., Forstner J., Innes D. J., Ravdin J. I. Mucin and nonmucin secretagogue activity of Entamoeba histolytica and cholera toxin in rat colon. Gastroenterology. 1991 Apr;100(4):986–997. doi: 10.1016/0016-5085(91)90274-o. [DOI] [PubMed] [Google Scholar]
- Chadee K., Meerovitch E. Entamoeba histolytica: early progressive pathology in the cecum of the gerbil (Meriones unguiculatus). Am J Trop Med Hyg. 1985 Mar;34(2):283–291. doi: 10.4269/ajtmh.1985.34.283. [DOI] [PubMed] [Google Scholar]
- Chadee K., Meerovitch E. The Mongolian gerbil (Meriones unguiculatus) as an experimental host for Entamoeba histolytica. Am J Trop Med Hyg. 1984 Jan;33(1):47–54. doi: 10.4269/ajtmh.1984.33.47. [DOI] [PubMed] [Google Scholar]
- Chadee K., Meerovitch E. The pathogenesis of experimentally induced amebic liver abscess in the gerbil (Meriones unguiculatus). Am J Pathol. 1984 Oct;117(1):71–80. [PMC free article] [PubMed] [Google Scholar]
- Chadee K., Meerovitch E. The pathology of experimentally induced cecal amebiasis in gerbils (Meriones unguiculatus). Liver changes and amebic liver abscess formation. Am J Pathol. 1985 Jun;119(3):485–494. [PMC free article] [PubMed] [Google Scholar]
- Chadee K., Ndarathi C., Keller K. Binding of proteolytically-degraded human colonic mucin glycoproteins to the Gal/GalNAc adherence lectin of Entamoeba histolytica. Gut. 1990 Aug;31(8):890–895. doi: 10.1136/gut.31.8.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadee K., Petri W. A., Jr, Innes D. J., Ravdin J. I. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Invest. 1987 Nov;80(5):1245–1254. doi: 10.1172/JCI113199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim R. E., Specian R. D., Forstner G. G., Forstner J. F. Characterization and localization of the putative 'link' component in rat small-intestinal mucin. Biochem J. 1987 May 1;243(3):631–640. doi: 10.1042/bj2430631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I., Branfoot A. C. Abnormal patterns of mucus secretion in apparently normal mucosa of large intestine with carcinoma. Cancer. 1974 Aug;34(2):282–290. doi: 10.1002/1097-0142(197408)34:2<282::aid-cncr2820340211>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Gold D. V., Miller F. Characterization of human colonic mucoprotein antigen. Immunochemistry. 1974 Jul;11(7):369–375. doi: 10.1016/0019-2791(74)90190-6. [DOI] [PubMed] [Google Scholar]
- Gold D. V., Shochat D., Miller F. Protease digestion of colonic mucin. Evidence for the existence of two immunochemically distinct mucins. J Biol Chem. 1981 Jun 25;256(12):6354–6358. [PubMed] [Google Scholar]
- Gum J. R., Hicks J. W., Swallow D. M., Lagace R. L., Byrd J. C., Lamport D. T., Siddiki B., Kim Y. S. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990 Aug 31;171(1):407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- Koninkx J. F., Mirck M. H., Hendriks H. G., Mouwen J. M., van Dijk J. E. Nippostrongylus brasiliensis: histochemical changes in the composition of mucins in goblet cells during infection in rats. Exp Parasitol. 1988 Feb;65(1):84–90. doi: 10.1016/0014-4894(88)90109-9. [DOI] [PubMed] [Google Scholar]
- LaMont J. T., Ventola A. S. Purification and composition of colonic epithelial mucin. Biochim Biophys Acta. 1980 Nov 20;626(1):234–243. doi: 10.1016/0005-2795(80)90214-7. [DOI] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Antigenic and structural features of goblet-cell mucin of human small intestine. Biochem J. 1984 Jan 1;217(1):159–167. doi: 10.1042/bj2170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. R. Gastrointestinal mucus, a medium for survival and for elimination of parasitic nematodes and protozoa. Parasitology. 1987;94 (Suppl):S77–100. doi: 10.1017/s0031182000085838. [DOI] [PubMed] [Google Scholar]
- Murty V. L., Downs F. J., Pigman W. Rat-colonic, mucus glycoprotein. Carbohydr Res. 1978 Mar;61:139–145. doi: 10.1016/s0008-6215(00)84474-2. [DOI] [PubMed] [Google Scholar]
- Petri W. A., Jr, Smith R. D., Schlesinger P. H., Murphy C. F., Ravdin J. I. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J Clin Invest. 1987 Nov;80(5):1238–1244. doi: 10.1172/JCI113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A., Lynch K. E. Human colonic goblet cells. Demonstration of distinct subpopulations defined by mucin-specific monoclonal antibodies. J Clin Invest. 1986 Apr;77(4):1263–1271. doi: 10.1172/JCI112429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Composition of human colonic mucin. Selective alteration in inflammatory bowel disease. J Clin Invest. 1983 Jul;72(1):142–153. doi: 10.1172/JCI110952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Glycoprotein composition of colonic mucosa. Specific alterations in ulcerative colitis. Gastroenterology. 1984 Nov;87(5):991–998. [PubMed] [Google Scholar]
- Podolsky D. K. Oligosaccharide structures of human colonic mucin. J Biol Chem. 1985 Jul 15;260(14):8262–8271. [PubMed] [Google Scholar]
- Podolsky D. K. Oligosaccharide structures of isolated human colonic mucin species. J Biol Chem. 1985 Dec 15;260(29):15510–15515. [PubMed] [Google Scholar]
- Ravdin J. I., Guerrant R. L. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J Clin Invest. 1981 Nov;68(5):1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton A. M., Mantle M., Fahim R. E., Specian R. D., Bennick A., Kawagishi S., Sherman P., Forstner J. F. The putative 'link' glycopeptide associated with mucus glycoproteins. Composition and properties of preparations from the gastrointestinal tracts of several mammals. Biochem J. 1989 Jul 15;261(2):637–647. doi: 10.1042/bj2610637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomiany B. L., Murty V. L., Slomiany A. Isolation and characterization of oligosaccharides from rat colonic mucus glycoprotein. J Biol Chem. 1980 Oct 25;255(20):9719–9723. [PubMed] [Google Scholar]
- Smith A. C., Podolsky D. K. Biosynthesis and secretion of human colonic mucin glycoproteins. J Clin Invest. 1987 Aug;80(2):300–307. doi: 10.1172/JCI113073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. C., Podolsky D. K. Colonic mucin glycoproteins in health and disease. Clin Gastroenterol. 1986 Oct;15(4):815–837. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse S. K., Chadee K. The interaction between intestinal mucus glycoproteins and enteric infections. Parasitol Today. 1991 Jul;7(7):163–172. doi: 10.1016/0169-4758(91)90121-4. [DOI] [PubMed] [Google Scholar]
- Tysk C., Riedesel H., Lindberg E., Panzini B., Podolsky D., Järnerot G. Colonic glycoproteins in monozygotic twins with inflammatory bowel disease. Gastroenterology. 1991 Feb;100(2):419–423. doi: 10.1016/0016-5085(91)90211-3. [DOI] [PubMed] [Google Scholar]
- Walsh J. A. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986 Mar-Apr;8(2):228–238. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]
- Wesley A., Forstner J., Qureshi R., Mantle M., Forstner G. Human intestinal mucin in cystic fibrosis. Pediatr Res. 1983 Jan;17(1):65–69. doi: 10.1203/00006450-198301000-00013. [DOI] [PubMed] [Google Scholar]
- Wesley A., Mantle M., Man D., Qureshi R., Forstner G., Forstner J. Neutral and acidic species of human intestinal mucin. Evidence for different core peptides. J Biol Chem. 1985 Jul 5;260(13):7955–7959. [PubMed] [Google Scholar]
- Zhu H., Bussey H., Thomas D. Y., Gagnon J., Bell A. W. Determination of the carboxyl termini of the alpha and beta subunits of yeast K1 killer toxin. Requirement of a carboxypeptidase B-like activity for maturation. J Biol Chem. 1987 Aug 5;262(22):10728–10732. [PubMed] [Google Scholar]