Abstract
Enterohemorrhagic Escherichia coli (EHEC) produce Shiga-like toxins and attach to certain tissue culture cells. T84 cells are human colonic carcinoma cells. Unlike previously studied cell lines, T84 cells grown on collagen-coated surfaces polarize and produce tight junctions and desmosomes, forming a colonic epithelial cell layer in vitro. The purpose of this study was to examine the attachment of EHEC strains to the T84 cell line as a possibly more relevant in vitro model of EHEC adherence. Twelve EHEC strains were grown overnight in Penassay broth, suspended in minimal essential medium with and without 0.5% mannose, and incubated for 1 to 3 h with 5- to 7-day-old T84 cell monolayers grown on collagen-coated coverslips. The bacteria were removed, and attachment was quantitated microscopically. For both E. coli O157:H7 and other EHEC serotypes, there were marked differences in adherence between strains (range of 152 to 3 bacteria per oil immersion field). Mannose partially inhibited the adherence of some EHEC strains. Adherence to the T84 cells appeared to be related to the amount of pili present and not to the serotype. Electron micrographs showed that a highly adherent strain (strain 43-12) tended to form microcolonies in the area of tight junctions on the T84 cell monolayers. In addition, the attachment of these EHEC strains to T84 cells correlated with their ability to adhere to isolated rabbit colonocytes (r = 0.91, P = 0.00004; without mannose) (r = 0.60, P = 0.04; with mannose). These data show that there are EHEC strain-related differences in adherence which can be demonstrated in a human-derived colonic epithelial cell line (T84) and that these cells can be used to study EHEC adherence.
Full text
PDF


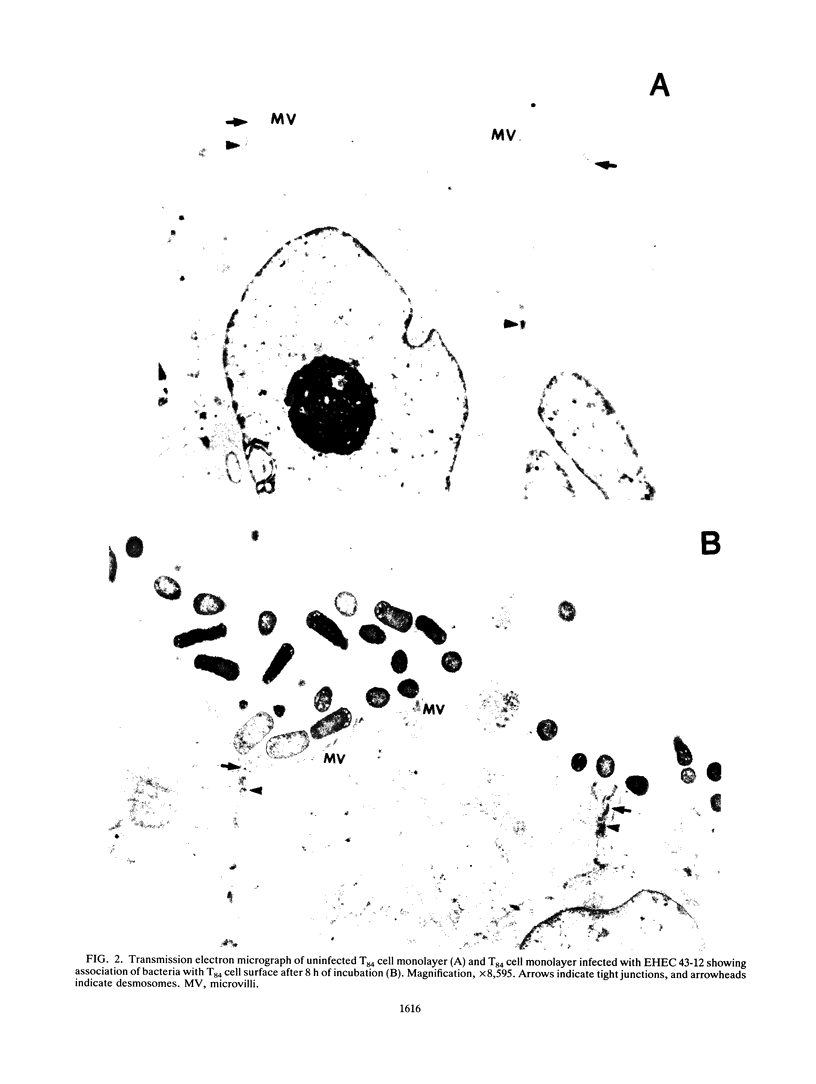

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi S., May L., LaRocco M., Lopez E. L., Cleary T. G. The effect of postnatal age on the adherence of enterohemorrhagic Escherichia coli to rabbit intestinal cells. Pediatr Res. 1991 Jan;29(1):14–19. doi: 10.1203/00006450-199101000-00004. [DOI] [PubMed] [Google Scholar]
- Audus K. L., Bartel R. L., Hidalgo I. J., Borchardt R. T. The use of cultured epithelial and endothelial cells for drug transport and metabolism studies. Pharm Res. 1990 May;7(5):435–451. doi: 10.1023/a:1015800312910. [DOI] [PubMed] [Google Scholar]
- Darfeuille-Michaud A., Aubel D., Chauviere G., Rich C., Bourges M., Servin A., Joly B. Adhesion of enterotoxigenic Escherichia coli to the human colon carcinoma cell line Caco-2 in culture. Infect Immun. 1990 Apr;58(4):893–902. doi: 10.1128/iai.58.4.893-902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durno C., Soni R., Sherman P. Adherence of vero cytotoxin-producing Escherichia coli serotype O157:H7 to isolated epithelial cells and brush border membranes in vitro: role of type 1 fimbriae (pili) as a bacterial adhesin expressed by strain CL-49. Clin Invest Med. 1989 Jun;12(3):194–200. [PubMed] [Google Scholar]
- Holmgren J., Fryklund J., Larsson H. Gamma-interferon-mediated down-regulation of electrolyte secretion by intestinal epithelial cells: a local immune mechanism? Scand J Immunol. 1989 Oct;30(4):499–503. doi: 10.1111/j.1365-3083.1989.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Karch H., Heesemann J., Laufs R., O'Brien A. D., Tacket C. O., Levine M. M. A plasmid of enterohemorrhagic Escherichia coli O157:H7 is required for expression of a new fimbrial antigen and for adhesion to epithelial cells. Infect Immun. 1987 Feb;55(2):455–461. doi: 10.1128/iai.55.2.455-461.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., Petric M., Lim C., Fleming P. C., Arbus G. S., Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985 May;151(5):775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- Knutton S., Baldwin T., Williams P. H., McNeish A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989 Apr;57(4):1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konowalchuk J., Speirs J. I., Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977 Dec;18(3):775–779. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez E. L., Diaz M., Grinstein S., Devoto S., Mendilaharzu F., Murray B. E., Ashkenazi S., Rubeglio E., Woloj M., Vasquez M. Hemolytic uremic syndrome and diarrhea in Argentine children: the role of Shiga-like toxins. J Infect Dis. 1989 Sep;160(3):469–475. doi: 10.1093/infdis/160.3.469. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Stafford J., Dharmsathaphorn K., Carlson S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology. 1987 May;92(5 Pt 1):1133–1145. doi: 10.1016/s0016-5085(87)91069-9. [DOI] [PubMed] [Google Scholar]
- Marcon M. A., McCool D., Forstner J., Forstner G. Inhibition of mucin secretion in a colonic adenocarcinoma cell line by DIDS and potassium channel blockers. Biochim Biophys Acta. 1990 Apr 9;1052(1):17–23. doi: 10.1016/0167-4889(90)90051-e. [DOI] [PubMed] [Google Scholar]
- Pai C. H., Gordon R., Sims H. V., Bryan L. E. Sporadic cases of hemorrhagic colitis associated with Escherichia coli O157:H7. Clinical, epidemiologic, and bacteriologic features. Ann Intern Med. 1984 Dec;101(6):738–742. doi: 10.7326/0003-4819-101-6-738. [DOI] [PubMed] [Google Scholar]
- Richardson S. E., Karmali M. A., Becker L. E., Smith C. R. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum Pathol. 1988 Sep;19(9):1102–1108. doi: 10.1016/s0046-8177(88)80093-5. [DOI] [PubMed] [Google Scholar]
- Riley L. W., Remis R. S., Helgerson S. D., McGee H. B., Wells J. G., Davis B. R., Hebert R. J., Olcott E. S., Johnson L. M., Hargrett N. T. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983 Mar 24;308(12):681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- Sajjan S. U., Forstner J. F. Characteristics of binding of Escherichia coli serotype O157:H7 strain CL-49 to purified intestinal mucin. Infect Immun. 1990 Apr;58(4):860–867. doi: 10.1128/iai.58.4.860-867.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. M., Soni R. Adherence of Vero cytotoxin-producing Escherichia coli of serotype O157:H7 to human epithelial cells in tissue culture: role of outer membranes as bacterial adhesins. J Med Microbiol. 1988 May;26(1):11–17. doi: 10.1099/00222615-26-1-11. [DOI] [PubMed] [Google Scholar]
- Sherman P., Cockerill F., 3rd, Soni R., Brunton J. Outer membranes are competitive inhibitors of Escherichia coli O157:H7 adherence to epithelial cells. Infect Immun. 1991 Mar;59(3):890–899. doi: 10.1128/iai.59.3.890-899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P., Soni R., Karmali M. Attaching and effacing adherence of Vero cytotoxin-producing Escherichia coli to rabbit intestinal epithelium in vivo. Infect Immun. 1988 Apr;56(4):756–761. doi: 10.1128/iai.56.4.756-761.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P., Soni R., Yeger H. Characterization of flagella purified from enterohemorrhagic, vero-cytotoxin-producing Escherichia coli serotype O157:H7. J Clin Microbiol. 1988 Jul;26(7):1367–1372. doi: 10.1128/jcm.26.7.1367-1372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth I., Cohen M. L., Rumschlag H. S., Riley L. W., White E. H., Carr J. H., Bond W. W., Wachsmuth I. K. Influence of the 60-megadalton plasmid on adherence of Escherichia coli O157:H7 and genetic derivatives. Infect Immun. 1990 May;58(5):1223–1231. doi: 10.1128/iai.58.5.1223-1231.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Wachsmuth I. K., Chapman C., Birden R., Brittingham J., Jackson C., Hogg J. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. J Infect Dis. 1986 Oct;154(4):712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- Tzipori S., Wachsmuth K. I., Smithers J., Jackson C. Studies in gnotobiotic piglets on non-O157:H7 Escherichia coli serotypes isolated from patients with hemorrhagic colitis. Gastroenterology. 1988 Mar;94(3):590–597. doi: 10.1016/0016-5085(88)90228-4. [DOI] [PubMed] [Google Scholar]
- Wasserman S. I., Barrett K. E., Huott P. A., Beuerlein G., Kagnoff M. F., Dharmsathaphorn K. Immune-related intestinal Cl- secretion. I. Effect of histamine on the T84 cell line. Am J Physiol. 1988 Jan;254(1 Pt 1):C53–C62. doi: 10.1152/ajpcell.1988.254.1.C53. [DOI] [PubMed] [Google Scholar]



