Abstract
Until the past few years, it has been thought that lymphoid and myeloid lineage segregation represents the first step of lineage restriction during hematopoiesis from hematopoietic stem cell. Recent investigation of the cell populations within multipotent progenitors in the bone marrow has led to new understanding of how hematopoietic stem cells diversify into different hematopoietic cell types. This review focuses on the recent advances in understanding the developmental events that occur during HSC specification into the T and B lymphocyte lineages in adult mice.
Keywords: hematopoietic differentiation, multipotent progenitors, lineage commitment, lymphocyte development, thymic immigrants
Introduction
All classes of blood cells are derived from bone marrow (BM) resident hematopoietic stem cells (HSCs) in adults, through progressive loss of differentiation potentials for other cell lineages. Due to the ease of isolation of HSCs and the established in vitro and in vivo assays in studying blood cell differentiation, hematopoiesis serves as a model system to study the molecular regulation of cell fate decisions and lineage commitment in stem and progenitor cells during mammalian development (1).
Hematopoietic cells can be broadly separated into two major lineages: the lymphoid lineage including T, B, and natural killer (NK) cells, or the myeloid lineage comprised of erythrocytes, megakaryocytes, granulocytes, and monocyte/macrophage. Dendritic cells are also of hematopoietic origin. However, their lineage affiliation remains unclear and controversial (2). Identification and characterization of HSCs as well as multiple progenitor populations in the BM have led to insights into the developmental hierarchy of each cell lineage. This review will focus on discussing the characterization of cell populations upstream lineage committed progenitors, which led to our current understanding of how HSCs diverge into the lymphoid or the myeloid lineages.
From HSCs to lineage committed progenitors
Upon receiving signals to undergo differentiation, the first biological change in HSCs is the gradual loss of self-renewal ability. This is revealed by the characterization of the progenitors immediately downstream of HSCs (3, 4). Like long-term (LT−) HSCs (Thy-1.1loFlt3− Lin−Sca-1+c-Kit+ (LSK) or CD34−Flt3− LSK or CD150+CD244−CD48−), short-term (ST−) HSCs (Thy-1.1loFlt3+ LSK or CD34+Flt3− LSK) contribute to multi-lineage differentiation and generate CFU-S (3, 5, 6). However, ST-HSCs could only sustain hematopoiesis in vivo for about 6 weeks (3). Multipotent progenitors, defined as Thy-1.1−Flt3+ LSK or CD34+Flt3+ LSK, have limited burst size and lose the ability to self-renew, but retain transient multi-lineage differentiation potential (3). These populations were found to be in a developmental sequence, such that LT-HSC -> ST-HSC -> MPP (4). Since MPPs do not self-renew, it was thought that the first step of lineage restriction occurs at this stage during hematopoiesis.
Early divergence of lymphoid and myeloid cell lineages from HSCs during hematopoiesis has long been proposed. This concept was reinforced through the discovery of common lymphoid progenitors (CLPs) and common myeloid progenitors (CMPs), which have differentiation potentials restricted to all cell types within their respective lineage on the clonal level (7, 8). Because of the indispensable role of IL-7/IL-7R signaling in both T and B cell development (9, 10), CLPs were identified using the expression of IL-7Rα on primitive hematopoietic progenitors, defined as IL-7Rα+Thy-1.1−Lin−Sca-1loc-Kitlo (7). By excluding the cell surface markers that identified HSCs and CLPs such as Sca-1 and IL-7Rα, CMPs were similarly isolated and defined as CD34+FcγRIII−/loThy-1.1−IL-7Rα−Lin−Sca-1−c-Kit+ (8). CMPs also give rise to megakaryocyte/erythroid (MegE) restricted and macrophage/granulocyte (GM) restricted bipotent progenitors, defined as CD34−FcγRIII−Thy-1.1−IL-7Rα−Lin−Sca-1−c-Kit+ and CD34+FcγRIII+Thy-1.1−IL-7Rα−Lin−Sca-1−c-Kit+, respectively (8).
Identification of these lineage-restricted progenitors separated the lymphoid and myeloid lineage on the progenitor level. Furthermore, the existence of a lymphoid lineage committed progenitor suggests a common origin of all lymphocytes, rather than a separate developmental pathway for each lymphocyte lineage from HSCs. The characterization of these early hematopoietic progenitors and their developmental hierarchy formed the basis of the “classical” model of hematopoiesis, where the lymphoid and myeloid lineages diverge from a single MPP in a symmetrical fashion, representing the first step of irreversible lineage commitment from HSCs during early hematopoietic ontogeny (Figure 1). More recent characterization of MPPs, however, provided a higher resolution of the hematopoietic tree upstream of CLPs and CMPs, and suggests that lymphoid and myeloid lineage commitment do not occur as previously proposed in the “classical” model.
Figure 1.
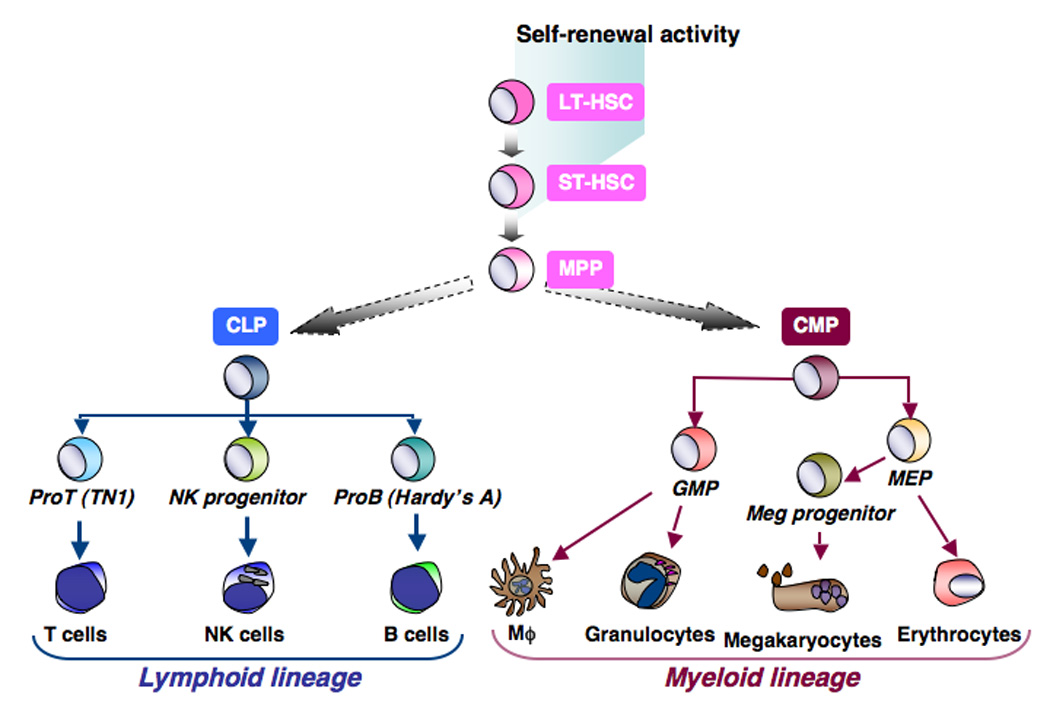
The classical model of hematopoiesis predicts that the first step of lineage restriction from HSC is lymphoid and myeloid lineage commitment. In this model, CLP and CMP are symmetrically derived from the same MPP.
Heterogeneity in MPPs: Step-wise loss of myeloid differentiation during early lymphoid lineage differentiation
In the past five years, multiple laboratories have characterized subfractions within the MPP population with different lineage differentiation potential. Adolfsson et al. reported that MPPs could be separated based on Flt3 expression level (11). They found that the 25% of MPPs that express the highest density of Flt3 do not have MegE differentiation potential in vitro and in vivo, but have robust GM in addition to T and B cell differentiation potential in vitro (11). Using VCAM-1 and Flt3 in combination, Lai and Kondo further subdivided the MPP populations into 3 distinct subsets, Flt3loVCAM-1+, Flt3hiVCAM-1+, and Flt3hiVCAM-1− MPPs (12, 13). Characterization of the in vivo differentiation potential of these cell populations indicated that Flt3loVCAM-1+ MPPs represent true multi-lineage progenitors, with the ability to give rise to MegE, GM, and lymphoid lineages (13). In agreement with the studies by Adolfsson et al., Flt3hiVCAM-1+ MPPs have lost MegE differentiation potential, but retain high GM and T/B differentiation potential both in vitro and in vivo (13). Although the vast majority of Flt3hiVCAM-1− MPPs also have GM differentiation potential in vitro on the clonal level, they preferentially give rise to lymphocytes when injected into lethally irradiated mice (12, 13). These studies also defined the relationship between the MPP subsets with CMPs and CLPs. Only the most primitive Flt3loVCAM-1+ MPPs can give rise to CMPs (13). In contrast, the most developmentally advanced Flt3hiVCAM-1− MPPs give rise to CLPs (12).
Together these studies demonstrated that the first step of lineage restriction is not simply lymphoid and myeloid lineage commitment. Adolfsson et al. proposed an alternative hematopoietic hiearchy to the classical model, where the MegE lineage first diverges from ST-HSC or MPPs, and this loss of MegE differentiation potential is a prerequisite lineage restriction step prior to GM or lymphoid lineage differentiation (11). However, since Flt3loVCAM-1+ MPPs can give rise to CMPs, the classical model is still valid, only that lymphoid-committed CLPs are not generated from the same MPP that give rise to CMPs. While myeloid lineage cells seem to be able to diverge off different subsets of MPPs, lymphoid lineage differentiation appears to go through multiple obligatory lineage restriction steps, through a step-wise loss of MegE and then GM differentiation potential prior to lymphoid lineage commitment (Figure 2).
Figure 2.
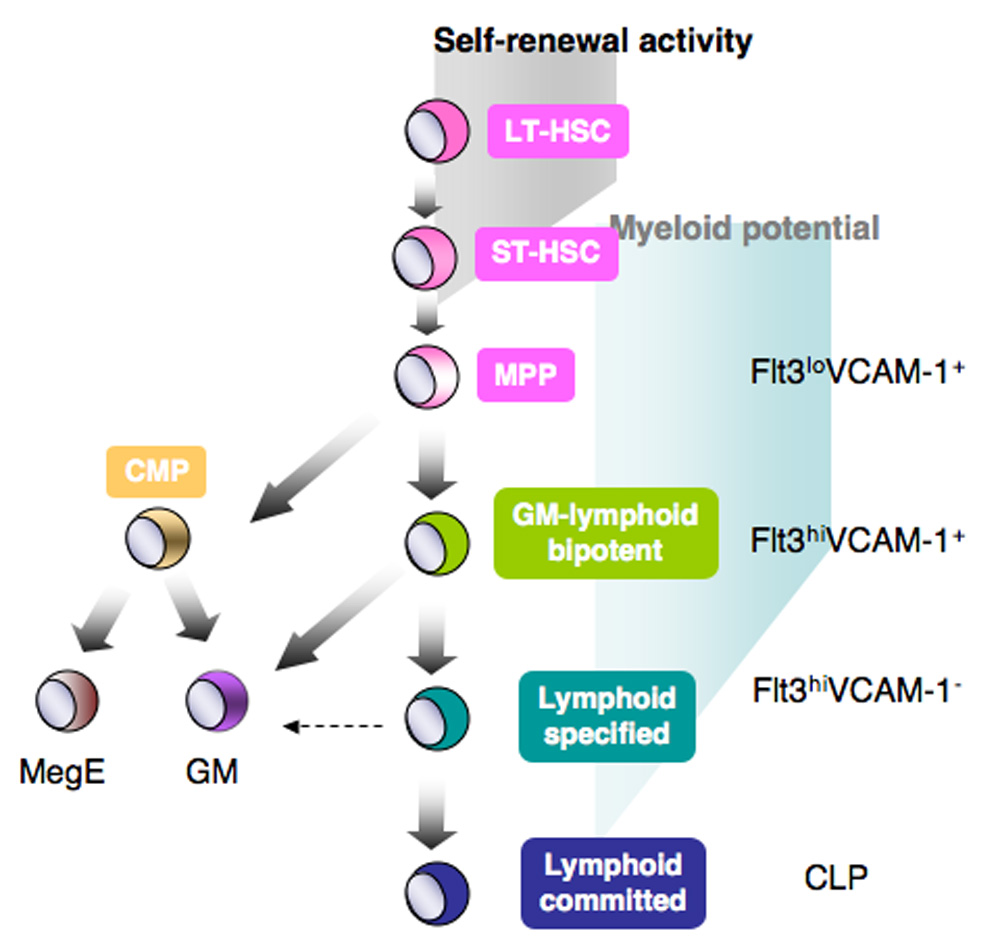
A revised version of the classical model of the hematopoietic tree. In this model, CLP and CMP are generated asymmetrically from different MPPs. Prior to lymphoid lineage commitment at the CLP stage, MPPs loses myeloid lineage differentiation potential in a step-wise fashion.
Lymphoid specification vs. lymphoid commitment
As mentioned above, one subset of MPPs (Flt3hiVCAM-1−) predominately give rise to lymphocytes in vivo, although they retain robust GM differentiation potential when cultured in vitro (12). These lymphoid-biased progenitors are very similar to early lymphoid progenitors (ELPs) previously characterized by Igarashi et al (14). In fact, 90% of the ELP population, defined as GFP+LSK in RAG1-GFP KI BM, overlaps with Flt3hiVCAM-1− MPPs (Lai and Kondo, unpublished observation). The existence of these lymphoid-biased progenitors fits with the idea of a gradual down-modulation of myeloid potential during the course of lymphocyte development. In this case, Flt3hiVCAM-1− MPPs or ELPs would be considered lymphoid specified, meaning they are programmed to differentiate into lymphocytes, but this occurs prior to the silencing of the myeloid differentiation program. Commitment to the lymphoid lineage, at the CLP stage, is established when the cell loses all abilities (without the exogenous introduction of genes) to generate myeloid lineage cells.
In addition to MPPs, other lineage-biased progenitors have been observed. For example, both CLPs and CLP-2 (hCD25+c-kit−B220+CD19− cells in pre-Tα-hCD25 reporter transgenic mice) potently differentiate into T cells when injected intrathymically or when cultured on OP9-DL1 stromal cells (7, 15, 16). When intravenously injected in vivo, however, CLPs preferentially develop into B cells (7). Similarly, immature thymocytes retain a weak ability to generate other cell types, including B, NK, and macrophages (17). There are two possible explanations for the discrepancy between in vitro and in vivo differentiation potentials of MPPs and lymphoid progenitors: 1) During in vitro culture, nonphysiologically high concentrations of cytokines or growth factors may be present to allow progenitors to differentiate into cell lineages that they do not normally become in vivo, or 2) upon specification to a certain lineage, progenitors preferentially migrate to distinct niches within the BM or to the thymus that only support certain lineage maturation. If progenitor niches do exist, we need to be careful when interpreting the in vivo differentiation potential of cells upon purification and transplantation, since cells may be removed from their niches during manipulation and may not home correctly upon intravenous injection.
Microenvironments for hematopoietic differentiation
Microenvironments, also known as niches, are specialized spatial structures or cellular components where stem or progenitor cells are localized, in order to receive the critical stimuli that support their differentiation and function. Osteoblasts at the trebacular region of the bone cavity serve as a niche for HSCs (18, 19). These osteoblasts express adhesion molecules such as N-cadherin and VCAM-1, which function to retain HSCs at their niche (19, 20), although the involvement of N-cadherin might be controversial (21). Osteoblasts also provide important signals to keep HSCs in a quiescent and undifferentiated state, such as angiopoietin (22), and Jagged-1 (18), respectively. A vascular niche for HSC has also been identified, where HSCs were found to associate with CXCL12 expressing reticular cells in the sinusoidal endothelium (6, 23). It is postulated that HSCs migrate away from their niche into the core of BM upon differentiation (24).
In addition to HSC niches, microenvironments supporting stage-specific differentiation during T and B cell development have also been found. A notable example of this is that developing thymocytes migrate to different regions of the thymus to undergo different maturation steps (25). While CD4 and CD8 double negative (DN) thymocytes are localized in the capsule and subcapsular region of the thymus, double positive (DP) thymocytes are generated in the outer cortex. Positive and negative selections of DP thymocytes then take place in the cortex, and positively selected DPs migrate to the medulla for further maturation to SP thymocytes. Specialized niches have also been shown to support B cell differentiation at different developmental stages in BM (26). While CXCL12/SDF-1α expressing cells in the BM form the niche for pre-proB cells, proB cells are preferentially localized with IL-7 producing stromal cells distinct from those secreting SDF-1α. It remains to be determined whether localization of MPPs have implications in lineage specification and commitment, and whether distinct microenvironments that support exclusively lymphoid or myeloid lineage differentiation exist.
Homing of stem cells and progenitors to their specialized niches is often directed by chemokines (24). For example, SDF-1 is involved in the retention of hematopoietic progenitors and colonization of HSCs in BM (27, 28). In the thymus, multiple chemokines, including SDF1α, CCL19, CCL21, and CCL25, have been shown to be important in the homing of developing thymocytes to their specific microenvironment (25). Disruption of these chemokine/chemokine receptor signaling (29−31), or the timing of the expression of chemokine receptors in thymocytes (32), can result in the perturbation of their maturation pathway. Therefore, identification of chemokine receptors expressed in early hematopoietic progenitors may lead to insights into their localization in the BM, and whether distinct microenvironments exist to support differentiation of different cell lineages. In fact, analysis of the expression of the receptor for a thymusspecific chemokine in MPPs have led to the identification of a population of thymic immigrants that contribute to T lineage differentiation (33). This will be further discussed in the following section.
Do T and B cell lineage diverge from a common or separate progenitor?
The thymus is the site of T cell differentiation and maturation. However, unlike BM HSCs, immature thymocytes do not have self-renewal ability. Thus, the thymus requires continuous replenishment of progenitors from the BM in order to sustain T cell production (34). The nature of thymic immigrants from BM remains a subject of debate.
In order to understand the connection between BM progenitors and the earliest T lineage progenitors (ETPs) in the thymus, significant research effort has been spent in characterizing the most immature thymocytes. During the earliest stages of T cell development in the thymus, progenitors which are CD4 and CD8 double negative (DN), can be subdivided into DN1-DN4 maturational stages (35). T lineage commitment is thought to occur at DN3 stage, as DN1 and DN2 progenitors retain differentiation potential for other hematopoietic cell types, including B cells, macrophages, NK, and DCs (17). The DN1 population, defined as CD44+CD25−, is highly heterogeneous (36). Subfractionation of this population suggest that progenitors with T cell developmental potential and the most proliferative capacity reside in c-Kithigh expressing cells, which is also known as early T lineage progenitors (ETPs) (36, 37). Since B cell differentiation potential is only found in DN1 but not in DN2 progenitors, it is thought that thymic progenitors that can differentiate into B cells are the most immature cells that first arrived in the thymus. Further characterization of ETPs suggests that only the Flt3+ or CCR9+ fraction has B lineage differentiation potential (38, 39). Flt3+ or CCR9+ ETPs are therefore currently viewed as the most immature T cell progenitors in the thymus.
Although immature thymocytes retain a weak myeloid differentiation potential, it has been thought that T and B lineage bipotent progenitors from the BM should be thymus-seeding cells. Notch signaling is a critical component during intrathymic T cell development. Inhibition of Notch 1 signaling in hematopoietic progenitors results in B cell development in the thymus (40). Conversely, expression of constitutively active Notch 1 causes extrathymic T cell development in the expense of B cell differentiation in BM (41). Myeloid lineage differentiation, however, is not affected in the absence of Notch signaling. More recently, the proto-oncogene LRF has been shown to repress the expression of Notch 1. Ablation of LRF resulted in aberrant T cell development in the BM and a decreased in early B cell progenitors (42). These findings led to the interpretation that Notch signaling provides the deterministic cue in dictating T vs. B lineage differentiation in T/B bipotent progenitors. The existence of CLPs in the adult BM further support the notion that T/B bipotent progenitors are thymus-seeding cells from the BM.
Several recent studies, however, indicate that the divergence of the T cell lineage should occur upstream of CLPs in BM. Since ETPs are unresponsive to IL-7 (37), and do not express IL-7Rα, the defining marker of CLPs (7), the idea of CLPs as physiological T cell progenitors was disputed. Although CLPs readily give rise to T cells when injected into thymus, intravenous injection of CLPs only weakly contributed to thymopoiesis (7, 33). In addition, while ETPs retains a weak GM differentiation potential, myeloid differentiation is silenced at the CLP stage (7). Genetic studies of Ikaros, which have shown to be critical for all lymphocyte lineage development, revealed the different stage requirement of this gene in T and B lineage differentiation. While the generation of CLPs requires Ikaros, some developing thymocytes are present in Ikaros-null mice, suggesting the possibility of an earlier divergence of the T cell lineage from B and NK precursors (43)
The nature of these findings revealed that the intrinsic T cell differentiation potential of CLPs do not necessarily reflect their physiological contribution to thymopoiesis. Thymic homing of progenitors from the BM adds another level of complexity to the nature of T lineage progenitors (44). In addition to intrinsic T cell differentiation potential, thymic immigrants need to have the ability to become mobilized from the BM and subsequently home to the thymus from circulation. Accumulating evidence implicates that subsets of MPPs, which are developmentally upstream of CLPs in the BM, are thymic seeding cells (45, 46). MPPs are phenotypically similar to ETPs (c-KithighFlt3+Thy1−), have robust T cell differentiation potential, and can be found in the peripheral blood (3, 47, 48).
In addition to clarifying the mechanism of lymphoid vs. myeloid lineage differentiation, characterization of MPPs have also led to insights into the divergence of T and B lineage in the BM. Perry et al. demonstrated that MPPs that express CD62L have enhanced thymic repopulating capacity (45). This MPP population overlaps significantly with ELPs (49). Since these cell populations, as well as Flt3hiVCAM−1− MPPs, much more efficiently generate T cells upon intravenous injection than CLPs, it was speculated that these MPPs have an enhanced thymus-homing mechanism that is lacking in CLPs. A small population of MPPs was found to express CCR9 (33, 50), the chemokine receptor for CCL25, which is expressed in the thymus but not BM. Importantly, CCR9 expression is not detected on CLPs (33). CCR9+ MPPs have robust thymus-homing and repopulating capability, and overlap with CD62L+ MPPs as well as ELPs (33). However, since a significant portion of CD62L+ MPPs or ELPs is CCR9−, this suggests that CCR9+ MPPs represent a more enriched population of thymic immigrants from the BM.
Dissecting the heterogeneity of MPPs in the BM has led to the identification of a rare population of thymic immigrants that contribute to T cell development. This finding provides the missing link that connects the progenitors in the BM and the thymus, and led to insights into the developmental potential of T lineage progenitors before they home to the thymus. Since CCR9+ MPPs, the BM precursor to ETPs, is developmentally more immature than lymphoid committed CLPs, thus confirming recent speculations that CLPs are not the major “physiological” T cell progenitors. Nonetheless, the characterization of CCR9+ MPPs demonstrated that these cells are lymphoid specified or highly lymphoid biased, which indicate that the divergent of the T cell lineage occurs after the major branching point of the lymphoid-myeloid pathways, as predicted by the “classical” model of hematopoiesis. With the identification of thymic immigrants in the BM, it is now possible to investigate the mechanism of their mobilization from BM and their subsequent homing to the thymus.
More comparative analysis between multiple BM progenitors with T cell differentiation potential will be important to clarify whether only one or multiple BM populations contribute to thymopoiesis in the steady-state. Results from these studies may have implications in improving BM transplantation strategies in the clinical setting. BM transplantation is clinically applied to treat leukemia and congenital immunodeficient patients (51). However, it takes months to fully reconstitute T cells after BM transplantation (52). During this period of time, patients are susceptible to opportunistic infections. Therefore, transplanting an increased number of BM progenitors that can contribute to T cell development may facilitate the reconstitution of the peripheral T cell compartment. It is also intriguing to know whether ectopic introduction of CCR9 into CLPs can enhance their thymic homing capability. Since the kinetics of T cell development from CLPs is 1–2 weeks faster than MPPs when injected intrathymically (7, 12), T cell reconstitution may be accelerated by enhancing the ability of CLPs to home to the thymus.
Future directions
Studies of the MPP subsets at the molecular level will be key to resolve the mechanism of lineage specification and commitment. It has been demonstrated that lymphoid and myeloid related genes could be co-expressed in lymphoid specified MPPs (11, 53). It is unclear how lymphoid and myeloid genes cross-regulate at this developmental stage to silence myeloid related genes, which may be a main drive to establish lymphoid lineage commitment in CLPs. Although lymphoid related genes such as RAG1, IL-7Rα, and EBF are expressed in MPPs (13), these genes are not involved in lymphoid specification. In the absence of these genes, developmental arrest occurs after lymphoid commitment in early B or T cell progenitor stages (9, 10, 54–56). Therefore, an unidentified transcription factor is likely to be involved in lymphoid specification. Gene expression profiling of the MPP subsets will be important in identifying novel genes involved in this process.
Identification of the earliest lymphoid specific gene expressed during early hematopoiesis may also provide a strategy for genetic marking of the earliest lymphoid specified MPPs in a reporter mouse. This will enable us to trace the lineage contributions of these lymphoid specified, but not committed, progenitors in vivo without any manipulations. Our studies thus far involve FACS purification of BM cell populations and injection back into irradiation conditioned recipients. In this system, we cannot ensure the proper homing of progenitors to their specific microenvironment in the BM, which may affect their differentiation to certain cell lineages. For example, it is not clear whether the weak differentiation ability of VCAM-1− MPPs to GM cells after in vivo injection is indeed the case under the physiological setting, or whether GM differentiation is simply due to improper homing of some VCAM-1− MPPs to a different microenvironment upon transplantation into the recipient mice. Furthermore, it is also possible that the differentiation potential of hematopoietic progenitors may be altered due to stress in conditioned hosts. Using a gene-marking system to analyze the differentiation potential of MPPs will also resolve an important drawback of the transplantation system as pointed out by Forsberg et al (57). Since the burst size of MPPs is small, a weak or low contribution to a certain cell lineage (especially myeloid lineage cells with short lifespan and high turnover rate) by MPP transplanted in small numbers may not be readily detected.
In addition to transcription factors, increasing evidence suggests that microenvironements in the BM may play a role in lymphoid specification and commitment. Therefore, it will be equally as important to characterize the different non-hematopoietic cells of the BM, in order to identify the cellular components that form the putative lymphoid progenitor niche. Since chemokines play important roles in the migration and homing of cells, identification of chemokines produced by non-hematopoietic cells, as well as chemokine receptors expressed on MPPs, may help identify these distinct microenvironments. These future studies will contribute to the understanding of how extrinsic and intrinsic factors in orchestrating lineage commitment during hematopoiesis.
Acknowledgments
We apologize to those whose work was not cited owing to space limitations. Our work has been supported by NIH R01 AI056123 and R01 CA098129. M.K. is a scholar of Leukemia & Lymphoma society.
Abbreviations
- HSC
hematopoietic stem cell
- CLP
common lymphoid progenitor
- CMP
common myeloid progenitor
- MPP
multipotent progenitor
- ELP
early lymphoid progenitor
- MegE
megakaryocyte/erythrocyte
- GM
granulocyte/macrophage
- NK
natural killer
- DC
dendritic cell
- ETP
early T lineage progenitor
- DN
double negative
- DP
double positive
- BM
bone marrow
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287:1442–1446. doi: 10.1126/science.287.5457.1442. [DOI] [PubMed] [Google Scholar]
- 2.Wu L, Vandenabeele S, Georgopoulos K. Derivation of dendritic cells from myeloid and lymphoid precursors. International Reviews of Immunology. 2001;20:117–135. doi: 10.3109/08830180109056726. [DOI] [PubMed] [Google Scholar]
- 3.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Bryder D, Adolfsson J, Nygren J, Mansson R, Sigvardsson M, Jacobsen SE. Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3-short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 6.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells.[see comment] Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 8.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 9.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. Journal of Experimental Medicine. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. Journal of Experimental Medicine. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, Anderson K, Sitnicka E, Sasaki Y, Sigvardsson M, Jacobsen SE. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Lai AY, Lin SM, Kondo M. Heterogeneity of flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- 13.Lai AY, Kondo M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J Exp Med. 2006;203:1867–1873. doi: 10.1084/jem.20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igarashi H, Gregory S, Yokota T, Sakaguchi N, Kincade P. Transcription from the RAG1 Locus Marks the Earliest Lymphocyte Progenitors in Bone Marrow. Immunity. 2002;17:117. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 15.Martin CH, Aifantis I, Scimone ML, von Andrian UH, Reizis B, von Boehmer H, Gounari F. Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nat Immunol. 2003;4:866–873. doi: 10.1038/ni965. [DOI] [PubMed] [Google Scholar]
- 16.Krueger A, Garbe AI, von Boehmer H. Phenotypic plasticity of T cell progenitors upon exposure to Notch ligands. J Exp Med. 2006;203:1977–1984. doi: 10.1084/jem.20060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo M, Scherer DC, King AG, Manz MG, Weissman IL. Lymphocyte development from hematopoietic stem cells. Curr Opin Genet Dev. 2001;11:520–526. doi: 10.1016/s0959-437x(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 18.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 20.Rafii S, Mohle R, Shapiro F, Frey BM, Moore MA. Regulation of hematopoiesis by microvascular endothelium. Leukemia & Lymphoma. 1997;27:375–386. doi: 10.3109/10428199709058305. [DOI] [PubMed] [Google Scholar]
- 21.Kiel MJ, Radice GL, Morrison SJ. Lack of Evidence that Hematopoietic Stem Cells Depend on N-Cadherin-Mediated Adhesion to Osteoblasts for Their Maintenance. Cell Stem Cell. 2007;1:204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche.[see comment] Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches.[see comment] Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Suda T, Arai F, Hirao A. Hematopoietic stem cells and their niche. Trends Immunol. 2005;26:426–433. doi: 10.1016/j.it.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nature Reviews. Immunology. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 26.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 28.Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19:257–267. doi: 10.1016/s1074-7613(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 29.Ara T, Itoi M, Kawabata K, Egawa T, Tokoyoda K, Sugiyama T, Fujii N, Amagai T, Nagasawa T. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. Journal of Immunology. 2003;170:4649–4655. doi: 10.4049/jimmunol.170.9.4649. [DOI] [PubMed] [Google Scholar]
- 30.Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, Kakiuchi T, Lipp M, Boyd RL, Takahama Y. CCR7 signals are essential for cortex-medulla migration of developing thymocytes.[see comment][erratum appears in J Exp Med. 2004 Oct 4;200(7):following 946] Journal of Experimental Medicine. 2004;200:493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 32.Uehara S, Hayes SM, Li L, El-Khoury D, Canelles M, Fowlkes BJ, Love PE. Premature expression of chemokine receptor CCR9 impairs T cell development. Journal of Immunology. 2006;176:75–84. doi: 10.4049/jimmunol.176.1.75. [DOI] [PubMed] [Google Scholar]
- 33.Lai AY, Kondo M. Identification of a bone marrow precursor of the earliest thymocytes in adult mouse. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6311–6316. doi: 10.1073/pnas.0609608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwarz BA, Bhandoola A. Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis. Immunological Reviews. 2006;209:47–57. doi: 10.1111/j.0105-2896.2006.00350.x. [DOI] [PubMed] [Google Scholar]
- 35.Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 36.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Allman D, Sambandam A, Kim S, Miller JP, Pagan A, Well D, Meraz A, Bhandoola A. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 38.Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein RM, Aster JC, Pear WS, Bhandoola A. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol. 2005;6:663–670. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- 39.Benz C, Bleul CC. A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J Exp Med. 2005;202:21–31. doi: 10.1084/jem.20050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson A, MacDonald HR, Radtke F. Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus.[see comment] Journal of Experimental Medicine. 2001;194:1003–1012. doi: 10.1084/jem.194.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC, Pear WS. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 42.Maeda T, Merghoub T, Hobbs RM, Dong L, Maeda M, Zakrzewski J, van den Brink MR, Zelent A, Shigematsu H, Akashi K, Teruya-Feldstein J, Cattoretti G, Pandolfi PP. Regulation of B versus Tlymphoid lineage fate decision by the proto-oncogene LRF.[see comment] Science. 2007;316:860–866. doi: 10.1126/science.1140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M, Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 44.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. Journal of Experimental Medicine. 2001;193:365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry SS, Wang H, Pierce LJ, Yang AM, Tsai S, Spangrude GJ. L-selectin defines a bone marrow analog to the thymic early T-lineage progenitor. Blood. 2004;103:2990–2996. doi: 10.1182/blood-2003-09-3030. [DOI] [PubMed] [Google Scholar]
- 46.Perry SS, Pierce LJ, Slayton WB, Spangrude GJ. Characterization of thymic progenitors in adult mouse bone marrow. J Immunol. 2003;170:1877–1886. doi: 10.4049/jimmunol.170.4.1877. [DOI] [PubMed] [Google Scholar]
- 47.Yang L, Bryder D, Adolfsson J, Nygren J, Mansson R, Sigvardsson M, Jacobsen S. Identification of Lin-Sca1+kit+CD34+Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated recipients. Blood. 2004 doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
- 48.Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004 doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- 49.Perry SS, Welner RS, Kouro T, Kincade PW, Sun XH. Primitive lymphoid progenitors in bone marrow with T lineage reconstituting potential. J Immunol. 2006;177:2880–2887. doi: 10.4049/jimmunol.177.5.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE, Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. Journal of Immunology. 2007;178:2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- 51.Sykes M, Nikolic B. Treatment of severe autoimmune disease by stem-cell transplantation. Nature. 2005;435:620–627. doi: 10.1038/nature03728. [DOI] [PubMed] [Google Scholar]
- 52.Schwinger W, Weber-Mzell D, Zois B, Rojacher T, Benesch M, Lackner H, Dornbusch HJ, Sovinz P, Moser A, Lanzer G, Schauenstein K, Ofner P, Handgretinger R, Urban C. Immune reconstitution after purified autologous and allogeneic blood stem cell transplantation compared with unmanipulated bone marrow transplantation in children. British Journal of Haematology. 2006;135:76–84. doi: 10.1111/j.1365-2141.2006.06244.x. [DOI] [PubMed] [Google Scholar]
- 53.Mansson R, Hultquist A, Luc S, Yang L, Anderson K, Kharazi S, Al-Hashmi S, Liuba K, Thoren L, Adolfsson J, Buza-Vidas N, Qian H, Soneji S, Enver T, Sigvardsson M, Jacobsen SE. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26:407–419. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 54.Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]
- 56.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 57.Forsberg EC, Serwold T, Kogan S, Weissman IL, Passegue E. New evidence supporting megakaryocyte-erythrocyte potential of flk2/flt3+ multipotent hematopoietic progenitors. Cell. 2006;126:415–426. doi: 10.1016/j.cell.2006.06.037. [DOI] [PubMed] [Google Scholar]


