Abstract
St. John's Wort is commonly known for its antiviral, antidepressant, and cytotoxic properties, but traditionally St. John's Wort has also been used to treat inflammation. In this study, we sought to characterize the mechanisms used by St. John's Wort to treat inflammation by examining the effect of the recently isolated protein from St. John's Wort, p27SJ on the expression of MCP-1. By employing an adenovirus expression vector, we demonstrate that a low concentration of p27SJ upregulates the MCP-1 promoter through the transcription factor C/EBPβ. In addition, we found that C/EBPβ-homologous protein (CHOP) or siRNA-C/EBPβ significantly reduced the ability of p27SJ to activate MCP-1 gene expression. Results from protein-protein interaction studies illustrate the existence of a physical interaction between p27SJ and C/EBPβ in microglial cells. The use of chromatin immunoprecipitation assay (ChIP) led to the identification of a new cis-element that is responsive to C/EBPβ within the MCP-1 promoter. Association of C/EBPβ with MCP-1 DNA was not affected by the presence of p27SJ. The biological activity of MCP-1 produced by cultures of adenovirus-p27SJ transduced cells was increased relative to controls as measured by the transmigration of human Jurkat cells. Thus, we conclude that at high concentration, p27SJ is a potential agent that may be developed as a modulator of MCP-1 leading to the inhibition of the cytokine-mediated inflammatory responses.
INTRODUCTION
Hypericum perforatum L. (Hypericaceae), popularly called St. John's Wort, has been used in popular medicine since ancient times for several disorders such as skin wounds, eczema, burns, and diseases of the alimentary tract, insomnia, and mental illness, among others [1]. H. perforatum extract contains flavonoids such as rutin, quercetin, and quercitrin, which have a free radical scavenging activity in a model of auto-oxidation of rat cerebral membranes [2]. Thus Hypericum extract has a potential antioxidant activity, which may be of value in treating dementia as well as other disorders of senility in which free radical generation is implicated. In addition, besides its antidepressant activities, H. perforatum also possesses anxiolytic, antiviral, wound healing, antimicrobial, analgesic, and anti-inflammatory effects [3]. Antidepressant, analgesic, anti-inflammatory, antioxidant, antimicrobial, and wound healing effects have also been found for other species of the genus Hypericum. [4]. More recently, H. perforatum extract has been reported to efficiently attenuate interferon-γ (IFN-γ)-elicited activation of STAT-1 in alveolar A549/8 and colon DLD-1 cells [5]. p27SJ is a biologically active protein that we have recently described, which extracted and purified from a laboratory callus culture of H. perforatum [6]. We recently demonstrated the ability of the C/EBPβ and p27SJ to physically and functionally associate and that this association leads to the suppression of HIV-1 gene expression [6].
C/EBPβ belongs to a family of basic region-leucine zipper (bZIP) transcription factors that bind to DNA in a sequence-specific manner as dimers and regulate the transcription of genes involved in proliferation and differentiation [7, 8]. The C/EBPβ gene is transcribed into a single 1.4 kb mRNA [9, 10]. At the protein level, however, multiple C/EBPβ isoforms, varying in size from 14 to 40 kDa, have been reported [10]. The C/EBPβ isoforms include full-length and LAP (Liver-enriched Activator Protein) isoform (40 and 35 kDa) and two truncated 14 and 21 kDa LIP (Liver-enriched Inhibitory Protein) isoform [10]. Another member of the C/EBP family is called CHOP (C/EBP-Homologous Protein), and acts in most, but not all circumstances as a dominant-negative inhibitor of DNA-binding when it is heterodimerized to another C/EBP partner [11]. C/EBPβ binding sites have been identified in the promoter regions of numerous genes, including HIV-1 LTR [12], IL-6 [13], TNF-α [14], and MCP-1 [15]. Moreover, the activity of C/EBPβ is influenced by a variety of inflammatory stimuli, including LPS [16], IL-6 [17], and TNF-α [18].
The monocyte chemoattractant protein (MCP-1) is a potent chemotactic factor for monocytes. MCP-1 is produced constitutively, or after induction by oxidative stress, cytokines, or growth factors, by a variety of cell types, including monocytes, smooth muscle cells, and endothelial cells. It regulates the migration and infiltration of monocytes, memory T lymphocytes, and natural killer cells (NK) cells [19]. Increased expression of MCP-1 mRNA or protein has been associated with a variety of human diseases (e.g. AIDS) [20]. MCP-1 expression is induced by inflammatory mediators, such as TNF-α, platelet-derived growth factor (PDGF) BB, IL-1β, and IFN-γ [19]. Agents that suppress inflammation, including retinoic acid, dexamethasone, and estrogen can suppress the induction of MCP-1 [21]. The MCP-1 promoter is composed of two upstream regulatory regions, distal and proximal, separated by 2.2 kb of DNA [22]. The proximal regulatory region, which is required for all aspects of MCP-1 gene expression contains two elements, κB [23] and a GC-rich domain [24] which are binding sites for NF-κB and Sp1 proteins, respectively. A third element is also found which is known as site B for which binding proteins have not yet been identified. The MCP-1 promoter also contains a classical CAAT box, which can serve as a target for the C/EBPβ transcription factor [15].
Since p27SJ was shown to be a potent suppressor of the HIV-1 gene expression, we sought to investigate the effect of p27SJ on MCP-1 regulation and whether p27SJ may be involved in suppressing inflammation via MCP-1. In light of our previous findings on MCP-1 induction [25], we also examined the role of C/EBPβ in this process.
MATERIALS AND METHODS
Plasmids
The MCP-1-CAT plasmid (−500/+6) and its deletion mutants (−400/+6, −300/+6, −200/+6, −100/+6), C/EBPβ, and CHOP expression plasmids were previously described [25]. pcDNA6/myc-His-B-p27SJ plasmid was previously described [6].
Recombinant adenoviruses
p27SJ cDNA (788 bp) excised from pcDNA6/myc-His-B-p27SJ and cloned into EcoRI and NheI sites of the adenovirus-shuttle plasmid pDC515 under the control of the murine cytomegalovirus promoter (purchased from Microbix Inc. Ontario, Canada). Adeno-p27SJ recombinant shuttle containing p27SJ sequence (pDC515-p27SJ) was transfected into HEK-293 cells with pBHGfrt (del) E1, 3FLP, a plasmid that provides adenovirus type-5 genome deleted in E1 and E3 genes. Plaques of recombinant adenovirus arising as a result of frt/ FLP recombination were isolated, grown and purified by cesium chloride density equilibrium banding as previously described [26]. Empty shuttle plasmid, pDC515, was used to construct control adenoviral vector (Adeno-null, a virus without any transgene). Adeno-p27SJ or adeno-null were used at an MOI of 0.5, 1, and 10 plaques forming units per cell. [MOI= multiplicity of infection].
Cell Culture, and Transfection Assays
Human astroglioma (U-87MG) or microglial cells [27] were maintained in DMEM + 10% FBS. Cells were transfected with 0.5 µg of reporter plasmid (MCP-1-CAT) or co-transfected with 0.5 µg of various expression cDNAs as previously described [28]. At 4 hours post transfection, the cells were transduced with adeno-p27SJ or adeno-null. The amount of DNA used for each transfection was normalized with pcDNA6 vector plasmids. Each transfection was repeated multiple times with different plasmid preparations. Cell extracts were prepared 24 h after transfection, and CAT assays were performed as previously described [29].
Immunoprecipitation and Western blotting
Microglial cells were infected with adeno-null or adeno--p27SJ virus at an MOI of 1 at 37°C. Twenty-four hours post-infection, 200 µg of cell extracts were immunoprecipitated with antibodies as indicated. Western blot analysis was performed as described previously [30] using anti-C/EBPβ (Santa Cruz), or anti-c-myc/His6 (Invitrogen) antibodies. Note that the pcDNA6/myc-His-B plasmid contains both tags, which gave us the flexibility to use antibodies against either one.
RNA interference
Transient knockdown of C/EBPβ was performed with a C/EBPβ-specific siRNA (Dharmacon Research Inc., Lafayette, CO) [31]. After 24 h of cell plating, microglial cells were rinsed once with Optimem. siRNA was added at a final concentration of 50 nm as previously described [28]. Western blot analysis was performed with protein extracts from untransfected cells or cells transfected with C/EBPβ specific siRNA using anti-C/EBPβ antibody. For a loading control, anti-Grb2 antibody was used. Control non-targeting siRNA was also obtained from Dharmacon.
Chromatin Immunoprecipitation (ChIP) Assay
Microglial cells were grown overnight in 100-mm dishes to 60–70% confluency; cells were then infected with adeno-null or adeno-p27SJ as described above. Plates were returned to the incubator for 40–48 h. Cells were cross-linked with formaldehyde, harvested, and ChIP was performed. For these studies, only 5 × 106 cells were used per immunoprecipitation reaction because the plasmid (MCP-1 CAT) is present at a high copy number. The remainder of the procedure followed standard protocols for ChIP analysis according to the manufacturer’s protocol (Upstate Biotechnology, Lake Placid, NY). The resulting DNA was analyzed by PCR reactions using MCP-1 primers. Antibody used in the ChIP procedure is anti-His or anti-C/EBPβ (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) as well as rabbit anti-mouse IgG. The following primers were used for ChIP assay:
F1 (−479/−457): 5’ TCCTACAGTTCTGCTAGGCTTC 3’
F2 (−390/−370): 5’ GCTTGAGAGCTCCTTCCTGG 3’
F3 (−273/−253): 5’ CGCTTCACAGAAAGGCAGAAT 3’
F4 (−190/−170): 5’ CCCCATTTGCTCATTTGGTC 3’
R1 (−360/−380): 5’ GGCCTCCCAGCCAGGAAGGA 3’
R2 (−273/−259): 5’ CTTTCTGTGAAGCGAAAACTG 3’
R3 (−160/−180): 5’ TCACTGCTGAGACCAAATGA 3’
R4 (−39/−59): 5’ AGGAGGGATCTTCCATGAGT 3’
Transmigration Assay
Microglial cells were infected with adeno-null or adeno-p27SJ in serum free media as previously described [32]. Media were collected and placed in wells of a 24 well plate. Jurkat cells, in serum-free medium, were placed in 3.0 µm pore sized fluorescence blocking PET track-etched membrane HTS FluoroBlock™ cell inserts (Becton Dickinson, Franklin Lakes, NJ). Jurkat cells migration towards serum-free medium or medium containing 2% serum and were used as negative and positive controls, respectively.
RESULTS
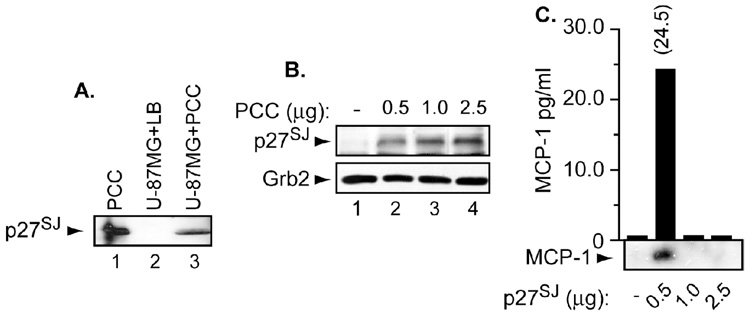
We first examined whether p27SJ can enter the cells under normal physiological conditions especially since p27SJ is a part of Hypericum perforatum L., which are normally taken orally. Therefore, we treated the cells with 8 µg of purified callus culture of H. perforatum or lysate buffer for three hours after which the cell lysates were prepared. Fifty micrograms of cell lysates were subjected for Western blot analysis using anti-p27SJ polyclonal antibody. Note that 200 ng of pure callus culture (PCC) were used as a control (lane 1). As shown in Figure 1A, p27SJ was detected in cell lysates (lane 3) and in the control (lane 1) but not in lysis buffer (LB) (lane 2). These results confirm the ability of p27SJ to enter the cells under normal physiological conditions.
Figure 1. Expression of p27SJ under normal physiological conditions and the status of MCP-1.
A, B. U-87MG (A) and microglial (B) cells were treated with purified callus culture of H. perforatum. Fifty micrograms of cell lysates were subjected for Western blot analysis using anti-p27SJ rabbit polyclonal antibody. Arrows depict the position of p27SJ protein. Anti-Grb2 was used for equal protein loading. C. MCP-1 levels were measured in supernatants collected from microglial cells treated with an increasing amount of p27SJ by ELISA and or Western blot. Number on the top of the bar represents the amount of MCP-1 secreted in the presence of p27SJ as measured by ELISA. Arrow depicts the position of MCP-1 protein. Histogram represents amount of MCP-1 secreted following p27SJ treatment.
Using an increasing concentration of PCC, we next sought to examine whether the levels of p27SJ protein change in these cells. Microglial cells were treated with an increasing concentration of PCC (0.5 – 2.5 µg) for 24 h, after which the proteins lysates were prepared. Fifty micrograms of cell lysates were subjected to Western blot analysis with anti-p27SJ and −Grb2 antibodies. As shown in Figure 1B, an increasing amount of p27SJ was detected in these cells (p27SJ panel). Anti-Grb2 was used for equal protein loading.
Following PCC treatment, we investigated the impact of increasing amount of p27SJ protein on MCP-1 secretion. Microglial cells were treated with an increasing amount of p27SJ for 24h after which the supernatant were collected from these cells and subjected to ELISA to examine the levels of MCP-1. MCP-1 ELISA kit was purchased from Amersham and contains anti-MCP-1 antibody. In parallel, Western blot analysis was performed using 30 µl of these supernatants. As shown in Figure 1C, MCP-1 was only detected in the supernatant prepared from cells treated with only 0.5 µg of p27SJ. These results were also confirmed using ELISA where 24.5 pg/ml of MCP-1 were only detected in the supernatant prepared from cells treated with 0.5 µg of p27SJ. These results confirm that p27SJ has the capability on inducing secretion of the MCP-1 protein in the supernatant at low concentration. However, at high concentration, p27SJ suppresses secretion of the MCP-1 protein.
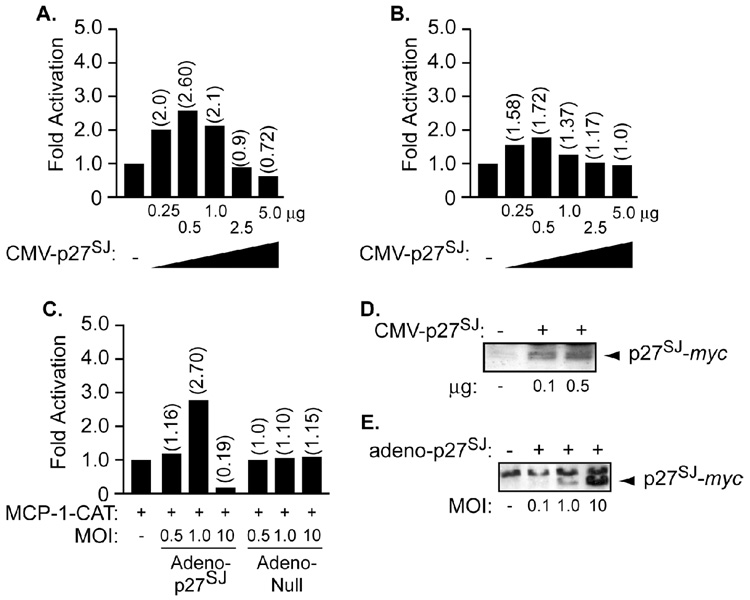
Next, we sought to examine whether p27SJ can affect MCP-1 at transcriptional levels. Since we examined the effect of p27SJ in U-87MG cells, we decided to include them in our study as a control. Microglial (Figure 2A) and U-87MG (Figure 2B) cells were transfected with 0.5 µg of the full-length MCP-1 promoter fused to the reporter gene CAT (MCP-1-CAT), alone or with an increasing amount of pCDNA-6 Myc/His-B-p27SJ (0.25, 0.5, 1.0, 2.5 and 5.0 µg). Twenty-four hours post-transfection, the cells were washed, harvested and assayed for CAT activity. As shown in Figure 2A and B, transfection of the cells with 0.25 µg of p27SJ was enough to upregulate the MCP-1 promoter by ~ 2 fold, while other concentration (2.5 and 5.0 µg) had no or a negative effect on the MCP-1 promoter. In parallel, we sought to examine the effect of adeno-p27SJ on the MCP-1 promoter.
Figure 2. Transcription regulation of MCP-1 by p27SJ.
A, B, C. Human microglial (A and C) and U-87MG (B) cells were transfected with 0.5 µg of MCP-1-CAT reporter plasmid alone or combined with increasing amounts of transfected (A, B) or infected with adeno-p27SJ (C) as indicated. Adeno-null was used as a negative control. CAT activity was determined 24 h after transfection. D, E. Twenty micrograms of extracts prepared from non-transfected, p27SJ-transfected U-87MG cells (D), or from uninfected or p27SJ-infected cells (E) were subjected to Western blot analysis using anti myc antibody. The arrows point to the position of p27SJ. The values shown on the top of each bar represent the fold activation over the basal promoter activity, which is arbitrarily set at one. The data represent the mean value of at least three separate transfection experiments.
To that end, the cells were transfected with 0.5 µg of MCP-1-CAT alone or with infection by an increasing amount of adeno-p27SJ or adeno-null (MOI = 0.5, 1, and 10). Note that the cells were infected with adeno-p27SJ or adeno-null at 4 h after the transfection. Twenty-four hours post-infection, the cells were washed, harvested and assayed for CAT activity. As shown in Figure 2C, infection of the cells with an MOI of 1 of adeno-p27SJ was enough to upregulate the MCP-1 promoter, while other concentration had no or a negative effect on the p27SJ promoter. No effect was observed in cells transfected with the MCP-1 promoter and infected with the adeno-null virus. Expression of p27SJ was examined by Western blot analysis using cellular extract prepared from non-transfected, U-87MG-transfected with pcDNA6/myc-His-B-p27SJ (0.1 and 0.5 µg) (Figure 2D), non-infected or infected with adeno-p27SJ (MOI = 0.1, 1, and 10) (Figure 2E) for 24 h. Anti c-myc antibody was used to detect expression of p27SJ in these extracts (Panels D and E).
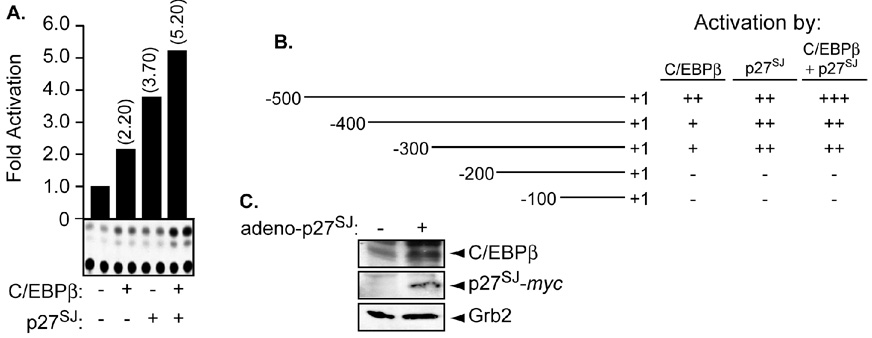
Later, we examined the ability of p27SJ to activate the MCP-1 promoter in the presence of C/EBPβ. The ability of C/EBPβ to physically and functionally cooperate with p27SJ [6] gave us rationale to examine its involvement, if any with p27SJ in regulating the MCP-1 promoter. Microglial cells were transfected in duplicate with 0.5 µg of MCP-1 promoter alone or in the presence of 0.5 µg of C/EBPβ expression plasmid followed by infection of 1 MOI of adeno-p27SJ. Twenty-four hours after transfection, the cells were harvested and processed for CAT assay. As shown in Figure 3A, transfection of C/EBPβ or p27SJ leads to a modest activation of the MCP-1 promoter (compare lane 1 to lanes 2 and 3). Interestingly, when the transfection was performed using both plasmids, activation of the MCP-1 promoter was higher than that observed with individual plasmid (lane 4) (compare lane 4 to lanes 2 and 3).
Figure 3. Transcription regulation of MCP-1 by p27SJ and C/EBPβ.
A, B. Microglial cells were transfected with 0.5 µg of MCP-1-CAT reporter plasmid (full length) (A) or deletion mutants (B) alone or in the presence of 0.5 µg of C/EBPβ and/or infected with adeno-p27SJ at an MOI of 1 as indicated. The values shown on the top of each bar represent the fold activation over the basal promoter activity, which is arbitrarily set at one. The values shown on the right represent significant activation (+++ or ++), modest activation (+), or no effect (−) of p27SJ and/or C/EBPβ over the basal promoter. The data represent the mean value of at least three separate transfection experiments. An example of CAT assay is also displayed (A). C. Twenty micrograms of extracts prepared from uninfected or p27SJ-infected microglial cells were subjected to Western blot analysis using anti-C/EBPβ, -myc, or −Grb2 antibodies. The arrows point to the positions of different proteins. Anti-Grb2 was used to control equal protein loading.
In order to identify the cis-element within the MCP-1 promoter responsible for p27SJ activation, we used a series of MCP-1 promoter deletion mutants. Microglial cells were transfected with 0.5 µg of the MCP-1 promoter deletion mutants either alone or in the presence of 0.5 µg of C/EBPβ expression plasmid followed by infection of adeno-p27SJ at an MOI of 1. The results from CAT assays demonstrate that the region between nucleotide −300 and − is responsible for C/EBPβ and/or p27SJ activation (Figure 3B). The levels of C/EBPβ in microglial cells transduced with p27SJ was also examined. As shown in Figure 3C, endogenous levels of C/EBPβ was induced in extracts prepared from p27SJ -tranduced cells but not from the mock. As a control for equal protein loading anti-Grb2 was used.
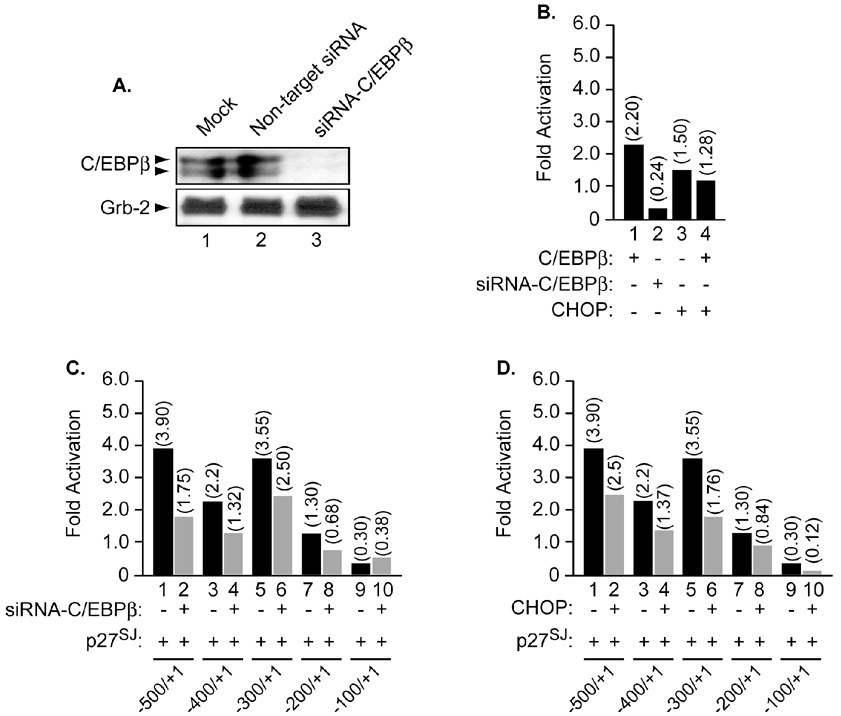
To further demonstrate the specificity of this functional interaction, and to examine whether p27SJ requires the presence of C/EBPβ to activate the MCP-1 promoter, similar experiments were performed using either a dominant negative form of C/EBPβ, CHOP, or a small interference RNA directed against C/EBPβ (siRNA-C/EBPβ). The efficiency of siRNA-C/EBPβ in abrogating endogenous C/EBPβ expression was determined by Western blot (Figure 4A, compare lanes 1 and 3). The use of non-target siRNA did not affect expression of C/EBPβ (compare lanes 1 and 2). Note that transfection of the cells with siRNA was performed as described in Materials and Methods.
Figure 4. Functional interplay between C/EBPβ and p27SJ in the presence of CHOP and siRNA-C/EBPβ.
A. Microglial cells were transfected with non-target siRNA or with siRNA-C/EBPβ as described in Materials and Methods. Cellular proteins were then prepared and subjected to Western blot analysis using anti-C/EBPβ antibody. Arrows point to the positions of C/EBPβ proteins. Equal loading of proteins was controlled using anti Grb-2 antibody (lower panel). Next, microglial cells were transfected with 0.5 µg of the MCP-1 promoter alone or in the presence of CHOP (B, D) or siRNA-C/EBPβ (B, C). Four hours post transfection, the cells were infected with adeno-p27SJ at an MOI of 1 (C, D) or adeno-null (data not shown). CAT activity was determined 24 h after transfection. The values shown on the top of each bar represent the fold activation over the basal promoter activity which is arbitrarily set at one. The data represent the mean value of at least three separate transfection experiments.
Next, we examined the ability and efficiency of CHOP or the siRNA-C/EBPβ to silence C/EBPβ gene. Microglial cells were transfected with 0.5 µg of the MCP-1 promoter alone or in the presence of 0.5 µg of CHOP expression plasmid or with 50 nM siRNA-C/EBPβ alone or in the presence of 0.5 µg of C/EBPβ expression plasmid. Twenty-four hours later, the cells were collected, washed and subjected to CAT assay. As shown in Figure 4B, addition of CHOP (lane 4) or siRNA-C/EBPβ (lane 2) inhibits the ability of C/EBPβ to activate the MCP-1 promoter (compare lane 1 to lanes 2 and 4).
We then examine the ability of p27SJ to activate the MCP-1 promoter in the presence of siRNA-C/EBPβ. Microglial cells were transfected with MCP-1 promoter deletion mutants alone or in the presence of p27SJ and/or siRNA-C/EBPβ. As illustrated in Figure 4C, addition of siRNA-C/EBPβ decreased the ability of p27SJ to activate the MCP-1 promoter (compare lanes 2, 4, 6, 8 and 10 to lanes 1, 3, 5, 7 and 9). Similar results were obtained when p27SJ was co-expressed with CHOP (Figure 4D). Interestingly, inhibition of C/EBPβ by RNAi or CHOP partially decreases activation of the MCP-1 promoter (panels C and D, lanes 2 and 4, respectively). Therefore, we concluded that regulation of the MCP-1 promoter by p27SJ partially depends on C/EBPβ and that other factors might be involved, which remain to be identified.
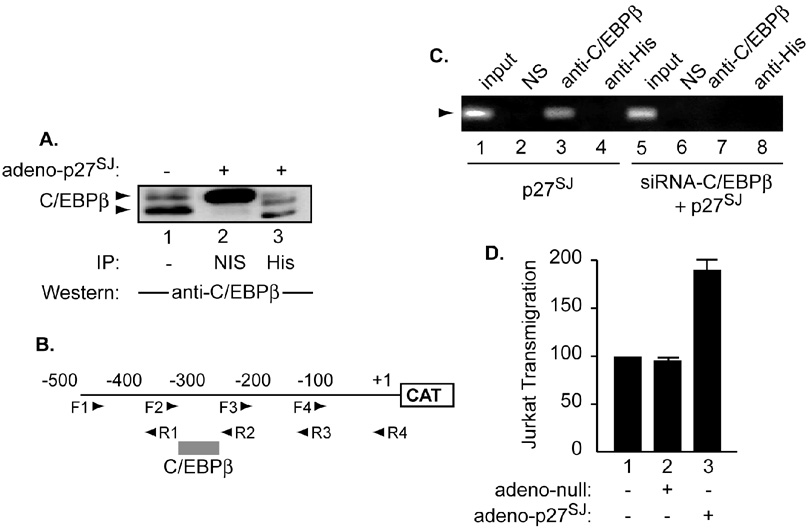
However, the existence of a functional interplay between p27SJ and C/EBPβ gave us a rationale to examine whether a physical interaction exists between these two proteins in microglial cells. The cells in serum free media were infected with adenovirus-myc-p27SJ at an MOI of 1 or with the control adeno-null virus. Two hours after the infection, the cells were washed with PBS, and complete media was added for 24 h, after which the cells were collected and 200 µg of extracts were subjected to immunoprecipitation using anti-His antibody (Figure 5A, lane 3) or with non-immune mouse serum (NIS) (lane 2). This experiment demonstrated the ability of endogenous C/EBPβ (lane 3) to interact with p27SJ (lane 3). The specificity of p27SJ:C/EBPβ interaction was confirmed using NIS. Interestingly, immunoprecipitation/Western analysis performed with extracts prepared from p27SJ-infected cells revealed that p27SJ associates with both the 38 and 35 kDa isoforms of C/EBP (compare lanes 1 and 3). Note that C/EBPγ migrates around 45 kDa as previously described [33]. No interaction was observed between extracts prepared from adeno-null infected cells and C/EBPβ (data not shown). Our data agree with previously published data regarding the physical interaction between p27SJ and C/EBPβ in astrocytes [6].
Figure 5. Association of C/EBPβ with p27SJ protein and with MCP-1 DNA.
A. Cell lysates were prepared from microglial cells infected with adeno-p27SJ or adeno-null at an MOI of 1. Two hundred micrograms of cell extract were immunoprecipitated followed by Western blot analysis utilizing anti-myc antibody (lane 3) or NIS (lane 2). Western blot analysis was performed using anti-C/EBPβ antibody. In parallel, 50 µg of total cell extracts were analyzed by direct Western blot assay (lane 1). The arrows depict the positions of the 38 and 35-kDa C/EBPβ. B. Schematic representation of the MCP-1 promoter with the positions of the various primers used for ChIP assay. Binding of C/EBPβ to MCP-1 DNA is also shown. C. Extracts prepared from microglial cells transfected with plasmid expressing C/EBPβ expression plasmid or infected with adeno-p27SJ were subjected to ChIP assay to evaluate the association of p27SJ and C/EBPβ with the DNA in the absence (lanes 1–4) and presence (lanes 5–8) of siRNA-C/EBPβ. D. p27SJ modulates MCP-1 activity in culture supernatant measured by Jurkat transmigration activity. Microglial cells were infected with adeno-null or -p27SJ for 24 h. Supernatants were harvested and analyzed by transmigration assay as described in Materials and Methods.
Next, we sought to test whether p27SJ also associates with MCP-1 DNA. Microglial cells were transfected with 1.0 µg of MCP-1-CAT. Four hours post transfection, the cells were infected with adeno-p27SJ. Forty-eight hours after transfection, the cells were harvested and processed for ChIP assay using several primers as described in Materials and Methods (Figure 5B). To examine the basal binding affinity of C/EBPβ, MCP-1-transfected/uninfected cells were also used (Figure 5C, lane 1). ChIP assay was performed using antibodies directed against C/EBPβ (lane 3) or His (lane 4). Rabbit anti-mouse serum was used as a negative control (NIS) (lane 2). As shown in Figure 5C, endogenous C/EBPβ binds to MCP-1 DNA (lane 3). It should be noted that C/EBPβ-DNA interaction was not affected by the presence of p27SJ (lane 3). The ChIP assay was also performed in the presence of siRNA-C/EBPβ (lanes 5–8). As expected, C/EBPβ failed to associate with MCP-1 DNA (lane 7), however, p27SJ was unable to associate with MCP-1 DNA in the presence or absence of C/EBPβ (lane 8). No interaction was observed when the, non-specific control, serum was used (lanes 2 and 6). These results led us to identify new cis-element within the MCP-1 promoter responsive to p27SJ activation through C/EBPβ-DNA association (Figure 5B). Further, it also demonstrated that p27SJ was unable to associate with MCP-1 DNA or to affect C/EBPβ-DNA association.
Transmigration assays have shown that MCP-1 is a potent chemoattractant agent that is believed to play an important role in inflammatory processes seen in AIDS patients [20]. Thus in order to gain information regarding the physiological effect of p27SJ on MCP-1, we performed a transmigration assay as previously described [34, 35]. Human microglial cells, maintained in serum containing media, were infected with adenovirus-p27SJ at an MOI of 1 or with the control adeno-null virus. After 24 h, the cells were removed, washed, and transferred to serum free media. Twelve hours later the conditional media was collected and incubated with Jurkat grown in serum-free medium labeled with calcein. The number of cells migrating toward the bottom chamber was determined after 0.5, 1, and 2 hours. As shown in Figure 5D, at 1 h, infection with null adenovirus did not cause any increase in the levels of Jurkat cell transmigration, however, expression of p27SJ by adenovirus-p27SJ showed a significant positive impact (80%) on the transmigration of the Jurkat cells (compare lanes 2 and 3). p27SJ failed to induce secretion of MCP-1 in cells where antibodies directed against MCP-1 or MCP-1 receptor, CCR2, were added (data not shown). These data further confirm the existence of a functional interplay between p27SJ and MCP-1.
DISCUSSION
The identification of St John's Wort as antiviral, antidepressant, and inhibitor of inflammation prompted us to decipher the mechanisms used by this plant to contain inflammation. We recently isolated a 27-kDa protein (p27SJ) from a laboratory callus culture of H. perforatum that has the capacity to inhibit expression of the HIV-1 LTR [6]. To further explore the role of p27SJ, we have now examined its effect on MCP-1 gene expression and production. We show that low concentration of p27SJ was enough to upregulate the MCP-1 promoter through its physical and functional association with the transcription factor C/EBPβ. In addition, the activity of MCP-1 produced by cultures of adenovirus-p27SJ transduced cells is upregulated as measured by the transmigration of human Jurkat cells. Thus, we conclude that p27SJ is a potential therapeutic agent that may be developed as a regulator of MCP-1 leading to the inhibition of the cytokine-mediated inflammatory responses.
Induction of MCP-1 by C/EBPβ is not without a precedent. The bZip domain of C/EBPβ was shown to mediate lipopolysaccharide (LPS) induction of MCP-1 [36]. Further, C/EBPβ was also shown to activate the MCP-1 promoter through its association with MCP-1 DNA [15, 25]. In the first study, two C/EBPβ binding sites were identified between 2.6 and 3.6 kb upstream of the MCP-1 gene, while in the second study, C/EBPβ binding site was shown to be located in the proximal region of the MCP-1 promoter between positions −200 and +1. Further, in aortas from hyper-insulinaemic rats, C/EBPβ was shown to bind to MCP-1 DNA and induce the MCP-1 promoter, which may lead to initiate the process of atherosclerosis [37]. Finally, in a recent study, it was shown phosphorylation of serine 64 of C/EBPβ is necessary for C/EBPβ-activation of the MCP-1 promoter [38]. All these data corroborate with our data regarding the ability of C/EBPβ to activate the MCP-1 promoter. However, in here, we identified a new C/EBPβ binding site with the MCP-1 promoter located between positions −300 and −200 as demonstrated by transfection and ChIP assays (Figure 3A and Figure 5C). Further, our data also confirmed the previously published data regarding the functional and physical interplay between p27SJ and C/EBPβ [6]. Though in both cases, it associates with C/EBPβ, p27SJ seems to play a dual role, at low concentration as an activator and at high concentration as a suppressor. It should be noted that the observed effect of p27SJ is not cell specific (data not shown). This dual role might be beneficial to keep MCP-1 secretion levels under control especially in diseases where induction of MCP-1 was shown to affect the development and progression of the disease [37]. In this regard, MCP-1 was shown to play a role in rheumatoid arthritis and inflammatory bone-resorbing diseases and that its effect can be partially suppressed through activation of peroxisome proliferator-activated receptor gamma [39]. Note that p27SJ joins a long list of chemical effectors that have the capability of suppressing MCP-1 such as D-Piscose and EGCG. D-Psicose was shown to inhibit the expression of MCP-1 induced by high-glucose stimulation in HUVECs, whereas (−)-Epigallocatechin-3-gallate (EGCG) was shown to suppress MCP-1 expression in endothelial cells via blocking NF-κB signaling [40, 41]. In accord with this last observation, it has been shown that hyperforin, an active component of St. John's Wort, induces IL-8 expression in human intestinal epithelial cells through a MAPK-dependent, NF-κB-independent pathway [42]. However, hyperforin also reduced chemotaxis and chemoinvasion and restrained inflammation-triggered angiogenesis and lung fibrosis [43]. In another study, extracts of St. John's Wort increased both 5-HT1A and 5-HT2A serotonergic receptors by 50% in rats [44]. Similar to St. John's Wort, MCP-1 was also shown to activate 5-HT-induced mitogenesis [45]. Therefore, one may suggest that modulation of MCP-1 by p27SJ through its cooperativity with C/EBPβ, at the transcriptional and translational levels, is one of the pathways used by Hypericum perforatum and/or its protein extracts to induce 5-HT leading to restrain inflammation (studies in progress). Finally, in addition to its involvement in several diseases, MCP-1 was also shown to play a role in inflammation often observed in AIDS. Taken together, these results suggest that a high concentration of p27SJ might be beneficial to suppress MCP-1-involvement in diseases (e.g. AIDS).
ACKNOWLEDGMENTS
We thank past and present members of the Center for Neurovirology and Department of Neuroscience for sharing ideas. We also thank Dr. Martyn K. White for editing assistance. This work is supported by NIH Grant awarded to BES and SA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barnes J, Anderson LA, Phillipson JD. St John's wort (Hypericum perforatum L.): a review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2001;53:583–600. doi: 10.1211/0022357011775910. [DOI] [PubMed] [Google Scholar]
- 2.Saija A, Scalese M, Lanza M, Marzullo D, Bonina F, Castelli F. Flavonoids as antioxidant agents: importance of their interaction with biomembranes. Free Radic Biol Med. 1995;19:481–486. doi: 10.1016/0891-5849(94)00240-k. [DOI] [PubMed] [Google Scholar]
- 3.Flausino OA, Jr, Zangrossi H, Jr, Salgado JV, Viana MB. Effects of acute and chronic treatment with Hypericum perforatum L. (LI 160) on different anxiety-related responses in rats. Pharmacol Biochem Behav. 2002;71:251–257. doi: 10.1016/s0091-3057(01)00665-7. [DOI] [PubMed] [Google Scholar]
- 4.Couladis M, Chinou IB, Tzakou O, Petrakis PV. Composition and antimicrobial activity of the essential oil of Hypericum rumeliacum subsp. apollinis (Boiss. & Heldr.) Phytother Res. 2003;17:152–154. doi: 10.1002/ptr.1093. [DOI] [PubMed] [Google Scholar]
- 5.Tedeschi E, Menegazzi M, Margotto D, Suzuki H, Forstermann U, Kleinert H. Anti-inflammatory actions of St. John's wort: inhibition of human inducible nitric-oxide synthase expression by down-regulating signal transducer and activator of transcription-1alpha (STAT-1alpha) activation. J Pharmacol Exp Ther. 2003;307:254–261. doi: 10.1124/jpet.103.054460. [DOI] [PubMed] [Google Scholar]
- 6.Darbinian-Sarkissian N, Darbinyan A, Otte J, Radhakrishnan S, Sawaya BE, Arzumanyan A, Chipitsyna G, Popov Y, Rappaport J, Amini S, Khalili K. p27(SJ), a novel protein in St John's Wort, that suppresses expression of HIV-1 genome. Gene Ther. 2006;13:288–295. doi: 10.1038/sj.gt.3302649. [DOI] [PubMed] [Google Scholar]
- 7.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timchenko NA, Harris TE, Wilde M, Bilyeu TA, Burgess-Beusse BL, Finegold MJ, Darlington GJ. CCAAT/enhancer binding protein alpha regulates p21 protein and hepatocyte proliferation in newborn mice. Mol Cell Biol. 1997;17:7353–7361. doi: 10.1128/mcb.17.12.7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 10.Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- 11.Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 12.Henderson AJ, Zou X, Calame KL. C/EBP proteins activate transcription from the human immunodeficiency virus type 1 long terminal repeat in macrophages/monocytes. J Virol. 1995;69:5337–5344. doi: 10.1128/jvi.69.9.5337-5344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, Nakajima T, Hirano T, Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pope RM, Leutz A, Ness SA. C/EBP beta regulation of the tumor necrosis factor alpha gene. J Clin Invest. 1994;94:1449–1455. doi: 10.1172/JCI117482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekine O, Nishio Y, Egawa K, Nakamura T, Maegawa H, Kashiwagi A. Insulin activates CCAAT/enhancer binding proteins and proinflammatory gene expression through the phosphatidylinositol 3-kinase pathway in vascular smooth muscle cells. J Biol Chem. 2002;277:36631–36639. doi: 10.1074/jbc.M206266200. [DOI] [PubMed] [Google Scholar]
- 16.An MR, Hsieh CC, Reisner PD, Rabek JP, Scott SG, Kuninger DT, Papaconstantinou J. Evidence for posttranscriptional regulation of C/EBPalpha and C/EBPbeta isoform expression during the lipopolysaccharide-mediated acute-phase response. Mol Cell Biol. 1996;16:2295–2306. doi: 10.1128/mcb.16.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poli V, Mancini FP, Cortese R. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell. 1990;63:643–653. doi: 10.1016/0092-8674(90)90459-r. [DOI] [PubMed] [Google Scholar]
- 18.Yin M, Yang SQ, Lin HZ, Lane MD, Chatterjee S, Diehl AM. Tumor necrosis factor alpha promotes nuclear localization of cytokine-inducible CCAAT/enhancer binding protein isoforms in hepatocytes. J Biol Chem. 1996;271:17974–17978. doi: 10.1074/jbc.271.30.17974. [DOI] [PubMed] [Google Scholar]
- 19.Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- 20.El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly RW, Carr GG, Riley SC. The inhibition of synthesis of a beta-chemokine, monocyte chemotactic protein-1 (MCP-1) by progesterone. Biochem Biophys Res Commun. 1997;239:557–561. doi: 10.1006/bbrc.1997.7502. [DOI] [PubMed] [Google Scholar]
- 22.Boekhoudt GH, Guo Z, Beresford GW, Boss JM. Communication between NF-kappa B and Sp1 controls histone acetylation within the proximal promoter of the monocyte chemoattractant protein 1 gene. J Immunol. 2003;170:4139–4147. doi: 10.4049/jimmunol.170.8.4139. [DOI] [PubMed] [Google Scholar]
- 23.Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, Fukushima J, Kawamoto S, Ishigatsubo Y, Okubo T. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153:2052–2063. [PubMed] [Google Scholar]
- 24.Ping D, Boekhoudt G, Zhang F, Morris A, Philipsen S, Warren ST, Boss JM. Sp1 binding is critical for promoter assembly and activation of the MCP-1 gene by tumor necrosis factor. J Biol Chem. 2000;275:1708–1714. doi: 10.1074/jbc.275.3.1708. [DOI] [PubMed] [Google Scholar]
- 25.Abraham S, Sweet T, Sawaya BE, Rappaport J, Khalili K, Amini S. Cooperative interaction of C/EBP beta and Tat modulates MCP-1 gene transcription in astrocytes. J Neuroimmunol. 2005;160:219–227. doi: 10.1016/j.jneuroim.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Mastrangeli A, Danel C, Rosenfeld MA, Stratford-Perricaudet L, Perricaudet M, Pavirani A, Lecocq JP, Crystal RG. Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J Clin Invest. 1993;91:225–234. doi: 10.1172/JCI116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janabi N, Peudenier S, Héron B, Ng KH, Tardieu M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci Lett. 1995;195:105–108. doi: 10.1016/0304-3940(94)11792-h. [DOI] [PubMed] [Google Scholar]
- 28.Mukerjee R, Sawaya BE, Khalili K, Amini S. Association of p65 and C/EBPbeta with HIV-1 LTR modulates transcription of the viral promoter. J Cell Biochem. 2007;100:1210–1216. doi: 10.1002/jcb.21109. [DOI] [PubMed] [Google Scholar]
- 29.Amini S, Clavo A, Nadraga Y, Giordano A, Khalili K, Sawaya BE. Interplay between cdk9 and NF-kappaB factors determines the level of HIV-1 gene transcription in astrocytic cells. Oncogene. 2002;21:5797–5803. doi: 10.1038/sj.onc.1205754. [DOI] [PubMed] [Google Scholar]
- 30.Claudio PP, Cui J, Ghafouri M, Mariano C, White MK, Safak M, Sheffield JB, Giordano A, Khalili K, Amini S, Sawaya BE. Cdk9 phosphorylates p53 on serine 392 independently of CKII. J Cell Physiol. 2006;208:602–612. doi: 10.1002/jcp.20698. [DOI] [PubMed] [Google Scholar]
- 31.Goransson M, Elias E, Stahlberg A, Olofsson A, Andersson C, Aman P. Myxoid liposarcoma FUS-DDIT3 fusion oncogene induces C/EBP beta-mediated interleukin 6 expression. Int J Cancer. 2005;115:556–560. doi: 10.1002/ijc.20893. [DOI] [PubMed] [Google Scholar]
- 32.Eldeen MB, Deshmane SL, Simbiri K, Khalili K, Amini S, Sawaya BE. MH2 domain of Smad3 reduces HIV-1 Tat-induction of cytokine secretion. J Neuroimmunol. 2006;176:174–180. doi: 10.1016/j.jneuroim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Roman C, Platero JS, Shuman J, Calame K. Ig/EBP-1: a ubiquitously expressed immunoglobulin enhancer binding protein that is similar to C/EBP and heterodimerizes with C/EBP. Genes Dev. 1990;4:1404–1415. doi: 10.1101/gad.4.8.1404. [DOI] [PubMed] [Google Scholar]
- 34.Staniszewska I, Zaveri S, Del Valle L, Oliva I, Rothman VL, Croul SE, Roberts DD, Mosher DF, Tuszynski GP, Marcinkiewicz C. Interaction of α9β1 integrin with thrombospondin-1 promotes angiogenesis. Circ Res. 2007;100:1308–1316. doi: 10.1161/01.RES.0000266662.98355.66. [DOI] [PubMed] [Google Scholar]
- 35.Huang ZH, Wang Y, Cao L, Su ZD, Zhu YL, Chen YZ, Yuan XB, He C. Migratory properties of cultured olfactory ensheathing cells by single-cell migration assay. Cell Res. 2008;18:479–490. doi: 10.1038/cr.2008.38. [DOI] [PubMed] [Google Scholar]
- 36.Hu HM, Tian Q, Baer M, Spooner CJ, Williams SC, Johnson PF, Schwartz RC. The C/EBP Bzip domain can mediate lipopolysaccharide induction of the proinflammatory cytokines interleukin-6 and monocyte chemoattractant protein-1. J Biol Chem. 2000;275:16373–16381. doi: 10.1074/jbc.M910269199. [DOI] [PubMed] [Google Scholar]
- 37.Sato Y, Nishio Y, Sekine O, Kodama K, Nagai Y, Nakamura T, Maegawa H, Kashiwagi A. Increased expression of CCAAT/enhancer binding protein-beta and -delta and monocyte chemoattractant protein-1 genes in aortas from hyperinsulinaemic rats. Diabetologia. 2007;50:481–489. doi: 10.1007/s00125-006-0480-4. [DOI] [PubMed] [Google Scholar]
- 38.Spooner CJ, Sebastian T, Shuman JD, Durairaj S, Guo X, Johnson PF, Schwartz RC. C/EBPbeta serine 64, a phosphoacceptor site, has a critical role in LPS-induced IL-6 and MCP-1 transcription. Cytokine. 2007;37:119–127. doi: 10.1016/j.cyto.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Hounoki H, Sugiyama E, Mohamed SG, Shinoda K, Taki H, Abdel-Aziz HO, Maruyama M, Kobayashi M, Miyahara T. Activation of peroxisome proliferator-activated receptor gamma inhibits TNF-alpha-mediated osteoclast differentiation in human peripheral monocytes in part via suppression of monocyte chemoattractant protein-1 expression. Bone. 2008;42:765–774. doi: 10.1016/j.bone.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Murao K, Yu X, Cao WM, Imachi H, Chen K, Muraoka T, Kitanaka N, Li J, Ahmed RA, Matsumoto K, Nishiuchi T, Tokuda M, Ishida T. D-Psicose inhibits the expression of MCP-1 induced by high-glucose stimulation in HUVECs. Life Sci. 2007;81:592–599. doi: 10.1016/j.lfs.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 41.Hong MH, Kim MH, Chang HJ, Kim NH, Shin BA, Ahn BW, Jung YD. (−)-Epigallocatechin-3-gallate inhibits monocyte chemotactic protein-1 expression in endothelial cells via blocking NF-kappaB signaling. Life Sci. 2007;80:1957–1965. doi: 10.1016/j.lfs.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Zhou C, Tabb MM, Sadatrafiei A, Grun F, Sun A, Blumberg B. Hyperforin, the active component of St. John's wort, induces IL-8 expression in human intestinal epithelial cells via a MAPK-dependent, NF-kappaB-independent pathway. J Clin Immunol. 2004;24:623–236. doi: 10.1007/s10875-004-6248-z. [DOI] [PubMed] [Google Scholar]
- 43.Dell'Aica I, Niero R, Piazza F, Cabrelle A, Sartor L, Colalto C, et al. Hyperforin blocks neutrophil activation of matrix metalloproteinase-9, motility and recruitment, and restrains inflammation-triggered angiogenesis and lung fibrosis. J Pharmacol Exp Ther. 2007;321:492–500. doi: 10.1124/jpet.106.116459. [DOI] [PubMed] [Google Scholar]
- 44.Teufel-Mayer R, Gleitz J. Effects of long-term administration of hypericum extracts on the affinity and density of the central serotonergic 5-HT1 A and 5-HT2 A receptors. Pharmacopsychiatry. 1997;30:113–116. doi: 10.1055/s-2007-979530. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe T, Pakala R, Katagiri T, Benedict CR. Monocyte chemotactic protein 1 amplifies serotonin-induced vascular smooth muscle cell proliferation. J Vasc Res. 2001;38:341–349. doi: 10.1159/000051065. [DOI] [PubMed] [Google Scholar]