The most popular hypothesis circulating within and beyond the scientific community is that viral infections enhance or elicit autoimmune disorders such as type 1 diabetes. Indeed, viruses can injure β-cells and have been isolated in pancreatic tissues from diabetic patients. However, accumulating evidence suggests that the opposite scenario, which is prevention or amelioration of type 1 diabetes, might be at least as common an outcome of viral infection. Here, we discuss epidemiological and experimental evidence for the main mechanisms accounting for the role of viruses in type 1 diabetes to better understand the complex relationship between viral infections and autoimmune diabetes.
INSIGHT FROM EPIDEMIOLOGY AND CLINICAL INVESTIGATIONS
The influence of the environment.
Type 1 diabetes is a genetic autoimmune disorder caused by autoreactive CD4+ and CD8+ T-cells that recognize pancreatic antigens such as insulin or GAD and subsequently destroy insulin-producing β-cells. The subject of very active research is the question of how endogenous β-cell antigens become immunogenic. Infiltration of the islets of Langerhans, where β-cells reside, by activated autoreactive T-cells is considered to be the major driving force in type 1 diabetes progression. The islet infiltrate in humans consists primarily of CD8+ T-cells and B-cells, followed by macrophages and dendritic cells of different subtypes (1). Interestingly, significantly fewer T-cells are found in human islets compared with islets from nonobese diabetic (NOD) mice. The reduced numbers of T-cells, and in this way a limited autoreactive component in human islets, leads one to consider whether other contributing factors may be involved in disease development. Otherwise, sufficient insulitic infiltrate to destroy islet β-cells might not be easily maintained in humans. Further supporting a role for nongenetic factors in the control of type 1 diabetes is the observation that disease concordance among monozygotic twins is below 50% (2). Migrant studies also suggest the involvement of an environmental factor in type 1 diabetes, since disease incidence in migrating populations appears to conform to the incidence of the region to which there is migration (3). There is an ever-increasing body of literature suggesting that the significant environmental component to type 1 diabetes development and progression is a viral infection. However, this has not been clearly demonstrated. In fact, viral infections appear to have both detrimental and protective effects on type 1 diabetes development, which might be contingent upon the nature of the virus, but also the immune status of the host and thus the timing of infection.
Certain viruses might promote autoimmunity.
A significant number of viruses have been associated with type 1 diabetes, including enteroviruses such as Coxsackievirus B (CVB) (4), but also rotavirus (5,6), mumps virus (7), and cytomegalovirus (8). Rubella virus has been suggested to cause type 1 diabetes, but so far only congenital rubella syndrome has conclusively been associated with the disease (9–11). The prime viral candidates for causing type 1 diabetes in humans are enteroviruses. Enterovirus infections are more frequent in siblings developing type 1 diabetes compared with nondiabetic siblings, and enterovirus antibodies are elevated in pregnant mothers whose children later develop type 1 diabetes (12). Interestingly, studies in the Finnish population demonstrated that appearance of autoantibodies in genetically susceptible children paralleled the seasonal pattern of enterovirus infections (13). More specifically, a temporal association has been observed between the appearance of the first autoantibodies and signs of enterovirus infection both among siblings of affected children and among children with increased HLA-conferred diabetes susceptibility (14).
CVB4 is the most common enteroviral strain found in pre-diabetic and diabetic individuals. CVB RNA has been detected in blood from patients at the onset or during the course of type 1 diabetes (15,16). Furthermore, cellular immune responses to CVB antigens were found to be enhanced in type 1 diabetic patients after the onset of the disease (17). One CVB4 strain was isolated from the pancreas of a deceased diabetic child, passaged through murine β-cells, and found to induce diabetes after inoculation in mice (18). Recently, Dotta et al. (19) also detected CVB4 in pancreatic tissue specimens from three of six type 1 diabetic patients. Elshebani et al. (20) recently found that enterovirus isolates obtained from newly diagnosed type 1 diabetic patients could infect and induce destruction of human islet cells in vitro. Recently, Oikarinen et al. (21) have isolated enteroviruses from intestinal biopsy samples in 75% of type 1 diabetes cases versus 10% of control patients, possibly reflecting persistent enterovirus infection of gut mucosa in type 1 diabetic patients. In sum, isolation of enteroviral antigens from diabetic individuals, particularly after recent onset, is becoming a fairly reproducible finding, supporting a role for these viruses in disease development. However, it is still unclear whether this phenomenon is indeed a common etiology for the majority of patients diagnosed with type 1 diabetes, or whether it can be found only in a particular subpopulation of individuals with perhaps higher genetic susceptibility to infection.
The molecular means by which enteroviruses could enhance type 1 diabetes is a topic of significant inquiry. Virus infections activate strong immune responses. CVB4 infection of islet cells was indeed reported to induce strong inflammation mediated by natural killer (NK) cells within the islets (19). If the virus can, in addition, promote direct cytolysis of β-cells, autoantigens are introduced in a context of heightened immune response and inflammation. This might be the case after direct infection of β-cells by the virus. Enteroviruses might target β-cells via surface molecules such as the poliovirus receptor and integrin αvβ3. Both of these molecules are expressed on human β-cells and can act as enterovirus receptors in established cell lines (22). Infections by viruses that target β-cells and promote strong inflammation within the islets may thus represent the initial step in the induction of autoimmunity. However, studies on human pancreata or cultured islets have shown that there are considerable variations in the adverse effects of enteroviruses on β-cells, not only between various viral serotypes, but also between strains of the same serotype (23–25). While the mechanism by which viruses might induce autoimmunity is not understood, viral infections might be capable of “unmasking” β-cells for recognition by CD8+ T-cells by promoting interferon production and upregulation of major histocompatibility complex (MHC) class I molecules on β-cells. These events combined may be sufficient to condition the pancreatic islets for autoimmune attack. In this respect, Foulis et al. (26) described MHC class I upregulation and interferon induction in noninfiltrated islets obtained from presumably pre-diabetic individuals. Although this may possibly be a postmortem artifact, such findings should prompt larger-scale investigations, for example, on freshly obtained tissues via nPOD (Network for Pancreatic Organ Donors with Diabetes; www.jdrfnpod.org). Access to fresh pre-diabetic pancreata is needed to conduct valid viral studies.
Viruses may be wrongly accused.
The possibility that enteroviruses promote autoimmunity suggests that vaccination, which will impair viral infection of the islets, may provide protection against type 1 diabetes. Accordingly, an earlier report suggested that lower type 1 diabetes incidence in Estonia compared with Finland may be associated with polio vaccination schedule, resulting in stronger immunity to diabetogenic enterovirus infections (27). Similarly, while a significant increase in the number of type 1 diabetes cases was observed in Finland 2–4 years after a mumps epidemic (7), the incidence of type 1 diabetes reached a plateau 6 years after introduction of the mumps-measles-rubella vaccine (28). However, there is significant epidemiological data contradicting the involvement of viruses as causative agents in type 1 diabetes. There exists a geographical north-south gradient indicative of an inverse correlation between “hygiene” and incidence of autoimmune disease (as well as allergy). Countries such as Finland versus Venezuela/China, or wider regions such as Northern versus Southern Europe (with the exception of Sardinia), represent areas where socioeconomics correlate closely with type 1 diabetes prevalence. Reduced type 1 diabetes incidence is observed in countries of lower socioeconomic status, which is associated with a higher rate of infection. This phenomenon may also be related to the use of particular vaccine strategies in countries exhibiting different sanitary standards. It is also interesting to note that many type 1 diabetic patients are first born of large families, possibly indicative of lower exposure to infections. In addition, while congenital infections have been proposed to account for type 1 diabetes development in the offspring, the use of antimicrobials by mothers before pregnancy and subsequently by the child was suggested to be associated with higher risk for type 1 diabetes (29). Increased diabetes incidence in the Western world may thus be reflective of the “ultra-clean living” phenomenon. In the “hygiene hypothesis,” reduced rates of infection contribute to increased type 1 diabetes incidence, not supporting a disease-inductive role for viruses. Alternatively, it has been argued that reduced frequencies of infection may result in increased susceptibility to the effect of diabetogenic viruses (30,31). Regardless, although there is significant evidence for viral penetration of pancreatic tissue from type 1 diabetic patients, exposure to viruses does not appear to be necessarily causative of type 1 diabetes and may in fact be beneficial in some cases. This could be an indication that the immune system can be educated to better deal with inflammatory disorders by being frequently exposed to inflammatory events over life.
INSIGHT FROM EXPERIMENTAL WORK
While epidemiological studies have shed important insight into the association between viral infections and autoimmune diabetes in humans, a significant body of evidence is derived from investigations using animal models for type 1 diabetes. Notably, NOD mice are susceptible to spontaneous type 1 diabetes that develops slowly over several weeks and mimics most aspects of human type 1 diabetes (32). In NOD mice, nondestructive insulitis develops in the pancreas during the pre-diabetic phase and, although this period is variable, most mice go on to develop T-cell–mediated destruction of β-cells leading to overt diabetes. NOD mice thus constitute a critical tool to address how exposure to viral infection during the pre-diabetic phase will affect subsequent disease development. In humans, the prime candidates for infectious causes of type 1 diabetes are enteroviruses such as CVB. In mice, and most notably in NOD mice, CVB also appears associated with the development of autoimmunity. Early studies have shown that infection of normal mice with CVB4 causes a diabetic state associated with low insulin levels consistent with islet cell destruction (33). CVB4 has since been shown to be tightly associated with the initiation of type 1 diabetes in the NOD mouse. However, the influence of the virus appears to be contingent upon the precise point in time at which infection occurs (34). The B3 strain of CVB3, in contrast, mediates significant protection against type 1 diabetes development in NOD mice regardless of the time of infection (35). However, as discussed below, CVB3 and CVB4 differ regarding tropism for pancreatic tissue, which may account for the differential effect of these two stains in autoimmune diabetes. Importantly in that respect, there might be fundamental difference between rodents and humans regarding tropism of enteroviruses for pancreatic β-cells. One should thus be cautious when extrapolating rodent data to human type 1 diabetes in the context of enterovirus infections. Interestingly, other viruses that are also thought to be associated with the pathogenesis of type 1 diabetes in animals have been shown to mediate protective effects in some instances. For example, the D variant of encephalomyocarditis virus (EMC-DV) was found to induce β-cell destruction and subsequent type 1 diabetes in the mouse, but this was found to occur in a T-cell–independent fashion. EMC-DV was also reported to diminish autoimmunity in diabetes-prone NOD mice (36–38). In the BioBreeding diabetes-resistant (BB-DR) rat, however, it was shown that infection with Kilham's rat virus induces autoimmune diabetes (39). But while the use of animals has clearly established that particular viruses are capable of inducing autoimmune diabetes, it has also evidenced that these viruses can play a preventive role in the development of the disease. Two different, yet non–mutually exclusive, mechanisms have been proposed to account for the causative role of viruses in type 1 diabetes. Detailed insight into these mechanisms could provide better understanding of the dual roles played by viral infections in autoimmune diabetes.
Molecular mimicry might enhance but not initiate autoimmunity.
One of the two mechanisms by which viruses may be capable of initiating type 1 diabetes is termed molecular mimicry. If the T-cell receptors expressed by particular autoreactive T-cells enable these cells to recognize viral antigens (or vice versa), both autoreactive and antiviral T-cells have the potential to become activated as a consequence of presentation of viral antigens by antigen-presenting cells (APCs). Activation of autoreactive T-cells upon CVB4 infection was proposed to occur by molecular mimicry, since the P2-C protein sequence of the virus partially resembles that of human GAD, a protein expressed in the islets as well as other nervous tissues (40). T-cells from patients at risk for type 1 diabetes were found to recognize a GAD determinant that shares significant sequence similarity with the P2-C protein of CVB4, and patients whose T-cells responded to this particular GAD determinant were found to also respond to a Coxsackie viral peptide (41). However, antibodies present in GAD-positive sera from patients with type 1 diabetes were not found to cross-react with P2-C (42). Cross-reactivity between GAD and P2-C was further assessed using the NOD mouse model for type 1 diabetes, where it was determined that the common region of these two proteins is immunodominant and presented to cross-reactive T-cells only in the context of a NOD diabetes susceptibility MHC allele (43). Cross-reactivity between P2-C and GAD was thus proposed to account for the capacity of CVB4 to induce type 1 diabetes in genetically predisposed humans. However, Horwitz et al. (44) found that congenic B10.H2g7 mice, which carry the NOD MHC allele but lack other type 1 diabetes susceptibility factors, do not develop diabetes upon CVB4 infection. Moreover, infection of BDC2.5 transgenic mice, which express a T-cell receptor specific for an islet antigen that does not cross-react with the CVB4 P2-C protein was able to induce type 1 diabetes in the majority of these otherwise non–diabetes-prone mice. Therefore, cross-reactivity between P2-C and GAD may not by itself account for initiation of type 1 diabetes but might act as an essential enhancer of disease once autoimmune attack of β-cells has been initiated.
The rat insulin promoter (RIP)–lymphocytic choriomeningitis virus (LCMV) system is a mouse model in which diabetes is initiated by viral infection (45–47). In this model, RIP-LCMV mice transgenically express the glycoprotein or nucleoprotein of LCMV as a target antigen in their islets under the control of the RIP. Infection of these mice with LCMV breaks peripheral responsiveness to glycoprotein/nucleoprotein, leading to attack of β-cells by T-cells and eventual development of type 1 diabetes. Importantly, this model suggests that viral infection is able to induce autoimmunity only if homology between viral and β-cell antigens is 100%, since a single amino acid change flanking a cytotoxic T-lymphocyte (CTL) epitope was found to interfere with the development of type 1 diabetes (48). These data further support the hypothesis that molecular mimicry alone might not be capable of inducing type 1 diabetes but rather be an essential precipitator once autoimmunity has been initiated. Indeed, we previously reported that an LCMV mimic ligand can accelerate preexisting autoimmunity by inducing autoreactive T-cells to proliferate and localize in the islets, yet does not generate sufficient autoreactive T-cells to initiate disease in naive mice (49). We propose that viral infections alone will not initiate autoimmunity but rather act to provide a “fertile field” for further expansion of activated autoreactive T-cells, leading to autoimmune disease (50).
Bystander mechanisms induce APC activation and initiate autoimmunity.
While molecular mimicry may enhance autoimmune responses, the central role in induction of autoimmunity by viruses might be played by the proinflammatory/inflammatory mediators produced upon infection. Hence, bystander activation of autoreactive T-cells could occur during a viral infection with heterologous antigenic specificity and result in autoimmune disease. This might be the consequence of inflammation inducing tissue damage and release of sequestered islet antigens, leading to enhanced autoantigen presentation by APCs. Accordingly, CVB4-induced type 1 diabetes was found to be associated with initial phagocytosis of CVB4-infected β-cells by macrophages, leading to increased presentation of islet antigens, which promoted type 1 diabetes (51). Limited injury to β-cells using the islet-damaging agent streptozotocin was found to induce type 1 diabetes in BDC2.5 mice similar to CVB4 infection, as a consequence of release of β-cell antigens followed by their presentation by macrophages (52). Of note, injection of insulin along with poly-IC (polyinosinic-polycytidylic acid), which mimics double-stranded viral RNA, was found to induce anti-islet autoimmunity (53). Thus, virally induced release of β-cell antigen under inflammatory conditions might promote type 1 diabetes through activation of APCs such as macrophages, which have been shown to play a crucial role in the development of spontaneous diabetes (54,55). In particular, APCs themselves can release inflammatory and proinflammatory mediators as a result of viral infection. Likewise in the RIP-LCMV model, in the absence of infection, the glycoprotein/nucleoprotein protein is not expressed by costimulation-competent APCs, and the autoimmune process is only initiated when sufficient numbers of activated autoreactive T-cells are generated, after activation of APCs in the pancreas (56,57). Interestingly, activated T-cells from BDC2.5 mice are unable to induce diabetes in the mouse in the absence of CVB4 infection, which supports the possibility that bystander activation of APCs through virally mediated inflammation is necessary for efficient activation of autoreactive T-cells (44). Accordingly, we reported that type 1 diabetes develops in the absence of infection in the RIP-LCMV system when APCs are rendered costimulation-competent through transgenic expression of the B7–1 (CD80) costimulatory molecule (58). Importantly, in the RIP-LCMV system, APCs presenting self-antigens not only mediate priming of autoreactive T-cells, but also help maintain a peripheral immune response in the pancreatic islets (59). Thus, antigen presentation appears to play a crucial role not only in inducing but also sustaining the diabetogenic response.
Viral infection and local β-cell injury.
Initial damage to β-cells and uptake of autoantigen by APCs appear crucial in the initiation of autoimmunity upon viral infection. If a particular virus is highly lytic for β-cells, insulin deficiency and type 1 diabetes will result when more than 90% of β-cells are destroyed (60,61). This scenario can notably be observed after high-dose infection with EMC-DV, in which case type 1 diabetes is nonautoimmune in nature (62,63). However, limited injury to β-cells, caused by the virus or antiviral mechanisms, can lead to initial release of sequestered self-antigens and ultimately their presentation by APCs, which in turn promote further β-cell damage by activating autoreactive T-cells. Inflammatory cytokines produced during viral infection may play a crucial role in initial destruction of β-cells. Using the RIP-LCMV model, we found that upon viral infection, systemic production of interferon (IFN)-γ can directly cause β-cell destruction in the islets (64). Furthermore, inflammatory cytokines such as type I and II interferons may indirectly contribute to β-cell death by inducing upregulation of MHC class I by these cells, thereby “unmasking” them for recognition by autoreactive T-cells. It was reported that immunization with an LCMV-derived peptide does not induce type 1 diabetes in RIP-LCMV mice in the absence of IFN-α production and MHC class I upregulation in the islets (65). Furthermore, our work has shown that upregulation of MHC class II and activation of APCs within the pancreatic islets is required for β-cell destruction by activated autoreactive T-cells (56). Thus, cytokines produced upon infection by viruses exhibiting pancreatic tropism might be capable of preconditioning the islets for initial autoimmune attack of β-cells, similar to the possible scenario in humans.
Studies in the mouse further indicate that activation of Toll-like receptor (TLR) signaling might play a crucial part in the process. TLR ligation was shown to induce type 1 diabetes by increasing IFN-α and MHC class I expression in the islets of RIP-LCMV mice immunized with an LCMV-derived peptide (65). Similarly, in the BB-DR rat, initiation of autoimmune diabetes by Kilham's rat virus was found to be enhanced through activation of TLR signaling and induction of inflammatory cytokine production by APCs (66,67). More recently, Kim et al. (68) reported that activation of the TLR2 signaling pathway by secondary apoptotic β-cells might participate in the initiation of type 1 diabetes by inducing tumor necrosis factor-α (TNF-α) production by macrophages. However, TLR2 signaling was also recently suggested to enhance immune regulation (69), and it was previously reported that limited apoptosis of β-cells decreases diabetes incidence in NOD mice (70). In addition, as discussed below, TNF-α may have a protective function in type 1 diabetes depending on the time of action. Yet, regardless of the underlying mechanism, the observation by Kim et al. suggests that the mode by which initial injury to β-cells occurs is a crucial determinant in the induction of autoimmunity. Therefore, direct viral tropism for β-cells could play a major role in the capacity of viruses to mediate type 1 diabetes. Of note, CVB4 has a direct tropism for β-cells and exhibits a differential effect in type 1 diabetes depending on the time of infection, whereas CVB3 infects the acinar cells of the exocrine pancreas and prevents disease regardless of the time of infection (34,35). Although CVB4 infection of β-cells may not directly cause their demise (51), CVB3 and CVB4 do not mediate similar injury to these cells, which may account for their differential role in type 1 diabetes (68). Modulation of autoimmune diabetes by viruses may thus depend in part on their capacity to influence the mode and extent of β-cell death, which both appear as crucial factors influencing the course of the disease (71).
The importance of timing.
Another major component determining virally mediated modulation of autoimmunity appears to be the time at which infection occurs during the pre-diabetic phase. Whereas type 1 diabetes is enhanced in 8-week-old NOD mice infected with CVB4, infection of younger mice has no effect on disease outcome (34). This suggests that the status of autoimmune progression is a crucial determinant in the diabetogenic potency of the virus. Importantly, just like viral infections, inflammatory cytokines appear to play a dual role in autoimmune diabetes. Previous work has shown that expression or neutralization of cytokines commonly produced during viral infections has opposing effects on type 1 diabetes outcome depending on the time of expression. For instance, we found that early neutralization of TNF-α abrogates type 1 diabetes in RIP-LCMV mice, while at later time points, this cytokine appears to play a beneficial role by diminishing the number and activity of autoreactive T-cells (72,73). Thus, the capacity of particular viral infections to modulate autoimmunity at a certain point in time might be the direct consequence of their ability to promote inflammation during the pre-diabetic process beyond a particular autoimmune threshold. Accordingly, it was reported that enhancement of diabetes by CVB4 infection occurs only after a critical mass of activated autoreactive T-cells has accumulated in the islets (74). However, CVB3 was reported to prevent disease in both young and older mice (35). This suggests that while timing is important, the state of advancement of autoimmunity at the time of infection is not the sole explanation for the dual role of viruses in type 1 diabetes.
Impairment of autoimmunity may occur through bystander effects.
APC activation and associated inflammation, whether or not induced by viral infection, may not always have detrimental consequences in autoimmune diabetes. As discussed above, epidemiological studies provide evidence that infectious events occurring during early childhood might have the ability to prevent or delay type 1 diabetes development (75). The ability of viral infections to abrogate autoimmune diabetes was also reported in different animal models using not only CVB3 (35), but also LCMV (46,76,77), EMC-DV (38), mouse hepatitis virus (78), and lactate dehydrogenase virus (79). Interestingly, both acute and persistent viral infections appear capable of modulating the immune system in a diabetes-preventive fashion. While the mechanisms accounting for the beneficial effect of viruses on the immune system are poorly understood and may vary from one individual to the next (or one mouse model to the next), a feature common to viral infections is their paradoxical capacity to induce inflammation. This is also the case for a number of nonviral infections, vaccines, or treatments reported to protect NOD mice against diabetes (80–89). In fact, in some cases, type 1 diabetes can be inhibited by direct treatment of pre-diabetic mice with proinflammatory or inflammatory cytokines such as type I interferons, IFN-γ, or interleukin-2 (81,90–93), or by inducing the production of similar factors via stimulation of innate immunity (81,82,85,94). In the RIP-LCMV system, although viral infection represents the event initiating autoimmunity and diabetes, we have shown that inflammation mediated upon viral challenge can prevent type 1 diabetes. Our results indicate that secondary infection of RIP-nucleoprotein mice with a different strain of LCMV during the pre-diabetic phase completely abrogates diabetes development (95). This phenomenon is dependent on IFN-γ and TNF-α production and results from the recruitment of activated T-cells away from the islet infiltrate toward the pancreatic draining lymph node, as a consequence of selective expression of the chemokine IP-10 (CXCL10). In these studies, the LCMV strain used to prevent diabetes shares a homologous nucleoprotein sequence with that used to initiate diabetes, and thus the two infections activate comparable T-cell responses. Therefore, inflammatory cytokines and chemokines produced during viral infection might play a crucial part in controlling the location of virally activated autoreactive T-cells and their subsequent capacity to infiltrate pancreatic islets.
Antiviral T-cell memory and autoimmune diabetes.
Previous reports suggest that part of the T-cells activated during viral infection can cross-react with new infectious agents or allo-antigens and modulate immunity to unrelated antigens (96–98). Consequently, such heterologous immunity might result in the accumulation of memory T-cells of specificity unrelated to the original viral agent (99). It is thus possible that the memory T-cell pool generated during life comprises autoreactive/cross-reactive T-cells induced nonspecifically as a consequence of cumulative or chronic viral infections. Since stimulated memory T-cells respond to antigen more rapidly and efficiently than naive cells, repeated or sustained antiviral immunity during life may eventually favor autoimmunity. While in most cases, this phenomenon will not result in autoimmune disease, it might be a prerequisite for overt diabetes in genetically predisposed individuals. As discussed above, in different mouse models, initiation of diabetes by viral infection requires a critical mass of autoreactive T-cells along with activated APCs (34,56–58,100), and it is possible that, in humans, such a mass is provided progressively over life by repeated or sustained viral infections. In other words, autoimmunity might not be induced de novo at type 1 diabetes onset, and the autoreactive T-cell pool is likely comprised of cells that have already responded to antigenic stimulation during viral infection in the past. On the other hand, previous work suggests that repeated or sustained encounters with viral antigen during chronic infection is associated with protection against type 1 diabetes (46,77). This may be due in part to exhaustion of T-cell immunity, which is commonly found in chronic viral infection and was notably reported in protracted LCMV infection (101). Alternatively, or in addition, diabetes abrogation during chronic viral infection may be the consequence of virally induced regulatory mechanisms suppressing antiviral immunity and possibly autoimmunity as well (102,103). In this respect, a number of chronic as well as acute infections have been reported to induce the activation of regulatory T-cells (Tregs), in particular naturally occurring Tregs (CD4+CD25+ Tregs), which are known to play a crucial role in the control of autoimmunity (104–108). Thus, repeated exposure to viral antigen during life may not necessarily be pathogenic in autoimmunity. Nonspecific activation of autoreactive T-cells as a consequence of repeated or protracted viral infections may even be beneficial in some cases. Notably, CD4+CD25+ Tregs, which are selected in the thymus and thought to react to self-antigens in the periphery (109,110), can in essence be considered “autoreactive” and may thus be beneficially activated in heterologous immunity. In fact, we found that CD4+CD25+ Tregs are modulated during viral infection and become capable of halting the course of type 1 diabetes (C.M.F., unpublished data). In addition, our results suggest that resemblance between viral and β-cell antigens can, in some cases, promote activation of diabetes-preventive CD4+CD25+ Tregs.
CLINICAL IMPLICATIONS: DO VIRAL INFECTIONS INDUCE OR PREVENT TYPE 1 DIABETES?
A number of epidemiological studies support the hypothesis that viral infections play a causative role in type 1 diabetes. However, systematic review of control studies published between 1966 and 2002 has shown no convincing evidence for or against an association between type 1 diabetes and the prime candidate for infectious cause, CVB (111). In animal models for type 1 diabetes, solid evidence supporting an inductive role for viruses is faced with just as solid evidence supporting a protective effect of viral infections. Based on mouse studies alone, there is no doubt that association between viruses and type 1 diabetes is extremely complex: while belonging to the same enteroviral group, CVB3 and CVB4 have opposing effects on type 1 diabetes in the same mouse model; LCMV initiates diabetes in the RIP-LCMV model but prevents disease in the NOD model; and to make matters more complicated, CVB4 and LCMV are capable of both inducing and preventing diabetes in the same mouse model depending on the time of infection. Thus, the reason for current failure to associate a particular virus with induction of autoimmune diabetes likely is that such an association might be impossible to make. Certain viruses might be capable of inducing diabetes and others of preventing diabetes, and type 1 diabetes inducers might be capable of preventing disease under certain conditions. This will depend of course on the nature of the considered virus (resemblance with β-cell antigens; tropism for β-cells; induction of a chronic infection), but also on the state of advancement of autoimmunity at the time of infection (generation of sufficient numbers of autoreactive T-cells; nature of the cytokine milieu systemically and in the islets). A given viral infection could thus be an essential disease precipitator once required predisposing events have occurred, but could on the other hand disrupt accumulation of these events.
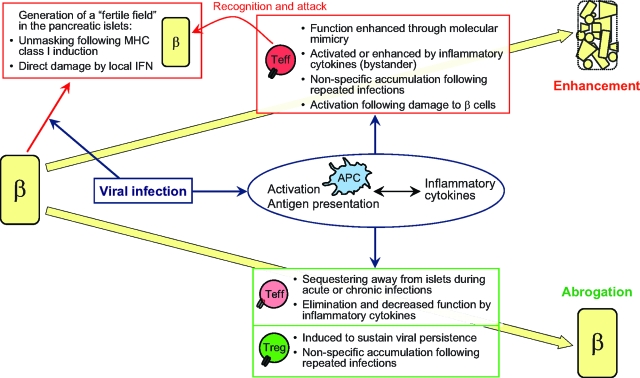
Most important is the indication from animal studies that modulation of autoimmunity during viral infection does not depend merely on inherent properties of the virus, but also significantly on intrinsic factors of the host. The close interplay between the two will dictate whether enhancement or abrogation of autoimmune diabetes occurs. While molecular mimicry might activate autoreactive T-cells, it could also segregate these cells away from the islets and/or induce the activation of protective Tregs. While inflammatory cytokines might promote bystander activation of APCs and autoreactive T-cells, infection could occur at a time where inflammation will induce the relocation or demise of these cells. Whereas β-cell lysis and presentation of islet antigen might promote activation of autoreactive T-cells, it could also suppress the function of these cells by promoting Treg activity. Whereas repeated/sustained infections might lead to the accumulation of autoreactive T-cells within the memory pool, they could also induce suppressor mechanisms that will hinder autoimmunity. These possibilities are illustrated in Fig. 1.
FIG. 1.
Interplay between virus- and host-intrinsic properties dictates whether enhancement or abrogation of type 1 diabetes occurs. β = β cells. Teff, effector (autoreactive) T-cells.
Based on current evidence, it thus appears impossible to assess the capacity of viruses to modulate type 1 diabetes without knowledge of the state of advancement of autoimmunity and infection history of affected individuals. This is no easy task, but tremendous effort is currently being made in the U.S. and Europe to closely monitor exposure to infections in individuals at risk for type 1 diabetes. In particular, the TEDDY (The Environmental Determinants of Diabetes in the Young) study is currently assessing the influence of environmental factors, among which are viral infections, on the development of autoimmune diabetes. In this study, blood from children with increased genetic risk for type 1 diabetes is assessed for viral exposure every 3 months for the first 4 years of life, and then every 6 months until the age of 15 years. Stool samples are also assessed for viral exposure at monthly intervals for the first 4 years of life and then biannually until the age of 15. Importantly, as the period of time between a particular viral infection and the initiation of autoimmunity appears variable, occurrence of a possibly critical infectious event might be extremely hard to detect. It thus appears crucial that children with increased genetic risk for type 1 diabetes are monitored not only at regular intervals, but also whenever they are undergoing a viral infection. Closer monitoring of individuals with high risk for type 1 diabetes should give us more convincing evidence for the contribution of an infectious agent to progression toward autoimmunity. In addition, newly obtained mechanisms from experimental investigations will be useful for the development of novel immunotherapy for type 1 diabetes.
Acknowledgments
The authors acknowledge support from the Brehm Coalition.
Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered. See http://creativecommons.org/licenses/by-nc-nd/3.0/ for details.
REFERENCES
- 1.In’t Veld P, Lievens D, De Grijse J, Ling Z, Van der Auwera B, Pipeleers-Marichal M, Gorus F, Pipeleers D: Screening for insulitis in adult autoantibody-positive organ donors. Diabetes 56: 2400–2404, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Redondo MJ, Rewers M, Yu L, Garg S, Pilcher CC, Elliott RB, Eisenbarth GS: Genetic determination of islet cell autoimmunity in monozygotic twin, dizygotic twin, and non-twin siblings of patients with type 1 diabetes: prospective twin study. BMJ 318: 698–702, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwright R: Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ 304: 1020–1022, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyoty H, Taylor KW: The role of viruses in human diabetes. Diabetologia 45: 1353–1361, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Honeyman MC, Stone NL, Harrison LC: T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: potential for mimicry with rotavirus and other environmental agents. Mol Med 4: 231–239, 1998 [PMC free article] [PubMed] [Google Scholar]
- 6.Honeyman MC, Coulson BS, Stone NL, Gellert SA, Goldwater PN, Steele CE, Couper JJ, Tait BD, Colman PG, Harrison LC: Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes 49: 1319–1324, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Hyoty H, Leinikki P, Reunanen A, Ilonen J, Surcel HM, Rilva A, Kaar ML, Huupponen T, Hakulinen A, Makela AL, et al.: Mumps infections in the etiology of type 1 (insulin-dependent) diabetes. Diabetes Res 9: 111–116, 1988 [PubMed] [Google Scholar]
- 8.Pak CY, Eun HM, McArthur RG, Yoon JW: Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet 2: 1–4, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Forrest JM, Menser MA, Burgess JA: High frequency of diabetes mellitus in young adults with congenital rubella. Lancet 2: 332–334, 1971 [DOI] [PubMed] [Google Scholar]
- 10.Menser MA, Forrest JM, Bransby RD: Rubella infection and diabetes mellitus. Lancet 1: 57–60, 1978 [DOI] [PubMed] [Google Scholar]
- 11.Devendra D, Liu E, Eisenbarth GS: Type 1 diabetes: recent developments. BMJ 328: 750–754, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyoty H, Hiltunen M, Knip M, Laakkonen M, Vahasalo P, Karjalainen J, Koskela P, Roivainen M, Leinikki P, Hovi T, et al.: A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM: Childhood Diabetes in Finland (DiMe) Study Group. Diabetes 44: 652–657, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Kimpimaki T, Kupila A, Hamalainen AM, Kukko M, Kulmala P, Savola K, Simell T, Keskinen P, Ilonen J, Simell O, Knip M: The first signs of beta-cell autoimmunity appear in infancy in genetically susceptible children from the general population: the Finnish Type 1 Diabetes Prediction and Prevention Study. J Clin Endocrinol Metab 86: 4782–4788, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Lonnrot M, Korpela K, Knip M, Ilonen J, Simell O, Korhonen S, Savola K, Muona P, Simell T, Koskela P, Hyoty H: Enterovirus infection as a risk factor for beta-cell autoimmunity in a prospectively observed birth cohort: the Finnish Diabetes Prediction and Prevention Study. Diabetes 49: 1314–1318, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Clements GB, Galbraith DN, Taylor KW: Coxsackie B virus infection and onset of childhood diabetes. Lancet 346: 221–223, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Andreoletti L, Hober D, Hober-Vandenberghe C, Belaich S, Vantyghem MC, Lefebvre J, Wattre P: Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type I diabetes mellitus. J Med Virol 52: 121–127, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Juhela S, Hyoty H, Roivainen M, Harkonen T, Putto-Laurila A, Simell O, Ilonen J: T-cell responses to enterovirus antigens in children with type 1 diabetes. Diabetes 49: 1308–1313, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Yoon JW, Austin M, Onodera T, Notkins AL: Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med 300: 1173–1179, 1979 [DOI] [PubMed] [Google Scholar]
- 19.Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P: Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 104: 5115–5120, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elshebani A, Olsson A, Westman J, Tuvemo T, Korsgren O, Frisk G: Effects on isolated human pancreatic islet cells after infection with strains of enterovirus isolated at clinical presentation of type 1 diabetes. Virus Res 124: 193–203, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Oikarinen M, Tauriainen S, Honkanen T, Oikarinen S, Vuori K, Kaukinen K, Rantala I, Maki M, Hyoty H: Detection of enteroviruses in the intestine of type 1 diabetic patients. Clin Exp Immunol 151: 71–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ylipaasto P, Klingel K, Lindberg AM, Otonkoski T, Kandolf R, Hovi T, Roivainen M: Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47: 225–239, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Roivainen M, Rasilainen S, Ylipaasto P, Nissinen R, Ustinov J, Bouwens L, Eizirik DL, Hovi T, Otonkoski T: Mechanisms of coxsackievirus-induced damage to human pancreatic beta-cells. J Clin Endocrinol Metab 85: 432–440, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Roivainen M, Ylipaasto P, Savolainen C, Galama J, Hovi T, Otonkoski T: Functional impairment and killing of human beta cells by enteroviruses: the capacity is shared by a wide range of serotypes, but the extent is a characteristic of individual virus strains. Diabetologia 45: 693–702, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Roivainen M: Enteroviruses: new findings on the role of enteroviruses in type 1 diabetes. Int J Biochem Cell Biol 38: 721–725, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Foulis AK, Jackson R, Farquharson MA: The pancreas in idiopathic Addison's disease: a search for a prediabetic pancreas. Histopathology 12: 481–490, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Juhela S, Hyoty H, Hinkkanen A, Elliott JF, Roivainen M, Kulmala P, Rahko J, Knip M, Ilonen J: T cell responses to enterovirus antigens and to beta-cell autoantigens in unaffected children positive for IDDM-associated autoantibodies. J Autoimmun 12: 269–278, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Hyoty H, Hiltunen M, Reunanen A, Leinikki P, Vesikari T, Lounamaa R, Tuomilehto J, Akerblom HK: Decline of mumps antibodies in type 1 (insulin-dependent) diabetic children and a plateau in the rising incidence of type 1 diabetes after introduction of the mumps-measles-rubella vaccine in Finland: Childhood Diabetes in Finland Study Group. Diabetologia 36: 1303–1308, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Kilkkinen A, Virtanen SM, Klaukka T, Kenward MG, Salkinoja-Salonen M, Gissler M, Kaila M, Reunanen A: Use of antimicrobials and risk of type 1 diabetes in a population-based mother-child cohort. Diabetologia 49: 66–70, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Viskari H, Ludvigsson J, Uibo R, Salur L, Marciulionyte D, Hermann R, Soltesz G, Fuchtenbusch M, Ziegler AG, Kondrashova A, Romanov A, Kaplan B, Laron Z, Koskela P, Vesikari T, Huhtala H, Knip M, Hyoty H: Relationship between the incidence of type 1 diabetes and maternal enterovirus antibodies: time trends and geographical variation. Diabetologia 48: 1280–1287, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Viskari H, Ludvigsson J, Uibo R, Salur L, Marciulionyte D, Hermann R, Soltesz G, Fuchtenbusch M, Ziegler AG, Kondrashova A, Romanov A, Knip M, Hyoty H: Relationship between the incidence of type 1 diabetes and enterovirus infections in different European populations: results from the EPIVIR project. J Med Virol 72: 610–617, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y: Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu 29: 1–13, 1980 [DOI] [PubMed] [Google Scholar]
- 33.Coleman TJ, Gamble DR, Taylor KW: Diabetes in mice after Coxsackie B 4 virus infection. Br Med J 3: 25–27, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serreze DV, Ottendorfer EW, Ellis TM, Gauntt CJ, Atkinson MA: Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T-cells in pancreatic islets. Diabetes 49: 708–711, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Tracy S, Drescher KM, Chapman NM, Kim KS, Carson SD, Pirruccello S, Lane PH, Romero JR, Leser JS: Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J Virol 76: 12097–12111, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craighead JE, McLane MF: Diabetes mellitus: induction in mice by encephalomyocarditis virus. Science 162: 913–914, 1968 [DOI] [PubMed] [Google Scholar]
- 37.Yoon JW, McClintock PR, Bachurski CJ, Longstreth JD, Notkins AL: Virus-induced diabetes mellitus: no evidence for immune mechanisms in the destruction of beta-cells by the D-variant of encephalomyocarditis virus. Diabetes 34: 922–925, 1985 [DOI] [PubMed] [Google Scholar]
- 38.Hermitte L, Vialettes B, Naquet P, Atlan C, Payan MJ, Vague P: Paradoxical lessening of autoimmune processes in non-obese diabetic mice after infection with the diabetogenic variant of encephalomyocarditis virus. Eur J Immunol 20: 1297–1303, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Guberski DL, Thomas VA, Shek WR, Like AA, Handler ES, Rossini AA, Wallace JE, Welsh RM: Induction of type I diabetes by Kilham's rat virus in diabetes-resistant BB/Wor rats. Science 254: 1010–1013, 1991 [DOI] [PubMed] [Google Scholar]
- 40.Kaufman DL, Erlander MG, Clare-Salzler M, Atkinson MA, Maclaren NK, Tobin AJ: Autoimmunity to two forms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J Clin Invest 89: 283–292, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atkinson MA, Bowman MA, Campbell L, Darrow BL, Kaufman DL, Maclaren NK: Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Invest 94: 2125–2129, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richter W, Mertens T, Schoel B, Muir P, Ritzkowsky A, Scherbaum WA, Boehm BO: Sequence homology of the diabetes-associated autoantigen glutamate decarboxylase with coxsackie B4–2C protein and heat shock protein 60 mediates no molecular mimicry of autoantibodies. J Exp Med 180: 721–726, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian J, Lehmann PV, Kaufman DL: T cell cross-reactivity between coxsackievirus and glutamate decarboxylase is associated with a murine diabetes susceptibility allele. J Exp Med 180: 1979–1984, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N: Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med 4: 781–785, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H: Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65: 305–317, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H: Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell 65: 319–331, 1991 [DOI] [PubMed] [Google Scholar]
- 47.von Herrath MG, Dockter J, Oldstone MB: How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity 1: 231–242, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Sevilla N, Homann D, von Herrath M, Rodriguez F, Harkins S, Whitton JL, Oldstone MB: Virus-induced diabetes in a transgenic model: role of cross-reacting viruses and quantitation of effector T cells needed to cause disease. J Virol 74: 3284–3292, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christen U, Edelmann KH, McGavern DB, Wolfe T, Coon B, Teague MK, Miller SD, Oldstone MB, von Herrath MG: A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J Clin Invest 114: 1290–1298, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Herrath MG, Fujinami RS, Whitton JL: Microorganisms and autoimmunity: making the barren field fertile? Nat Rev Microbiol 1: 151–157, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Horwitz MS, Ilic A, Fine C, Balasa B, Sarvetnick N: Coxsackieviral-mediated diabetes: induction requires antigen-presenting cells and is accompanied by phagocytosis of beta cells. Clin Immunol 110: 134–144, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Horwitz MS, Ilic A, Fine C, Rodriguez E, Sarvetnick N: Presented antigen from damaged pancreatic beta cells activates autoreactive T cells in virus-mediated autoimmune diabetes. J Clin Invest 109: 79–87, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moriyama H, Wen L, Abiru N, Liu E, Yu L, Miao D, Gianani R, Wong FS, Eisenbarth GS: Induction and acceleration of insulitis/diabetes in mice with a viral mimic (polyinosinic-polycytidylic acid) and an insulin self-peptide. Proc Natl Acad Sci U S A 99: 5539–5544, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hutchings P, Rosen H, O'Reilly L, Simpson E, Gordon S, Cooke A: Transfer of diabetes in mice prevented by blockade of adhesion-promoting receptor on macrophages. Nature 348: 639–642, 1990 [DOI] [PubMed] [Google Scholar]
- 55.Jun HS, Yoon CS, Zbytnuik L, van Rooijen N, Yoon JW: The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med 189: 347–358, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Herrath M, Holz A: Pathological changes in the islet milieu precede infiltration of islets and destruction of beta-cells by autoreactive lymphocytes in a transgenic model of virus-induced IDDM. J Autoimmun 10: 231–238, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Garza KM, Chan SM, Suri R, Nguyen LT, Odermatt B, Schoenberger SP, Ohashi PS: Role of antigen-presenting cells in mediating tolerance and autoimmunity. J Exp Med 191: 2021–2027, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Herrath MG, Guerder S, Lewicki H, Flavell RA, Oldstone MB: Coexpression of B7–1 and viral (“self”) transgenes in pancreatic beta cells can break peripheral ignorance and lead to spontaneous autoimmune diabetes. Immunity 3: 727–738, 1995 [DOI] [PubMed] [Google Scholar]
- 59.Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM: Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med 188: 1493–1501, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oldstone MB, Southern P, Rodriquez M, Lampert P: Virus persists in beta cells of islets of Langerhans and is associated with chemical manifestations of diabetes. Science 224: 1440–1443, 1984 [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez M, Garrett RS, Raitt M, Lampert PW, Oldstone MB: Virus persists in beta cells of islets of Langerhans and infection is associated with chemical manifestations of diabetes. II. Morphologic observations. Am J Pathol 121: 497–504, 1985 [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon JW, Morishima T, McClintock PR, Austin M, Notkins AL: Virus-induced diabetes mellitus: mengovirus infects pancreatic beta cells in strains of mice resistant to the diabetogenic effect of encephalomyocarditis virus. J Virol 50: 684–690, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoon JW, Jun HS: Viruses cause type 1 diabetes in animals. Ann N Y Acad Sci 1079: 138–146, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Seewaldt S, Thomas HE, Ejrnaes M, Christen U, Wolfe T, Rodrigo E, Coon B, Michelsen B, Kay TW, von Herrath MG: Virus-induced autoimmune diabetes: most beta-cells die through inflammatory cytokines and not perforin from autoreactive (anti-viral) cytotoxic T-lymphocytes. Diabetes 49: 1801–1809, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S, Odermatt B, Conrad C, Ittner LM, Bauer S, Luther SA, Uematsu S, Akira S, Hengartner H, Zinkernagel RM: Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med 11: 138–145, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Zipris D, Lien E, Nair A, Xie JX, Greiner DL, Mordes JP, Rossini AA: TLR9-signaling pathways are involved in Kilham rat virus-induced autoimmune diabetes in the biobreeding diabetes-resistant rat. J Immunol 178: 693–701, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Zipris D, Lien E, Xie JX, Greiner DL, Mordes JP, Rossini AA: TLR activation synergizes with Kilham rat virus infection to induce diabetes in BBDR rats. J Immunol 174: 131–142, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS: Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity 27: 321–333, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR: Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest 116: 2022–2032, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Hugues S, Mougneau E, Ferlin W, Jeske D, Hofman P, Homann D, Beaudoin L, Schrike C, Von Herrath M, Lehuen A, Glaichenhaus N: Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic beta cells. Immunity 16: 169–181, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Filippi CM, von Herrath MG: Islet beta-cell death: fuel to sustain autoimmunity? Immunity 27: 183–185, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Christen U, Wolfe T, Mohrle U, Hughes AC, Rodrigo E, Green EA, Flavell RA, von Herrath MG: A dual role for TNF-alpha in type 1 diabetes: islet-specific expression abrogates the ongoing autoimmune process when induced late but not early during pathogenesis. J Immunol 166: 7023–7032, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Yang XD, McDevitt HO: Role of TNF-alpha in the development of autoimmunity and the pathogenesis of insulin-dependent diabetes mellitus in NOD mice. Circ Shock 43: 198–201, 1994 [PubMed] [Google Scholar]
- 74.Serreze DV, Wasserfall C, Ottendorfer EW, Stalvey M, Pierce MA, Gauntt C, O'Donnell B, Flanagan JB, Campbell-Thompson M, Ellis TM, Atkinson MA: Diabetes acceleration or prevention by a coxsackievirus B4 infection: critical requirements for both interleukin-4 and gamma interferon. J Virol 79: 1045–1052, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bach JF: Protective role of infections and vaccinations on autoimmune diseases. J Autoimmun 16: 347–353, 2001 [DOI] [PubMed] [Google Scholar]
- 76.Oldstone MB: Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science 239: 500–502, 1988 [DOI] [PubMed] [Google Scholar]
- 77.Oldstone MB, Ahmed R, Salvato M: Viruses as therapeutic agents. II. Viral reassortants map prevention of insulin-dependent diabetes mellitus to the small RNA of lymphocytic choriomeningitis virus. J Exp Med 171: 2091–2100, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilberz S, Partke HJ, Dagnaes-Hansen F, Herberg L: Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia 34: 2–5, 1991 [DOI] [PubMed] [Google Scholar]
- 79.Takei I, Asaba Y, Kasatani T, Maruyama T, Watanabe K, Yanagawa T, Saruta T, Ishii T: Suppression of development of diabetes in NOD mice by lactate dehydrogenase virus infection. J Autoimmun 5: 665–673, 1992 [DOI] [PubMed] [Google Scholar]
- 80.McInerney MF, Pek SB, Thomas DW: Prevention of insulitis and diabetes onset by treatment with complete Freund's adjuvant in NOD mice. Diabetes 40: 715–725, 1991 [DOI] [PubMed] [Google Scholar]
- 81.Serreze DV, Hamaguchi K, Leiter EH: Immunostimulation circumvents diabetes in NOD/Lt mice. J Autoimmun 2: 759–776, 1989 [DOI] [PubMed] [Google Scholar]
- 82.Quintana FJ, Rotem A, Carmi P, Cohen IR: Vaccination with empty plasmid DNA or CpG oligonucleotide inhibits diabetes in nonobese diabetic mice: modulation of spontaneous 60-kDa heat shock protein autoimmunity. J Immunol 165: 6148–6155, 2000 [DOI] [PubMed] [Google Scholar]
- 83.Shehadeh N, Calcinaro F, Bradley BJ, Bruchim I, Vardi P, Lafferty KJ: Effect of adjuvant therapy on development of diabetes in mouse and man. Lancet 343: 706–707, 1994 [DOI] [PubMed] [Google Scholar]
- 84.Kim JY, Cho SH, Kim YW, Jang EC, Park SY, Kim EJ, Lee SK: Effects of BCG, lymphotoxin and bee venom on insulitis and development of IDDM in non-obese diabetic mice. J Korean Med Sci 14: 648–652, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iguchi M, Inagawa H, Nishizawa T, Okutomi T, Morikawa A, Soma GI, Mizuno D: Homeostasis as regulated by activated macrophage. V. Suppression of diabetes mellitus in non-obese diabetic mice by LPSw (a lipopolysaccharide from wheat flour). Chem Pharm Bull (Tokyo) 40: 1004–1006, 1992 [DOI] [PubMed] [Google Scholar]
- 86.Qin HY, Sadelain MW, Hitchon C, Lauzon J, Singh B: Complete Freund's adjuvant-induced T cells prevent the development and adoptive transfer of diabetes in nonobese diabetic mice. J Immunol 150: 2072–2080, 1993 [PubMed] [Google Scholar]
- 87.Bras A, Aguas AP: Diabetes-prone NOD mice are resistant to Mycobacterium avium and the infection prevents autoimmune disease. Immunology 89: 20–25, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toyota T, Satoh J, Oya K, Shintani S, Okano T: Streptococcal preparation (OK-432) inhibits development of type I diabetes in NOD mice. Diabetes 35: 496–499, 1986 [DOI] [PubMed] [Google Scholar]
- 89.Sobel DO, Yankelevich B, Goyal D, Nelson D, Mazumder A: The B-subunit of cholera toxin induces immunoregulatory cells and prevents diabetes in the NOD mouse. Diabetes 47: 186–191, 1998 [DOI] [PubMed] [Google Scholar]
- 90.Tanaka-Kataoka M, Kunikata T, Takayama S, Iwaki K, Fujii M, Ohashi K, Ikeda M, Kurimoto M: Oral use of interferon-alpha delays the onset of insulin-dependent diabetes mellitus in nonobese diabetes mice. J Interferon Cytokine Res 19: 877–879, 1999 [DOI] [PubMed] [Google Scholar]
- 91.Brod SA, Malone M, Darcan S, Papolla M, Nelson L: Ingested interferon alpha suppresses type I diabetes in non-obese diabetic mice. Diabetologia 41: 1227–1232, 1998 [DOI] [PubMed] [Google Scholar]
- 92.Sobel DO, Ahvazi B: Alpha-interferon inhibits the development of diabetes in NOD mice. Diabetes 47: 1867–1872, 1998 [DOI] [PubMed] [Google Scholar]
- 93.Sobel DO, Han J, Williams J, Yoon JW, Jun HS, Ahvazi B: Gamma interferon paradoxically inhibits the development of diabetes in the NOD mouse. J Autoimmun 19: 129–137, 2002 [DOI] [PubMed] [Google Scholar]
- 94.Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, Miura T, Haba T, Scherer DC, Wei J, Kronenberg M, Koezuka Y, Van Kaer L: The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med 7: 1052–1056, 2001 [DOI] [PubMed] [Google Scholar]
- 95.Christen U, Benke D, Wolfe T, Rodrigo E, Rhode A, Hughes AC, Oldstone MB, von Herrath MG: Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J Clin Invest 113: 74–84, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nahill SR, Welsh RM: High frequency of cross-reactive cytotoxic T lymphocytes elicited during the virus-induced polyclonal cytotoxic T lymphocyte response. J Exp Med 177: 317–327, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Selin LK, Nahill SR, Welsh RM: Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med 179: 1933–1943, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Selin LK, Lin MY, Kraemer KA, Pardoll DM, Schneck JP, Varga SM, Santolucito PA, Pinto AK, Welsh RM: Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity 11: 733–742, 1999 [DOI] [PubMed] [Google Scholar]
- 99.Selin LK, Brehm MA, Naumov YN, Cornberg M, Kim SK, Clute SC, Welsh RM: Memory of mice and men: CD8+ T-cell cross-reactivity and heterologous immunity. Immunol Rev 211: 164–181, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohashi PS, Oehen S, Aichele P, Pircher H, Odermatt B, Herrera P, Higuchi Y, Buerki K, Hengartner H, Zinkernagel RM: Induction of diabetes is influenced by the infectious virus and local expression of MHC class I and tumor necrosis factor-alpha. J Immunol 150: 5185–5194, 1993 [PubMed] [Google Scholar]
- 101.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R: Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 188: 2205–2213, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB: Interleukin-10 determines viral clearance or persistence in vivo. Nat Med 12: 1301–1309, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, von Herrath MG: Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med 203: 2461–2472, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T: Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance: deficit of a T cell subset as a possible cause of autoimmune disease J Exp Med 161: 72–87, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT: CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol 172: 4123–4132, 2004 [DOI] [PubMed] [Google Scholar]
- 106.Iwashiro M, Messer RJ, Peterson KE, Stromnes IM, Sugie T, Hasenkrug KJ: Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc Natl Acad Sci U S A 98: 9226–9230, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.MacDonald AJ, Duffy M, Brady MT, McKiernan S, Hall W, Hegarty J, Curry M, Mills KH: CD4 T helper type 1 and regulatory T cells induced against the same epitopes on the core protein in hepatitis C virus-infected persons. J Infect Dis 185: 720–727, 2002 [DOI] [PubMed] [Google Scholar]
- 108.Robertson SJ, Messer RJ, Carmody AB, Hasenkrug KJ: In Vitro Suppression of CD8+ T Cell Function by Friend Virus-Induced Regulatory T Cells. J Immunol 176: 3342–3349, 2006 [DOI] [PubMed] [Google Scholar]
- 109.Apostolou I, Sarukhan A, Klein L, von Boehmer H: Origin of regulatory T cells with known specificity for antigen. Nat Immunol 3: 756–763, 2002 [DOI] [PubMed] [Google Scholar]
- 110.Shevach EM, McHugh RS, Piccirillo CA, Thornton AM: Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev 182: 58–67, 2001 [DOI] [PubMed] [Google Scholar]
- 111.Green J, Casabonne D, Newton R: Coxsackie B virus serology and type 1 diabetes mellitus: a systematic review of published case-control studies. Diabet Med 21: 507–514, 2004 [DOI] [PubMed] [Google Scholar]