For nonimmunologists, a daunting and rapidly evolving immunologic vocabulary, our incomplete understanding of both normal and abnormal immune function, and multiple interrelated complex immune cellular pathways can be a barrier to using basic immunology to understand and improve care for patients with type 1 diabetes. Our task in this review is to introduce current immune concepts specifically relevant to type 1 diabetes. Because our understanding is not complete, as evidenced perhaps by our lack of standard immunotherapy to prevent type 1 diabetes, we can only provide a partial framework. Perhaps the simplest framework (which may be wrong) is to consider the development of type 1 diabetes as the balance between regulatory and effector T lymphocytes. We know that in the absence of a major portion of regulatory T lymphocytes in a rare syndrome caused by mutation of the FOXP3 gene, most infants (even neonates) develop type 1 diabetes. Central to the development of type 1 diabetes are T lymphocytes with specific T-cell receptors that recognize islet molecules. When a T-cell is activated through its receptor, it can orchestrate protection from infection or autoimmunity, depending on the target. Other T-cells can suppress or enhance autoimmunity (either in general or only for T lymphocytes in islets). The activation of a T-cell involves multiple different cell types and genes, as we will discuss.
IMMUNE RESPONSE
The modern era of immunology began with the clonal selection theory independently expressed by David W. Talmage and Sir Frank Macfarlane Burnet (1,2). The clonal selection theory postulates that a foreign antigen entering the body binds to one unique antibody selected from an unlimited repertoire of antibodies formed early in the organism's life. This explains how the immune system is able to recognize and respond to a virtually inestimable number of foreign antigens.
The immune system is a complex network of cells and organs that functions to protect the body against pathogens. This network uses multiple specialized cell types communicating via cellular interactions and humoral factors such as cytokines. The immune system is composed of the adaptive and the innate immune system. The adaptive immune system is an antigen-specific system that generates immunological memory and T-cell and antibody responses specific to pathogens or infected cells. The innate immune system is the first line of defense against pathogens, working to recognize common components of pathogens so that further immune responses can be signaled in the presence of foreign pathogens. The natural mechanisms involved in host defense can turn against self, promoting the development of an autoimmune response to antigens of the host's own tissue. Importantly, the majority of autoimmune responses against self-antigens do not result in disease progression. Only when sustained autoimmune responses cause tissue damage is the consequence of this destructive process identified as autoimmune disease.
ADAPTIVE IMMUNITY AND TYPE 1 DIABETES
The adaptive immune system is an antigen-specific structure that discriminates non-self molecules through the recognition of peptide antigens using receptor interactions between T-cells and antigen-presenting cells (APCs). This highly specific system uses receptor interaction between T-cells and APCs to discriminate self from nonself. Adaptive immunity establishes long-term immunological memory responses that trigger clonal expansion of T lymphocytes, which in turn cross-talk to B-cells to produce antigen-specific antibodies. The components of adaptive immunity are T and B lymphocytes, each with their own structurally unique cell receptors, which are somatically generated during thymic cell development. The adaptive immune system depends on the ability to assemble rearranged genes for both the T-cell receptor (TCR) and the immunoglobulin gene. This ability results from two genes known as RAG-1 and RAG-2 and their gene products that encode a recombinase involved in somatic recombination. The adaptive immune system allows T- and B-cells to generate an enormously diverse response to different pathogens. Both the naive T- and B-cell receptor repertoire are generated by interaction with self-ligands, such as the major histocompatibility complex (MHC), which in turn can signal to T- and B-cells to mature and survive. T-cells that are selected on self-ligands and sustained on self-ligands are termed “autoreactive T-cells.”
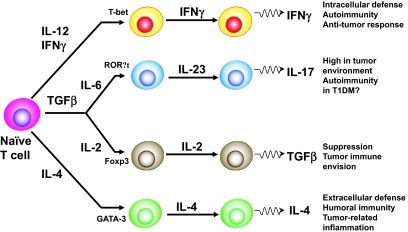
T-cells secrete large quantities of cytokines in response to antigen-specific activation and, based on their cytokine secretion profiles, are defined as T-helper type 1 (TH1), TH2, or TH17 (Fig. 1). TH1 cells mature in response to interleukin (IL)-12 and produce interferon (IFN)-γ, which enhances cellular immunity and is important for intracellular defense, autoimmunity, and anti-tumor response. TH2 cells develop in response to IL-4 and produce IL-4, IL-5, and IL-13, which enhance humoral immunity and are important for extracellular defense. IL-2 is essential for transforming growth factor-β–mediated induction of Foxp3+ regulatory T-cells (Tregs) and for the survival of Foxp3+ Tregs in the periphery (3,4). Interest in Tregs has been heightened by evidence that anti-CD3 monoclonal antibody treatment reverses hyperglycemia in newly diagnosed NOD mice, and perhaps also in humans, as a result of the induction of regulatory T-cells (5,6). Tregs can be expanded in vitro and in vivo and could be harnessed therapeutically to treat type 1 diabetes or facilitate tolerance to an allogeneic graft (7). An exhaustive review of Tregs in type 1 diabetes is provided in this issue of Diabetes.
FIG. 1.
CD4+ T-cells have been subdivided into different subsets on the basis of their cytokine production and their functions. TH1, TH2, and Tregs have been well characterized with respect to factors influencing their development. Recently, another subset of T-cells has been identified, namely TH17, which is distinct from TH1 or TH2 and is characterized by its production of IL-17. TH17 cells may play a crucial role in the induction of autoimmune tissue injury. In contrast, CD4+CD25+Foxp3+ regulatory T-cells inhibit autoimmunity and protect against tissue injury. IL-17–expressing T-cells are largely found in the tumor environment, particularly in advanced tumors, but not in the tumor-draining lymph nodes. Both IL-6 and transforming growth factor (TGF)-β induce the differentiation of pathogenic TH17 cells from naive T-cells (3). TH17 cells may play a role in a range of inflammatory conditions including uveitis, multiple sclerosis, experimental autoimmune encephalomyelitis, psoriasis, and rheumatoid arthritis (9), but there is not yet conclusive evidence that TH17 cells are somehow involved in the pathogenesis of type 1 diabetes (T1DM).
The TH-17 subset of T helper cells was identified on the basis of its ability to produce IL-17A, IL-17F, and IL-22. TH-17 cells were first recognized during assessment of the involvement of IL-23 in autoimmune disease. IL-23 is a member of the IL-6 family, and the nuclear receptor RORt acts as a key transcription factor in this lineage-commitment process (8) (Fig. 1). TH-17 cells provide protection in certain infections but have also been linked to a number of autoimmune diseases, a function previously assigned to TH1 cells and IFN-γ. TH-17 cells seem to mediate pathology in uveitis, multiple sclerosis, experimental autoimmune encephalomyelitis (EAE) psoriasis, and rheumatoid arthritis (9), but it is not entirely clear if the TH-17 pathway is influenced in type 1 diabetes. A recent study in NOD mice indicated that IFN-γ induced by an adjuvant-free antigen can reverse hyperglycemia, possibly as a result of suppression of pathogenic IL-17–producing cells (10). However, additional research is necessary to establish to what extent TH-17 cells play a role in type 1 diabetes pathogenesis.
The HLA complex.
Type 1 diabetes is a complex heterogeneous disease for which there is a small number of genes with large effects (i.e., HLA) and a large number of genes with small effects (11). There are probably many genetic forms of type 1 diabetes, and most forms are influenced by genes within the HLA region on chromosome 6p21 (IDDM1). Certain combinations of HLA alleles are found to be associated with each other on the same chromosome with a frequency greater than expected, and, consequently, they are not randomly distributed within the general population. This phenomenon is known as linkage disequilibrium, and it is quantified by the difference between the observed and the expected frequencies of certain combinations of alleles. It is the combination of these alleles on single chromosomes (haplotypes) and combinations of both chromosomes (one from each parent: genotype) that predominantly determines diabetes risk.
The principal genes localized within the MHC code for human leukocyte antigens, or HLA, two molecular classes of cell surface glycoproteins differing in structure, function, and tissue distribution. The genes that encode class I MHC consist of HLA-A, -B, and -C, whereas class II molecules are encoded by the DR, DQ, and DP genes.
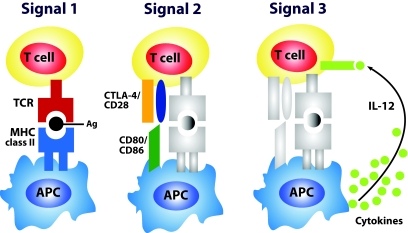
APCs are cell populations specialized to take up pathogens and other antigens, present them to lymphocytes, and provide signals that stimulate the proliferation and differentiation of lymphocytes, generally T lymphocytes (Fig. 2). The main type of APC that gives rise to T-cell responses is the dendritic cell. Other cells functioning as APCs include macrophages, B-cells (12), and, recently, stellate cells (Ito cells), which appear to be new actors in antigen presentation (13). T-cell activation requires a sustained interaction between a naïve T-cell's TCR and the MCH-peptide complex on an antigen presenting (signal 1, signal 2, signal 3; Fig. 2).
FIG. 2.
Exogenous antigens are taken into the APCs, cleaved into peptides, and coupled with MHC molecules for presentation and recognition by naïve T-cells. T-cells bear on their surface unique receptors created by genetic recombinatorial processes. Signal 1 is delivered through the TCR when it engages an appropriate peptide-MHC complex. To facilitate this interaction, adhesion structures on both the APC and T-cell interact; for example, intracellular adhesion molecule-1 (ICAM-1) on the APC interacts with leukocyte function–associated antigen-1 (LFA-1) on the T-cell. Another adhesion receptor pair is formed between CD58 (LFA-3) on the APC and CD2 on the T-cell. For the process of T-cell activation to continue, the T-cell requires an activation signal from the APC. This “second signal” can be provided by cell surface molecules such as CD80 or CD86, which bind to CD28 on the T-cell. Signal 2 is referred to as “co-stimulation” and is taken to mean an accessory signal(s) that, along with signal 1, induces “immunity.” This is often measured as T-cell clonal expansion, differentiation into effector cells, and a long-term increase in precursor frequency (“memory”). Alternatively, CTLA-4 can competitively bind CD80 or CD86, which results in a dampening of the T-cell response. The requirement for two signals to activate a T-cell is a mechanism for preventing nonspecific or self-specific T-cell inflammatory reactions; in the absence of both signal 1 and signal 2, TCR ligation results in anergy rather than activation. APCs therefore have the important function of determining when an activating second signal should be initiated. They do so by displaying pattern recognition receptors, which recognize highly conserved molecules expressed by infectious organisms called pathogen-associated molecular patterns. The interaction between an APC pattern recognition receptor and the pathogen-associated molecular pattern of a microorganism causes the APC to upregulate MHC and co-stimulatory molecule expression (i.e., CD80 and CD86). Recent observations indicate that APCs also determine the class of immune response by providing naïve T-cells in the draining lymphoid tissues with signals (signal 3), regulating the development of TH1, TH2, or Tr effector T-cells or cytotoxic T lymphocytes (CTL). The best-studied TH1-driving factor is IL-12, which binds the IL-12 receptor. Engagement of the IL-12 receptor co-stimulates Th cell proliferation while selectively upregulating and priming for high IFN-γ production. IL-12 is an example of a mediator that delivers a signal 3 that can promote Th1-cell or CTL development.
Susceptibility to type 1 diabetes is conferred by specific HLA DR/DQ alleles (e.g., DRB1*03-DQB1*0201 [DR3] or DRB1*04-DQB1*0302 [DR4]) (14,15). Each allele is simply given a number that represents a unique amino acid sequence. Each sequence binds only certain peptides and thus helps direct targeting of the immune system. The genotype associated with the highest risk for type 1 diabetes is the DR3/4-DQ8 (DQ8 is DQA1*0301, DQB1*0302) heterozygous genotype. In addition, HLA alleles such as DQB1*0602 are associated with dominant protection from type 1 diabetes in multiple populations (16). There is a different disease risk for each MHC genotype, and although it is possible that only a single peptide epitope will relate to disease with multiple MHC genotypes, this remains to be experimentally evaluated.
Aly et al. (17) provided evidence that risk for islet autoimmunity drastically increased in DR3/4-DQ2/DQ8 siblings who shared both HLA haplotypes identical by descent with their diabetic proband sibling (63% by age 7 years, and 85% by age 15 years) compared with siblings who did not share both HLA haplotypes with their diabetic proband sibling. These data suggest that HLA genotyping at birth may identify individuals at very high risk of developing type 1 diabetes before the occurrence of clear signs of islet autoimmunity and, eventually, overt disease.
Class I MHC molecules are expressed in virtually all nucleated cells, whereas class II molecule expression is restricted to B lymphocytes, dendritic cells, macrophages, and activated T lymphocytes. Peptides presented via MHC class I structures interact with CD8+ T-cells, while those peptides presented via MHC class II structures interact with CD4+ T-cells. Once a processed peptide is recognized through an MHC-TCR interaction, a cascade of signaling events occurs dependent upon the class of MHC structure recognized and the T-cell type that is activated. Once activated, CD4+ T-cells promote T-cell activation and differentiation, along with the ability to signal B-cells to generate an antibody response. CD8+ T-cells, when activated, produce inflammatory mediators and can directly target the destruction of specific peptide-presenting cells. Specific HLA class I alleles also influence diabetes risk, after correcting for linkage disequilibrium with DR and DQ alleles (18,19).
Similar to other autoimmune disorders, in type 1 diabetes, CD4+ and CD8+ T-cells contribute to immune-mediated β-cell destruction. Accumulating evidence indicates that multiple islet antigens, including insulin, are targeted by MHC class II–restricted autoreactive T-cells (20,21). In particular, a high degree of T-cell clonal expansion was observed in pancreatic lymph nodes from two long-term diabetic patients but not from control subjects. The oligoclonally expanded T-cells from diabetic subjects with DR4, which is a notorious susceptibility allele for type 1 diabetes, recognized the insulin A 1–15 epitope restricted by DR4. These experiments indicated that clonally expanded, autoreactive T-cells could be cloned out directly from the pancreatic draining lymph nodes of NOD mice. Although intriguing, there are a number of caveats to be considered in the interpretation of the abovementioned data. The two diabetic subjects had high glycemic levels and had longstanding insulin-dependent diabetes. Thus, it is possible that daily administration of exogenous insulin could initiate and sustain a systemic anti-insulin T-cell response. There is a need to have access to pancreatic and lymphoid tissue from cadaveric donors with signs of autoimmunity before disease onset to uncover the role for T-cell responses against islet autoantigens in disease pathogenesis.
By using MHC class II tetramers to probe the TCR specificity and avidity of GAD65-reactive T-cell clones isolated from patients with type 1 diabetes, Reijonen et al. (22) identified high-avidity CD4+ T-cells from patients’ peripheral blood. The presence of autoreactive T-cells with potential preferential usage of TCR to diabetes-related autoantigens may serve as both a potential marker for disease progression and a target for immune manipulation in autoimmune diabetes.
Class I–restricted T-cells may also play a critical role in the development of autoimmune diabetes, as suggested by the observation that, in newly diagnosed diabetic patients, islet-infiltrating CD8+ T-cells represent the prevalent cell type of insulitis. The autoantigens targeted by autoreactive CD8+ T-cells in NOD mice appear to be insulin (23) and the islet-specific glucose-6-phosphatase catalytic subunit–related protein (IGRIP) (24).
Another intriguing observation provided evidence that peptide 10–18 of the insulin B-chain is related with recurrence of autoimmunity and loss of β-cell function in islet-grafted type 1 diabetes recipients (25). Other investigations indicated that islet destruction is caused by autoreactive T-cells, whereas the tolerant nondiabetic state is characterized by autoreactive T-cells that secrete the immune suppressive cytokine, IL-10 (26).
How much of type 1 diabetes pathoetiology is genetic and how much is environmental?
Perhaps the most convincing advancement in our knowledge of the genetics of type 1 diabetes derives from the discoveries of an autosomal recessive mutation on chromosome 21 causing the autoimmune polyendocrine syndrome type 1 (APS-I) (27) and an X chromosome mutation leading to an X-linked autoimmunity-allergic dysregulation syndrome (XLAAD; also termed IPEX) (28). The autoimmune polyendocrine syndrome type 1 is a rare syndrome with a relatively high incidence in Finland and Sardinia and among Iranian Jews, and it is characterized by type 1 diabetes, mucocutaneous candidiasis, hypoparathyroidism, Addison's disease, and hepatitis. This disease is caused by a mutation of the autoimmune regulator (AIRE) gene, which encodes a transcription factor. The gene product of AIRE is expressed in the thymus, and it might play an important role in maintaining self-tolerance to peripheral antigens such as insulin and other tissue-specific self-antigens in normal individuals (29). Loss-of-function mutations in the AIRE gene in autoimmune polyendocrine syndrome type 1 patients and, for the most part, in mutant mice lead to progressive autoimmune destruction of many tissues, including the pancreatic islets, adrenal cortex, parathyroid glands, and gonads.
The syndrome XLAAD is associated with severe neonatal autoimmunity, which is characterized by mononuclear infiltration of multiple organs including pancreatic β-cells. The causative gene, FOXP3 (Foxp3 in mice), and its protein product that encodes a transcription repressor are specifically expressed in CD25+CD4+ T-cells in the thymus and in the periphery. Lack of such regulatory T-cells lead to overwhelming autoimmunity in humans and mice. This is an important syndrome to diagnose because bone marrow transplantation is an effective therapeutic approach restoring regulatory T-cells in these patients and, possibly, preventing type 1 diabetes.
The mechanisms by which class II genes influence susceptibility to or protection from type 1 diabetes have been a subject of endless discussions. The crystal structure of DQ8 and I-Ag7 revealed important similarities between these two MHC class II molecules, and this implies that antigen presentation may occur in a comparable fashion in both humans and NOD mice. As a matter of fact, both DQ8 and IA-g7 bind similar sets of peptides, including those representing immunodominant epitopes in NOD mice. Interestingly, in a transgenic NOD mouse model, the expression of an I-Aβ (the equivalent to the human class II DQB allele) transgene carrying Asp 57 instead of Ser 57 prevents these mice from developing diabetes (30).
Brown et al. (31) characterized the structure of the crystallized HLA class II molecule. One hypothesis is that effective antigen binding depends on the conformation of the antigen binding site on the DQ dimer. It has been postulated that a substitution of an amino acid residue at these positions of the DQ molecule leads to conformational changes of the antigen-binding site and, consequently, to a modification of the affinity of the class II molecule for the “diabetogenic” peptide(s). As support for this hypothesis, it is known that Asp-57 is involved in hydrogen and salt bonding with both the peptide main chain and the DRα Arg-76 side chain. There are several highly diabetogenic class II DQ molecules with aspartic acid at position 57, and thus it is the complete amino acid sequence rather that any single amino acid residue that is relevant (32).
Autoimmunity is thought to result from an imbalance between the two functionally opposite processes, namely tolerance induction and immune responsiveness, each of which is dependent on the presence of MHC class I and class II molecules with appropriate structures (dictated by the genes encoding them) that are able to present antigenic peptides. In genetically susceptible individuals, certain class II molecules may poorly present self-peptides because of inefficiencies in the peptide-MHC structural interaction of these molecules, thereby leading to inadequate negative selection of T-cell populations that could later become activated to elicit an islet-specific destructive autoimmune response. Nepom and Kwok (33) explained the molecular basis of HLA-DQ associations with type 1 diabetes exactly on this basis. Paradoxically, some self-peptides that normally negatively select T-cells are likely to lead to positive selection when the MHC molecule is, for example, the HLA-DQ3.2. There are many non-MHC genes associated with type 1 diabetes, including polymorphisms influencing thymic insulin expression and T-cell receptor signaling (34), with essentially all related to immune function.
Environmental factors such as congenital rubella and enteroviruses (particularly Coxsackie B virus) have been related to type 1 diabetes pathogenesis. The presence of a viral infection can lead to immune cell activation through numerous mechanisms. Viruses may directly alter a host cell that may be lysed, releasing self-peptides and fragments of the host cell into the extracellular milieu, whereby they may be processed and presented via APCs. Upon reacting to a viral infection, the immune system may process and present a homologous viral protein in such a manner that the epitope targeted by the immune system can interact with both self-antigens and viral proteins. This process is termed “molecular mimicry.” GAD, a well-defined autoantigen in type 1 diabetes, shares similarities with the P2-C viral sequence of the Coxsackie B virus and the major outer capsid protein of Rotavirus (35,36). Viral infections or immunostimulators such as poly I:C, which is used to stimulate viral infections, can trigger islet autoimmunity by activating the innate immune system alone, as demonstrated in the Kilham Rat virus–induced autoimmune diabetes model (37).
Recent observations suggest that, in the Aire-deficient mice model, which causes a number of autoimmune diseases including autoimmune diabetes, the stochastic genesis of pathogenic T-cells can initiate autoimmune disease without the need for environmental stimulation, underlining the importance of Aire-dependent thymic deletion rather than an environmental triggering event (38).
Overall studies on viral elements in the pathogenesis of type 1 diabetes have been conflicting and have failed to prove conclusively that any of the environmental factors has an undisputable role in the development of type 1 diabetes in genetically and nongenetically susceptible individuals. To date, clear conclusions are limited because most of the studies were not adequately powered to detect differences in exposure and disease associations, had inaccurate exposure estimates, and had confounding exposures.
WHAT IS THE ANTIGEN?
A common peculiarity of many autoimmune diseases, such as type 1 diabetes, is the presence of humoral as well as T-cellular responses directed against multiple autoantigens. Since the early 1980s, many molecular targets of type 1 diabetes–related autoimmune responses have been identified, and these include insulin (39), GAD, islet cell antibody (ICA)512/IA-2 (40), I-A2β (phogrin), and, recently, the zinc transporter Znt8 (Slc30A8) (41). The majority of studies have largely focused on insulin and GAD65. Insulin-specific CD4+ and CD8+ T-cells have been isolated from islets from young NOD mice and the insulin peptide (B-chain, amino acid residues 9–23), which is immunodominant in NOD mice and is also recognized by human CD4+ and CD8+ cells from pre-diabetics (20,25).
As autoimmunity in type 1 diabetes progresses from initial activation to a chronic state, there is often an increase in the number of islet autoantigens targeted by T-cells and autoantibodies (42,43). This condition is termed “epitope spreading.” There is convincing evidence that islet autoantibody responses against multiple islet autoantigens are associated with progression to overt disease (42). More recently, we provided evidence suggesting that a subset of cytoplasmic ICA is related to a more rapid progression to insulin-requiring diabetes in GAD65 and IA-2 antibody–positive relatives compared with relatives with GAD65 and IA-2 antibodies without ICA (44). We believe that this ICA response is more than likely caused by a subset of the ICA reacting with unidentified islet autoantigen(s).
Recent studies have suggested a sequential hierarchy in reactivity to these islet autoantigens (45). Although the occurrence of immune responses against multiple autoantigens is proportionally associated with the risk of type 1 diabetes progression, the elimination of autoimmune responses to insulin prevents the development of the disease in NOD mice. In contrast, transgenic overexpression of islet-specific glucose-6-phosphatase catalytic subunit–related protein (IGRP) resulted in loss of intra-islet IGRP-specific T-cells but did not protect NOD mice from insulitis or type 1 diabetes. These data provide evidence that the response against IGRP is downstream of the response to proinsulin (45).
In summary, it is reasonable to hypothesize that the process of antigenic and epitope spreading is applicable to autoreactive T-cell responses, which can kill β-cells and in turn lead to release of additional antigens, which can then be presented to the immune system and give rise to new T-cell responses reactive to these antigens and further spreading to new epitopes and antigens.
T-cell antigen receptor.
The TCR for MHC-restricted CD4+ helper and CD8+ cytolytic lymphocytes is a membrane-anchored heterodimeric glycoprotein comprised of an α-chain covalently linked to a β-chain. TCR-α and -β genes are assembled by somatic DNA recombination during T-cell development in the thymus. Many different α- and β-chains are expressed within a single individual, and each T-cell expresses just two α-chains and two β-chains. The extracellular part of both the α- and β-chains consists of a variable (V) and a constant (C) domain.
T-cell activation requires a sustained interaction between a naïve T-cell, TCR, and the MCH-peptide complex on an APC. Exogenous antigens are taken into the APC, cleaved into peptides, and coupled with MHC molecules for presentation and recognition by naïve T-cells. T-cells bear on their surface unique receptors created by genetic recombinatorial processes. T-cell α- and β-chains (or γ and δ) can be rearranged and paired to produce an estimated 107-108 different TCRs in humans.
T-cells from “humanized” TCR transgenic mice are functional.
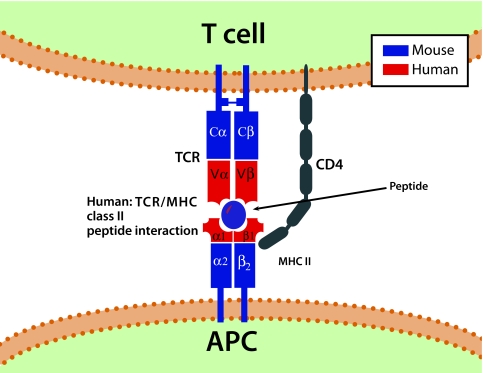
At least 200 therapeutic strategies can prevent diabetes in the NOD mice, and some of them might eventually work in humans. Thus, there is a necessity to develop a more “robust” murine model that mimics the opponent difficulty of altering the human disease. The NOD mouse represents a relevant animal model of autoimmunity in type 1 diabetes. Immunologists analyzed the effect of genes on immunity using this model of spontaneous diabetes, and they generated numerous transgenic mice on the NOD background to address specific immunologic questions. Although these models provided important clues in understanding autoimmunity in diabetes, they have significant limitations such as numerous differences in the structure of the immune system between mouse and humans, which likely result in discrepancies in how the immune system responds to physiologic and pathologic stimuli. For instance, the mouse MHC is distinct in many aspects from that of humans in that the MHC class II is expressed on activated human but not murine CD4+ T-cells. Moreover, human and mouse dendritic cell subsets express different cell surface markers and receptors, including different Toll-like receptors (46). Predisposing HLA alleles have been introduced and crossed onto multiple mouse strains that were engineered to develop autoimmunity. This approach was used to generate transgenic mice, which would express human components: HLA-DR2 (DRB*0101/DRB1*1501) (Fig. 3), CD4 co-receptor, and a TCR from a patient-derived T-cell clone recognizing the dominant myelin basic protein epitope (47). The resulting mice developed a disease that, in many aspects, resembled multiple sclerosis.
FIG. 3.
Exemplification of a human-mouse TCR-MHC class II transgenic mouse model [i.e., HLA-DR2 (DRB1*1501)]. Illustration of a human TCR class II complex in a TCR transgenic mouse.
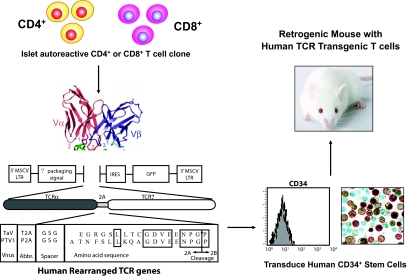
Recently, Vignali and colleagues (48) developed a novel approach for the rapid generation of TCR “retrogenic” mice and established TCR transgenic mice (among them NOD mice) to be used in studies of autoimmune diabetes pathogenesis (Fig. 4). Vignali and colleagues generated mice possessing a monoclonal population of T-cells expressing 1 of 17 TCRs specific for known autoantigens (GAD65, IA2, IA2β/phogrin, or insulin), unknown islet antigens, or control antigens on a NODscid background using retroviral-mediated stem cell gene transfer and 2A linked multicistronic retroviral vectors. This TCR retrogenic approach provides a mechanism by which T-cells with broad phenotypic differences can be directly compared. Importantly, recent data generated by this approach suggest that relatively few autoantigen-specific TCRs can mediate islet infiltration and β-cell destruction. These data strongly advocate that T-cell autoreactivity is not synonymous with pathogenicity (D. Vignali, personal communication).
FIG. 4.
“Retrogenic” is a term used for TCR transgenic mice generated by a retrovirus-mediated stem-cell gene transduction of hematopoietic stem cells with a vector carrying linked TCR α- and β-chains. The TCR α- and β-chains are expressed from a single “2A” peptide-linked multicistronic retroviral vector. The short 2A peptide inserted between the α- and β-chains encodes a sequence that impairs the formation of a normal glycine-proline peptide bond at the end of the sequence. This occurs via a “ribosomal skip” mechanism without affecting translation of the second protein. Naturally occurring 2A sequences are found in many viruses and some parasites. Mouse hematopoietic stem cells transduced with 2A retroviral vectors are then injected into conditioned mice to reconstitute the mouse with T-cells expressing the transgenic TCR.
A remarkable effect of the MHC complex in type 1 diabetes susceptibility is conferred by the highest-risk class II genotype (DR3-DQ2:DR4-DQ8) making up one-third of individuals who develop the disease versus a population frequency of 2.4% in Denver, Colorado (17). In addition, HLA molecules such as DQB1*0602 provide dominant protection from type 1 diabetes in multiple populations (16).
The general consensus is that the specificity for β-cell destruction lies in the failure of the host to eradicate or silence pathogenic T-cells with corresponding TCRs that recognize epitopes from β-cell–derived antigens. In the NOD mouse model, we have direct data regarding conservation of only the Vα and Jα gene segments of T-cell clones that react with the B:9–23 insulin peptide (49,50). Mutating this peptide prevents all diabetes (20). Simply putting back into these double insulin gene knockout mice a transgene with the normal insulin B:9–23 sequence (in contrast to transgene with B:9–23 sequence mutated at position B16) restores development of insulin autoantibodies and insulitis (follow-up to evaluate development of diabetes is under way). The T-cell α-receptor is relatively simple, with conservation of only two elements (Vα and Jα) and lacking conservation of the α-chain N region and all of the β-chain. Zekzer et al. (51) described a CD4+ T-cell clone (2H6) derived from pancreatic lymph nodes of NOD mice that 1) recognized the insulin B:9–23 and B:12–25 epitopes, 2) produced both transforming growth factor-β and IFN-γ, 3) were able to home to islets, and 4) prevented spontaneous diabetes in NOD mice. Remarkably, the TCR of this CD4+ T-cell clone shared a common Jα chain motif found in three of 50 germ-line Jα sequences (Table 1, Fig. 5) but used by the majority of pathogenic clones reacting with insulin peptide B:9–23.
TABLE 1.
Anti-insulin B:9–23 TCR α-chain conservation
| Clone | Designation | Vα | N | Jα | |
|---|---|---|---|---|---|
| Yale clone | |||||
| 2H6 | TRAV6S-1 | TRAJ53*01 | VYHCILR | VD | SGGSNYKLTFGKGTLLTVTP |
| BDC (Barbara Davis Center) clones | |||||
| 12-4.1 | TRAV5D-4*04 | TRAJ53*01 | MYFCAAS | G A N | SGGSNYKLTFGKGTLLTVTP |
| 12-4.4 | TRAV5D-4*04 | TRAJ53*01 | MYFCAAS | A | SGGSNYKLTFGKGTLLTVTP |
| 6-10.14 | TRAV5D-4*04 | TRAJ53*01 | MYFCAAS | S R | GGSNYKLTFGKGTLLTVTP |
| 4-7.2 | TRAV5D-4*04 | TRAJ53*01 | MYFCAAS | A N | GGSNYKLTFGKGTLLTVTP |
| 6-4.3 | TRAV5D-4*04 | TRAJ53*01 | MYFCAAS | A S G | SGGSNYKLTFGKGTLLTVTP |
| 8-1.3(LN) | TRAV5D-4*04 | TRAJ42 | MYFCAAS | A R G | SGGSNYKLTFGKGTKLSVKS |
| 12-3.20 | TRAV5D-4*04 | TRAJ42 | MYFCAAS | K I | GGSNYKLTFGKGTKLSVKS |
| 8-1.9(LN) | TRAV5D-4*04 | TRAJ42 | MYFCAAS | R P | GGSNYKLTFGKGTKLSVKS |
| 8-1.1(LN) | TRAV5D-4*04 | TRAJ56 | MYFCAAS | K | TGGNNKLTFGQGTVLSVIP |
| 8-1.5(LN) | TRAV5D-4*04 | TRAJ13 | MYFCAAS | A | NSGTYQRFGTGTKLQVVP |
| 12-1.19 | TRAV13 | TRAJ11 | TYLCAME | R S | SGYNKLTFGKGTVLLVSP |
| 12-2.35 | TRAV12 | TRAJ23 | LYFCAAI | Q | NYNOGKL I FGQGTKLSIKP |
| 6-6.4 | TRAV7 | TRAJ57 | LYYCAPN | Q | GGSAKL I FGEGTKLTVSS |
| 2/88 Vα = MYFCAAS | 3/50 Jα = KLTFG | ||||
Reprinted with permission from the Journal of Clinical Investigation (49). The α-chain used to generate the mice described by Homann and Eisenbarth (49) uses the dominant conserved Jα chain (TRAJ53*01). Gene segments are in italics. Gene segments that are conserved across multiple α-chains are in italics and boldface. Specific sequences that are shared across α-chains are in boldface but not italics, as they are not genes.
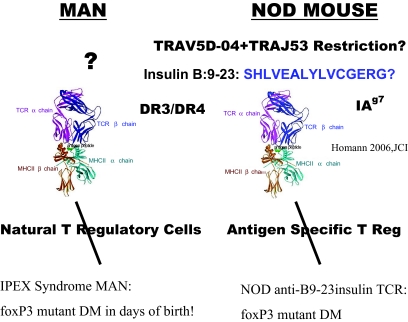
FIG. 5.
Hypothesis for type 1 diabetes (DM) for humans and the NOD mouse emphasizing recognition of critical islet autoantigenic peptide presented in groove of class II major histocompatibility antigen (e.g., DR or DQ). For the NOD mouse, insulin is a primary target autoantigen and, in particular, a peptide of insulin (insulin B:9–23 peptide SHLVEALY) targeted by a conserved T-cell receptor α-chain (TRAV5D-04 TRAJ53). The peptide is presented by a specific HLA-like molecule (I-Ag7). With loss or abnormalities of antigen-specific regulatory T-cell function, development of diabetes is enhanced both for NOD mice and humans (i.e., in the IPEX syndrome, mutation of FOXP3 leads to neonatal autoimmune diabetes, at times presenting within days of birth).
CENTRAL AND PERIPHERAL TOLERANCE
The concept of breaking of tolerance is fundamental to the development of autoimmunity. The first and perhaps most vital stage of tolerance induction to self-antigens occurs in the thymus during T-cell development. Because proteins with tissue-restricted or peripheral expression are traditionally thought to be unavailable for presentation in the thymus, it has been proposed that tolerance to such proteins can only be achieved through mechanisms of peripheral tolerance. Indeed, one attractive hypothesis is that type 1 diabetes is essentially due to failure of negative selection of autoreactive T-cells, either in the thymus or in the periphery, or because of a breakdown in tolerance to β-cell–specific antigens. This hypothesis has received support after thymic transplantation of islet antigens or expression of putative islet cell autoantigens resulting in prevention of diabetes in both NOD mouse and BB rat models (52,53).
There is evidence suggesting that molecules with tissue-restricted expression are also being expressed in the thymus (54). Genes encoding the type 1 diabetes–related autoantigens insulin, IA-2, and GAD and the neuroendocrine antigen ICA69 are transcribed in human thymus throughout fetal life and childhood. Insulin gene transcription in human thymus was also reported by others, and similarly, insulin, glucagon, GAD, and ICA69 transcripts were detected in mouse and rat thymus (55,56). If certain antigens could not be expressed in the thymus, the process of selection would be “blind” to such self-reactive T-cell clones, which could therefore escape negative selection. More recent work has identified AIRE as the gene responsible for this ectopic expression of many self-antigens in the thymus, which causes autoimmune polyendocrinopathy-candidasis-ectodermal dystrophy (27,57). This rare syndrome is an example demonstrating that a mutation of a single gene can give rise to an array of autoimmune polyendocrine disorders, including autoimmune insulin-requiring diabetes. These observations underline the importance of central tolerance in controlling the generation of autoimmunity.
Peripheral tolerance occurs in secondary lymphoid organs (i.e., lymph nodes and spleen) and regulates the activation of naïve T-cells via two mechanisms: anergy and regulatory T-cells. Anergy involves the clonal inactivation of T-cells with the potential to respond to self-antigens, resulting in cells that are resistant to activation upon antigen encounter. The initial step is selection of T-cells in the thymus followed by migration to the periphery, where T-cells encounter the antigen in pancreatic lymph nodes (58). If primed correctly, activated T-cells traffic to pancreatic islets, giving rise to a variety of inflammatory lesions from mild insulitis to a rapidly destroying immune-mediated β-cell response, leading to fulminant diabetes.
PROINFLAMMATORY CYTOKINES AND CHEMOKINES: KILLING OF β-CELLS
Multiple mechanisms have been invoked to elucidate how insulin-producing cells are destroyed. T-cells can directly kill β-cells through a cytotoxic process, but they can also influence β-cell destruction through multiple factors, including the release of proinflammatory cytokines, granzyme B, or perforin and, possibly, signaling through pathways of programmed cell death (59–61). Several observations suggest that proinflammatory cytokines, such as IL-1β, IFN-γ, and free radicals, are mediators of pancreatic β-cell death (62). There is compelling evidence that cytokines influence the expression of inducible nitric oxide (NO) synthase (iNOS), leading to NO production. In particular, IL-1β + IFN-γ, via NO synthesis, markedly decreased SERCA2b protein expression, depleted Ca2+ stores, and activated the ER stress pathway, which is a potential contributing mechanism to β-cell death (59). Furthermore, cytokine-induced (IL-1β + IFN-γ) apoptosis of INS-1 cells seems to be dependent on NO production, as demonstrated by the use of the NO blocker NG-methyl-l-arginine (63). NO contributes to cytokine-induced apoptosis through potentiation of Jun NH2-terminal kinase (JNK) activity and suppression of members of the serine/threonine-specific protein kinase (Akt) family (63). Although the role of oxidative stress in the pathogenesis of type 1 diabetes (64) is still a subject of debate, a reduced antioxidant capacity has been demonstrated in type 1 diabetic patients compared with healthy control subjects.
Accumulating evidence suggests that pancreatic β-cells are highly susceptible to a situation of protein folding imbalance termed endoplasmic reticulum (ER) stress (65). In other words, unfolded proteins accumulate in the ER as a result of ER stress, triggering apoptosis if this imbalanced condition is not reversed. Cytokines IL-1β and IFN-γ induce severe ER stress through, respectively, NO-mediated depletion of ER calcium and inhibition of ER chaperones, inhibiting β-cell defense and augmenting proapoptotic pathways (59). In pancreatic β-cells, components of an adaptive pathway termed the unfolded protein response can act as beneficial regulators under physiological conditions or promote β-cell dysfunction and apoptosis under situations of chronic stress. The adaptive unfolded protein response pathway naturally reduces ER stress, raising the question of whether enhancing the unfolded protein response pathway could protect β-cells from death in type 1 diabetes (60). It would be of importance to further explore the relationship between ER stress and β-cell attrition during autoimmune diabetes directly in living animals.
Chemokines are chemotactic cytokines that attract leukocytes to tissues. They are subdivided into four families termed CXC (CXCL 1–16), CX3C (CX3CL1), C (XCL1–2), and CC (CCL1–28) based on the position of the NH2-terminal cysteine residues (66). As many as half of known chemokines have been implicated in the pathogenesis of type 1 diabetes. Early studies found that pancreas-infiltrating CD4+ T-cells produce a wide range of chemokines including CCL2, CCL3, CCL4, CCL5, CCL7, CCL12, CXCL10, and XCL1 (67). More recently, a number of studies have implicated the CXCR3-binding chemokine, CXCL10, in the pathogenesis of type 1 diabetes (66). Just before or at the onset of diabetes, serum CXCL10 levels seem to be elevated in both human and animal models, suggesting an accumulation of Th1 lymphocytes. The wide range of findings regarding the role of chemokines in the pathogenesis of type 1 diabetes emphasizes the complex nature of disease development, and the integration of these findings into a coherent perspective on pathogenesis has been hampered by the fact that the majority of published expression analyses are limited to quantitation of chemokine transcripts without corresponding data on chemokines protein expression as well as mechanistic in vivo studies.
INNATE IMMUNITY AND TYPE 1 DIABETES
The first line of defense against pathogens is obtained through the response of the innate immune system (68). Unlike the adaptive immune system, the innate immune system recognizes pathogens and foreign molecules without having been previously exposed to them and without generating long-term immunological memory. The innate immune system achieves this by the use of invariant pattern recognition molecules, which recognize conserved molecular patterns present in foreign particles that enter the host, as well as several cell products that can be associated with a breach in defenses. These pattern recognition molecules can be soluble (e.g., collectins, pentraxins), membrane-bound (e.g., Toll-like receptors), or cytosolic (e.g., NOD-like receptors). It has been shown how deregulation of NOD-like receptors can lead to several inflammatory conditions, such as inflammatory bowel disease (69) and, possibly, type 1 diabetes.
This innate system uses multiple cell types, including macrophages, dendritic cells, NK cells, neutrophils, and epithelial cells, each of which has their own specific function in an innate response, from phagocytosis of infectious pathogens to direct targeted lysis of infected host cells. Natural killer (NK) cells are involved in killing target cells, and they interact with APCs and T-cells. It has been reported that, in longstanding type 1 diabetes, there is a reduced activation of NK cells (70). It is not clear if this anomaly is a consequence rather than a cause of disease, since prolonged hyperglycemia could also explain this phenomenon. Candidate targets for NK cell recognition in the pancreatic β-cells are ligands for the NKG2D receptor expressed by NK cells and CD8+ T-cells. A number of observations suggest that NKG2D plays a role in the development of autoimmune diabetes in NOD mice (71), raising the concept that blocking NKG2D interactions with its ligands or blocking NKG2D signaling could be potential therapeutic alternatives for type 1 diabetes by preventing the expansion and function of CD8 T-cells.
Recent observations suggest that innate immunity might play a role in the development of islet autoimmunity in a virus-induced murine model of autoimmune diabetes (37). It has been postulated that TLRs also have the potential to recognize self-antigens and trigger systemic autoimmune disease such as systemic lupus erythematosus, rheumatoid arthritis, and autoimmune myocarditis (72).
In summary, additional studies are necessary to unravel the role of innate immunity in type 1 diabetes. Cellular immunology might provide clues that are indispensable for further progress. The necessary studies include research on putative abnormalities within the TLR and NOD-like receptor pathways, which could be present many years before the clinical onset of type 1 diabetes.
CONCLUSION
Autoimmunity in general and type 1 diabetes in particular are the end result of altered pathogenicity and regulation. In physiologic conditions, there is balance between pathogenic T-cells that mediate disease such as effector T-cells with marked conservation of their TCRs (i.e., insulin) and regulatory T-cells that control autoimmunity. In type 1 diabetes and other autoimmune disorders, there is an altered balance between pathogenic and regulatory T-cells. In the next decade, research should target two specific pathways: 1) blocking the ability to generate a pathogenic T-cell response to antigen(s) thought to initiate the disease and 2) developing genetically engineered Treg-based cellular therapeutics to suppress pathogenic autoimmune responses.
In parallel with the experimental work, mathematical modeling has played a substantial role in the understanding of various disease pathways related to cancer immunology (73). Thus far, the appliance of modeling in diabetes research has focused mostly on the kinetics of glucose-induced insulin secretion (74), and only recently has mechanistic modeling begun to explore specific pathways related to disease, such as the effect of T-cells in β-cell destruction. Several groups are applying multiple complex system modeling and biosimulation to type 1 diabetes research (Entelos, Archimedes), and our group believes that there are central elements of this complex interplay between pathogenic, regulatory T-cells and β-cell dysfunction in the pathoetiology of the disease process. To this end, in silico research will likely be complementary to current experimental approaches in type 1 diabetes research and has the potential to enhance our understanding of the disease process and assist researchers in designing laboratory experimentation and in building a framework for the data collection in an effort to solve biological uncertainty.
Acknowledgments
This work was supported by the National Institutes of Health (grants RO1 DK53456, DK56200, and NIDDK PA-04-081, to M.P., and grants DK32083, DK32493, and DK057538), the Diabetes Autoimmunity Study in the Young, Autoimmunity Prevention Center (grant AI050864), Diabetes Endocrine Research Center (grant P30 DK57516), Clinical Research Centers (grants MO1 RR00069 and MO1 RR00051), the Immune Tolerance Network (grant AI15416), the American Diabetes Association, the Juvenile Diabetes Research Foundation, the Children's Diabetes Foundation (to G.S.E.), and the University of Michigan Center for Computational Medicine and Biology (CCMB) Pilot Research Program (to P.W.N.).
We gratefully acknowledge the support of the Brehm Coalition. We thank Drs. Emil Unanue, Gilbert Omenn, and Dario Vignali for helpful discussions. The skill of Allison Picinotti in electronically generating the images for this manuscript is appreciated.
REFERENCES
- 1.Talmage DW: Immunological specificity, unique combinations of selected natural globulins provide an alternative to the classical concept. Science 129: 1643–1648, 1959 [DOI] [PubMed] [Google Scholar]
- 2.Burnet FM: The new approach to immunology. N Engl J Med 264: 24–34, 1961 [DOI] [PubMed] [Google Scholar]
- 3.McGeachy MJ, Cua DJ: T cells doing it for themselves: TGF-beta regulation of Th1 and Th17 cells. Immunity 26: 547–549, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM: Cutting edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol 178: 4022–4026, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM: CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med 199: 1467–1477, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA: Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 346: 1692–1698, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Masteller EL, Tang Q, Bluestone JA: Antigen-specific regulatory T cells: ex vivo expansion and therapeutic potential. Semin Immunol 18: 103–110, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR: The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, O'Shea JJ: Th17 cells: a new fate for differentiating helper T cells. Immunol Res Jan 3 (Epub ahead of print), 2008 [DOI] [PubMed]
- 10.Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, Zaghouani H: Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med 205: 207–218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F, Lowe CE, Szeszko JS, Hafler JP, Zeitels L, Yang JH, Vella A, Nutland S, Stevens HE, Schuilenburg H, Coleman G, Maisuria M, Meadows W, Smink LJ, Healy B, Burren OS, Lam AA, Ovington NR, Allen J, Adlem E, Leung HT, Wallace C, Howson JM, Guja C, Ionescu-Tîrgovişte C; Genetics of Type 1 Diabetes in Finland, Simmonds MJ, Heward JM, Gough SC; Wellcome Trust Case Control Consortium, Dunger DB, Wicker LS, Clayton DG.: Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 39: 857–864, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noorchashm H, Noorchashm N, Kern J, Rostami SY, Barker CF, Naji A: B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice. Diabetes 46: 941–946, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH: Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity 26: 117–129, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Todd JA, Bell JI, McDevitt HO: HLA-DQb gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 329: 599–604, 1987 [DOI] [PubMed] [Google Scholar]
- 15.Morel PA, Dorman JS, Todd JA, McDevitt HO, Trucco M: Aspartic acid at position 57 of the HLA-DQ beta chain protects against type I diabetes: a family study. Proc Natl Acad Sci U S A 85: 8111–8115, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenbaum CJ, Schatz DA, Cuthbertson D, Zeidler A, Eisenbarth GS, Krischer JP: Islet cell antibody-positive relatives with human leukocyte antigen DQA1*0102, DQB1*0602: identification by the Diabetes Prevention Trial-type 1. J Clin Endocrinol Metab 85: 1255–1260, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Aly TA, Ide A, Jahromi MM, Barker JM, Fernando MS, Babu SR, Yu L, Miao D, Erlich HA, Fain PR, Barriga KJ, Norris JM, Rewers MJ, Eisenbarth GS: Extreme genetic risk for type 1A diabetes. Proc Natl Acad Sci U S A 103: 14074–14079, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdes AM, Erlich HA, Noble JA: Human leukocyte antigen class I B and C loci contribute to type 1 diabetes (T1D) susceptibility and age at T1D onset. Hum Immunol 66: 301–313, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, Hulme J, Maier LM, Smyth D, Bailey R, Cooper JD, Ribas G, Campbell RD, Clayton DG, Todd JA: Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature 450: 887–892, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS: Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435: 220–223, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kent SC, Chen Y, Bregoli L, Clemmings SM, Kenyon NS, Ricordi C, Hering BJ, Hafler DA: Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature 435: 224–228, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Reijonen H, Mallone R, Heninger AK, Laughlin EM, Kochik SA, Falk B, Kwok WW, Greenbaum C, Nepom GT: GAD65-specific CD4+ T-cells with high antigen avidity are prevalent in peripheral blood of patients with type 1 diabetes. Diabetes 53: 1987–1994, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Wong FS, Karttunen J, Dumont C, Wen L, Visintin I, Pilip IM, Shastri N, Pamer EG, Janeway CA Jr: Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med 5: 1026–1031, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Lieberman SM, Evans AM, Han B, Takaki T, Vinnitskaya Y, Caldwell JA, Serreze DV, Shabanowitz J, Hunt DF, Nathenson SG, Santamaria P, DiLorenzo TP: Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A 100: 8384–8388, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO: Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci U S A 102: 18425–18430, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M: Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest 113: 451–463, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJE, Lalioti MD, Mullis PE, Antonarkis SE, Kawasaki K, Asakawa S, Ito F, Shimizu N: Positional cloning of the APECED gene. Nat Genet 17: 393–398, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, Azzari C, Ziegler SF, Levings MK, Roncarolo MG: Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest 116: 1713–1722, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gavanescu I, Kessler B, Ploegh H, Benoist C, Mathis D: Loss of Aire-dependent thymic expression of a peripheral tissue antigen renders it a target of autoimmunity. Proc Natl Acad Sci U S A 104: 4583–4587, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyazaki T, Uno M, Uehira M, Kikutani H, Kishimoto T, Kmoto M, Nishimoto H, Miyazaki JI: Direct evidence for the contribution of unique I-ANOD to the development of insulitis in non-obese diabetic mice. Nature 345: 722–724, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Brown JH, Jardetzky TS, Gorga JC, Stern LF, Urban RG, Strominger JL, Wiley DC: Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature 364: 33–39, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, Lavant E, Louey A, Moonsamy P: HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the Type 1 Diabetes Genetics Consortium Families. Diabetes Feb 5 (Epub ahead of print), 2008 [DOI] [PMC free article] [PubMed]
- 33.Nepom GT, Kwok WT: Molecular basis for HLA-DQ association in IDDM. Diabetes 47: 1177–1184, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, et al.: Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316: 1336–1341, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkinson MA, Bowman MA, Campbell L, Darrow BL, Kaufman DL, Maclaren NK: Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Invest 94: 2125–2129, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honeyman MC, Coulson BS, Stone NL, Gellert SA, Goldwater PN, Steele CE, Couper JJ, Tait BD, Colman PG, Harrison LC: Association between rotavirus infection and pancreatic islet autoimmunity in children at risk of developing type 1 diabetes. Diabetes 49: 1319–1324, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Zipris D, Lien E, Nair A, Xie JX, Greiner DL, Mordes JP, Rossini AA: TLR9-signaling pathways are involved in Kilham rat virus-induced autoimmune diabetes in the biobreeding diabetes-resistant rat. J Immunol 178: 693–701, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Gray DH, Gavanescu I, Benoist C, Mathis D: Danger-free autoimmune disease in Aire-deficient mice. Proc Natl Acad Sci U S A 104: 18193–18198, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL: Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 222: 1337–1339, 1983 [DOI] [PubMed] [Google Scholar]
- 40.Lan MS, Lu J, Goto Y, Notkins AL: Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol 13: 505–514, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC: The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 104: 17040–17045, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase PH, Eisenbarth GS: Prediction of type I diabetes mellitus in first degree relatives using a combination of insulin, glutamic acid decarboxylase and ICA512bdc/IA-2 autoantibodies. Diabetes 45: 926–933, 1996 [DOI] [PubMed] [Google Scholar]
- 43.von Herrath M, Sanda S, Herold K: Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol 7: 988–994, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Pietropaolo M, Yu S, Libman IM, Pietropaolo SL, Riley K, LaPorte RE, Drash AL, Mazumdar S, Trucco M, Becker DJ: Cytoplasmic islet cell antibodies remain valuable in defining risk of progression to type 1 diabetes in subjects with other islet autoantibodies. Pediatr Diabetes 6: 184–192, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Krishnamurthy B, Dudek NL, McKenzie MD, Purcell AW, Brooks AG, Gellert S, Colman PG, Harrison LC, Lew AM, Thomas HE, Kay TW: Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest 116: 3258–3265, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A: The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood 102: 956–963, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Madsen LS, Andersson EC, Jansson L, krogsgaard M, Andersen CB, Engberg J, Strominger JL, Svejgaard A, Hjorth JP, Holmdahl R, Wucherpfennig KW, Fugger L: A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat Genet 23: 343–347, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Holst J, Szymczak-Workman AL, Vignali KM, Burton AR, Workman CJ, Vignali DA: Generation of T-cell receptor retrogenic mice. Nat Protoc 1: 406–417, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Homann D, Eisenbarth GS: An immunologic homunculus for type 1 diabetes. J Clin Invest 116: 1212–1215, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levisetti MG, Suri A, Petzold SJ, Unanue ER: The insulin-specific T cells of nonobese diabetic mice recognize a weak MHC-binding segment in more than one form. J Immunol 178: 6051–6057, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Zekzer D, Wong FS, Wen L, Altieri M, Gurlo T, von Grafenstein H, Sherwin RS: Inhibition of diabetes by an insulin-reactive CD4 T-cell clone in the nonobese diabetic mouse. Diabetes 46: 1124–1132, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Gerling IC, Serreze DV, Christianson SW, Leiter EH: Intrathymic islet cell transplantation reduces β-cell autoimmunity and prevents diabetes in NOD/Lt Mice. Diabetes 41: 1672–1676, 1992 [DOI] [PubMed] [Google Scholar]
- 53.Posselt AM, Barker CF, Friedman AL, Naji A: Prevention of autoimmune diabetes in the BB rat by intrathymic islet transplantation at birth. Science 256: 1321–1324, 1992 [DOI] [PubMed] [Google Scholar]
- 54.Pugliese A, Zeller M, Fernandez A Jr, Zalcberg LJ, Barlett RJ, Ricordi C, Pietropaolo M, Eisenbarth GS, Bennett ST, Patel DD: The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet 15: 293–297, 1997 [DOI] [PubMed] [Google Scholar]
- 55.Mathews CE, Pietropaolo SL, Pietropaolo M: Reduced thymic expression of islet antigen contributes to loss of self tolerance. Ann N Y Acad Sci 1005: 412–417, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Pietropaolo M, Giannoukakis N, Trucco M: Cellular environment and freedom of gene expression. Nat Immunol 3: 335, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D: The cellular mechanism of Aire control of T cell tolerance. Immunity 23: 227–239, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Hoglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D: Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med 189: 331–339, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL: Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 54: 452–461, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Kopito RR, Ron D: Conformational disease. Nat Cell Biol 2: E207–E209, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Estella E, McKenzie MD, Catterall T, Sutton VR, Bird PI, Trapani JA, Kay TW, Thomas HE: Granzyme B-mediated death of pancreatic beta-cells requires the proapoptotic BH3-only molecule bid. Diabetes 55: 2212–2219, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Haskins K, Kench J, Powers K, Bradley B, Pugazhenthi S, Reusch J, McDuffie M: Role for oxidative stress in the regeneration of islet beta cells? J Investig Med 52: 45–49, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Størling J, Binzer J, Andersson AK, Zullig RA, Tonnesen M, Lehmann R, Spinas GA, Sandler S, Billestrup N, Mandrup-Poulsen T: Nitric oxide contributes to cytokine-induced apoptosis in pancreatic beta cells via potentiation of JNK activity and inhibition of Akt. Diabetologia 48: 2039–2050, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Vessby J, Basu S, Mohsen R, Berne C, Vessby B: Oxidative stress and antioxidant status in type 1 diabetes mellitus. J Intern Med 251: 69–76, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M: Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci U S A 98: 10845–10850, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shigihara T, Oikawa Y, Kanazawa Y, Okubo Y, Narumi S, Saruta T, Shimada A: Significance of serum CXCL10/IP-10 level in type 1 diabetes. J Autoimmun 26: 66–71, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Bradley LM, Asensio VC, Schioetz LK, Harbertson J, Krahl T, Patstone G, Woolf N, Campbell IL, Sarvetnick N: Islet-specific Th1, but not Th2, cells secrete multiple chemokines and promote rapid induction of autoimmune diabetes. J Immunol 162: 2511–2520, 1999 [PubMed] [Google Scholar]
- 68.Medzhitov R, Janeway CA: Innate immunity: impact on the adaptive immune response. Curr Opin Immunol 9: 4–9, 1997 [DOI] [PubMed] [Google Scholar]
- 69.Corbaz A, ten Hove T, Herren S, Graber P, Schwartsburd B, Belzer I, Harrison J, Plitz T, Kosco-Vilbois MH, Kim SH, Dinarello CA, Novick D, van Deventer S, Chvatchko Y: IL-18-binding protein expression by endothelial cells and macrophages is up-regulated during active Crohn's disease. J Immunol 168: 3608–3616, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Rodacki M, Svoren B, Butty V, Besse W, Laffel L, Benoist C, Mathis D: Altered natural killer cells in type 1 diabetic patients. Diabetes 56: 177–185, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Ogasawara K, Hamerman JA, Ehrlich LR, Bour-Jordan H, Santamaria P, Bluestone JA, Lanier LL: NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity 20: 757–767, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S, Odermatt B, Conrad C, Ittner LM, Bauer S, Luther SA, Uematsu S, Akira S, Hengartner H, Zinkernagel RM: Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med 11: 138–145, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Ciupe SM, Ribeiro RM, Nelson PW, Perelson AS: Modeling the mechanisms of acute hepatitis B virus infection. J Theor Biol 247: 23–35, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergman RN, Ider YZ, Bowden CR, Cobelli C: Quantitative assessment of insulin sensitivity. Am J Physiol 236: E667–E677, 1979 [DOI] [PubMed] [Google Scholar]