Abstract
OBJECTIVE—We determined whether muscle AMP-activated protein kinase (AMPK) has a role in the development of insulin resistance.
RESEARCH DESIGN AND METHODS—Muscle-specific transgenic mice expressing an inactive form of the AMPK α2 catalytic subunit (α2i TG) and their wild-type littermates were fed either a high-fat (60% kcal fat) or a control (10% kcal fat) diet for 30 weeks.
RESULTS—Compared with wild-type mice, glucose tolerance in α2i TG mice was slightly impaired on the control diet and significantly impaired on the high-fat diet. To determine whether the whole-body glucose intolerance was associated with impaired insulin sensitivity in skeletal muscle, glucose transport in response to submaximal insulin (450 μU/ml) was measured in isolated soleus muscles. On the control diet, insulin-stimulated glucose transport was reduced by ∼50% in α2i TG mice compared with wild-type mice. High-fat feeding partially decreased insulin-stimulated glucose transport in wild-type mice, while high-fat feeding resulted in a full blunting of insulin-stimulated glucose transport in the α2i TG mice. High-fat feeding in α2i TG mice was accompanied by decreased expression of insulin signaling proteins in gastrocnemius muscle.
CONCLUSIONS—The lack of skeletal muscle AMPK α2 activity exacerbates the development of glucose intolerance and insulin resistance caused by high-fat feeding and supports the thesis that AMPK α2 is an important target for the prevention/amelioration of skeletal muscle insulin resistance through lifestyle (exercise) and pharmacologic (e.g., metformin) treatments.
The development of insulin resistance in skeletal muscle precedes the onset of type 2 diabetes by decades (1). Although the underlying mechanism is not fully understood, in recent years there has been growing interest in AMP-activated protein kinase (AMPK) as a potential target to attenuate skeletal muscle insulin resistance. As examples, two well-known therapies for type 2 diabetes, physical exercise and metformin, both activate AMPK in skeletal muscle (2). Despite this emphasis on AMPK, there is little direct evidence showing that AMPK is in fact critical in the development of skeletal muscle insulin resistance.
AMPK is an energy-sensing enzyme that is activated by acute increases in the cellular AMP-to-ATP ratio. In skeletal muscle, AMPK activity is increased by stimuli such as exercise, hypoxia, ischemia, and osmotic stress, all of which reduced cellular energy level (2). When intracellular ATP decreases, AMPK acts to switch off ATP-consuming pathways, such as glycogen, fatty acid, and protein synthesis pathways, and acts to switch on alternative pathways for ATP regeneration, such as glucose transport, glycolysis, and fatty acid oxidation. AMPK may also play a role in enhancing insulin sensitivity and/or responsiveness for glucose transport (3–6) in skeletal muscle.
AMPK is a heterotrimeric serine/threonine kinase that consists of a catalytic α-subunit and regulatory β- and γ-subunits (7–11). In skeletal muscle, α2 (12,13) is the major catalytic isoform expressed. To examine a possible role of AMPK in the development of insulin resistance, muscle-specific transgenic mice expressing an inactive form of the AMPK α2 catalytic subunit isoform (α2i TG mice) (14) and their wild-type littermates were subjected to a high-fat diet. Here, we show that ablation of muscle AMPK α2 activity worsens the glucose intolerance induced by high-fat feeding. This exacerbated glucose intolerance is associated with a marked decrease in muscle glucose transport in response to insulin, measured in vitro in isolated muscles. These results demonstrate that AMPK α2 activity is an important factor contributing to insulin action on glucose transport in skeletal muscle. Therefore, activation of muscle AMPK α2 by exercise and/or pharmacological stimulation can be a significant strategy to improve insulin resistance in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Transgenic mice.
To render the catalytic subunit inactive, the aspartic acid at amino acid residue 157 of rat AMPK α2 subunit was substituted for alanine (15). Mice expressing the inactive α2 tagged at the amino terminus with a HA epitope were generated by injecting the recombinant DNA driven by a muscle creatine kinase promoter into fertilized FVB (Friend virus B-type) mouse oocytes, and the initial characterization of these mice has been described previously (14). All procedures used were approved by the institutional animal care and use committee of the Joslin Diabetes Center.
Diet treatments.
Six-week-old male α2i TG mice and their wild-type littermates were housed in plastic cages in animal quarters maintained at 22°C with a 12:12-h dark-light cycle. Animals were fed a high-fat or standard diet for 30 weeks. The high-fat diet consisted of 19.7% (of energy) casein, 54.4% lard, 5.5% soybean oil, 12.3% maltodextrin, and 6.7% sucrose (D12492; Research Diets, New Brunswick, NJ). The control diet consisted of 19.7% casein, 4.4% lard, 5.5% soybean oil, 3.5% maltodextrin, 34.5% sucrose, and 31.1% corn starch (D12450B; Research Diets). Both control and high-fat diets were supplemented with 1.3% (wt/wt) vitamin mix, 0.3% (wt/wt) choline bitartrate, 1.3% (wt/wt) mineral mix, 1.7% (wt/wt) dicalsium phosphate, 0.7% (wt/wt) calcium carbonate, and 2.1% (wt/wt) potassium citrate.
Body weights and blood glucose concentrations.
The mice were weighed weekly, and blood glucose concentrations were measured in the fasting state once every 2 weeks. To measure blood glucose concentrations, food was removed at 2100 h and the mice were kept in a new cage for 12 h before blood collection. A drop of blood was taken from the tail between 0900 and 1000 h and blood glucose concentrations were determined using a glucometer (One Touch Ultra, LifeScan, West Chester, PA).
Glucose tolerance tests.
Mice underwent glucose tolerance tests at weeks 4, 18, 26, and 29. Glucose (1 g/kg) was injected intraperitoneally and blood was collected from the tail before (0 min) and after glucose injection (15, 30, 60, and 120 min). Blood glucose was determined using the glucometer.
Blood parameters.
The mice were fasted for 12 h before the experiment. Mice were killed by decapitation and trunk blood collected. Serum insulin concentrations were assessed using a rat/mouse insulin enzyme-linked immunosorbent assay kit with a mouse insulin standard (Linco Research, St. Charles, MO). Serum triglycerides were determined by kit assay (Sigma, St. Louis, MO) and serum free fatty acids using the NEFA C kit (Wako, Dallas, TX).
Measurement of muscle lipid and glycogen content.
Muscle triglycerides were estimated by measuring glycerol fluorimetrically after hydrolysis (16). Diacylglycerol and ceramide content in the extracts were determined using a diglyceride kinase reaction-based method (17). For glycogen measurements, muscle was hydrolyzed and glycogen concentrations were determined by the hexokinase enzymatic method, using the glucose HK reagent (Sigma). Tibialis anterior muscles were used for these measurements.
Muscle incubation and glucose transport.
After 30 weeks of dietary treatment, the mice were fasted overnight and killed. The soleus muscles were rapidly removed and treated as previously described (14). Briefly, each soleus muscle was mounted on an incubation apparatus and preincubated in Krebs-Ringer bicarbonate (KRB) buffer containing 2 mmol/l pyruvate for 20 min. The muscles were then incubated in KRB buffer in the absence or presence of 450 μU/ml insulin for 30 min. The buffers were kept at 37°C throughout the experiment and gassed continuously with 95% O2 and 5% CO2. Immediately after muscle incubation, the muscles were transferred to KRB buffer containing the same concentration of insulin and 1 mmol/l 2-[3H]-deoxy-d-glucose (1.5 μCi/ml) and 7 mmol/l d-[14C]-mannitol (0.3 μCi/ml), and glucose transport was measured as described (14).
Immunoblotting.
Immunoblotting was done using standard procedures as previously described (18). Antibodies were from commercial sources and consisted of insulin receptor β-subunit (Cell Signaling), insulin receptor substrate-1 (IRS-1), Akt (Upstate Biotechnology, Lake Placid, NY), and GLUT1 and GLUT4 (Chemicon, Temecula, CA). Gastrocnemius muscles were used for immunoblotting.
Measurement of isoform-specific AMPK activity.
AMPK activity was measured as previously described (14). Briefly, muscle lysates (150 μg protein) were immunoprecipitated with specific antibodies against the α1 or α2 catalytic subunits and protein A beads. The kinase reaction was carried out in 40 mmol/l HEPES, pH 7.0; 0.1 mmol/l synthetic SAMS peptide; 0.2 mmol/l AMP; 80 mmol/l NaCl; 0.8 mmol/l dithiothreitol; 5 mmol/l MgCl2; and 0.2 mmol/l ATP (2 μCi of [γ-32P]ATP) for 20 min at 30°C. Reaction products were spotted on Whatman P81 filter paper, the papers were extensively washed in 1% phosphoric acid, and radioactivity was assessed with a scintillation counter. Kinase activity was assessed by incorporated ATP (pmol) per immunoprecipitated protein (mg) per min.
Noninvasive physiological and behavioral characterization.
Wild-type and α2i TG mice were subjected to noninvasive physiological and behavioral characterization using the Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) at the Physiology Core Laboratory of the Joslin Diabetes Center. The mice were monitored for 24 h and assessed for oxygen consumption (Vo2; ml · kg−1 · h−1), carbon dioxide generation (Vco2; ml · kg−1 · h−1), heat generation calculated from gas exchange data (kcal/h), respiratory exchange ratio calculated from gas exchange data, food consumption (g/24 h), water consumption (ml/24 h), and locomotive activity evaluated by three-dimensional fixed-point observation (counts/h). Monitoring started at 1000 h, and CLAMS assessment was made during both the light cycle (0700–1900 h) and dark cycle (1900–700 h).
Statistics.
Statistical evaluation was performed by two-way ANOVA or Student's two-tailed t test. When ANOVA revealed significant differences, the Fisher's t test was used as a post hoc test for multiple comparisons.
RESULTS
Body weight and blood glucose concentrations.
Body weights and blood glucose concentrations are summarized in Table 1. The effects of the high-fat diet on body weight emerged by week 4 and remained for the remaining experimental period in both wild-type and α2i TG mice. The high-fat diet–induced increase in body weight was similar between wild-type and α2i TG mice. Blood glucose concentrations in the fasted state tended to be higher with the high-fat diet compared with the control diet in both wild-type and α2i TG mice (main effect of the diet, P < 0.05 at week 26 and P = 0.07 at week 29). The relatively minor effect of the high-fat diet on blood glucose may be due to the mouse strain used in this study. It has been shown that in FVB mice, the background strain used in the current study are resistant to high-fat diets compared with other mouse strains (19,20). No significant differences in blood glucose concentrations were observed between genotypes in both control and high-fat diet–treated mice. There was a tendency for blood glucose concentrations to decline from week 0 to week 4, which is likely due to acclimation of the mice to handling during blood collection.
TABLE 1.
Change in body weight and blood glucose
| Week
|
|||||
|---|---|---|---|---|---|
| 0 | 4 | 18 | 26 | 29 | |
| Body weight (g) | |||||
| Control diet | |||||
| Wild type | 26.5 ± 0.7 | 30.6 ± 1.0 | 34.9 ± 1.8 | 37.8 ± 1.7 | 37.9 ± 1.4 |
| α2i TG | 25.0 ± 0.6 | 29.4 ± 0.6 | 32.7 ± 1.2 | 35.5 ± 1.3 | 36.4 ± 1.2 |
| High-fat diet | |||||
| Wild type | 24.8 ± 0.7 | 34.1 ± 0.8* | 41.3 ± 1.9* | 47.3 ± 2.5* | 47.6 ± 2.5* |
| α2i TG | 24.9 ± 0.5 | 34.9 ± 0.8* | 42.7 ± 1.7* | 49.2 ± 1.0* | 48.6 ± 1.5* |
| Blood glucose (mg/dl) | |||||
| Control diet | |||||
| Wild type | 190.6 ± 10.8 | 127.8 ± 5.1 | 136.4 ± 6.9 | 130.8 ± 6.9 | 126.6 ± 20.3 |
| α2i TG | 165.4 ± 11.4 | 155.0 ± 11.1 | 133.6 ± 12.7 | 122.4 ± 9.3 | 143.5 ± 29.4 |
| High-fat diet | |||||
| Wild type | 171.8 ± 16.6 | 165.0 ± 12.8 | 149.0 ± 28.6 | 154.5 ± 7.9 | 162.0 ± 17.6* |
| α2i TG | 182.5 ± 12.3 | 160.3 ± 10.8 | 157.2 ± 6.4 | 150.3 ± 14.3‡ | 191.5 ± 18.3‡ |
Data are means ± SE. n = 5–6 for all points.
P < 0.01 vs. control diet in same genotype.
P < 0.05 vs. control diet in same genotype.
AMPK activity.
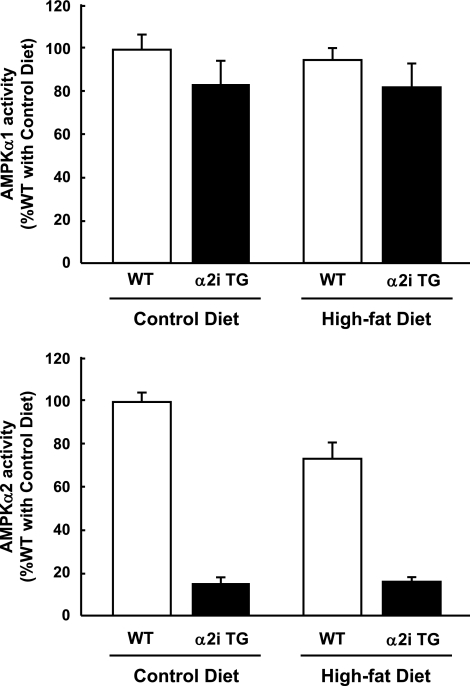
To confirm that muscle AMPK α2 activity was inhibited in muscles of α2i TG mice, isoform-specific AMPK activities were measured in gastrocnemius muscle at the end of the experimental period. As expected, α2 activity was blunted in α2i TG mice, and there was no compensatory increase in α1 activity in the α2i TG mice (Fig. 1). Interestingly, there was a tendency for AMPK α2 activity to diminish with long-term high-fat feeding in wild-type mice, whereas there was no effect of high-fat feeding on AMPK α1 activity (Fig. 1).
FIG. 1.
AMPK activity. Thirty weeks after the dietary treatments, the mice were fasted overnight. AMPK α1 and α2 activities were analyzed in extracts from gastrocnemius muscles. Data are represented as means ± SE. n = 5–6 for all groups.
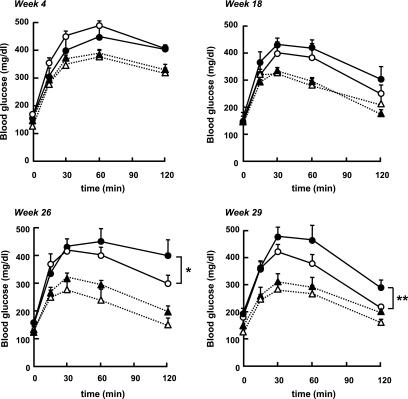
Glucose tolerance.
To test whether the lack of muscle AMPK α2 activity induces glucose intolerance in vivo, wild-type and α2i TG mice fed the control or high-fat diet were subjected to glucose tolerance tests. Blood glucose concentrations were monitored for 120 min after intraperitoneal glucose injection. As shown in Fig. 2, the high-fat diet resulted in impaired glucose tolerance in both wild-type and α2i TG mice by week 4, an effect that persisted for the remainder of the study. There was no difference between wild-type and α2i TG mice with both control and high-fat diet treatments at weeks 4 and 18. However, with 26 and 29 weeks of high-fat feeding, glucose tolerance in α2i TG mice was significantly impaired compared with wild-type mice. The area under the curve of the glucose tolerance test at week 29 was significantly higher (P < 0.05) in the α2i TG mice that were fed the high-fat diet (17.6 ± 7.2 vs. 22.1 ± 22.9; mg/dl × 120 min × 103). This shows that the lack of skeletal muscle AMPK α2 activity exacerbates the development of glucose intolerance.
FIG. 2.
Effect of high-fat diet and lack of AMPK α2 activity on glucose tolerance test. Glucose tolerance tests were performed in wild-type and α2i TG mice fed a control or a high-fat diet at weeks 4, 18, 26, and 29. Blood glucose concentration was measured at time points 0, 15, 30, 60, and 120 min after the glucose injection. Data are represented as means ± SE. n = 5–6 for all groups. *P < 0.05 vs. wild type with high-fat diet; **P < 0.01 vs. wild type with high-fat diet. ○, high-fat diet wild type; •, high-fat diet α2i TG; ▵, control diet wild type; ▴, control diet α2i TG.
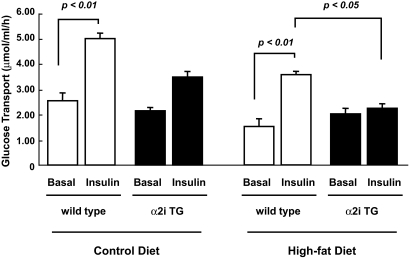
Glucose transport in isolated muscle.
We next examined muscle insulin sensitivity for glucose transport using the in vitro muscle incubation system (Fig. 3). At week 30, soleus muscles were isolated from the mice and incubated in the absence or presence of a submaximal dose of insulin (450 μU/ml). In mice fed the control diet, insulin increased glucose transport by twofold in wild-type mice. This increase in insulin-stimulated glucose transport tended to be diminished in α2i TG compared with wild-type mice (P = 0.07). Insulin significantly increased glucose transport in wild-type mice fed both the standard and high-fat diet, but this increase was blunted by 40% with the high-fat feeding. In α2i TG mice, insulin-stimulated glucose transport was totally abolished on the high-fat diet, showing that a lack of AMPK activity further worsens high-fat diet–induced muscle insulin resistance. These results suggest that impaired insulin-stimulated glucose transport in α2i TG mice on the high-fat diet contributes to the deterioration of glucose intolerance observed in these animals. Isolated extensor digitorum longus muscles were also used to determine glucose transport in vitro (data not shown). However, the increase in insulin-stimulated glucose transport was very small even in control diet–fed wild-type mice, making it difficult to compare the difference between genotype and fed conditions and demonstrating that extensor digitorum longus muscles become insulin resistant with age.
FIG. 3.
Effect of lack of AMPK α2 activity and high-fat diet on insulin-stimulated glucose transport. Thirty weeks after the dietary treatments, the mice were fasted overnight. Isolated soleus muscles were incubated in KRB buffer with or without submaximal doses of insulin (450 μU/ml) for 30 min. Muscle lysates were used for the measurement of glucose transport. Data are represented as means ± SE. n = 5–6 for all groups.
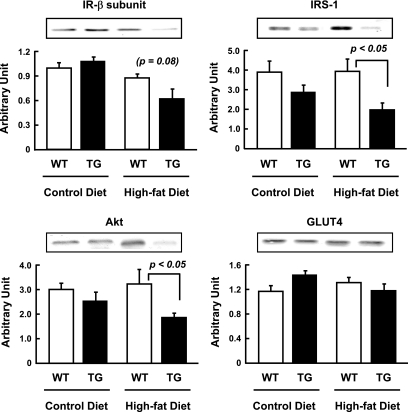
Expression of insulin signaling molecules.
Because α2i TG mice showed decreased insulin sensitivity for glucose transport, we also determined the effects of diet and genotype on protein expression of key insulin signaling molecules. Since use of the entire soleus muscle was necessary to measure glucose transport, we used gastrocnemius muscles for these analyses. The decrease in insulin-stimulated glucose transport in the α2i TG mice fed the high-fat diet was accompanied by reductions in insulin receptor-β subunit, IRS-1, and Akt compared with wild-type mice fed the high-fat diet (Fig. 4). Expression of these molecules was not significantly different between α2i TG and wild-type mice fed the control diet. Therefore, lack of AMPK α2 activity itself does not seem to be a trigger for the downregulation of insulin signaling molecules. Instead, there may be synergic effects of high-fat feeding and a lack of AMPK α2 activity on the expression of these proteins. Interestingly, GLUT4 expression was not different among the four groups (Fig. 4). Although it has been reported that activation of AMPK by 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR) increases GLUT4 expression in skeletal muscle (21,22), our results show that ablation of AMPK activity does not affect GLUT4 protein expression.
FIG. 4.
Effect of lack of AMPK α2 activity and high-fat diet on expression of molecules related with insulin-dependent glucose transport. Thirty weeks after the dietary treatments, the mice were fasted overnight. Immunoblotting of gastrocnemius muscle lysate from wild-type and α2i TG mice was performed. Quantification of insulin receptor-β, IRS-1, Akt, and GLUT4 were displayed. Data are represented as means ± SE. n = 5–6 for all groups.
Muscle lipid and glycogen content.
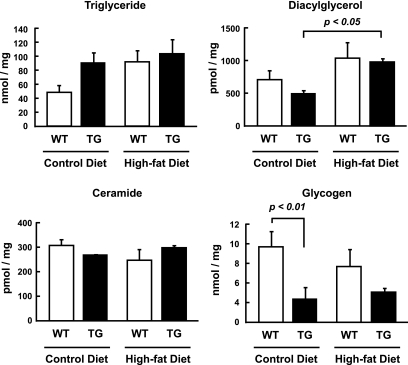
Muscle lipid concentrations are considered to be an important determinant of insulin resistance in skeletal muscle (23). Therefore, we measured muscle triglyceride, diacylglycerol, and ceramide content in tibialis anterior muscles (Fig. 5). Muscle triglyceride concentrations tended to be higher in α2i TG mice compared with wild-type mice on the control diet, although the difference did not reach statistical significance (P = 0.09). The high-fat diet also tended to increase triglyceride content in wild-type mice compared with the control diet (P = 0.08). There was no additive effect of the high-fat diet and lack of AMPK α2 activity on triglyceride content. Muscle diacylglycerol concentrations were not different between wild-type and α2i TG mice on the control diet. The high-fat diet increased diacylglycerol content, and this increase was statistically significant only in the α2i TG mice. There were no differences in muscle ceramide concentrations among the four groups. Muscle glycogen concentrations are also considered to be an important factor in the regulation of insulin-stimulated glucose transport (24). Muscle glycogen content in the α2i TG mice was lower compared with wild-type mice under both the standard and high-fat diet conditions. The high-fat diet did not significantly affect muscle glycogen content in both wild-type and α2i TG mice.
FIG. 5.
Effect of lack of AMPK α2 activity and high-fat diet on muscle lipid and glycogen content. Thirty weeks after the dietary treatments, the mice were fasted overnight. Triglyceride, diacylglycerol, ceramide, and glycogen content were analyzed in extracts from tibialis anterior muscles. Data are represented as means ± SE. n = 5–6 for all groups.
Serum insulin, free fatty acid, and triglyceride concentrations.
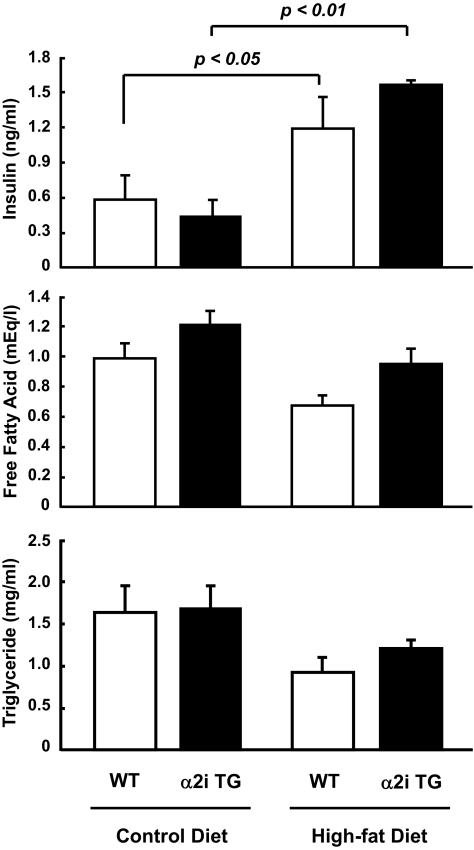
As shown in Fig. 6, the high-fat diet significantly increased serum insulin in both the wild-type and α2i TG mice, with no difference between genotypes. Both serum free fatty acids and triglycerides tended to decrease with the high-fat diet compared with the control diet in both wild-type and α2i TG mice, although these changes did not reach statistical significance.
FIG. 6.
Effect of lack of AMPK α2 activity and high-fat diet on serum metabolic parameters. Thirty weeks after the dietary treatments, the mice were fasted overnight. Serum insulin, free fatty acids, and triglycerides were measured. Data are represented as means ± SE. n = 5–6 for all groups.
Respiratory exchange ratio.
As expected, respiratory exchange ratio was significantly reduced (P < 0.05) with the high-fat diet in both wild-type (control 0.86 ± 0.03; high fat 0.76 ± 0.03) and α2i TG (control 0.84 ± 0.03; high fat 0.75 ± 0.01) mice. There were no differences of respiratory exchange ratio between genotypes in both control and high-fat diet conditions.
DISCUSSION
AMPK has been proposed to be a key regulator of glucose metabolism in skeletal muscle and a therapeutic target for the treatment of type 2 diabetes (10,25). Although direct evidence for a role of AMPK in the long-term regulation of skeletal muscle insulin sensitivity is lacking, there are several lines of evidence to support the concept that AMPK may be important for normal insulin action in this tissue. As examples, acute activation of AMPK is followed by an increase in insulin action on glucose transport in skeletal muscle (3,4,6,26,27). Prior incubation of isolated rat epitrochlearis muscles with the AMPK activator AICAR enhanced insulin-stimulated glucose transport by twofold (4). It was reported that the insulin-sensitizing effects of both AICAR and hyperosmolarity were diminished by inhibition of AMPK activity with compound C in C2C12 myotubes (6). The effects of chronic AMPK activation via long-term AICAR treatment have also been investigated. AICAR treatment of Zucker diabetic fatty rats for 8 weeks improved insulin sensitivity measured by a hyperinsulinemic-euglycemic clamp, an affect due mainly to increased glucose transport in skeletal muscle (28). In contrast, there was no effect of 1 h of AICAR pretreatment on insulin-stimulated glucose transport in primary human muscle cells (29), and administration of AICAR for 1 week did not increase insulin-stimulated glucose transport in isolated muscles from KKAy-CEPT (30) and db/db (31) mice. The differences between the studies might come from the distinct types of muscles used for each of the investigations, since chronic AICAR administration was shown to improve insulin-stimulated glucose transport in rat skeletal muscle in a fiber-type–specific manner (5). Although current observations are not entirely consistent, studies using AICAR generally suggest that AMPK has a role in insulin sensitivity for glucose transport in skeletal muscle.
AICAR is taken up into skeletal muscle and metabolized to ZMP, an analog of AMP. Therefore, AICAR is not completely specific for AMPK because ZMP regulates other AMP-sensitive enzymes such as fructose-1,6-bisphosphates (32) and muscle glycogen phosphorylase (33). Given the nonspecificity of AICAR to AMPK activation and the inconsistent findings discussed above, it is important to use an animal model in which AMPK activity is eliminated to determine the role of AMPK in insulin sensitivity. In the current study, we showed that ablation of skeletal muscle AMPK α2 activity aggravates the development of whole-body glucose intolerance caused by high-fat feeding. Although we cannot fully rule out the possibility that glucose intolerance in high-fat–fed α2i TG mice was due to defects in insulin secretion or hepatic glucose production, this seems unlikely because the ablation of AMPK α2 activity blunted insulin-stimulated glucose transport measured in isolated muscles from high-fat–fed mice. These results demonstrate that AMPK is a necessary factor in order to maintain normal insulin action in skeletal muscle. Our results also suggest that a lack of muscle AMPK activity may increase the risks of insulin resistance and type 2 diabetes. Since glucose tolerance in α2i TG mice was not significantly different compared with wild-type mice on the control diet, ablation of AMPK α2 activity itself may not be a direct trigger in the development of insulin resistance. Rather, lack of AMPK activity may work as a precipitating factor for the development of insulin resistance. Importantly, AMPK activity in skeletal muscle from patients with type 2 diabetes appears to be intact. Basal activity is similar to matched controls (13,34), and the enzyme can be activated normally by acute exercise (13) and metformin in vivo (13,35) and by AICAR in vitro (36). These reports also suggest that skeletal muscle insulin resistance is unlikely to be initiated by dysfunction of the AMPK trimer.
Other studies have shown that obese insulin-resistant Zucker rats have reduced AMPK activity and related signaling abnormalities in skeletal muscle (37–39). These reports indicate that skeletal muscle AMPK activity can be altered if the obesity and/or insulin resistance is severe, and, in this situation, lower AMPK activity may contribute to the obese and insulin-resistant phenotype (39). Importantly, exercise training can reverse abnormalities of impaired AMPK signaling in obese Zucker rats, which may contribute to the beneficial effect of exercise on improvement of insulin resistance (39). Interestingly, it has also been reported that suppression of AMPK signaling is involved in tumor necrosis factor–induced skeletal muscle insulin resistance (40). Furthermore, the antiobesity effects of ciliary neurotrophic factor have been recently shown to be mediated by AMPK in skeletal muscle, and these effects are not suppressed by diet-induced obesity. Thus, AMPK may be a significant contributing factor to the development of insulin resistance in obesity.
Recently, it has been reported that there are age-associated reductions in AMPK activity in skeletal muscle (41). In this report, acute activation of AMPK α2 by AICAR infusion or exercise observed in young rats (3 months old) was blunted in skeletal muscle of old rats (28 months old). This blunted AMPK α2 activation was associated with reduced mitochondrial biogenesis, suggesting that aging-associated reductions in muscle AMPK activity may be an important contributing factor in the reduced mitochondrial function and resultant insulin resistance (41). Interestingly, treatment with the antidiabetes agent rosiglitazone for 1 week enhanced AICAR-induced AMPK α2 activation and glucose uptake in insulin-resistant high-fat–fed rats, suggesting that rosiglitazone potentiates AMPK α2 activity (27). Since rosiglitazone acutely activates AMPK (42), chronic or repetitive activation of AMPK, such as exercise training, may potentiate its function/activity. It has been reported that basal AMPK activity is elevated with endurance training in human skeletal muscle (43). Therefore, activation of muscle AMPK by exercise and/or pharmacological stimulation can be an important strategy to improve insulin resistance (44).
Accumulation of muscle triglyceride is recognized as a consistent marker of insulin resistance (45). Interestingly, in our study, muscle triglyceride content tended to increase in the α2i TG mice fed the standard diet, and, in conjunction, insulin-stimulated glucose transport also tended to be lower in the α2i TG mice. One of the major roles of AMPK in skeletal muscle is fatty acid oxidation (8,46). Therefore, it is possible that ablation of AMPK activity inhibited fatty acid oxidation and facilitated accumulation of triglycerides in α2i TG mice. The high-fat diet increased muscle triglyceride content in both wild-type and α2i TG mice. The high-fat diet and AMPK activity ablation, however, did not have an additive effect on triglyceride content. Since insulin-stimulated glucose transport was further impaired by a high-fat diet in the α2i TG mice, triglyceride content is not the factor that exacerbates muscle insulin resistance in this study. Diacylglycerol has been shown to accumulate in the insulin-resistant muscle of obese animal models, including high-fat–fed rats (23). Since the diacylglycerol-sensitive novel protein kindase Cs, ɛ and/or θ, are associated with reduced insulin action in skeletal muscle (47,48), intracellular accumulation of diacylglycerol may be a factor contributing to insulin resistance. Consistent with previous reports, the high-fat diet used in this study increased diacylglycerol content in both wild-type and α2i TG mice. No difference, however, was observed between wild-type and α2i TG mice, similar to the triglyceride finding. Ceramide, a lipid implicated in reduced insulin signaling (23), did not change in response to the high-fat diet or lack of AMPK activity.
We do not know the exact mechanism by which ablation of AMPK activity exacerbates insulin resistance induced by a high-fat diet. However, the reduced expression of insulin receptor β-subunit, IRS-1, and Akt may be an essential component for the blunted insulin action on glucose transport. Interestingly, high fat feeding and ablation of AMPK activity per se did not alter expression of those molecules. In future studies, it will be important to investigate the signaling mechanisms that induce the synergistic effects of combining a high-fat diet and ablation of AMPK.
It has been reported that muscle glycogen content is inversely correlated with insulin-stimulated glucose transport (49–51). However, in the current study, glycogen content in the gastrocnemius muscles was lowered by ablation of AMPK activity in α2i TG mice compared with wild-type mice in both control and high-fat diet (Fig. 4). Consistent with our results, decreased muscle glycogen content was also observed in AMPK α2 knockout (soleus muscle) (52) and AMPK dominant-negative TG mice (gastrocnemius muscle) (53). Therefore, glycogen cannot be a major factor responsible for the impaired insulin-stimulated glucose transport in α2i TG mice (Fig. 2).
The greater impairment of glucose tolerance in the high-fat–fed α2iTG mice compared with wild-type mice did not occur immediately upon commencement of high-fat feeding. This may be explained by the findings that FVB mice have a higher degree of resistance to high-fat diets compared with other mouse strains (19,20). It is possible that the effects of blunted AMPK activity on glucose tolerance, glucose transport, and expression of insulin signaling proteins would have been more pronounced if the animals were on a different background strain.
In summary, the present study shows for the first time that ablation of AMPK α2 activity specifically in skeletal muscle exacerbates the development of glucose intolerance and insulin resistance caused by high-fat feeding. We conclude that AMPK functions in the regulation of insulin-stimulated glucose transport in skeletal muscle. Keeping AMPK activity intact by regular exercise or using an AMPK activator such as rosiglitazone or metformin may be important in the prevention and treatment of skeletal muscle insulin resistance.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1 AR45670 and RO1 DK068626 (to L.J.G.), DERC DK36836 at the Joslin Diabetes Center, and the Individual National Research Service Award F32 AR049662 (to R.H.).
We thank Yangfeng Li, Matthew M. Seifert, and Lauren E. Peter for technical assistance.
Published ahead of print at http://diabetes.diabetesjournals.org on 26 August 2008.
N.F. and R.C.H. contributed equally to this article.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR: Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 113: 909–915, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Fujii N, Jessen N, Goodyear LJ: AMP-activated protein kinase and the regulation of glucose transport. Am J Physiol Endocrinol Metab 291: E867–E877, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW: AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat–fed rats. Diabetes 51: 2886–2894, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA: Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 282: E18–E23, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Jessen N, Pold R, Buhl ES, Jensen LS, Schmitz O, Lund S: Effects of AICAR and exercise on insulin-stimulated glucose uptake, signaling, and GLUT-4 content in rat muscles. J Appl Physiol 94: 1373–1379, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Smith JL, Patil PB, Fisher JS: AICAR and hyperosmotic stress increase insulin-stimulated glucose transport. J Appl Physiol 99: 877–883, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Hardie DG, Carling D, Carlson M: The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem 67: 821–855, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Ruderman NB, Saha AK, Vavvas D, Witters LA: Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol 276: E1–E18, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA: Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci 24: 22–25, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Winder WW: Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J Appl Physiol 91: 1017–1028, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Carling D: The AM: P-activated protein kinase cascade: a unifying system for energy control. Trends Biochem Sci 29: 18–24, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D: Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J 346: 659–669, 2000 [PMC free article] [PubMed] [Google Scholar]
- 13.Musi N, Fujii N, Hirshman MF, Ekberg I, Froberg S, Ljungqvist O, Thorell A, Goodyear LJ: AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes 50: 921–927, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Fujii N, Hirshman MF, Kane EM, Ho RC, Peter LE, Seifert MM, Goodyear LJ: AMP-activated protein kinase {alpha}2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem 280: 39033–39041, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Stein SC, Woods A, Jones NA, Davison MD, Carling D: The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 345: 437–443, 2000 [PMC free article] [PubMed] [Google Scholar]
- 16.Le Marchand Y, Singh A, Assimacopoulos-Jeannet F, Orci L, Rouiller C, Jeanrenaud B: A role for the microtubular system in the release of very low density lipoproteins by perfused mouse livers. J Biol Chem 248: 6862–6870, 1973 [PubMed] [Google Scholar]
- 17.Perry DK, Bielawska A, Hannun YA: Quantitative determination of ceramide using diglyceride kinase. Methods Enzymol 312: 22–31.:22–31, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Fujii N, Boppart MD, Dufresne SD, Crowley PF, Jozsi AC, Sakamoto K, Yu H, Aschenbach WG, Kim S, Miyazaki H, Rui L, White MF, Hirshman MF, Goodyear LJ: Overexpression or ablation of JNK in skeletal muscle has no effect on glycogen synthase activity. Am J Physiol Cell Physiol 287: C200–C208, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Hamann A, Flier JS, Lowell BB: Decreased brown fat markedly enhances susceptibility to diet-induced obesity, diabetes, and hyperlipidemia. Endocrinology 137: 21–29, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Hu CC, Qing K, Chen Y: Diet-induced changes in stearoyl-CoA desaturase 1 expression in obesity-prone and -resistant mice. Obes Res 12: 1264–1270, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Holmes BF, Kurth-Kraczek EJ, Winder WW: Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol 87: 1990–1995, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Ojuka EO, Nolte LA, Holloszy JO: Increased expression of GLUT-4 and hexokinase in rat epitrochlearis muscles exposed to AICAR in vitro. J Appl Physiol 88: 1072–1075, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Hegarty BD, Furler SM, Ye J, Cooney GJ, Kraegen EW: The role of intramuscular lipid in insulin resistance. Acta Physiol Scand 178: 373–383, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Richter EA, Derave W, Wojtaszewski JF: Glucose, exercise and insulin: emerging concepts. J Physiol 535: 313–322, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winder WW, Hardie DG: AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277: E1–E10, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Iglesias MA, Furler SM, Cooney GJ, Kraegen EW, Ye JM: AMP-activated protein kinase activation by AICAR increases both muscle fatty acid and glucose uptake in white muscle of insulin-resistant rats in vivo. Diabetes 53: 1649–1654, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Ye JM, Dzamko N, Hoy AJ, Iglesias MA, Kemp B, Kraegen E: Rosiglitazone treatment enhances acute AMP-activated protein kinase-mediated muscle and adipose tissue glucose uptake in high-fat-fed rats. Diabetes 55: 2797–2804, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, Lund S: Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 54: 928–934, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Al Khalili L, Krook A, Zierath JR, Cartee GD: Prior serum- and AICAR-induced AMPK activation in primary human myocytes does not lead to subsequent increase in insulin-stimulated glucose uptake. Am J Physiol Endocrinol Metab 287: E553–E557, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Fiedler, m, Zierath JR, Selen G, Wallberg-Henriksson H, Liang Y, Sakariassen KS: 5-aminoimidazole-4-carboxy-amide-1-β-D-ribofuranoside treatment ameliorates hyperglycaemia and hyperinsulinaemia but not dyslipidaemia in KKAy-CETP mice. Diabetologia 44: 2180–2186, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Song XM, Fiedler, m, Galuska D, Ryder JW, Fernstrom M, Chibalin AV, Wallberg-Henriksson H, Zierath JR: 5-Aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice. Diabetologia 45: 56–65, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Vincent MF, Marangos PJ, Gruber HE, Van den Berghe G: Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes 40: 1259–1266, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Longnus SL, Wambolt RB, Parsons HL, Brownsey RW, Allard MF: 5-Aminoimidazole-4-carboxamide 1-beta-D-ribofuranoside (AICAR) stimulates myocardial glycogenolysis by allosteric mechanisms. Am J Physiol Regul Integr Comp Physiol 284: R936–R944, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Hojlund K, Mustard KJ, Staehr P, Hardie DG, Beck-Nielsen H, Richter EA, Wojtaszewski JF: AMPK activity and isoform protein expression are similar in muscle of obese subjects with and without type 2 diabetes. Am J Physiol Endocrinol Metab 286: E239–E244, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ: Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51: 2074–2081, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Koistinen HA, Galuska D, Chibalin AV, Yang J, Zierath JR, Holman GD, Wallberg-Henriksson H: 5-Amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes 52: 1066–1072, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Barnes BR, Ryder JW, Steiler TL, Fryer LG, Carling D, Zierath JR: Isoform-specific regulation of 5′ AMP-activated protein kinase in skeletal muscle from obese Zucker (fa/fa) rats in response to contraction. Diabetes 51: 2703–2708, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Bergeron R, Previs SF, Cline GW, Perret P, Russell RR III, Young LH, Shulman GI: Effect of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes 50: 1076–1082, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Sriwijitkamol A, Ivy JL, Christ-Roberts C, DeFronzo RA, Mandarino LJ, Musi N: LKB1-AMPK signaling in muscle from obese insulin-resistant Zucker rats and effects of training. Am J Physiol Endocrinol Metab 290: E925–E932, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Gorgun CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE: Tumor necrosis factor alpha-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 4: 465–474, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI: Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab 5: 151–156, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fryer LG, Parbu-Patel A, Carling D: The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 277: 25226–25232, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF: 5′-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab 286: E411–E417, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Smith AC, Mullen KL, Junkin KA, Nickerson J, Chabowski A, Bonen A, Dyck DJ: Metformin and exercise reduce muscle FAT/CD36 and lipid accumulation and blunt the progression of high-fat diet induced hyperglycemia. Am J Physiol Endocrinol Metab 293: E172–E181, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Kelley DE, Mandarino LJ: Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49: 677–683, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Long YC, Zierath JR: AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest 116: 1776–1783, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI: Free fatty acid–induced insulin resistance is associated with activation of protein kinase C è and alterations in the insulin signaling cascade. Diabetes 48: 1270–1274, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI: Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230–50236, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Jensen J, Aslesen R, Ivy JL, Brors O: Role of glycogen concentration and epinephrine on glucose uptake in rat epitrochlearis muscle. Am J Physiol 272: E649–E655, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Derave W, Lund S, Holman GD, Wojtaszewski J, Pedersen O, Richter EA: Contraction-stimulated muscle glucose transport and GLUT-4 surface content are dependent on glycogen content. Am J Physiol 277: E1103–E1110, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Kawanaka K, Han DH, Nolte LA, Hansen PA, Nakatani A, Holloszy JO: Decreased insulin-stimulated GLUT-4 translocation in glycogen- supercompensated muscles of exercised rats. Am J Physiol 276: E907–E912, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF: Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279: 1070–1079, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ: A role for AMP-activated protein kinase in contraction-and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094, 2001 [DOI] [PubMed] [Google Scholar]