Abstract
OBJECTIVE—Blockade of the CB1 receptor is one of the promising strategies for the treatment of obesity. Although antagonists suppress food intake and reduce body weight, the role of central versus peripheral CB1 activation on weight loss and related metabolic parameters remains to be elucidated. We therefore specifically assessed and compared the respective potential relevance of central nervous system (CNS) versus peripheral CB1 receptors in the regulation of energy homeostasis and lipid and glucose metabolism in diet-induced obese (DIO) rats.
RESEARCH DESIGN AND METHODS—Both lean and DIO rats were used for our experiments. The expression of key enzymes involved in lipid metabolism was measured by real-time PCR, and euglycemic-hyperinsulinemic clamps were used for insulin sensitivity and glucose metabolism studies.
RESULTS—Specific CNS-CB1 blockade decreased body weight and food intake but, independent of those effects, had no beneficial influence on peripheral lipid and glucose metabolism. Peripheral treatment with CB1 antagonist (Rimonabant) also reduced food intake and body weight but, in addition, independently triggered lipid mobilization pathways in white adipose tissue and cellular glucose uptake. Insulin sensitivity and skeletal muscle glucose uptake were enhanced, while hepatic glucose production was decreased during peripheral infusion of the CB1 antagonist. However, these effects depended on the antagonist-elicited reduction of food intake.
CONCLUSIONS—Several relevant metabolic processes appear to independently benefit from peripheral blockade of CB1, while CNS-CB1 blockade alone predominantly affects food intake and body weight.
The incidence of obesity and the metabolic syndrome have grown to epidemic proportions, making increased research efforts toward discovery of novel anti-obesity therapies increasingly important. Endocannabinoids are key modulators of feeding behavior through the activation of the CB1 receptor (1,2), which is localized in the periphery as well as in many brain areas involved in the regulation of energy homeostasis and reward processes (3,4). Recent studies (5–11) have demonstrated that blocking the activity of the endogenous cannabinoid system may be a successful strategy for the treatment of obesity and the metabolic syndrome.
It is well known that CB1 receptors in the hypothalamus might regulate food intake through the disinhibition of the release of melanin-concentrating hormone from lateral hypothalamic neurons (12) and the inhibition of the release and/or expression of corticotrophin-releasing hormone in the paraventricular nucleus (13). Both these effects are under the negative control of leptin, which is known to negatively control endocannabinoid tone in the hypothalamus (2). On the other hand, the effects of CB1 activation on α-melanocyte–stimulating hormone are controversial, since both inhibition and stimulation were reported in the study by Hentges et al. (14), and no downstream effects of α-melanocyte–stimulating hormone on endocannabinoid levels were found in the hypothalamus (15).
There is compelling evidence that beneficial effects of CB1 blockade on metabolism may exceed the anorexigenic response (16–18). For instance, the treatment with the CB1 antagonist SR141716 caused only a transient suppression in food intake but a maintained reduction in body weight in diet-induced obese (DIO) mice (19) and Zucker rats (20). Those findings were consistent with other observations suggesting that the blockade of CB1 increases energy expenditure (21). These pharmacological data have been supported by data from genetic models. CB1 receptor knockout mice have significantly less fat mass than wild-type animals and are resistant to diet-induced obesity, even though their caloric intake is similar to that of wild-type animals (22,23). Collectively, these results suggest that changes in food intake are not critical and imply that endocannabinoids may regulate peripheral metabolism directly by binding to CB1 receptors expressed in peripheral tissues involved in the regulation of energy metabolism, such as white adipose tissue (WAT) (22), liver (24,25), skeletal muscle (26), and pancreas (27). Consistent with this, the endocannabinoid system has been reported to have a direct role in the modulation of adipocyte metabolism. Activation of the CB1 receptor in isolated mouse adipocytes increases the activity of lipoprotein lipase (22), increases the number of intracellular lipid droplets, and decreases adiponectin expression (27). CB1 receptor activation also increases hepatic fatty acid synthesis by upregulating the lipogenic transcription factor sterol regulatory element–binding protein-1c and its targets acetyl-CoA carboxylase α (ACCα) and fatty acid synthase (FAS) expression (24,25), suggesting the potential involvement of the endocannabinoid system in the pathogenesis of fatty liver. Finally, the CB1 antagonist SR141716 increases glucose uptake in isolated soleus muscle of ob/ob mice (26).
It is well known that the endocannabinoid/CB1 tone is upregulated in obesity, at both the central and peripheral levels (27–31). The important point is that CB1 activation has multiple direct metabolic actions on tissues that are consistent with storing more fat, and these appear to occur independently of any transient change of energy intake. So whereas the CB1 receptor subtype mediates both central and peripheral actions of endocannabinoids on energy balance, it is often unclear if endocannabinoid-mediated changes of lipid and glucose metabolism depend on central and/or peripheral CB1 activation (32–34). In fact, even though chronic CB1 blockade in obese animals and humans improves several symptoms of the metabolic syndrome (35), it still remains to be determined whether beneficial metabolic effects can directly result from CB1 receptor antagonism in the absence of changes in food intake. To address these questions, we infused a CB1 antagonist centrally or peripherally into normal and DIO rats for 1 week. This design allowed us to define the relative contribution of peripheral CB1 receptors by specifically comparing the consequences of central versus global CB1 antagonism. Pair-fed control groups allowed us to determine which metabolic consequences are independent of changes in food intake. To our knowledge, this is the first study to systematically examine these complex, but clinically relevant, questions. We find that chronic global, but not isolated central, CB1 antagonism has beneficial effects on glucose and lipid metabolism beyond the secondary effects of decreased food intake.
RESEARCH DESIGN AND METHODS
In a first experiment, 300- to 350-g male Wistar rats, purchased from Charles River Laboratories (L’Arbresle, France), were housed in individual cages under controlled temperature (23°C) and illumination (12-h light/12-h dark cycle). They were allowed ad libitum access to water and to a standard diet (RMI; Hersteller, Essex, U.K.). In a second experiment, male Sprague-Dawley rats were fed a 45% high-fat diet (Ssniff RM/H; Ssniff Spezialdiäten, Soest, Germany) for 8 weeks before the beginning of the experiments. The animals were handled regularly, and food intake and body weight were measured daily during the experimental period. The animals were killed at 16 weeks of age. Rats were tested either for euglycemic-hyperinsulinemic clamps or for measurement of hormones, metabolites, and tissue-specific mRNA or protein expression. All procedures were approved by the institutional animal care and use committees at the University of Cincinnati, in accordance with the National Institutes of Health guide for the care and use of laboratory animals; the office vétérinaire fédéral et cantonal, Geneva; or by the animal welfare committee at the Institute of Experimental Medicine of the Hungarian Academy of Sciences.
Intracerebroventricular and peripheral infusions.
To assess the effects of semichronic central CB1 antagonism, SR141716 (kindly provided by Procter and Gamble Pharmaceuticals, Mason, OH) was delivered over 6 days by intracerebroventricular infusion, using osmotic minipumps (model 2001D, Alzet Osmotic Pumps; Durect, Cupertino, CA). This was done under anesthesia with intramuscular ketamine-xylazine (ketalar-rompun; Parke-Davis and Bayer, Leverkusen, Switzerland) used at 50–80 and 9–13 mg/kg, respectively, by placing a cannula tip in the right lateral cerebral ventricle, fixed on the skull with dental cement. Following surgery, the animals received a single dose of 0.28 mg/kg buprenorphin (Buprenex; Reckitt Benckiser Healthcare, Hull, U.K.). After 1 week of recovery, the drinking response to intracerebroventricular angiotensin II injection (5 ng/μl) (Novabiochem, Laüfelfingen, Switzerland) was measured to confirm the correct placement of the cannula, and after 1 more week, the cannula was connected to an osmotic minipump using a polypropylene catheter, as previously described (36). Two doses of SR141716 (3 and 5 μg/rat per day) were tested. The vehicle used as a solvent was isotonic saline with 0.3% Tween 80. For peripheral delivery of the CB1 antagonist, we performed daily intraperitoneal injection of SR141716 at a dose of 10 mg · kg−1 · day−1 for 6 days. This dose has been extensively used in previous work (19,37,38). For both treatments, control rats received vehicle (NaCl 0.9% plus 0.3% Tween 80). To differentiate between the effects of the CB1 antagonist per se and those related to decreased food intake, groups of vehicle-treated rats were pair fed the amount of food consumed by CB1 antagonist–treated animals. The pair-feeding regimen consisted of giving the daily amount of food just before the onset of the dark phase. Thus, each experiment included three groups of animals: an ad libitum–fed control group treated intracerebroventricularly or intraperitoneally with vehicle, an intracerebroventricular or intraperitoneal CB1 antagonist–treated group, and a vehicle pair-fed control group. To exclude that the difference in the final effect on food intake—body weight and metabolic alterations were due to the different dosages used (5 μg/rat per day i.c.v. and 10 mg · kg−1 · day−1 i.p.)—a dose of 5 μg/rat per day was infused subcutaneously using osmotic minipumps (model 2001D).
Preparation of mouse serum and brain samples.
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO), J.T. Baker (Phillipsburg, NJ), EM Science (Gibbstown, NJ), and Pierce (Rockford, IL). Standards and quality controls (QCs) were prepared in whole blank or vehicle-treated homogenized mouse brain, one individual tissue per standard or QC. Blank mouse brain was homogenized by adding thawed tissue to a 3-ml syringe and pushing it through an 18-gauge needle into an individual 2-ml plastic conical tube. Internal standard in mouse serum (100 ng per 20 μl) was added. Spiking standard stocks were prepared in mouse serum. Working standards (22–22,222 ng/g) were made by spiking an aliquot of the spiking standard stock (50 μl total volume) in the 2-ml plastic screw cap vial containing the blank homogenized mouse brain, and 20 μl internal standard in mouse serum stock solution and samples were vortexed. For in vivo samples, whole mouse brains were homogenized individually as above. Internal standard (100 ng per 20 μl) and 50 μl blank serum was added and samples were vortexed. Methanol (600 μl) was added and samples were mixed for ∼10 min on a multitube vortex. Samples were centrifuged using a Hermle 2360 K centrifuge at 10,000 rpm for 10 min. The aliquot of the supernatant was transferred to a Gilson vial containing water/methanol (70/30; vol/vol) for analysis by liquid chromatography/mass spectrometry.
Liquid chromatography/mass spectrometry analysis.
Samples were analyzed using an Applied Biosystems (Foster City, CA) PE-Sciex API 4000 mass spectrometer with gradient reversed-phase high-performance liquid chromatography/electrospray–tandem mass spectrometry with a Waters (Milford, MA) Symmetry Shield C18 column (2.1 × 50 mm, 3.5 μm) and selected reaction–monitoring detection. The selected reaction–monitoring schemes were 463 → 363 and 555 → 455 for Rimonabant and the internal standard, respectively. Mobile phases contained acetronitrile, water, formic acid, and n-heptafluorobutyric acid (10/90/0.2%/1 mmol/l for A and 90/10/0.2%/1 mmol/l for B). The gradient allowed the internal standard and the analytes to be completely resolved.
Euglycemic-hyperinsulinemic clamps.
Global and tissue-specific glucose utilization rates were measured by performing euglycemic-hyperinsulinemic clamps associated with the labeled 2-deoxyglucose technique (39,40). In these studies, rats were fasted overnight and intraperitoneally anesthetized with Nembutal (50 mg/kg sodium pentobarbital; Abbott Laboratories, Chicago, IL). Total glucose utilization and hepatic glucose production rates were measured during euglycemic-hyperinsulinemic clamps under basal and insulin-stimulated states (200 mU/ml, Actrapid HM; Novo Nordisk, Bagsvaerd, Denmark) by infusing d-[U-14C]glucose (5 μCi/ml; Anawa Trading, Zürich, Switzerland), as previously described in detail (39,40). Immediately following euglycemic-hyperinsulinemic clamps, the in vivo insulin-stimulated glucose utilization index of individual tissues was determined by delivering a single bolus of labeled 2-deoxy-d-[1-3H]glucose (30 μCi/rat; Amersham Biosciences U.K., Buckinghamshire, U.K.) as previously described (39,40). Rats were killed by rapid decapitation, and tissues were rapidly removed, freeze clamped, and stored at −80°C until use.
Analytical procedures relative to clamp studies.
The 2-deoxy-d-[1-3H]glucose–and d-[U-14C]glucose–specific activities were determined in deproteinized blood samples (38,39). Measurement of tissue concentration of 2-deoxy-d-[1-3H]glucose-6-phosphate allowed calculation of the in vivo glucose utilization index of individual tissues and was expressed in nanograms per minute per milligram of tissue.
Quantitative RT-PCR procedure.
Various tissues were sampled, freeze- clamped, and stored at −80°C for measurement of mRNA expression of lipoprotein lipase (LPL), acetyl-CoA carboxylase α (ACCα), fatty acid synthase (FAS), stearoyl-CoA desaturase-1 (SCD-1), carnitine palmitoyltransferase-1 (CPT-1), hormone-sensitive lipase, adipose triglyceride lipase, ribosomal protein S29, and cyclophilin A by real-time quantitative PCR (Applied Biosystems, Warrington, U.K.). For primer sequences, see Table 1. Total RNA was extracted from frozen tissue samples using TRIzol Reagent (Invitrogen, Carlsbad, CA). RNA integrity was assessed by performing a 1% agarose gel electrophoresis in 1 × Tris-borate-EDTA, and its concentration was determined by spectrophotometry. cDNA templates for RT-PCR were synthesized using 2 μg of total RNA, random hexamers (Microsynth, Balgach, Switzerland), deoxyribonucleotide triphosphates, RNase inhibitor, RNAsin (Catalys; Promega, Madisson, WI), and Superscript III (Invitrogen). Quantitative PCR was performed using SYBR Green I DNA master mixture (Applied Biosystems) according to the standard protocol and using ∼70 ng template cDNA. All primers were used at a final concentration of 0.5 μmol/l. A standard curve allowed obtaining the relative concentration of each experimental gene. Values were normalized by the expression of ribosomal protein S29 in WAT and by that of cyclophilin A in the liver.
TABLE 1.
Primer sequences used for real-time quantitative PCR
| Gene | Accession number | Forward sequence | Reverse sequence | PCR product | Annealing T |
|---|---|---|---|---|---|
| ACCα (rat) | J03808 | 5’-TCC GGC TTG CAC CAT GAT AA-3’ | 5’-CCC CCA AAA CGA GTA ACA A-3’ | 104 | 54 |
| FAS (rat) | M76767 | 5’-AGG ATG TCA ACA AGC CCA AG-3’ | 5’-ACA GAG GAG AAG GCC ACA AA-3’ | 100 | 55 |
| SCD-1 (rat) | AF509569 | 5’-TGA AAG CTG AGA AGC TGG TG-3’ | 5’-CAG TGTGGG CAG GAT GAA G-3’ | 83 | 57 |
| LPL (rat) | L03294 | 5’-TCT CCT GAT GAT GCG GAT TT-3’ | 5’-CAA CAT GCC CAT CTG GTT TC-3’ | 97 | 54 |
| CPT-1α (rat) | L07736 | 5’-GGA TGG CAT GTG GGT AAA AG-3’ | 5’-TAC TGA CAC AGG CAG CCA AA-3’ | 203 | 55 |
| Hormone-sensitive | |||||
| lipase (rat) | NM012859 | 5’-TCT ATG CGC AGG AGT GTG TC-3’ | 5’-CTG GTT TCA GCC TCT TCC TG-3’ | 227 | 60 |
| Adipose triglyceride | |||||
| lipase (rat) | XM341960 | 5’-ACC TGT GCC TTA CCG TTC AC-3’ | 5’-GGC AAG AGT GAC ATG CAG AA-3’ | 186 | 60 |
| Ribosomal protein | |||||
| S29 (rat) | X59051 | 5’-GCT GAA CAT GTG CCG ACA GT-3’ | 5’-GGT CGC TTA GTC CAA CTT AAT GAA G-3’ | 73 | 58 |
| Glucose-6-phosphate (rat) | NM013098 | 5’-ACC CTG GTA GCC CTG TCT TT-3’ | 5’-GGG CTT TCT CTT CTG TGT CG-3’ | 150 | 60 |
| Cyclophilin A (rat) | M19533 | 5’-AGC ACT GGG GAG AAA GGA TT-3’ | 5’-CAT GCC TTC TTT CAC CTT CC-3’ | 291 | 55 |
Plasma measurements.
Plasma glucose was measured by the glucose oxidase method (Glu; Roche Diagnostics, Rotkreuz, Switzerland). Plasma nonesterified fatty acid and triglyceride levels were determined using Wako Chemicals (Neuss, Germany) and Biomérieux (Marcy l’Etoile, France) commercial kits, respectively. Plasma corticosterone (Immunodiagnostic Systems, Boldon, U.K.) and insulin (Linco Research, St. Charles, MO) levels were determined using a double-antibody radioimmunoassay kit. Plasma insulin levels were also measured by radioimmunoassay, as previously described (41).
Triglyceride content in adipose tissue.
The extraction procedure for tissue triglycerides was adapted from methods described previously (42,43). The triglyceride content of each sample was measured in duplicate after evaporation of the organic solvent, using an enzymatic method (Randox Laboratories, Crumlin, U.K.).
Statistics.
Results are given as means ± SE. Statistical analysis was performed using one-way ANOVA, followed by the post hoc Tukey test. A two-tailed P value <0.05 was considered statistically significant.
RESULTS
Chronic central CB1 antagonism suppresses food intake and decreases body weight in a dose-dependent manner in normal rats.
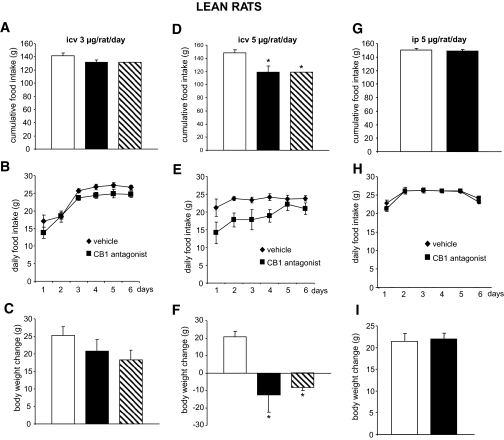
Although it has been reported that an acute intracerebroventricular injection of a CB1 antagonist at 3 μg/rat per day decreases food intake (37), central CB1 blockade with that dose for 6 days had no effect on food intake or body weight in normal rats in our paradigm (Fig. 1A–C). The higher dose of the CB1 antagonist (5 μg/rat per day for 6 days) elicited a decrease in food intake relative to vehicle-infused controls (Fig. 1D and E). Decreased food consumption was accompanied by a loss of body weight. Body weight loss of pair-fed vehicle-infused controls equaled that of intracerebroventricular SR141716-treated rats, implicating decreased food intake as the key mechanism for this effect (Fig. 1F).
FIG. 1.
Effects of CB1 antagonism on food intake and body weight in lean rats. A and B: Effect of a 6-day intracerebroventricular (icv) CB1 antagonist (3 μg/day) infusion on food intake in lean rats. C: Effect of a 6-day intracerebroventricular CB1 antagonist (3 μg/day) infusion on body weight in lean rats. D and E: Effect of a 6-day intracerebroventricular CB1 antagonist (5 μg/day) infusion on food intake in lean rats. F: Effect of a 6-day intracerebroventricular CB1 antagonist (5 μg/day) infusion on body weight in lean rats. G and H: Effect of a 6-day intraperitoneal (ip) CB1 antagonist (5 μg/day) infusion on food intake in lean rats. I: Effect of a 6-day intraperitoneal CB1 antagonist (5 μg/day) infusion on body weight in lean rats. Values are means ± SE of 5–7 animals per group. *P < 0.05. A and D: □, vehicle; ▪, CB1 antagonist; , vehicle-pf. B, E, and H: ♦, vehicle; ▪, CB1 antagonist.
, vehicle-pf. B, E, and H: ♦, vehicle; ▪, CB1 antagonist.
Interference of peripheral CB1 activity.
To rule out the possibility that centrally infused CB1 antagonist leaks out of the central nervous system (CNS) into the circulation and elicits a response by directly acting at peripheral CB1, we chronically administered the CB1 antagonist peripherally (6 days, via subcutaneous minipumps). Using the same dose as that infused centrally (5 μg/rat per day), we were unable to detect changes in body weight or food intake (Fig. 1G–I). We therefore conclude that the suppressive effects on food intake and body weight in response to the intracerebroventricular administration of 5 μg/rat per day were entirely due to CNS-CB1.
Central and peripheral CB1 antagonism suppresses food intake and body weight in DIO rats.
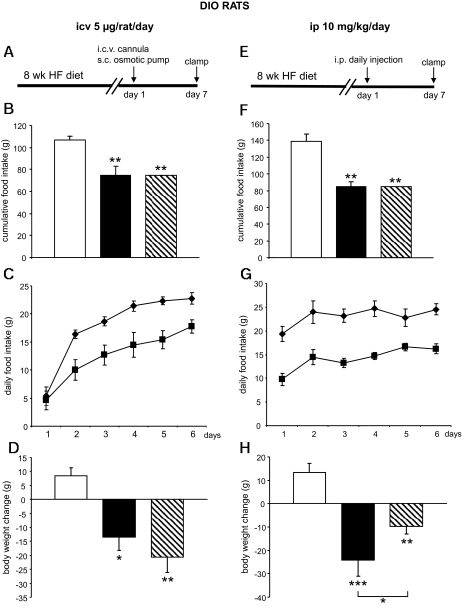
After observing that a dose of 5 μg/rat per day when administered intracerebroventricularly for 6 days significantly decreased food intake and body weight gain in normal rats, we assessed whether this dose would be effective in DIO animals (Fig. 2A, experimental timeline). Chronic CNS-CB1 blockade also suppressed food intake in DIO rats, and the decrease was of greater magnitude on a percentage basis than in normal rats (31 vs. 17% decrease, respectively) (Fig. 2B and C). The SR141716-induced loss of body weight was again mimicked by pair feeding. Unexpectedly, pair feeding produced a slightly more pronounced body weight loss than CB1 blockade in this cohort (Fig. 2D).
FIG. 2.
Effect of CB1 antagonism on food intake and body weight in DIO rats. A: Experimental timeline of intracerebroventricular (icv) studies. HF, high fat; s.c., subcutaneous. B and C: Effect of a 6-day intracerebroventricular CB1 antagonist (5 μg/day) infusion on food intake in DIO rats. D: Effect of a 6-day intracerebroventricular CB1 antagonist (5 μg/day) infusion on body weight in DIO rats. E: Experimental timeline of intraperitoneal (ip) studies. F and G: Effect of a 6-day intraperitoneal CB1 antagonist (10 mg/kg) administration on food intake in DIO rats. H: Effect of a 6-day intraperitoneal CB1 antagonist (10 mg/kg) administration on body weight in DIO rats. Values are means ± SE of five to seven animals per group. *P < 0.05; **P < 0.01; ***P < 0.001. B, F, D, and H: □, vehicle; ▪, CB1 antagonist; , vehicle-pf. C and G: ♦, vehicle; ▪, CB1 antagonist.
, vehicle-pf. C and G: ♦, vehicle; ▪, CB1 antagonist.
We subsequently compared the effects of a peripheral administration of CB1 antagonist (10 mg · kg−1 · day−1), leading to global CB1 antagonism, with the effects of CNS-CB1 blockade in DIO rats (Fig. 2E, experimental timeline). Peripheral infusion of the CB1 blocker SR141716 caused a decrease in food consumption (40% decrease, P < 0.01) (Fig. 2F and G) and body weight (Fig. 2H). In contrast to what occurred following central administration, body weight reduction induced by the peripherally administered CB1 antagonist was more marked than that of pair-fed controls (Fig. 2H) (P < 0.05 CB1-treated rats vs. vehicle pair fed). This observation is consistent with the conclusion that blockade of peripheral, but not central, CB1 receptors leads to metabolic changes driving body weight loss independent from food intake. No significant differences were detected in circulating glucose or insulin levels (Table 2).
TABLE 2.
Effect of central and global CB1 blockade on plasma glucose, insulin, nonesterified fatty acid, and corticosterone levels in DIO rats
| Central CB1 blockade
|
Global CB1 blockade
|
|||||
|---|---|---|---|---|---|---|
| Vehicle (ad libitum fed) | CB1 antagonist | Vehical (pair fed) | Vehicle (ad libitum fed) | CB1 antagonist | Vehical (pair fed) | |
| Glucose (mmol/l) | 5.35 ± 0.77 | 3.78 ± 0.12 | 4.11 ± 0.24 | 4.07 ± 0.62 | 5.32 ± 0.59 | 4.53 ± 0.38 |
| Insulin (ng/ml) | 1.67 ± 0.35 | 0.58 ± 0.12 | 1.04 ± 0.17 | 2.25 ± 0.36 | 1.38 ± 0.23 | 3.45 ± 0.73 |
| Nonesterified fatty acids (mmol/l) | 0.87 ± 0.12 | 0.66 ± 0.13 | 0.98 ± 0.17 | 1.78 ± 0.49 | 0.95 ± 0.14 | 0.94 ± 0.1 |
| Corticosterone (ng/ml) | 59.84 ± 9.28 | 59.77 ± 7.26 | 63.91 ± 7.14 | 38.67 ± 4.65 | 73.35 ± 10.1* | 75.08 ± 9.62* |
Data are means ± SE of five to seven animals per group. Rats were intracerebroventricularly infused (5 μg/rat per day) or peripherally injected (10 mg · kg−1 · day−1) with either CB1 antagonist or vehicle (controls) for 6 days. Vehicle pair-fed animals were restricted to the same amount of food as that consumed by CB1 antagonist–infused rats.
P < 0.05 vs. controls.
The peripheral injections have been made diluting the CB1 antagonist in Tween 80 (0.3%), and it is known that low concentrations of Tween may induce cellular damage. In addition to use in the vehicle group (saline plus 0.3% Tween 80), we have also assessed if Tween had any effect on food intake or body weight per se. Our data indicate that rats centrally infused with vehicle had the same food intake and body weight as saline-infused rats (online appendix Fig. 1 [available at http://dx.doi.org/10.2337/db08-0161]). Thus, the metabolic actions of the CB1 antagonist were not altered by Tween 80.
Analysis of the CB1 antagonist.
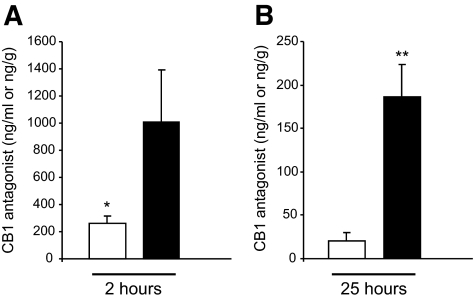
Having established that the CB1 antagonist showed a clear effect at both central and peripheral level in reducing food intake and body weight, we wanted to assess if this compound would reach the brain after peripheral injection. As shown in Fig. 3 and Table 3, mice treated with Rimonabant for 10 days at 10 mg/kg showed higher concentrations of the compound in the brain than in the serum at the corresponding times. This suggests an equilibrium delay between the brain and the serum and is the likely source of the longer apparent half-life in the brain versus the serum.
FIG. 3.
Concentrations of Rimonabant in serum and brain. The levels of the compound were determined following intraperitoneal dosing once daily for 10 days at 10 mg/kg. On the final day, mice were injected with compound and killed at ∼2 h (A) and ∼25 h (B) postdose and serum and brain collected and processed. □, serum; ▪, brain. *P < 0.05; **P < 0.01.
TABLE 3.
Summary tissue–to–serum concentration ratio after dosing in mice (C57BL/6J, male, day 10)
| Tissue
|
Tissue-to-serum ratio (k)
|
||||
|---|---|---|---|---|---|
| 3.85 ± 0.66
| |||||
| Brain
|
8.59 ± 3.71
|
||||
| Tissue | QD dose (mg/kg) | Sample | Time (h) | Concentration ± SD (ng/ml or ng/g) | Pseudo T1/2(h) |
| Serum | 10 | Cmax | 1.97 | 258 ± 58.9 | 6.33 |
| Cmin | 25.1 | 20.5 ± 9.25 | |||
| Brain | 10 | Cmax | 1.97 | 1010 ± 384 | 9.44 |
| Cmin | 25.1 | 186 ± 37.5 | |||
Data are means ± SE, unless otherwise indicated. Serum and brain concentrations of Rimonabant were determined following intraperitoneal dosing once daily for 10 days at 10 mg/kg. On the final day, mice were injected with compound and killed at ∼2 h and ∼25 h postdose and serum and brain collected and processed. The concentrations in the brain are 4–10 times higher than those in the serum at the corresponding time. The ratios at the 25-h collection times are almost two times higher than those observed at the 2-h collection. This suggests an equilibrium delay between the brain and the serum and is the likely source of the longer apparent half-life in the brain versus the serum. The pseudo T1/2 values were determined from the 2- and 25-h time points and are an approximation of the compound's half-life.
Differential effects of central versus global CB1 antagonism on lipid metabolism in DIO rats.
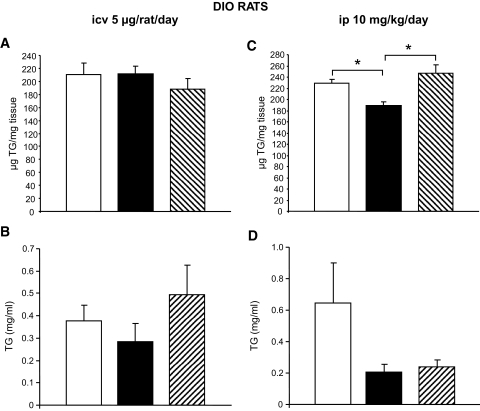
To dissect the effects of central and peripheral CB1 antagonism on lipid metabolism, we first determined the amount of triglycerides in epididymal WAT (Fig. 4A and C) and plasma (Fig. 4B and D) of DIO rats. Intracerebroventricular infusion of the CB1 antagonist did not modify triglyceride levels in WAT or in plasma (Fig. 4A and B). In contrast, WAT triglyceride content was decreased after peripheral CB1 blockade (Fig. 4C), a decrease that was not observed in the vehicle pair-fed control group and therefore cannot be secondary to CB1-induced changes in food intake. Circulating triglyceride levels were decreased in both the peripheral CB1 antagonist–infused animals and the pair-fed controls (Fig. 4D), although these differences did not reach statistical significance versus ad libitum–fed vehicle-infused controls.
FIG. 4.
Effect of CB1 antagonism on triglyceride levels in DIO rats. A: Effect of a 6-day intracerebroventricular (icv) CB1 antagonist (5 μg/day) infusion on triglyceride (TG) content in WAT in DIO rats. B: Effect of a 6-day intracerebroventricular CB1 antagonist (5 μg/day) infusion on circulating TG levels of DIO rats. C: Effect of a 6-day intraperitoneal (ip) CB1 antagonist (10 mg/kg) administration on TG content in the WAT of DIO rats. D: Effect of a 6-day intraperitoneal CB1 antagonist (10 mg/kg) administration on circulating TG levels of DIO rats. Values are means ± SE of five to seven animals per group. *P < 0.05. □, vehicle; ▪, CB1 antagonist; , vehicle-pf.
, vehicle-pf.
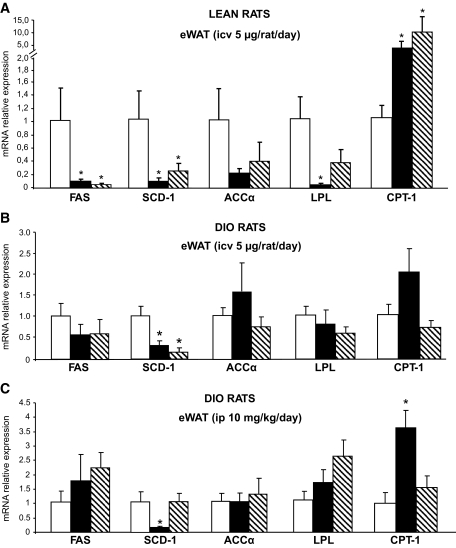
To better understand the molecular mechanisms responsible for the decreased triglyceride levels in the WAT of DIO rats after peripheral treatment with CB1 antagonist, we analyzed the expression profile of key enzymes involved in lipid metabolism. In WAT of normal rats centrally infused with the CB1 antagonist, the expression of genes promoting lipogenesis and triglyceride storage (i.e., FAS, SCD-1, ACCα, and LPL) was markedly reduced (Fig. 5A), and the expression of the fat-oxidizing enzyme CPT-1 was increased. These changes were linked to the reduction of food intake, since the vehicle pair-fed group had similar gene expression patterns as those observed in the CB1 antagonist–infused group. When the same dose of CB1 antagonist (5 μg/rat per day) was given peripherally instead of centrally, there was no change in the mRNA expression of any of these enzymes (online appendix Fig. 2).
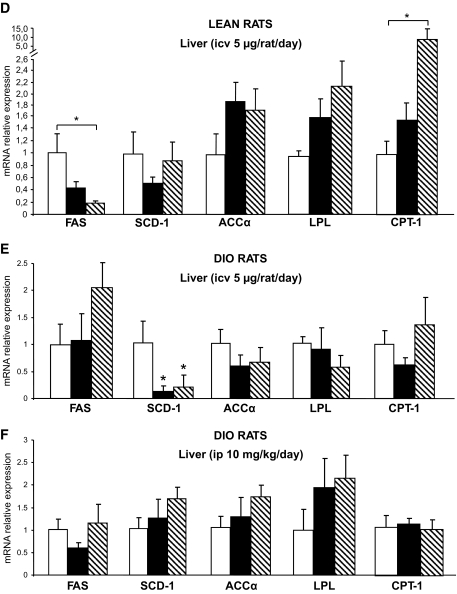
FIG. 5.
Effect of CB1 antagonism on peripheral lipid metabolism. A: Effect of a 6-day intracerebroventricular (icv) CB1 antagonist (5 μg/day) infusion on epidydimal white adipose tissue mRNA expression of FAS, SCD-1, ACCα, LPL, and CPT-1 in lean rats. B: Effect of a 6-day intracerebroventricular (icv) CB1 antagonist (5 μg/day) infusion on eWAT mRNA expression of FAS, SCD-1, ACCα, LPL, and CPT-1 in DIO rats. C: Effect of a 6-day intraperitoneal (ip) CB1 antagonist (10 mg/kg) administration on eWAT mRNA expression of FAS, SCD-1, ACCα, LPL, and CPT-1 in DIO rats. D: Effect of a 6-day intracerebroventricular CB1 antagonist (5 μg/day) infusion on liver mRNA expression of FAS, SCD-1, ACCα, LPL, and CPT-1 in lean rats. E: Effect of a 6-day intracerebroventricular CB1 antagonist (5 μg/day) infusion on liver mRNA expression of FAS, SCD-1, ACCα, LPL, and CPT-1 in DIO rats. F: Effect of a 6-day intraperitoneal CB1 antagonist (10 mg/kg) administration on liver mRNA expression of FAS, SCD-1, ACCα, LPL, and CPT-1 in DIO rats. Values are means ± SE of five to seven animals per group. *P < 0.05. □, vehicle; ▪, CB1 antagonist; , vehicle-pf.
, vehicle-pf.
In WAT of DIO rats centrally infused with the CB1 antagonist, the only two changes observed were a decrease in SCD-1 expression and a trend toward an increase in the expression of CPT-1. The decrease in SCD-1 expression was also present in the pair-fed vehicle-infused control group, indicating that it is driven by the hypophagic effect of SR141716 (Fig. 5B). A similar decrease in SCD-1 and increase in CPT-1 expression were observed in DIO rats receiving peripheral SR141716. In this case, the changes appeared to be independent of the hypophagia induced by the treatment, since the vehicle pair-fed group had similar levels of expression as ad libitum–fed control rats (Fig. 5C). The expression of other fat oxidation–promoting enzymes, hormone-sensitive lipase, and adipose triglyceride lipase was not modified by drug treatment or by pair feeding (data not shown).
No significant difference in gene expression of the key enzymes regulating triglyceride metabolism was observed between the livers of treated and normal control rats (Fig. 5D). The livers of DIO rats centrally infused with the CB1 antagonist had decreased expression of SCD-1, and this was paralleled by a similar decrease in vehicle pair-fed animals (Fig. 5E). No modification of gene expression was detected in the livers of DIO rats peripherally infused with the CB1 antagonist (Fig. 5F).
Peripheral, but not central, CB1 antagonism affects insulin sensitivity and glucose homeostasis in DIO rats.
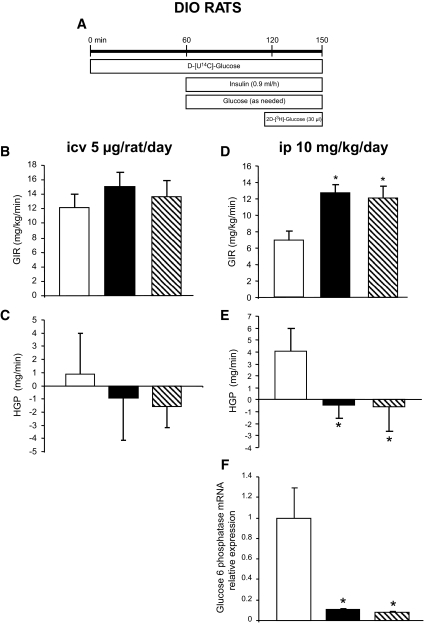
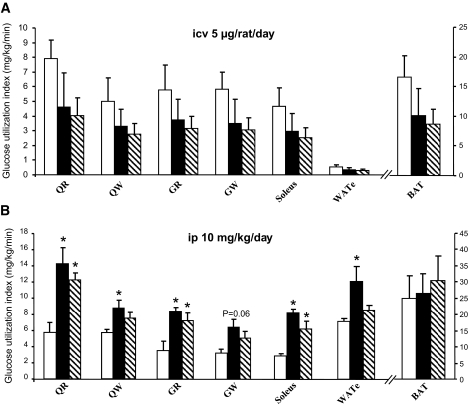
To determine whether the CB1 antagonist–induced changes in peripheral lipid metabolism may be associated with altered insulin sensitivity, we used euglycemic-hyperinsulinemic clamps associated with the labeled 2-deoxyglucose technique in DIO rats with central or peripheral CB1 blockade (Fig. 6A, experimental timeline). When infused centrally, the CB1 antagonist failed to elicit any change in overall insulin sensitivity. The glucose infusion rate (GIR) (Fig. 6B), hepatic glucose production (HGP) (Fig. 6C), and glucose uptake in skeletal muscle and WAT (Fig. 7A) were identical in vehicle and centrally SR141716-infused animals. In contrast, peripheral CB1 blockade enhanced overall insulin sensitivity, as indicated by higher GIR (Fig. 6D). Such an effect was likely linked to the reduced food intake induced by the treatment, since the vehicle pair-fed group had a similar increase in GIR (Fig. 6D). Consistent with this result, HGP was comparably suppressed in both peripherally CB1 antagonist–treated and vehicle pair-fed rats (Fig. 6E). The suppression of HGP in the peripherally treated rats may be explained by the marked decrease in the expression of glucose-6-phosphatase in both groups (Fig. 6F). The 2-deoxyglucose technique revealed an increase in glucose uptake by all skeletal muscles studied (red quadriceps, white quadriceps, red gastrocnemius, white gastrocnemius, and soleus) in the rats peripherally treated with the CB1 antagonist (Fig. 7B). This increase in glucose utilization in skeletal muscles was also secondary to the reduction of food intake, as it was also observed in vehicle-infused pair-fed controls (Fig. 7B). In contrast, glucose utilization by adipose tissue was increased only in the peripherally CB1 antagonist–infused group, indicating a food-independent effect of the treatment (Fig. 7B).
FIG. 6.
Effect of CB1 antagonism in the regulation of insulin action and hepatic glucose production in DIO rats. A: Schematic representation of the euglycemic-hyperinsulinemic clamps. B: Effect of a 6-day intracerebroventricular (icv) CB1 antagonist (5 μg/day) infusion on GIR in DIO rats. C: Effect of a 6-day intracerebroventricular CB1 antagonist (5 μg/day) infusion on hepatic glucose production (HPG) in DIO rats. D: Effect of a 6-day intraperitoneal (ip) CB1 antagonist (10 mg/kg) administration on GIR in DIO rats. E: Effect of a 6-day intraperitoneal CB1 antagonist (10 mg/kg) administration on hepatic glucose production (HPG) in DIO rats. F: Effect of a 6-day intraperitoneal CB1 antagonist (10 mg/kg) administration on liver mRNA expression of glucose 6 phosphatase in DIO rats. Values are means ± SE of five to seven animals per group. *P < 0.05. B–F: □, vehicle; ▪, CB1 antagonist; , vehicle-pf.
, vehicle-pf.
FIG. 7.
Effect of CB1 antagonism on peripheral glucose uptake in DIO rats. A: Effect of a 6-day intracerebroventricular (icv) CB1 antagonist (5 μg/day) infusion on insulin-stimulated glucose utilization measured during euglycemic-hyperinsulinemic clamps in several types of muscles: quadriceps red (QR), quadriceps white (QW), gastrocnemius red (GR), gastrocnemius white (GW), soleus, eWAT, and brown adipose tissue (BAT) of DIO rats. B: Effect of a 6-day intraperitoneal (ip) CB1 antagonist (10 mg/kg) administration on insulin-stimulated glucose utilization measured during euglycemic-hyperinsulinemic clamps in several types of muscles: quadriceps red, quadriceps white, gastrocnemius red (GR), gastrocnemius white, soleus, eWAT, and brown adipose tissue of DIO rats. Values are means ± SE of five to seven animals per group. *P < 0.05. □, vehicle; ▪, CB1 antagonist; , vehicle-pf.
, vehicle-pf.
DISCUSSION
The suppression of food intake and body weight gain induced by CB1 antagonists has been described in both rodents and humans (6,8,10,11,35,44,45). It is also known that CB1 antagonists improve glucose homeostasis and insulin sensitivity, outcomes that may be secondary to decreased body weight and/or food intake. Very recently, it was shown that engagement of the CB1 receptor by taranabant, a CB1 inverse agonist, leads to weight loss by reducing food intake and increasing energy expenditure and fat oxidation (10,11). However, the precise mechanisms regulating these effects and the involvement of central versus peripheral CB1 receptors leading to metabolic consequences for liver metabolism and lipid storage in adipose tissue are not well understood.
To address the relevance of central versus peripheral CB1 activation, we first compared the effects of central CB1 blockade on food intake and body weight in normal and DIO rodents. We observed that the CNS-CB1 antagonism was more effective in suppressing food intake in DIO rats than in normal rats. This is consistent with previous reports (17,46) demonstrating that peripheral CB1 antagonist infusion is more effective in obese than in lean Zucker rats. Those findings are also in agreement with the hypothesis that the endocannabinoid/CB1 tone is upregulated in obesity, both at the central and peripheral levels (2,27–31). The mechanisms of endocannabinoid activation in obesity are still not clear, but pathophysiological stimulation of cannabinoid receptors may, in part, explain obesity-associated metabolic changes and also offers an explanation for the successful use of the CB1 receptor inverse agonist/antagonist rimonabant for weight reduction and metabolic disorders of the obese (47). The hypothesis that the endocannabinoid/CB1 tone is upregulated in obesity is fully supported by our data, particularly by the finding of a stronger effect of centrally administered CB1 antagonist on food intake in DIO rats, as these rats being very likely leptin insensitive have higher hypothalamic levels of endocannabinoids (2) than lean rats.
An important further aim of these experiments was to compare the effects of central and peripheral CB1 blockade in DIO rats. Peripheral CB1 antagonist administration combines peripheral and central actions since the CB1 antagonist reaches the central nervous system, as previously shown by the Fos activation in different areas of the brain (48,49) and our present findings (Fig. 3 and Table 3), and therefore blocks not only the peripheral CB1 population but also CNS-CB1 receptors. We were therefore able to compare selective CNS-CB1 receptor blockade (due to intracerebroventricular infusion) with global CB1 blockade in order to infer the differential contributions of CNS and peripheral CB1 populations.
Our results indicate that both CNS- and global-CB1 blockade are effective at suppressing food intake and body weight gain in our rodent models. That said, the reduction of body weight in peripheral CB1 antagonist–treated rats was significantly more robust than that of the vehicle pair-fed group. Therefore, these data strongly imply that peripheral CB1 antagonism has additional catabolic actions, presumably due to interaction with peripheral CB1 receptors, that result in enhanced weight loss that cannot be entirely explained by the suppression of food intake. Our results also suggest that body weight and the metabolic amelioration after CB1 antagonist treatment depends on the dosage used, but the effects observed after the central infusion of the CB1 antagonist cannot be explained by the actions of CB1 in the periphery. Indeed, it was shown that the anorectic effect of Rimonabant usually disappears with time (19,38), but our experiments were designed to elucidate which metabolic actions are independent of food intake while the drug exerted anorectic effects.
We next dissected the central and peripheral actions of CB1 blockade on lipid and glucose metabolism in normal and DIO rats. In normal animals, CNS-CB1 blockade markedly decreased the gene expression of enzymes involved in lipid synthesis and storage in the periphery, while increasing expression of the lipid-oxidizing enzyme, CPT-1α. These results were presumably driven by the anorexigenic effect of the CB1 antagonist, as they could be reproduced by mimicking the decreased food intake with a pair-feeding protocol. With regard to DIO rats, it was previously reported that a CB1 antagonist is able to reverse the obese phenotype through the regulation of lipolysis and energy balance (38). However, in that study, it was not ascertained whether those actions were secondary to the anorexigenic action of the compound. An interesting finding that emerged from the present study is the food intake–independent effect of global CB1 antagonism on adipocyte metabolism in DIO rats. Peripheral infusion of the CB1 antagonist decreased the amount of triglycerides in WAT, decreased the expression of SCD-1, and increased the expression of CPT-1 in adipose tissue, and the effect was greater than occurred in pair-fed animals. Hence, hypophagia cannot account for all of the altered lipid metabolism. Again, the hypothesis that the endocannabinoid/CB1 tone is upregulated in obesity is fully supported by our data, particularly by the finding of food intake–independent metabolic effects of the CB1 antagonist on WAT triglyceride levels but not in the skeletal muscle and liver and only on peripherally versus centrally administered DIO rats, which is consistent with a possibly stronger association of elevated endocannabinoid tone with visceral WAT than with other tissues (27–29).
In the liver, on the other hand, global CB1 blockade in DIO rats appeared to have no effect on lipid metabolism. This is in keeping with the recent report that administration of CB1 antagonist alone did not modify the expression of lipogenic enzymes in the liver but only prevented the lipogenic effects induced by a CB1 agonist (24). Contrary to our data, one study (50) has found a food intake–independent effect on hepatic lipid metabolism when obese Zucker rats were treated with 30 mg/kg Rimonabant for 8 weeks, and another report (25) has suggested that alcohol-induced steatosis was attenuated in mice treated with 10 mg/kg Rimonabant for 3 weeks due to actions of this compound on hepatic CB1 receptors. The discrepancies between those studies and our current data might be explained by the different methodological approaches used, particularly the time of the treatment and the doses used. In addition, we used rats fed a high-fat diet instead of liquid ethanol diets or rats with genetic alterations. Collectively, therefore, our results are consistent with the conclusion that peripherally administered CB1 antagonist acts directly on adipose tissue metabolism by increasing lipid mobilization and decreasing lipid storage, thereby contributing to enhanced body weight loss. These changes might explain the more pronounced weight reduction observed in the CB1 antagonist–infused rats compared with pair-fed vehicle controls. In these experiments, it must be acknowledged that some of the administered CB1 antagonist crossed the blood-brain barrier, affected CNS-CB1 receptor populations, and reduced food intake. However, when we specifically blocked CNS-CB1 receptors alone, these specific effects on adipocyte metabolism did not occur. Our data therefore imply that such metabolic actions are likely mediated by peripheral CB1 receptors. Nonetheless, there are alternative possibilities. It may be, for example, that the systemically administered antagonist reaches populations of brain CB1 that are not reached by intracerebroventricular administration. While this seems unlikely due to the high lipid solubility of the compound, it cannot be ruled out. Definitively answering this issue will therefore await the availability of CB1 antagonists that can be administered systemically and that do not penetrate the blood-brain barrier.
Several reports indicate that the endocannabinoid system also affects glucose homeostasis and insulin sensitivity. Clinical trials (6,8) with a CB1 receptor antagonist have shown an improvement of insulin sensitivity after 1 year of treatment, and reports (23) on rodents indicate that the infusion of a CB1 receptor antagonist improved glycemia in DIO mice. Consistent with those reports, CB1 receptor activation was recently reported (51) to promote glucose intolerance in rats. None of those studies, however, addressed the relative importance of central versus peripheral CB1 receptor populations on insulin sensitivity, nor did they determine the extent to which effects of CB1 blockade on glucose metabolism were mediated by changes in food intake. The present data indicated that CNS-CB1 blockade did not significantly alter overall insulin sensitivity or tissue-specific glucose uptake in our paradigm. In contrast, global CB1 blockade enhanced overall insulin sensitivity, decreased HGP, and increased glucose utilization in muscles and WAT. The scientific value of such a comparison is obviously weakened by potential influences of dose dependencies as well as the different pharmacokinetics of central versus peripheral administration. Nonetheless, the fact that both administration patterns led to similar suppression of food intake suggests principal comparability. Although most of the effects on glucose metabolism can be explained by the anorexigenic properties of the CB1 antagonist, the effect on WAT was independent of food intake. Contrary to our results, there are two in vitro studies (30,52) indicating that glucose uptake in adipocytes is increased by CB1 receptor stimulation. Although we do not have a clear explanation for this discrepancy, it is very common that in vivo and in vitro studies do not show similar data. Further studies are needed to explore the precise role of cannabinoids on glucose homeostasis. Contrary to global CB1 blockade, CNS-CB1 blockade alone did not modify insulin sensitivity or glucose utilization, indicating that peripheral CB1 receptors are likely mediating the insulin-sensitizing effects of CB1 blockade as well.
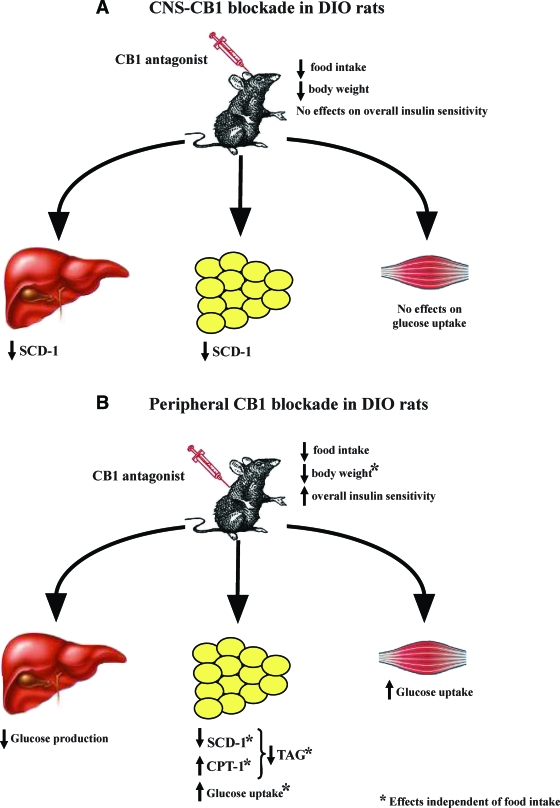
In summary and as schematized by Fig. 8, this series of experiments demonstrates that CNS-CB1 blockade in normal rats has several effects on body weight and metabolism and that many of these are secondary to a reduction of food intake in our rodent models. These effects include reduced efficiency of lipid storage in adipose tissue, decreased expression of SCD-1 in the liver and in WAT, and decreased body weight. They occur without any alteration in insulin sensitivity. In contrast, when the same CB1 antagonist was administered peripherally in DIO rats, there was an enhanced reduction of body weight and lipid mobilization from WAT, and these effects appear to be, at least in part, independent from food intake. These data are in keeping with, and might even represent, one of the mechanisms for the weight loss–independent effects on metabolic parameters (for example, on adiponectin and triglyceride levels) that have been suggested in several clinical trials (5,6,8). Our results might also support the possible use of peripherally restricted versus global CB1 antagonists in both obesity and metabolic disorders. In view of the adverse psychiatric effects reported to occur during the treatment of obesity and accompanying metabolic disorders with CB1 antagonists, these data are of importance because they suggest that CB1 antagonists do not need to act centrally to be effective.
FIG. 8.
A: Schematic overview summarizing the metabolic effects of CNS-CB1 receptor blockade on peripheral tissues. Blockade of CNS-CB1 decreases the expression of the lipid-promoting enzyme SCD-1 in liver and WAT, whereas it does not alter glucose utilization. B: Schematic overview summarizing the metabolic effects of peripheral CB1 receptor blockade on peripheral tissues. Blockade of peripheral CB1 decreases hepatic glucose production, promotes lipid mobilization independent of food intake, and increases glucose utilization. Combined, these parallel metabolic changes in multiple tissues represent a synergistic shift in substrate choice and nutrient partitioning, resulting in decreased energy storage. TAG, triglycerides.
Supplementary Material
Acknowledgments
This work was supported by the European Community FP6 Funding (contract no. LSHMCT-2003-503041) (to C.F., F.R.J., and M.H.T.), by RO1DK069987 (to S.C.W. and M.H.T.), and by the Swiss National Science Foundation (grant no. 310000-120147) (to F.R.J.). R.N. is a current recipient of a Marie Curie Outgoing International Fellowship.
The authors thank the excellent technical support of Karen Hodge, Cindy Obringer, and Mary Kay Dirr.
Published ahead of print at http://diabetes.diabetesjournals.org on 20 August 2008.
R.N. and C.V.-D. contributed equally to this article.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Williams CM, Kirkham TC: Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 143: 315–317, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G: Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410: 822–825, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Breivogel CS, Sim LJ, Childers SR: Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J Pharmacol Exp Ther 282: 1632–1642, 1997 [PubMed] [Google Scholar]
- 4.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM: Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83: 393–411, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S: Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365: 1389–1397, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Despres JP, Golay A, Sjostrom L: Effects of Rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353: 2121–2134, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Pagotto U, Pasquali R: Fighting obesity and associated risk factors by antagonising cannabinoid type 1 receptors. Lancet 365: 1363–1364, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J: Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 295: 761–775, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF: Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 368: 1660–1672, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohorquez SM, Stoch A, Stevens C, Fong TM, De Lepeleire I, Cilissen C, Cote J, Rosko K, Gendrano IN 3rd, Nguyen AM, Gumbiner B, Rothenberg P, de Hoon J, Bormans G, Depre M, Eng WS, Ravussin E, Klein S, Blundell J, Herman GA, Burns HD, Hargreaves RJ, Wagner J, Gottesdiener K, Amatruda JM, Heymsfield SB: The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab 7: 68–78, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Kirkham TC: Taranabant cuts the fat: new hope for cannabinoid-based obesity therapies? Cell Metab 7: 1–2, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Jo YH, Chen YJ, Chua SC Jr, Talmage DA, Role LW: Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron 48: 1055–1066, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG: Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci 26: 6643–6650, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hentges ST, Low MJ, Williams JT: Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci 25: 9746–9751, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matias I, Vergoni AV, Petrosino S, Ottani A, Pocai A, Bertolini A, Di Marzo V: Regulation of hypothalamic endocannabinoid levels by neuropeptides and hormones involved in food intake and metabolism: insulin and melanocortins. Neuropharmacology 54: 206–212, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Kunos G: Understanding metabolic homeostasis and imbalance: what is the role of the endocannabinoid system? Am J Med 120: S18–S24, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Serrano A, Del Arco I, Javier Pavon F, Macias M, Perez-Valero V, Rodriguez de Fonseca F: The cannabinoid CB1 receptor antagonist SR141716A (Rimonabant) enhances the metabolic benefits of long-term treatment with oleoylethanolamide in Zucker rats. Neuropharmacology 54: 226–234, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Tallett AJ, Blundell JE, Rodgers RJ: Behaviourally-selective hypophagic effects of naloxone in non-deprived male rats presented with palatable food. Behav Brain Res 187: 417–427, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Ravinet Trillou C, Arnone M, Delgorge C, Gonalons N, Keane P, Maffrand JP, Soubrie P: Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 284: R345–R353, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Bensaid M, Gary-Bobo M, Esclangon A, Maffrand JP, Le Fur G, Oury-Donat F, Soubrie P: The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 63: 908–914, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Kunz I, Meier MK, Bourson A, Fisseha M, Schilling W: Effects of rimonabant, a cannabinoid CB1 receptor ligand, on energy expenditure in lean rats. Int J Obes (Lond) 32: 863–870, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thone-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U: The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112: 423–431, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P: CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 28: 640–648, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G: Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115: 1298–1305, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong WI, Osei-Hyiaman D, Park O, Liu J, Batkai S, Mukhopadhyay P, Horiguchi N, Harvey-White J, Marsicano G, Lutz B, Gao B, Kunos G: Paracrine activation of hepatic CB1 receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab 7: 227–235, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Liu YL, Connoley IP, Wilson CA, Stock MJ: Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes (Lond) 29: 183–187, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R, Roche R, Maj M, Pagotto U, Monteleone P, Di Marzo V: Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 91: 3171–3180, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Bluher M, Engeli S, Kloting N, Berndt J, Fasshauer M, Batkai S, Pacher P, Schon MR, Jordan J, Stumvoll M: Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 55: 3053–3060, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cote M, Matias I, Lemieux I, Petrosino S, Almeras N, Despres JP, Di Marzo V: Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 31: 692–699, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Pagano C, Pilon C, Calcagno A, Urbanet R, Rossato M, Milan G, Bianchi K, Rizzuto R, Bernante P, Federspil G, Vettor R: The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab 92: 4810–4819, 2007 [DOI] [PubMed] [Google Scholar]
- 31.D’Eon TM, Pierce KA, Roix JJ, Tyler A, Chen H, Teixeira SR: The role of adipocyte insulin resistance in the pathogenesis of obesity-related elevations in endocannabinoids. Diabetes 57: 1262–1268, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Pagotto U, Cervino C, Vicennati V, Marsicano G, Lutz B, Pasquali R: How many sites of action for endocannabinoids to control energy metabolism? Int J Obes (Lond) 30 (Suppl. 1): S39–S43, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Horvath TL: The unfolding cannabinoid story on energy homeostasis: central or peripheral site of action? Int J Obes (Lond) 30 (Suppl. 1): S30–S32, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Nogueiras R, Rohner-Jeanrenaud F, Woods SC, Tschop MH: The endocannabinoid system and the control of glucose homeostasis. J Neuroendocrinol 20 (Suppl. 1): 147–151, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R: The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 27: 73–100, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Rohner-Jeanrenaud F, Walker CD, Greco-Perotto R, Jeanrenaud B: Central corticotropin-releasing factor administration prevents the excessive body weight gain of genetically obese (fa/fa) rats. Endocrinology 124: 733–739, 1989 [DOI] [PubMed] [Google Scholar]
- 37.Verty AN, McFarlane JR, McGregor IS, Mallet PE: Evidence for an interaction between CB1 cannabinoid and melanocortin MCR-4 receptors in regulating food intake. Endocrinology 145: 3224–3231, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le Fur G, Galiegue S, Casellas P: The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J 19: 1567–1569, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Vettor R, Zarjevski N, Cusin I, Rohner-Jeanrenaud F, Jeanrenaud B: Induction and reversibility of an obesity syndrome by intracerebroventricular neuropeptide Y administration to normal rats. Diabetologia 37: 1202–1208, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Terrettaz J, Assimacopoulos-Jeannet F, Jeanrenaud B: Severe hepatic and peripheral insulin resistance as evidenced by euglycemic clamps in genetically obese fa/fa rats. Endocrinology 118: 674–678, 1986 [DOI] [PubMed] [Google Scholar]
- 41.Herbert V, Lau KS, Gottlieb CW, Bleicher SJ: Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab 25: 1375–1384, 1965 [DOI] [PubMed] [Google Scholar]
- 42.Lopez M, Lelliott CJ, Tovar S, Kimber W, Gallego R, Virtue S, Blount M, Vazquez MJ, Finer N, Powles TJ, O’Rahilly S, Saha AK, Dieguez C, Vidal-Puig AJ: Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes 55: 1327–1336, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schurmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O’Rahilly S, Rohner-Jeanrenaud F, Tschop MH: The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 117: 3475–3488, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matias I, Di Marzo V: Endocannabinoids and the control of energy balance. Trends Endocrinol Metab 18: 27–37, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Di Marzo V, Matias I: Endocannabinoid control of food intake and energy balance. Nat Neurosci 8: 585–589, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA: Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl) 167: 103–111, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Xie S, Furjanic MA, Ferrara JJ, McAndrew NR, Ardino EL, Ngondara A, Bernstein Y, Thomas KJ, Kim E, Walker JM, Nagar S, Ward SJ, Raffa RB: The endocannabinoid system and rimonabant: a new drug with a novel mechanism of action involving cannabinoid CB1 receptor antagonism–or inverse agonism–as potential obesity treatment and other therapeutic use. J Clin Pharm Ther 32: 209–231, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F: Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science 276: 2050–2054, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Alonso R, Voutsinos B, Fournier M, Labie C, Steinberg R, Souilhac J, Le Fur G, Soubrie P: Blockade of cannabinoid receptors by SR141716 selectively increases Fos expression in rat mesocorticolimbic areas via reduced dopamine D2 function. Neuroscience 91: 607–620, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Gary-Bobo M, Elachouri G, Scatton B, Le Fur G, Oury-Donat F, Bensaid M: The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits cell proliferation and increases markers of adipocyte maturation in cultured mouse 3T3 F442A preadipocytes. Mol Pharmacol 69: 471–478, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Bermudez-Siva FJ, Serrano A, Diaz-Molina FJ, Sanchez Vera I, Juan-Pico P, Nadal A, Fuentes E, Rodriguez de Fonseca F: Activation of cannabinoid CB1 receptors induces glucose intolerance in rats. Eur J Pharmacol 531: 282–284, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Gasperi V, Fezza F, Pasquariello N, Bari M, Oddi S, Agro AF, Maccarrone M: Endocannabinoids in adipocytes during differentiation and their role in glucose uptake. Cell Mol Life Sci 64: 219–229, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.