Abstract
OBJECTIVE—To define the mechanisms underlying the accumulation of monocytes/macrophages in the islets of Langerhans.
RESEARCH DESIGN AND METHODS—We tested the hypothesis that macrophage accumulation into the islets is caused by overexpression of the chemokine CCL2. To test this hypothesis, we generated transgenic mice and evaluated the cellular composition of the islets by immunohistochemistry and flow cytometry. We determined serum levels of CCL2 by enzyme-linked immunosorbent assay, determined numbers of circulating monocytes, and tested whether CCL2 could mobilize monocytes from the bone marrow directly. We examined development of diabetes over time and tested whether CCL2 effects could be eliminated by deletion of its receptor, CCR2.
RESULTS—Expression of CCL2 by β-cells was associated with increased numbers of monocytes in circulation and accumulation of macrophages in the islets of transgenic mice. These changes were promoted by combined actions of CCL2 at the level of the bone marrow and the islets and were not seen in animals in which the CCL2 receptor (CCR2) was inactivated. Mice expressing higher levels of CCL2 in the islets developed diabetes spontaneously. The development of diabetes was correlated with the accumulation of large numbers of monocytes in the islets and did not depend on T- and B-cells. Diabetes could also be induced in normoglycemic mice expressing low levels of CCL2 by increasing the number of circulating myeloid cells.
CONCLUSIONS—These results indicate that CCL2 promotes monocyte recruitment by acting both locally and remotely and that expression of CCL2 by insulin-producing cells can lead to insulitis and islet destruction.
Type 1 diabetes is a chronic inflammatory disorder characterized by infiltration of the islets of Langerhans by mononuclear cells and autoimmune destruction of insulin-producing β-cells (1,2). Several lines of evidence suggest that macrophages play a role in the development of diabetes. Macrophages are the first cells that appear within the islets of NOD mice (3) and are also implicated in late phases of disease development (4). Administration of clodronate-loaded liposomes, which leads to disappearance of macrophages from the endocrine pancreas and periphery of NOD mice, delays the onset of diabetes (5). Moreover, macrophage depletion inhibits the development of β-cell–cytotoxic T-cells and prevents autoimmune diabetes (6). It has been suggested that the presentation of self-antigens to autoreactive T-cells by dendritic cells and macrophages recruited and activated by transgenic tumor necrosis factor-α (TNF-α) expression accounts for the development of diabetes (7). Macrophages and dendritic cells are found within islets from recent-onset type 1 diabetic patients (8).
Tissue macrophages originate from monocytes produced in the bone marrow. Current studies suggest that bone marrow–derived monocytes give rise to two subsets of peripheral blood monocytes (9). One subset (GR-1−, CX3CR1high, CCR2−, and CCL62L− monocytes) gives origin to tissue macrophages (splenic macrophages, Kupffer cells, alveolar macrophages, microglia, and osteoclasts). The second subset (GR-1+, CX3CR1low, CCR2+, and CD62L+ monocytes) is preferentially recruited to inflamed tissues and gives rise to macrophages and dendritic cells. This inflammatory subset also expresses CD115 (granulocyte macrophage colony–stimulating factor receptor) and Ly6C (10,11).
Several studies implicate the chemokine CCL2 in monocyte recruitment in vivo (12). CCL2 promotes recruitment of monocytes, macrophages, dendritic cells, and activated T-cells via its receptor, CCR2 (13). Numerous cell types, including fibroblasts, endothelial cells, epithelial cells, leukocytes, and smooth muscle cells express CCL2 in the presence of serum or specific stimuli (14,15). In addition to chemotaxis, CCL2 contributes to activation of monocytes and macrophages because CCL2 induces production of TNF-α and interleukin-1β in murine peritoneal macrophages (16). Macrophage recruitment caused by CCL2 expression has been strongly linked to several inflammatory conditions, such as atherosclerosis (17), development of intimal hyperplasia after arterial injury (18), obesity, and insulin resistance (19).
CCL2 expression has also been related to diabetes. Primary cultures of murine and human pancreatic islets express and secrete CCL2 (20). CCL2 expression has been detected in islets of NOD mice during cyclophosphamide treatment (21), and CCL2 expression parallels disease progression in NOD mice (22,23). Low-level secretion of CCL2 by islets before transplantation is associated with a higher rate of insulin independence, suggesting an important role for CCL2 in the clinical outcome of islet transplantation in patients with type 1 diabetes (24).
Our laboratory and others have shown that CCL2 expression by insulin-producing cells induces the accumulation of macrophages in the islets of transgenic mice (25,26). We have also found that transgenic expression of CCL2 induces migration of dendritic cells to the islets and that the number of inflammatory cells recruited is dependent on the levels of CCL2 produced by the β-cells (26). The mechanisms controlling accumulation of monocytes in nonlymphoid tissues in response to increased levels of CCL2 have not been examined. It is unclear whether the accumulation of monocytes in inflamed tissues in response to CCL2 reflects increased recruitment of circulating monocytes or monocyte precursors, changes in the number of circulating monocytes, or both. Finally, it is unclear whether changes in local levels of CCL2 may promote development of diabetes.
Here, we show that transgenic expression of CCL2 by insulin-producing cells is associated with changes in the number of monocytes in circulation. The monocytes are mobilized from the bone marrow in a CCR2-dependent mechanism. We also found spontaneous development of diabetes in two of the four transgenic lines expressing the highest levels of CCL2 in the pancreas. The development of diabetes required the accumulation of a large number of monocytes in the islets and did not depend on T- or B-cells. Animals expressing low levels of CCL2 in the islets did not spontaneously develop diabetes but became diabetic if the number of circulating monocytes was increased. These results indicate that CCL2 promotes monocyte recruitment by acting both locally and remotely and that dysregulated expression of CCL2 by insulin-producing cells can lead to insulitis and islet destruction.
RESEARCH DESIGN AND METHODS
RIPCCL2 mice were generated as described by Martin et al. (26) in B6D2 (C57BL/6 × DBA) background. CCR2−/− mice were described by Boring et al. (13). Rag-1−/− mice were obtained from The Jackson Laboratories (Bar Harbor, ME). In all experiments, transgenic mice were compared with their corresponding littermates. All mice were housed under specific pathogen-free conditions in individually ventilated cages at the Mount Sinai School of Medicine Animal Facility. All experiments were performed following institutional guidelines.
Diabetes.
The blood glucose was monitored weekly using a one-touch blood Ascensia Contour glucometer (Bayer, Elkhart, IN). Animals were considered diabetic when their blood glucose levels were >250 mg/dl in two consecutive daily measurements.
Histology.
Tissues for light microscopic examination were fixed by immersion in 10% phosphate-buffered formalin and then processed for paraffin sections. Routinely, 5-μm sections were cut and stained with hematoxylin-eosin (H-E). For immunohistochemical staining, fresh frozen sections were first fixed with ice-cold acetone for 20 min, dried, and stored at −20°C. Slides were incubated for 1 h at room temperature with purified primary antibodies followed by incubation with the appropriate labeled secondary antibodies for 30 min. Primary antibodies used were anti-CD45 (550539), CD3 (550275), CD11c (550283), B220 (550286), and CD11b (553308) from BD Biosciences Pharmingen (San Diego, CA); F4/80 (MCAP497) from Serotec (Oxford, U.K.); and guinea pig polyclonal anti-insulin (A0564) from Dako (Carpentaria, CA). Secondary antibodies used were Alexa Fluor 488 and 594 goat anti-rat IgG (A-11006 and A-11007) from Molecular Probes (Eugene, OR) and Cy3 goat anti–Armenian hamster (127-165-160), fluorescein isothiocyanate anti–guinea pig IgG (706-095-148), and Cy5 goat anti-rat (112-175-167) from Jackson ImmunoResearch (West Grove, PA).
To determine the degree of islet infiltration in RIPCCL2 mice, groups of mice (n = 5) were analyzed at 4 weeks of age. The total number of islets was counted in each section (assessing 40–100 islets per animal), and semiquantitative analysis was performed on insulin/CD45-stained sections. Insulitis was scored as follows: 0, no lesions; 1, peri-insular leukocytic aggregates; 2, moderate insulitis with mononuclear cells infiltrating <50% of the islet architecture; and 3, severe insulitis with >50% of the islet tissue infiltrated by mononuclear cells. An insulitis score for each mouse was obtained by dividing the total score for each pancreas by the number of islets examined. Data are presented as mean insulitis score ± SE for the indicated experimental group.
In situ perfusion of femoral bone marrow.
Female BALB/c mice were anesthetized with urethane (25% 0.2 ml i.p.), and the femoral artery and vein were exposed. The hind limb was isolated by occlusion of the external iliac artery, superficial epigastric, and muscular branch with 5/0 braided silk suture. Polyethylene cannulae (0.61-mm outer diameter; Portex, London) were immediately inserted into the femoral artery and vein and tied in place with 5/0 braided silk suture. Perfusion buffer (modified Krebs-Ringer bicarbonate buffer) at 37°C was infused (0.15 ml/min) via the arterial cannula and removed from the venous cannula using a Minipuls peristaltic pump (Anachem, Luton, U.K.). The hind limb was perfused for an initial 10 min to remove remaining blood from the vasculature and then perfused for a further 60 min with vehicle alone or 80 nmol/l CCL2 (250-10; Peprotech, London) infused over the first 20 min using an infusion/withdrawal pump (Harvard Instruments, Edenbridge, U.K.). Resulting perfusate was kept on ice.
Intraperitoneal glucose tolerance test.
After a 16-h fast, glucose (1.5 g/kg body wt in saline [0.9% NaCl]) was administered intraperitoneally. The blood glucose was monitored at 0, 30, 60, 120, and 240 min using a one-touch blood Ascensia Contour glucometer (Bayer). The intraperitoneal glucose tolerance test was performed on 8-week-old control and normoglycemic RIPCCL2 mice.
Isolation of pancreatic islets of Langerhans.
Islets of Langerhans were isolated as previously described (27). Briefly, the common bile duct was clamped distal to the pancreatic duct junction at its hepatic insertion. The proximal common bile duct was then cannulated using a 27-gauge needle, and the pancreas was infused by retrograde injection of 2 ml 1.0 mg/ml ice-cold collagenase solution (Sigma, St. Louis, MO) in Hanks’ balanced salt solution (HBSS; Invitrogen, Carlsbad, CA). Pancreatic tissue was recovered and subjected to a 12-min digestion at 37°C. Subsequently, ice-cold HBSS was added, and the suspension was vortexed at full speed for 10 s. Islets were purified on a discontinuous Ficoll gradient (Sigma) and hand-picked under a dissection microscope. Islets were used immediately after isolation to obtain RNA.
Evaluation of cellular stress.
To evaluate whether the islets of transgenic mice showed signs of distress, we determined the levels of expression of several “stress genes” by quantitative PCR. Primers used were sarcoendoplasmatic reticulum CA/ATPase type 2, GGCAAGATCCGGGATGAAAT (forward) and TCCAGACTGCAATGCAAATGA (reverse); GADD153/CHOP [CCAAT/enhancer binding protein], TCTCATCCCCAGGAAACGAA (forward) and ATCTGGAGAGCGAGGGCTTT (reverse); XBP-1, CTTTCATCCAGCCATTGTCTGA (forward) and GCCCTCATATCCACAGTCACTGT (reverse); activating transcription factor-4, CGAGTTAAGCACATTCCTGGA (forward) and TTCGCTGTTCAGGAAGCTCAT (reverse); immunoglobulin heavy-chain binding protein, ACCCCGAGAACACGGTCTT (forward) and GCTGCA CCGAAGGGTCATT (reverse); and tumor necrosis factor receptor–associated, AAGATGGAGGCCAAGAATTCC (forward) and AGTCCTGTTAGGTCCACAATAGCTTT (reverse).
As positive controls, we used islets from wild-type mice (8 weeks of age) that were treated with a single intraperitoneal injection of streptozotocin (STZ) (250 mg/kg body wt). Eight hours later, islets were isolated as described above.
Quantitative real-time PCR.
Total RNA was extracted from pooled islets using the RNeasy maxi kit (Qiagen) according to the manufacturer's instructions. Reverse transcription was performed for 3 μg RNA. Quantitative PCR was conducted in duplicate from 25 ng cDNA and with each primer at 0.4 μmol/l in a 30-μl final reaction volume of 1× SYBR Green PCR Master Mix (Applied Biosystems). PCR cycling conditions were 50°C for 2 min, 95°C for 15 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. Relative expression levels were calculated as 2Ct ubiquitin−Ct gene (where Ct is cycle threshold; for details see ABI PRISM 7700 User Bulletin no. 2; Applied Biosystems) using ubiquitin RNA as endogenous control.
Protein analysis.
CCL2 was measured in serum by ELISA (R&D Systems, Minneapolis, MN). Enzyme-linked immunosorbent assay (ELISA) assays were done according to manufacturer's instructions.
Flow cytometry analysis.
Peripheral blood was stained for 45 min with the antibody mixture, erythrocytes were lysed, and cells were fixed with flow cytometry analysis (FACS) lysis solution (Becton Dickinson, San Jose, CA). Bone marrow cells were obtained from the tibia and femur bones. Contaminating erythrocytes were lysed with ammonium chloride lysis buffer and samples were stained with antibody mixture for 45 min at 4°C. Antibodies used in the experiments described here were as follows: anti-CD115 (AFS98), anti-CD11b (M1/70), anti-CD11c (N418), and anti-F4/80 (BM8) from e-Bioscience (San Diego CA) and anti-mouse Ly6C (AL-21) from BD Pharmingen (San Diego, CA). Samples were analyzed in a FACSCanto instrument (Becton Dickinson). Up to 100,000 events were acquired.
Flow cytometry analysis of the bone marrow perfusate was performed as follows: The perfusate was centrifuged (200 × g for 5 min, 4°C). Erythrocytes were lysed using hypotonic shock, and the cell pellets were resuspended in FACS buffer. An aliquot of cells was taken to establish the total number of leukocytes mobilized over the 60-min period by staining with 2% methylene blue in 1% acetic acid. Cells were enumerated using an improved Neubauer Hemocytometer. The remaining leukocytes were stained with the following monoclonal antibodies: anti-mouse CD115, Gr1, CD3e, and B220. Appropriately conjugated isotype control antibodies were used to calculate background fluorescence. The samples were then analyzed by flow cytometry (BD FACSCalibur), and the relative number of cells expressing CD115/Gr1 intermediate (monocytes), Gr1high (neutrophils), CD3 (T-lymphocytes), and B220 (B-lymphocytes) was calculated.
Statistical analysis.
Data are expressed as means ± SE. One-way ANOVA and unpaired t test were used to determine statistical significance. Differences were considered significant when P < 0.05.
RESULTS
Levels of CCL2 and frequency of mononuclear infiltrates in the islets correlate with development of diabetes.
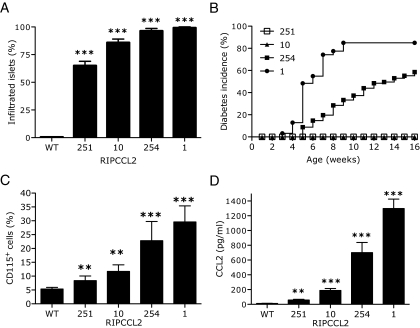
We have previously described the generation of five transgenic lines expressing different levels of CCL2 in the islets (26). We have observed that the number of inflammatory cells recruited to the islets is dependent on the levels of CCL2 produced by the β-cells (26). To investigate the frequency of mononuclear infiltration, semiquantitative analysis of infiltrated islets was performed in pancreata from 4-week-old mice (n = 8/group). No infiltrates were found in islets of wild-type mice regardless of age. In marked contrast, 60, 80, 95, and 100% of the islets in RIPCCL2 pancreata from lines 251, 10, 254, and 1, respectively, were infiltrated by leukocytes (largely monocytes and dendritic cells) (Fig. 1A) (26). The infiltrates were observed predominantly in the periphery of the islets in lines 251 and 10. Pancreata from mice of lines 254 and 1 showed a severe insulitis, with leukocytes present in the periphery and within the islets (data not shown) (26). The frequency and the size of these infiltrates did not change with age. To examine whether an increased number of infiltrated islets led to development of diabetes, we monitored RIPCCL2 mice and their control littermates weekly for hyperglycemia. No hyperglycemia was observed in control mice or in transgenic mice of lines 10 and 251 during the first 20 weeks of life. In contrast, animals from lines 254 and 1 (which had higher levels of CCL2 and an increased number of infiltrated islets) became hyperglycemic at 4–5 weeks of age. By 16 weeks of age, 80% of RIPCCL2 mice from line 1 and 60% of RIPCCL2 mice from line 254 were diabetic (Fig. 1B).
FIG. 1.
Augmented levels of CCL2 in islets of Langerhans lead to an increased number of monocytes in blood, infiltrated islets, and spontaneous diabetes. A: Semiquantitative analysis of islet infiltrates in the pancreata from control and RIPCCL2 transgenic mice at 4 weeks of age (n = 8/group). B: Cumulative incidence of diabetes in RIPCL2 mice from lines 251 (n = 60), 10 (n = 40), 254 (n = 95), and 1 (n = 31) and from nontransgenic littermates (n = 20). C: Relative numbers of CD115+ cells in blood from wild-type and RIPCCL2 mice (n = 20 mice in each line; t test **P < 0.005, ***P < 0.0001). D: CCL2 levels in the serum of RIPCCL2 mice of lines 251, 10, 254, and 1 and wild-type littermates was analyzed by ELISA (n = 20/line; t test **P < 0.005, ***P < 0.0001).
The development of diabetes in several transgenic lines has been linked to toxicity induced by transgene expression (28). To test this, we isolated islets from 6- to 8-week-old wild-type and normoglycemic RIPCCL2 mice and evaluated the expression of genes induced during cellular stress by quantitative PCR. As a positive control, we used islets isolated from wild-type mice injected with STZ. As shown in supplemental Fig. 1A (available in an online appendix at http://dx.doi.org/10.2337/db08-0625), islets isolated from mice administered STZ, but not those isolated from RIPCCL2 mice, had increased expression of stress genes. To exclude the possibility that transgene expression of CCL2 by β-cells led to impaired glucose tolerance, we performed a glucose tolerance test on 8-week-old control and normoglycemic RIPCCL2 mice. The glucose levels after intraperitoneal injection of glucose were comparable between wild-type and RIPCCL2 mice (supplemental Fig. 1B), suggesting that overexpression of CCL2 in the islets did not affect their ability to respond to a glucose challenge. Together, the results indicate that expression of CCL2 per se did not lead to cellular stress or affect the function of the β-cells. We suggest that the development of diabetes was dependent on the recruitment of myeloid cells by CCL2.
Levels of CCL2 in the islets and blood correlate with the number of monocytes in circulation.
The increased number of monocytes in the islets could be due to increased efficiency in recruitment of a fixed number of circulating cells or to an increased number of monocytes in circulation. To determine whether RIPCCL2 mice had more circulating monocytes, we performed FACS analysis of peripheral blood (Fig. 1C). Blood monocytes were defined as a leukocyte population with low side scatter that expressed the markers CD115, Ly6C, F4/80, and CD11b. Using these parameters, we found that RIPCCL2 mice had higher numbers of monocytes in circulation than control mice (10–30 and 5–6%, respectively) (Fig. 1C). We hypothesized that the increased number of cells in circulation was promoted by CCL2 produced by the islets of the RIPCCL2 mice. Serum from RIPCCL2 animals from different lines (n = 20 in each line, nondiabetic) had 3- to 35-fold more CCL2 than their nontransgenic littermates (n = 20) (Fig. 1D). The levels of CCL2 in serum correlated directly with the amount of CCL2 produced in the transgenic islets (26). These results confirm a correlation between increased levels of CCL2 in the tissue and blood and the number of monocytes in circulation.
CCL2 induces release of monocytes from the bone marrow.
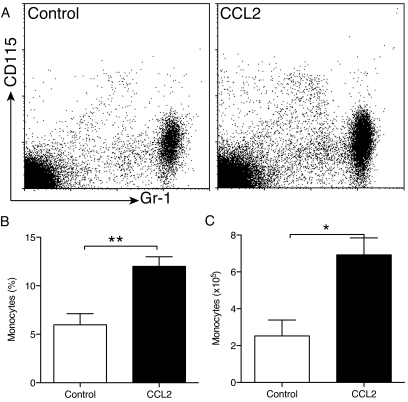
To determine whether CCL2 could exert a direct effect on the release of monocytes from the bone marrow, we used an in situ perfusion system of the mouse femoral bone marrow (29). The femoral artery and vein were cannulated in situ such that the femoral bone marrow could be perfused and the leukocytes released from the bone marrow collected as they exited via the femoral vein. Vehicle alone (PBS, control mice) or CCL2 was infused for 20 min directly into the mouse femoral artery using a Minipuls peristaltic pump, and the leukocytes mobilized from the femoral bone marrow were collected over a subsequent 60-min perfusion period. The numbers of monocytes, neutrophils, T-cells, and B-cells released into the perfusate were determined by flow cytometry. As shown in Fig. 2, CCL2 induced an increased release of monocytes from the bone marrow over the 60-min perfusion period compared with controls. The relative number of monocytes changed from 6% in PBS-infused mice to 13% in CCL2-infused mice (Fig. 2B). Moreover, CCL2 induced bone marrow release of 7 ×105 monocytes/ml perfusate, whereas PBS controls had 2.5 ×105 monocytes/ml perfusate. However, CCL2 infusion did not result in significant changes in the relative numbers of neutrophils (CD115−/GR-1+), T-cells (CD3+), or B-cells (B220+) (data not shown). These results support the hypothesis that an increased concentration of CCL2 in circulation can induce monocyte release from the bone marrow.
FIG. 2.
CCL2 induces release of monocytes from the bone marrow. The perfusion of femoral bone marrow was performed as described in research design and methods. A: Representative dot plot of monocyte release from bone marrow induced by CCL2 perfusion. B and C: Relative (B) and absolute (C) number of monocytes (CD115+/Gr-1intermediate cells) after PBS (control) or CCL2 perfusion (n = 5 mice in each treatment; t test *P < 0.05, **P < 0.005).
CCL2 promotes monocyte release from the bone marrow via CCR2.
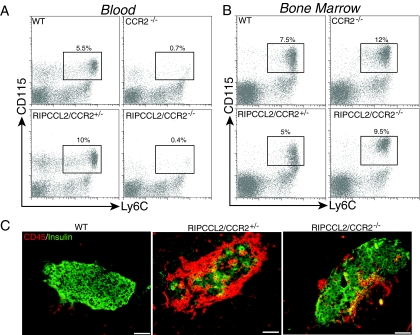
CCL2 is the ligand for the chemokine receptor CCR2. To demonstrate a tight link between the monocytosis in the RIPPCCL2 mice and the production of CCL2 in the periphery, we crossed RIPCCL2 mice (line 254) with CCR2-deficient animals to generate RIPCCL2/CCR2−/− mice. Deletion of CCR2 led to a significant decrease in the circulating numbers of CD115+/Ly6C+ monocytes (Fig. 3A), consistent with previous results (30). Genetic deletion of CCR2 in RIPCCL2 mice completely abrogated the monocytosis observed in these mice. FACS analysis of bone marrow showed that the number of monocytes in the bone marrow of wild-type mice varies between 5 and 9%. Similar numbers of monocytes were obtained in the bone marrow of RIPCCL2 mice. On the other hand, RIPCCL2/CCR2−/− mice had a larger number of CD115+/Ly6c+ monocytes than RIPCCL2/CCR2+/− mice (Fig. 3B). Analysis of islets infiltrates by immunostaining with anti-CD45 and anti-insulin showed that deletion of CCR2 significantly reduced the number of CD45+ cells in islets of RIPCCL2/CCR2−/− mice relative to RIPCCL2/CCR2+/− mice (Fig. 3C). Despite the marked reduction in the number of CD45+ cells, RIPCCL2/CCR2−/− mice still showed higher numbers of leukocytes in the pancreatic islets than wild-type mice, suggesting that leukocytes could still migrate to CCL2-expressing tissue in the absence of CCR2 as suggested by others (30,31). None of the RIPCCL2/CCR2−/− mice analyzed developed diabetes by 16 weeks of age (n = 30). These results indicate that CCR2 is required for effective mobilization of monocytes from the bone marrow in response to systemically elevated levels of CCL2.
FIG. 3.
Deletion of CCR2 abrogates peripheral monocytosis in RIPCCL2 mice. A and B: Relative number of CD115+Ly6C+ monocytes in the blood (A) and bone marrow (B) of wild-type, CCR2−/−, RIPCCL2/CCR2+/−, and RIPCCL2/CCR2−/− mice. Data are representative of one mouse in each group (n = 10/group). C: Immunostaining for CD45 (red) and insulin (green) in the pancreata of wild-type, RIPCCL2/CCR2+/−, and RIPCCL2/CCR2−/− mice at 8 weeks of age. Scale bars = 100 μm. (Please see http://dx.doi.org/10.2337/db08-0625 for a high-quality digital representation of this figure.)
Modulation of the number of circulating monocytes promotes diabetes in mice expressing low levels of CCL2 in the islets.
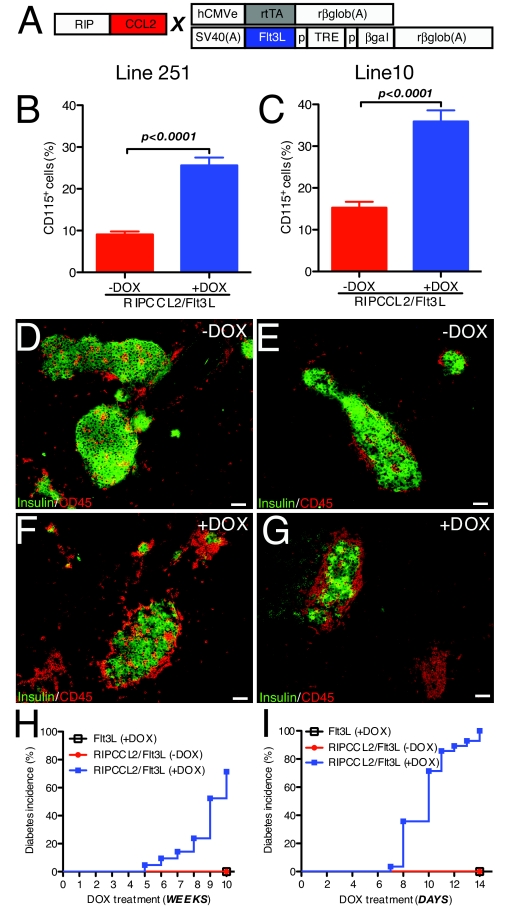
One of the predictions of the previous studies was that an increased number of circulating monocytes could lead to diabetes development if CCL2 was expressed in the islets. To test this hypothesis, we crossed RIPCCL2 mice from lines 251 and 10 with animals that conditionally express the myeloid growth factor Flt3L upon administration of doxycycline (DOX) (32) (Fig. 4A). Flt3L is a known growth factor for pluripotent hemopoietic stem cells and progenitor cells (33). Flt3L is also reported to increase numbers of lymphocytes, granulocytes, and monocytes in peripheral blood (34,35). Animals with the CCL2 and Flt3L transgenes are referred to as RIPCCL2/Flt3L mice. First, we analyzed the peripheral blood from untreated and treated RIPCCL2/Flt3L mice from lines 251 and 10. As shown in Fig. 4B, the relative number of CD115+ monocytes changed from 10% in untreated mice to 28% in DOX-treated mice in mice from line 251 (unpaired t test P < 0.0001). Evaluation of CD115+ monocytes in blood also showed an increase in the relative number of those cells from 16 to 35% when RIPCCL2/Flt3L mice from line 10 were treated for 2 weeks (Fig. 4C; unpaired t test P < 0.0001).
FIG. 4.
Increased availability of circulating monocytes promotes spontaneous diabetes in RIPCCL2 mice with lower production of CCL2. A: Outline of the RIPCCL2 transgene and of the activator and responder transgenes in the Flt3L mice. In Flt3L mice, in the activator transgene (top), the human cytomegalovirus enhancer (hCMVe) was juxtaposed to the chicken β-actin promoter to control the expression of reverse tetracycline-controlled transactivator. The responder transgene encodes β-galactosidase and mFlt3L in opposite orientation. Transcription of the β-galactosidase and mFlt3L genes is strongly induced when DOX and reverse tetracycline-controlled transactivator are present. TRE, tetracycline-responsive element; CMV, minimal CMV promoter; and rglob, rabbit β-globin. RIPCCL2 mice from lines 251 and 10 were crossed with Flt3L mice to generate the RIPCCL2/Flt3L mice. B: Relative numbers of CD115+ cells in blood from untreated RIPCCL2/Flt3L mice (RIPCCL2/Flt3L −DOX, n = 13) and DOX-treated RIPCCL2/Flt3L (n = 21) mice from line 251 (t test). C: Relative numbers of CD115+ cells in blood from untreated RIPCCL2/Flt3L mice (RIPCCL2/Flt3L −DOX, n = 15) and DOX-treated RIPCCL2/Flt3L (n = 11) mice from line 10 (t test). D–G: Immunostaining for CD45 (red) and insulin (green) in the pancreata of untreated (−DOX; D and E) or treated (+DOX; F and G) RIPCCL2/Flt3L mice from line 251 (D and F) or line 10 (E and G). H: Cumulative incidence of diabetes in untreated RIPCCL2/Flt3L mice (RIPCCL2/Flt3L −DOX, n = 13), DOX-treated Flt3L mice (n = 10), and RIPCCL2/Flt3L mice (n = 21) from line 251. Note that DOX-treated RIPCCL2/Flt3L mice developed diabetes after weeks of treatment. I: Cumulative incidence of diabetes in untreated RIPCCL2/Flt3L mice (RIPCCL2/Flt3L −DOX, n = 15), DOX-treated Flt3L mice (n = 10), and RIPCCL2/Flt3L mice (n = 29) from line 10. Note that DOX-treated RIPCCL2/Flt3L mice developed diabetes after days of treatment. Scale bars = 100 μm. (Please see http://dx.doi.org/10.2337/db08-0625 for a high-quality digital representation of this figure.)
Because the circulating monocyte population increased, we then determined whether islets from DOX-treated mice would show increased mononuclear cell infiltration. We examined histological sections of pancreata from untreated (−DOX) and treated (+DOX) double transgenic mice from both lines. Immunostaining with antibodies against CD45 and insulin showed infiltrates of varying sizes in islets of untreated (−DOX) RIPCCL2/Flt3L mice from line 251 (Fig. 4D) and line 10 (Fig. 4E), similar to what was observed in pancreata from RIPCCL2 animals of each line. In striking contrast, DOX treatment of RIPCCL2/Flt3L mice led to an increased number of leukocytes infiltrating the islets of double-transgenic mice from lines 251 and 10 (Fig. 4F and G, respectively). Subsequently, DOX-untreated and -treated RIPCCL2/Flt3L mice were monitored weekly for onset of hyperglycemia. As expected, none of the untreated RIPCCL2/Flt3L mice from line 251 developed diabetes after 10 weeks (Fig. 4H). However, 5 weeks after DOX treatment, RIPCCL2/Flt3L mice from line 251 started to show diabetes (70% of the animals in this group were diabetic after 10 weeks of treatment). No disease was observed in DOX-treated Flt3L littermates during this period (Fig. 4H). Strikingly, 100% of DOX-treated RIPCCL2/Flt3L mice from line 10 developed diabetes 14 days after DOX treatment (Fig. 4I). None of the untreated RIPCCL2/Flt3L mice or treated Flt3L mice developed diabetes during this time (Fig. 4I). Together, these results suggest that development of diabetes in RIPCCL2 mice depended both on the levels of expression of CCL2 in the islets and on the number of circulating cells.
Development of diabetes in RIPCCL2 mice does not depend on T- and B-cells.
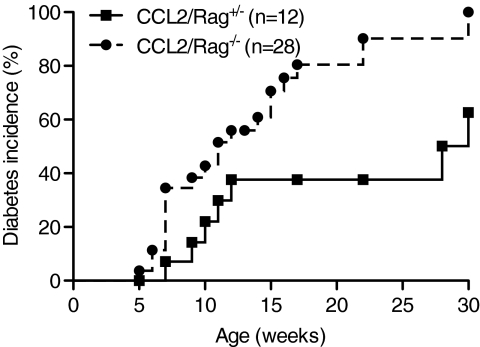
Because type 1 diabetes is a Th1-mediated autoimmune process induced by activated diabetogenic T-cells, the increase in the myeloid cells in the tissue could favor priming of T-cells or could itself lead to β-cell destruction. To investigate whether T- and B-cells were required to induce diabetes in RIPCCL2 mice, we crossed RIPCCL2 mice from line 1 (a line that develops diabetes with high incidence) with Rag-1−/− mice, which lack mature T- and B-cells, to generate RIPCCL2/Rag−/− mice. RIPCCL2/Rag−/− mice and their control littermates (RIPCCL2/Rag+/−) were monitored weekly for hyperglycemia. As expected, RIPCCL2/Rag+/− mice started to develop diabetes by 6 weeks of age and reached 60% of incidence by 30 weeks of age (Fig. 5). Although RIPCCL2/Rag−/− mice developed diabetes with a similar course, the incidence of diabetes was significantly increased compared with RIPCCL2/Rag+/− mice. This result suggests that mature T- and B-cells are not necessary for diabetes in this model, which is dependent primarily on myeloid cells to induce disease.
FIG. 5.
Diabetes in RIPCCL2 mice does not depend on T- and B-cells. Cumulative incidence of diabetes in RIPCL2/Rag+/− (n = 12) and RIPCL2/Rag−/− (n = 28) littermates from line 1 (P = 0.007).
DISCUSSION
Type 1 diabetes is an autoimmune disease characterized by a local inflammatory reaction in and around islets followed by selective destruction of insulin-secreting β-cells (36). Here, we show that accumulation of monocytes in islets of Langerhans is sufficient for induction of type 1 diabetes. In the model that we have developed, monocytes accumulate in the islet because of expression in the β-cells of the chemokine CCL2, which acts both locally and remotely to promote monocyte influx.
While local chemotactic activity has been demonstrated previously, only recently have chemokines been shown to modulate release of cells from the bone marrow (29,37). For instance, the chemokines CCL11 and CXCL1 have been shown to selectively mobilize eosinophils and neutrophils from the bone marrow, respectively (29,37). Mobilization of monocytes from the bone marrow is controlled mainly by CCR2 (30,31). During inflammation it has been suggested that CCL2 may be involved in this process (30), but the absence of a reductionist model has precluded firm conclusions. Here, we have directly tested the hypothesis that CCL2 can mobilize cells from the marrow in a transgenic model in which this chemokine is overexpressed in a single site (islets of Langerhans). We show a direct correlation between circulating numbers of monocytes and levels of CCL2 in tissue and in the blood. Furthermore, we show that infusion of CCL2 into the femoral bone marrow of control mice leads to monocyte mobilization from the femoral bone marrow into the bloodstream, strongly suggesting that the main mechanism accounting for the monocytosis is the direct release of monocytes from the bone marrow. The fact that deletion of CCL2 receptor CCR2 in RIPCCL2 mice abrogated monocytosis further supports an important role for CCR2 in monocyte release from the bone marrow (30).
There are several inflammatory conditions in which CCL2 serum levels correlate with pathogenesis and/or disease activity, including ischemic stroke and myocardial infarction (38), rheumatoid arthritis (39), chronic autoimmune thyroiditis (40), and HIV (41). Although many of these studies suggest that CCL2 serum levels could serve as a parameter to monitor the stage and severity of the disease, correlations between CCL2 serum levels, the number of monocytes in circulation, and the extent of mononuclear cell infiltration in the tissues in these patients have not been analyzed. However, a positive correlation between plasma levels of CCL2 and the number of circulating CD11b+ cells has been observed in obese mice (42). In humans, the number of monocytes increases by ∼10% in obese and overweight subjects (43). Furthermore, systemic administration of CCL2 in mice causes accumulation of MOMA-2+ monocytes in collateral arteries and increases neointimal formation (44). These observations strongly suggest that changes in plasma levels of CCL2 may lead to changes in the number of monocytes in circulation and that both of these parameters are indexes of disease severity.
The mechanisms accounting for monocyte accumulation in chronically inflamed tissues are not completely understood. We propose that one of the factors contributing to this process is CCL2. In this study, we show that CCL2 can induce recruitment of monocytes to the islets and that the number of infiltrated islets depends on CCL2 concentration and monocyte availability. These findings may have relevance in the context of diabetes because they suggest a direct role for these cells in the process of islet destruction. Indirect evidence suggests such a role. For example, inhibition of macrophage infiltration by silica treatment abolishes lymphocytic insulitis and diabetes development in the model featuring multiple low doses of STZ (MLDS) (45). Diabetes in the MLDS model can also be induced in NOD scid/scid mice lacking functional lymphocytes (46), which offers evidence for the importance of monocytes.
Although the preponderance of the evidence points to a critical importance of T-cells in the pathogenesis of type 1 diabetes, we show here that damage to the islets can be directly caused by monocytes and dendritic cells. Interestingly, the incidence of diabetes in the RIPCCL2/Rag−/− mice was actually higher than that in RIPCCL2/Rag+/− mice. The reasons for the higher incidence of diabetes in the RIPCCL2/Rag−/− group are not clear, but an intrinsic augmented activity of monocytes or other innate immune cells in the Rag−/− background may be reflected. In type 1 diabetes, an initial attack by monocytes and dendritic cells may put in motion a cascade of events leading to expansion of an autoreactive T-cell pool and complete destruction of the islets. In humans, circulating monocytes from type 1 and type 2 diabetic patients show an aberrant cytokine profile when stimulated (47), suggesting that they may contribute to the initiation or continuation of an immune attack against the pancreatic β-cells. We suggest that T-cells and macrophages may synergize to mediate islet pathology. Although our results suggest a key role for monocytes in the development of diabetes, an alternative explanation for our findings might be that the transgenic expression of CCL2 was itself toxic to the islets. Arguing against this hypothesis is the finding that expression of several transcription factors associated with β-cell apoptosis (rev. in 48) did not differ in transgenic or control islets, suggesting that CCL2 expression did not induce stress in the islets. Furthermore, transgenic expression of CCL2 did not impair glucose homeostasis. Before development of disease, transgenic mice responded to a glucose tolerance test similarly to control mice. Finally, RIPCCL2 mice lacking CCR2 did not develop diabetes over time, which rules out a direct toxic effect of CCL2 as the cause of diabetes in the RIPCCL2 mice.
We propose that the local concentration of CCL2 and the number of circulating monocytes play an important role in the onset of autoimmune diseases. Our group has shown that expression of CCL2 in the central nervous system drives monocyte/macrophage accumulation in the central nervous system without any symptom of neurological disease (49). However, those mice develop a severe demyelinating encephalomyelitis if the numbers of monocytes/macrophages and dendritic cells are altered in the periphery (34). Here, we show that elevating the number of monocytes by transgenic expression of Flt3L in RIPCCL2 lines with lower expression of CCL2 (RIPCCL2/Flt3L mice) results in increased monocyte infiltration of the islets and diabetes.
In summary, we find that the ability of CCL2 to promote tissue-specific influx of monocytes is due to two factors: its capacity to trigger mobilization of monocytes from bone marrow into circulation and its ability to mediate influx of monocytes into tissues. These properties are key for the infiltration and subsequent destruction of the islets of Langerhans in transgenic mice. The relevance of these mechanisms for autoimmune disease in NOD mice and humans is currently under investigation.
Supplementary Material
Acknowledgments
S.A.L. has received National Institutes of Health Grant DK-067381. G.C.F. has received the Dana Foundation Grant. S.P. has received British Heart Foundation Grant PG/05/-92.
We thank Peter Heeger, Bernd Schroppel, Miriam Merad, and Jay Unkeless for comments and suggestions to the manuscript and Claudia Canasto-Chibuque for expert technical assistance.
Published ahead of print at http://diabetes.diabetesjournals.org on 15 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
See accompanying commentary, p. 2922.
REFERENCES
- 1.Kolb H, Kolb-Bachofen V, Roep BO: Autoimmune versus inflammatory type I diabetes: a controversy? Immunol Today 16: 170–172, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS: Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358: 221–229, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA: Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and β-cell destruction in NOD mice. Diabetes 43: 667–675, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Rosmalen JG, Martin T, Dobbs C, Voerman JS, Drexhage HA, Haskins K, Leenen PJ: Subsets of macrophages and dendritic cells in nonobese diabetic mouse pancreatic inflammatory infiltrates: correlation with the development of diabetes. Lab Invest 80: 23–30, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Nikolic T, Geutskens SB, van Rooijen N, Drexhage HA, Leenen PJ: Dendritic cells and macrophages are essential for the retention of lymphocytes in (peri)-insulitis of the nonobese diabetic mouse: a phagocyte depletion study. Lab Invest 85: 487–501, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Jun HS, Santamaria P, Lim HW, Zhang ML, Yoon JW: Absolute requirement of macrophages for the development and activation of β-cell cytotoxic CD8+ T-cells in T-cell receptor transgenic NOD mice. Diabetes 48: 34–42, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Green EA, Eynon EE, Flavell RA: Local expression of TNFalpha in neonatal NOD mice promotes diabetes by enhancing presentation of islet antigens. Immunity 9: 733–743, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Uno S, Imagawa A, Okita K, Sayama K, Moriwaki M, Iwahashi H, Yamagata K, Tamura S, Matsuzawa Y, Hanafusa T, Miyagawa J, Shimomura I: Macrophages and dendritic cells infiltrating islets with or without beta cells produce tumour necrosis factor-alpha in patients with recent-onset type 1 diabetes. Diabetologia 50: 596–601, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Geissmann F, Jung S, Littman DR: Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, Sanchez-Torres C, Bromberg J, Charo IF, Jung S, Lira SA, Randolph GJ: Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med 200: 1231–1241, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ: Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol 172: 4410–4417, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Daly C, Rollins BJ: Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation 10: 247–257, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV Jr, Broxmeyer HE, Charo IF: Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest 100: 2552–2561, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walch L, Massade L, Dufilho M, Brunet A, Rendu F: Pro-atherogenic effect of interleukin-4 in endothelial cells: modulation of oxidative stress, nitric oxide and monocyte chemoattractant protein-1 expression. Atherosclerosis 187: 285–291, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Charo IF, Taubman MB: Chemokines in the pathogenesis of vascular disease. Circ Res 95: 858–866, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Biswas SK, Sodhi A: In vitro activation of murine peritoneal macrophages by monocyte chemoattractant protein-1: upregulation of CD11b, production of proinflammatory cytokines, and the signal transduction pathway. J Interferon Cytokine Res 22: 527–538, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Coll B, Alonso-Villaverde C, Joven J: Monocyte chemoattractant protein-1 and atherosclerosis: is there room for an additional biomarker? Clin Chim Acta 383: 21–29, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kim WJ, Chereshnev I, Gazdoiu M, Fallon JT, Rollins BJ, Taubman MB: MCP-1 deficiency is associated with reduced intimal hyperplasia after arterial injury. Biochem Biophys Res Commun 310: 936–942, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Sartipy P, Loskutoff DJ: Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A 100: 7265–7270, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen MC, Proost P, Gysemans C, Mathieu C, Eizirik DL: Monocyte chemoattractant protein-1 is expressed in pancreatic islets from prediabetic NOD mice and in interleukin-1 beta-exposed human and rat islet cells. Diabetologia 44: 325–332, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Reddy S, Bai Y, Robinson E, Ross J: Immunolocalization of monocyte chemoattractant protein-1 in islets of NOD mice during cyclophosphamide administration. Ann N Y Acad Sci 1079: 103–108, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Cameron MJ, Arreaza GA, Grattan M, Meagher C, Sharif S, Burdick MD, Strieter RM, Cook DN, Delovitch TL: Differential expression of CC chemokines and the CCR5 receptor in the pancreas is associated with progression to type I diabetes. J Immunol 165: 1102–1110, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Chen SC, Mehrad B, Deng JC, Vassileva G, Manfra DJ, Cook DN, Wiekowski MT, Zlotnik A, Standiford TJ, Lira SA: Impaired pulmonary host defense in mice lacking expression of the CXC chemokine lungkine. J Immunol 166: 3362–3368, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Piemonti L, Leone BE, Nano R, Saccani A, Monti P, Maffi P, Bianchi G, Sica A, Peri G, Melzi R, Aldrighetti L, Secchi A, Di Carlo V, Allavena P, Bertuzzi F: Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes 51: 55–65, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Grewal IS, Rutledge BJ, Fiorillo JA, Gu L, Gladue RP, Flavell RA, Rollins BJ: Transgenic monocyte chemoattractant protein-1 (MCP-1) in pancreatic islets produces monocyte-rich insulitis without diabetes: abrogation by a second transgene expressing systemic MCP-1. J Immunol 159: 401–408, 1997 [PubMed] [Google Scholar]
- 26.Martin AP, Canasto-Chibuque C, Shang L, Rollins BJ, Lira SA: The chemokine decoy receptor M3 blocks CC chemokine ligand 2 and CXC chemokine ligand 13 function in vivo. J Immunol 177: 7296–7302, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP: An improved method for isolation of mouse pancreatic islets. Transplantation 40: 437–438, 1985 [DOI] [PubMed] [Google Scholar]
- 28.Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L: RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J Biol Chem 281: 2649–2653, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Palframan RT, Collins PD, Williams TJ, Rankin SM: Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood 91: 2240–2248, 1998 [PubMed] [Google Scholar]
- 30.Serbina NV, Pamer EG: Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7: 311–317, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF: Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 117: 902–909, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manfra DJ, Chen SC, Jensen KK, Fine JS, Wiekowski MT, Lira SA: Conditional expression of murine Flt3 ligand leads to expansion of multiple dendritic cell subsets in peripheral blood and tissues of transgenic mice. J Immunol 170: 2843–2852, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Maraskovsky E, Pulendran B, Brasel K, Teepe M, Roux ER, Shortman K, Lyman SD, McKenna HJ: Dramatic numerical increase of functionally mature dendritic cells in FLT3 ligand-treated mice. Adv Exp Med Biol 417: 33–40, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Furtado GC, Pina B, Tacke F, Gaupp S, van Rooijen N, Moran TM, Randolph GJ, Ransohoff RM, Chensue SW, Raine CS, Lira SA: A novel model of demyelinating encephalomyelitis induced by monocytes and dendritic cells. J Immunol 177: 6871–6879, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Brasel K, McKenna HJ, Morrissey PJ, Charrier K, Morris AE, Lee CC, Williams DE, Lyman SD: Hematologic effects of flt3 ligand in vivo in mice. Blood 88: 2004–2012, 1996 [PubMed] [Google Scholar]
- 36.Foulis AK: C.L. Oakley lecture: The pathogenesis of beta cell destruction in type I (insulin-dependent) diabetes mellitus. J Pathol 152: 141–148, 1987 [DOI] [PubMed] [Google Scholar]
- 37.Wengner AM, Pitchford SC, Furze RC, Rankin SM: The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation. Blood 111: 42–49, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arakelyan A, Petrkova J, Hermanova Z, Boyajyan A, Lukl J, Petrek M: Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Mediators Inflamm 2005: 175–179, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klimiuk PA, Sierakowski S, Latosiewicz R, Skowronski J, Cylwik JP, Cylwik B, Chwiecko J: Histological patterns of synovitis and serum chemokines in patients with rheumatoid arthritis. J Rheumatol 32: 1666–1672, 2005 [PubMed] [Google Scholar]
- 40.Kokkotou E, Marafelia P, Mantzos EI, Tritos NA: Serum monocyte chemoattractant protein-1 is increased in chronic autoimmune thyroiditis. Metabolism 51: 1489–1493, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, Kulkarni H, Bamshad MJ, Telles V, Anderson SA, Walter EA, Stephan KT, Deucher M, Mangano A, Bologna R, Ahuja SS, Dolan MJ, Ahuja SK: HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci U S A 99: 13795–13800, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi K, Mizuarai S, Araki H, Mashiko S, Ishihara A, Kanatani A, Itadani H, Kotani H: Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J Biol Chem 278: 46654–46660, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Kullo IJ, Hensrud DD, Allison TG: Comparison of numbers of circulating blood monocytes in men grouped by body mass index (<25, 25 to <30, > or =30). Am J Cardiol 89: 1441–1443, 2002 [DOI] [PubMed] [Google Scholar]
- 44.van Royen N, Hoefer I, Bottinger M, Hua J, Grundmann S, Voskuil M, Bode C, Schaper W, Buschmann I, Piek JJ: Local monocyte chemoattractant protein-1 therapy increases collateral artery formation in apolipoprotein E-deficient mice but induces systemic monocytic CD11b expression, neointimal formation, and plaque progression. Circ Res 92: 218–225, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Papaccio G, Frascatore S, Esposito V, Pisanti FA: Early macrophage infiltration in mice treated with low-dose streptozocin decreases islet superoxide dismutase levels: prevention by silica pretreatment. Acta Anat (Basel) 142: 141–146, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Gerling IC, Friedman H, Greiner DL, Shultz LD, Leiter EH: Multiple low-dose streptozocin-induced diabetes in NOD-scid/scid mice in the absence of functional lymphocytes. Diabetes 43: 433–440, 1994 [DOI] [PubMed] [Google Scholar]
- 47.Giulietti A, Stoffels K, Decallonne B, Overbergh L, Mathieu C: Monocytic expression behavior of cytokines in diabetic patients upon inflammatory stimulation. Ann N Y Acad Sci 1037: 74–78, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL: Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54 (Suppl. 2): S97–S107, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira SA: Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol 155: 5769–5776, 1995 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.