Abstract
To investigate the distribution of lipids through the Golgi complex, we analyzed the envelopes of several viruses that assemble in different subcompartments of the Golgi, as well as subcellular fractions. Our results indicate that each Golgi subcompartment has a distinct phospholipid composition due mainly to differences in the relative amounts of semilysobisphosphatidic acid (SLBPA), sphingomyelin, phosphatidylserine, and phosphatidylinositol. Interestingly, SLBPA is enriched in the adjacent Golgi networks compared with the Golgi stack, and this enrichment varies with cell type. The heterogeneous distribution of SLBPA through the Golgi complex suggests it may play an important role in the structure and/or function of this organelle.
INTRODUCTION
The Golgi complex ensures the proper sorting and delivery of newly synthesized materials in eukaryotic cells. This organelle consists of stacks of cisternal membranes flanked on either side by two tubulovesicular regions, the intermediate compartment/cis-Golgi network (IC/CGN) and the trans-Golgi network (TGN; Mellman and Simons, 1992). The underlying organization of these subcompartments is not fully known, but it must be maintained in the face of continuous bidirectional traffic. Although it is clear that resident proteins are localized to discrete compartments in the organelle (Dunphy and Rothman, 1985), their distribution may substantially overlap (Nilsson et al., 1993; Velasco et al., 1993). Also, the subcompartment localization of some Golgi-associated proteins varies with cell type (Brown and Farquhar, 1987; Colley, 1997). Far less is known about the lipid environment of Golgi subcompartments despite a growing awareness of the importance of lipids in Golgi structure and function (Orci et al., 1981; Dunphy and Rothman, 1985; Schweizer et al., 1994; Pagano et al., 1989; Luzio et al., 1990; Bednarek et al., 1997). Existing data on the lipid composition of Golgi membranes were generated primarily from analysis of rat hepatocytes (e.g., Keenan and Morré, 1970), but these studies did not address the composition of the Golgi networks. Cholesterol may be heterogeneously distributed through the Golgi stack in pancreatic acinar cells (Orci et al., 1981), and a similar distribution of sphingomyelin is seen in fibroblasts (Pagano et al., 1989). Otherwise, we know very little about the distribution of lipids through the Golgi complex or if the composition and distribution of its lipids vary with cell type.
Unfortunately, the complex organization and dynamic nature of Golgi membranes make it difficult to study the lipid composition of its subcompartments by conventional fractionation techniques. However, enveloped viruses offer a convenient alternative because their envelopes may reflect the lipid composition of the membrane from which they were derived. When used together, subcellular fractionation and analysis of viral envelopes complement each other and help to validate studies of complex membrane regions.
We previously analyzed the two intracellular forms of the poxvirus vaccinia (intracellular mature virus form of vaccinia virus [VV-IMV] and intracellular enveloped virus form of vaccinia virus [VV-IEV]) grown in HeLa cells (Cluett and Machamer, 1996). The assembly sites of VV-IMV and VV-IEV (IC/CGN and TGN, respectively; Sodeik et al., 1993; Schmelz et al., 1994) allowed us to infer the lipid composition of the pleiomorphic Golgi networks in HeLa cells. However, the difficulties encountered in the fractionation of HeLa cells prevented a conclusive analysis of Golgi stacks. Herein we extend our studies by using other cell lines and additional enveloped viruses that assemble in the Golgi region to analyze the distribution of phospholipids through the entire Golgi complex, including the Golgi stacks.
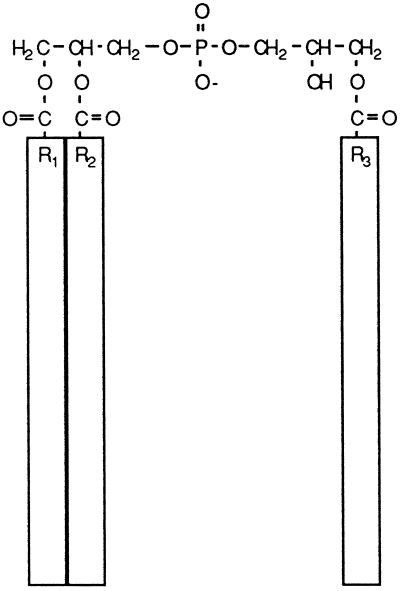
We were particularly interested in the lipid semilysobisphosphatidic acid (SLBPA). The unusual structure of SLBPA (Figure 1) suggests that it might have a significant effect on the structure of membranes in which it resides. SLBPA was previously found in vaccinia virus envelopes before the contribution of the Golgi to virus assembly was known (Hiller et al., 1981). We subsequently showed that SLBPA was present in Golgi membranes from uninfected cells (Cluett and Machamer, 1996). Since the distribution of SLBPA in uninfected HeLa cells paralleled that of galactosyltransferase, a Golgi marker, we wished to determine the location of this lipid more precisely. Here, we report that SLBPA is enriched in the Golgi networks compared with the stacks.
Figure 1.
Structure of SLBPA. The acyl chains are indicated by boxes because their molecular composition is not known. The small head group and three acyl chains may have significant effects on the lipid bilayer.
MATERIALS AND METHODS
Virus Infection and Purification
All viruses were propagated in baby hamster kidney cells BHK-21 (grown in DMEM with 5% fetal calf serum). Vaccinia virus was also grown in Madin-Darby canine kidney (MDCK) cells (DMEM with 10% fetal calf serum). Cells were infected with the IHD-J strain of vaccinia virus at 5 plaque-forming units (pfu) per cell for 24 h. The two intracellular forms of vaccinia virus were purified essentially as described (Cluett and Machamer, 1996). Additionally, the virus preparation from each cell type was briefly sonicated to separate virions from cellular membranes before loading on sucrose gradients. Vaccinia virus from BHK-21 cells was separated on linear gradients of 25–45% sucrose, whereas the virus isolated from MDCK cells was separated on a 25–50% sucrose gradient. Uukuniemi virus was purified from the medium 72 h after infection essentially as described (Pettersson and Kaariainen, 1973). The Beaudette strain of avian infectious bronchitis virus, adapted for growth in Vero cells (Machamer and Rose, 1987), was adapted to BHK-21 cells by three passages. Subsequently, cells were infected with virus at approximately 1 pfu per cell and cultured for 40 h before purifying virus from the medium as described (Stern et al., 1982), except that the second gradient was eliminated. Cells were infected with 10 pfu per cell of vesicular stomatitis virus (VSV, San Juan strain) for 17 h and virions were isolated from the medium by centrifuging through 10% sucrose onto a 60% sucrose cushion. Purity of the viruses was assessed by SDS-PAGE. In addition, the purity of the two forms of vaccinia virus was confirmed by immunoblotting with an antibody specific for VV-IEV (Cluett and Machamer, 1996). Finally, aliquots of purified virus were negatively stained with 2% aqueous uranyl acetate and observed by electron microscopy.
Isolation of Golgi Membranes
Six plates (24 × 24 cm) of confluent BHK-21 cells were used to isolate stacked Golgi membranes essentially as described (Cluett and Brown, 1992). A ball-bearing clearance of 0.001 inches and a sucrose step gradient of 8-ml steps of 0.8 M, 1.0 M, and 1.2 M sucrose were used. For MDCK cells, a clearance of 0.0011 inches and gradient of 0.7 and 1.1 M sucrose were used. Galactosyltransferase was enriched about 25-fold in the MDCK Golgi membranes and about 20-fold in the BHK Golgi membranes.
Fractionation of BHK-21 cells was performed as above with the following modifications. After homogenization, a very low speed centrifugation (500 × g) was carried out to separate nuclei from the mitochondrial fraction, as described by Suprynowicz and Gerace (1986). Then the supernatant was centrifuged at 5000 × g to generate a pellet, highly enriched in mitochondria, and a postmitochondrial supernatant. The supernatant was fractionated by centrifugation at 90,000 × g for 2.5 h on a discontinuous sucrose gradient with 0.6 M, 0.8 M, 1.2 M, 1.6 M, and 2.0 M steps. Bands, visible at all interfaces, were harvested and assayed for enzymatic activity (Cluett and Machamer, 1996). IC/CGN and TGN/endosomes were monitored by immunoblotting 30 μg of each fraction with antibodies to p58 (Saraste et al., 1987) or the cation-independent mannose-6-phosphate receptor (Brown and Farquhar, 1984), respectively. Approximately 40% of endoplasmic reticulum (ER), Golgi, IC/CGN, and TGN/endosome markers and 15% of lysosome markers were recovered in gradient fractions.
Lipid Analysis
Lipids were extracted and analyzed by high-performance thin layer chromatography (HPTLC) and digital densitometry as described (Cluett and Machamer, 1996). SLBPA was identified by comigration with standards in several different solvent systems and by molecular mass determination by mass spectrometry. Values are expressed as percent of total phospholipids. Because only the six major phospholipids were quantitated, the totals in Figure 4 do not add up to 100%. Calculation of the lipid composition of the TGN was performed as described (Cluett and Machamer, 1996). The amount of each phospholipid was extrapolated from concentration curves and normalized to the number of virions. The contribution of VV-IMV was subtracted from VV-IEV and the resulting value, when expressed as a percentage of the total phospholipid, represented the phospholipid composition of the TGN. To ascertain whether the lipid profiles of each virus and Golgi membranes were statistically different, an analysis of variance was performed for each phospholipid. The p values for each phospholipid were less than 0.0004 with the exception of phosphatidylcholine (p = 0.054, F < Fcrit) and phosphatidylethanolamine (p = 0.002). Further, the following correlation coefficients were determined for the lipid profiles: VV-IMV and infectious bronchitis virus (IBV) = 0.95; IBV and Uukuniemi = 0.85; VV-IMV and Uukuniemi = 0.74. The data for each lipid in VV-IMV and IBV were analyzed using a t test after determining the equality of variances with an F-test. Only sphingomyelin (p = 0.001) and phosphatidylinositol (p = 0.01) were statistically different. The same analysis was performed on data from Uukuniemi virus and Golgi membranes, and at a confidence level of 95%, there were no statistical differences. The lipid composition of Golgi membranes and Golgi networks were analyzed by analysis of variance and were found to be significantly different. In addition, the Golgi stacks and CGN of BHK cells were analyzed by t test (p = 0.02).
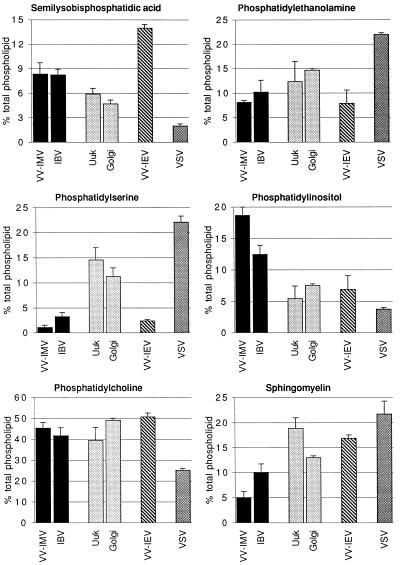
Figure 4.
Distribution of phospholipids in the Golgi complex of BHK-21 cells. The percent of total phospholipid (±SEM) is shown for six major phospholipids. VV-IMV and IBV assemble in the IC/CGN (solid bars). The Golgi stacks (shaded bars) were assayed by using Uukuniemi virus and an enriched fraction of Golgi membranes. The TGN (plus IC/CGN) (hatched bars) is represented by VV-IEV, and the plasma membrane (stippled bars) was sampled by VSV. Statistical analysis indicates the phospholipid profile of IBV is more similar to VV-IMV than to Uukuniemi virus. There were no statistical differences between the phospholipid composition of Uukuniemi virus and isolated Golgi membranes. Details are given in MATERIALS AND METHODS.
RESULTS
Phospholipid Distribution through the Golgi Complex
To overcome the difficulty of isolating pleiomorphic structures such as the IC/CGN and TGN by subcellular fractionation, we analyzed the lipids of enveloped viruses as a way to determine the lipid composition of these cellular compartments. We extended our previous work in HeLa cells (Cluett and Machamer, 1996) by using other viruses, in addition to vaccinia, that acquire their envelopes from distinct regions of the Golgi complex. Among these are the coronavirus IBV and the bunyavirus Uukuniemi (Figure 2). These enveloped viruses allowed us to sample the lipids of both Golgi networks and Golgi stacks. VV-IMV enwraps the membranes of the IC/CGN to obtain its membranes (Sodeik et al., 1993). IBV, a much smaller virus than vaccinia, obtains its envelope by budding into the IC/CGN (Griffiths and Rottier, 1992), the same compartment enwrapped by VV-IMV. By contrast, Uukuniemi virus buds predominantly into the cisternae of the Golgi stack (Kuismanen et al., 1982; Jantti et al., 1997). VV-IEV is formed when VV-IMV enwraps the membrane of the TGN (Schmelz et al., 1994). We compared the lipid composition of viral envelopes of VV-IMV and VV-IEV, IBV, and Uukuniemi virus to that of VSV, which buds from the plasma membrane. We used BHK-21 cells because they are permissive for all of these viruses. We also prepared Golgi membranes from these cells by conventional fractionation for comparison.
Figure 2.
Assembly sites of the viruses used in this study. UUK, Uukuniemi virus.
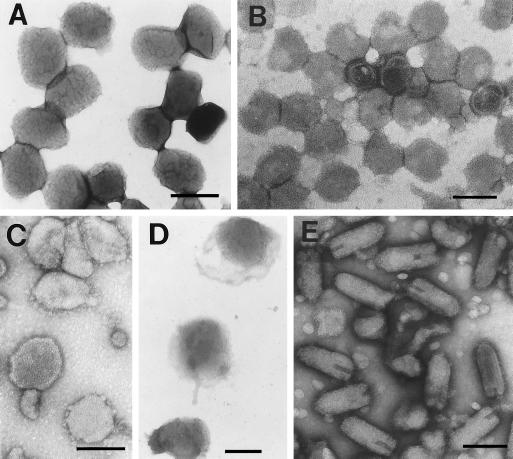
Electron microscopy of negatively stained virions purified from BHK-21 cells (Figure 3), and SDS-PAGE indicated that Uukuniemi, VSV, and both forms of vaccinia virus were highly purified. Although some contaminating membranes were present in the IBV preparation (Figure 3), it contained >75% viral proteins by SDS-PAGE (our unpublished results). Isolated Golgi membranes were enriched in galactosyltransferase activity about 20-fold over the postnuclear supernatant and contained less than 10% of ER and lysosomal marker activities.
Figure 3.
Electron microscopy of purified viruses after negative staining. Representative fields are shown for each virus. (A) VV-IMV. (B) Uukuniemi virus. (C) IBV. (D) VV-IEV. (E) VSV. Bars: A and D, 300 nm; B, C, and E, 150 nm.
The profiles of the major phospholipids from vaccinia virus, Uukuniemi, and VSV propagated in BHK-21 cells (Figure 4) were similar to published results obtained with radiolabeled lipids (Renkonen et al., 1972; Stern and Dales, 1974; Pal et al., 1980). Statistical analysis of the phospholipid composition of the viral envelopes indicated that each Golgi region had a distinct lipid composition, which differed significantly from the plasma membrane lipid composition (measured by VSV). The phospholipid composition of the IC/CGN was similar when assessed by two different enveloped viruses, VV-IMV and IBV (Figure 4, solid bars). Similarly, the two independent means of determining the composition of Golgi stacks gave statistically similar phospholipid compositions (gray bars in Figure 4). These results suggest that the enveloped viruses used herein provide a nonbiased sample of the membrane at which they assemble and validate our approach. To further support this, the total cellular phospholipid profile was not changed by infection with any of the viruses (our unpublished data). Interestingly, VV-IMV and IBV, representing the IC/CGN, had as much as threefold more phosphatidylinositol than the two distal Golgi regions, whereas Golgi stacks contained a higher percentage of phosphatidylserine than the Golgi networks. Sphingomyelin was enriched in the stacks and TGN, consistent with the reported localization of sphingomyelin synthase in the cis and medial cisternae of Golgi stacks (Futerman et al., 1990). The differences in the phospholipid profiles of Golgi subcompartments suggested that the IC/CGN and TGN do not copurify with Golgi stacks.
The Distribution of SLBPA through the Golgi Complex
We were most interested in the distribution of SLBPA. As shown in Figure 4, the envelope of VV-IEV, which contains membranes from the TGN, had a greater percentage of SLBPA than viral envelopes derived from IC/CGN, Golgi stacks, or plasma membrane. Importantly, SLBPA was about 8% of total phospholipids in both IBV and VV-IMV envelopes even though the two viruses assemble by completely different mechanisms. Because the level of this lipid may vary with cell type (Cluett and Machamer, 1996), we included in our analysis another cell type (MDCK) from which enriched Golgi membranes could be isolated. After normalizing the amount of lipid in VV-IMV and VV-IEV, we subtracted the contribution of the IC/CGN from VV-IEV envelopes to derive a lipid composition for the TGN (Cluett and Machamer, 1996). In BHK-21 cells, SLBPA represented about 15% of total phospholipid in the TGN, compared with 8% in the IC/CGN and almost 5% in Golgi stacks (Figure 5). Although the difference between the SLBPA content of the IC/CGN and Golgi stacks was small, it was statistically significant (see MATERIALS AND METHODS). By contrast, in MDCK cells SLBPA accounted for about 20% of the phospholipid in both Golgi networks compared with almost 7% in the Golgi stack (Figure 5). Although the percentage of SLBPA may differ between cell types, the same trend is observed: SLBPA is a component of Golgi membranes, and it is enriched in the juxta Golgi networks relative to the Golgi stacks.
Figure 5.
SLBPA is heterogeneously distributed through the Golgi complex. The contribution of the IC/CGN was subtracted from VV-IEV to give the TGN phospholipid composition (Cluett and Machamer, 1996). Although the amount of SLBPA in membranes from different Golgi regions differs with cell type, the same trend is apparent in both BHK-21 cells and MDCK cells. Analysis of BHK-21 cell data indicated a small but statistically significant (p = 0.02) difference between CGN and Golgi stack.
Cellular Distribution of SLBPA
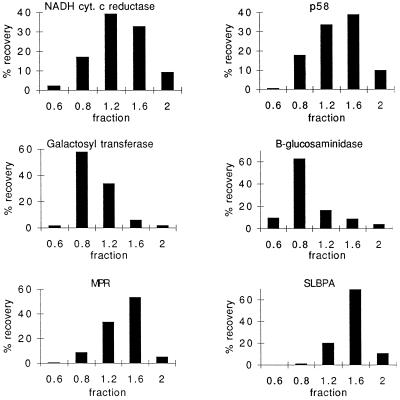
In whole cell extracts of BHK-21 and MDCK cells, SLBPA represents about 6.5% and 4.5%, respectively, of the total phospholipids. On the basis of previous estimates of percentage of Golgi membranes in BHK-21 cells (Griffiths et al., 1989), our data indicate that the Golgi complex cannot account for all the SLBPA in the cell. To determine other potential intracellular locations of SLBPA, we fractionated BHK-21 cells by using a sucrose step gradient. The amount of SLBPA found in nuclear or mitochondrial fractions was less than 1% of total phospholipid (our unpublished results). As seen in Figure 6, SLBPA was enriched in a dense 1.6 M sucrose fraction. Its distribution most closely followed that of the cation-independent mannose-6-phosphate receptor that is found in both the TGN and endosomes. However, this fraction also contained significant amounts of p58 (a marker of the IC/CGN) and an ER marker, NADH cytochrome c reductase. Interestingly, the distribution of galactosyltransferase and β-glucosaminidase did not coincide with the distribution of SLBPA. Although Golgi membranes, particularly the Golgi networks, appear to contain the highest level of SLBPA, a lower percentage in the ER (which accounts for a substantial portion of total cellular membrane) could account for the remaining SLBPA. The phospholipid profile of this 1.6 M fraction was consistent with that of the Golgi networks as determined by analysis of viral envelopes (our unpublished results).
Figure 6.
SLBPA is found in a “heavy” fraction with a distribution similar to the mannose-6-phosphate receptor. BHK-21 cells were fractionated as described in MATERIALS AND METHODS. Galactosyltransferase (a Golgi marker), NADH cytochrome c reductase (ER), and β-glucosaminidase (lysosomes) were assayed by enzymatic activity. β-Glucosaminidase activity paralleled the distribution of a lysosomal membrane protein as assayed by immunoblotting (our unpublished data). The distribution of p58 and the cation-independent mannose-6-phosphate receptor (MPR) were assayed by immunoblot and quantitated by digital densitometry.
DISCUSSION
Mounting evidence supports the notion of three distinct subcompartments in the Golgi complex (Mellman and Simons, 1992), but little is known regarding the lipid composition of these membranes. This work presents a phospholipid profile of the Golgi complex. Analyzing the envelopes of different purified viruses and fractionated membranes allowed us to compare the lipid composition of the Golgi networks to the Golgi stacks.
It is hard to assess how accurately viral envelopes reflect the membrane from which they were derived because it is difficult to obtain sufficient amounts of pure subcompartment membranes for comparison. Furthermore, identification of organelles with a limited battery of markers may also prove problematic for organelles such as the plasma membrane that is composed of different domains (Simons and Ikonen, 1997). For the plasma membrane, it is not clear whether a fraction containing 20% or less of recovered marker activity accurately represents the bulk lipid composition of the organelle (e.g., Pessin and Glaser, 1980). Consequently, it is not surprising that conflicting conclusions are drawn when the lipid composition of a plasma membrane fraction is compared with that of enveloped viruses (Pessin and Glaser, 1980; Van Meer and Simons, 1982). Our results suggest that the viruses used herein sample Golgi subcompartments nondiscriminately. The envelopes of two structurally different viruses (VV-IMV and IBV) that assemble by two completely different mechanisms in the IC/CGN had very similar phospholipid profiles, including similar amounts of SLBPA. Furthermore, the lipid profile of Uukuniemi virus closely resembled that of isolated Golgi membranes, which are derived from the stack. The composition of vaccinia virus envelopes purified from BHK-21 cells differed from those purified from HeLa cells (Cluett and Machamer, 1996), again supporting the idea that viral envelopes reflect the composition of cellular membranes from which they were derived. Finally, the distribution of SLBPA in membranes obtained from subcellular fractionation was consistent with the distribution found in viral envelopes.
We found that each Golgi subcompartment has a unique phospholipid composition. The amount of phosphatidylserine was higher in Golgi stacks than in either juxta Golgi network. Phosphatidylinositol was significantly enriched in the IC/CGN but was found in much lower amounts in the stacks and TGN. This observation is particularly intriguing because the SEC14 gene, which encodes a phosphatidylinositol/phosphatidylcholine transfer protein, is necessary for protein transport through the Golgi (Bankaitis et al., 1990). By contrast, sphingomyelin made up a lower percentage of the phospholipid in the CGN compared with the stacks or TGN, consistent with its proposed site of synthesis (Futerman et al., 1990; Jeckel et al., 1990). Furthermore, the sphingomyelin levels in the stacks and TGN are quite similar. In view of the proposed association of sphingomyelin and cholesterol in cellular membranes (Simons and Ikonen, 1997), it will be important to determine whether cholesterol has the same distribution. Interestingly, these same differences between the lipid profiles of the CGN and TGN in BHK-21 cells are also seen in HeLa (Cluett and Machamer, 1996) and MDCK cells (our unpublished data).
Our most important finding is that SLBPA is differentially enriched in the tubulovesicular networks on either side of the Golgi stack. The data presented herein illustrate the two distribution patterns of SLBPA seen in all cell types examined thus far. In HeLa and MDCK cells, SLBPA was found in similar amounts in both the IC/CGN and TGN (Cluett and Machamer, 1996; Figure 5). In contrast, SLBPA accounted for a higher percentage of phospholipid in the TGN compared with the IC/CGN in BHK-21 and Vero cells (Figure 5; our unpublished results). Surprisingly, we found much higher levels of SLBPA in BHK-21 cells than reported in earlier studies (Brotherus and Renkonen, 1974). This may be due to different growth conditions, extraction protocols, resolution of our HPTLC system, or radiolabeled versus unlabeled samples.
The data from the fractionation of BHK-21 cells support the contention that SLBPA is more enriched in Golgi networks than in Golgi stacks. SLBPA was found in a heavier fraction that was enriched in the mannose-6-phosphate receptor as well as p58 and ER. The lipid profile of this fraction features higher percentages of SLBPA and phosphatidylinositol and lower percentages of sphingomyelin and phosphatidylserine than other fractions. However, the levels of sphingomyelin and phosphatidylserine are higher than those found in the IC/CGN-associated viruses. The distribution of SLBPA was consistent with the data obtained from the viral envelopes and suggests that the bulk of SLBPA is indeed found in transitional regions between organelles of the intracellular transport pathway where coated vesicle production is high. If SLBPA is involved in membrane dynamics, it is not surprising that SLBPA is also found in the ER because coated vesicles form from ER membranes (Orci et al., 1994; Bednarek et al., 1995). Although at this time we cannot conclusively identify all the organelles in which SLBPA is found, it is clear that as a percent of total phospholipid, the amount of SLBPA is highest in Golgi membranes.
The unusual structure of SLBPA may have important consequences for Golgi structure and function. The three acyl chains and small charged head group suggest that the lipid may be curvature-inducing (Powell and Hui, 1996), but in fact, little is known about the biophysical properties of SLBPA. It is especially intriguing that SLBPA is localized to the most dynamic pleiomorphic regions of the Golgi complex. A related lipid, bisphosphatidic acid, may be formed from diacylglycerol and phosphatidic acid by a transphosphatidylation reaction catalyzed by phospholipase D (van Blitterswijk and Hilkmann, 1993). Phospholipase D is activated by ADP-ribosylation factor, an important protein in intracellular transport (Brown et al., 1993). Furthermore, phospholipase D activity is present in Golgi membranes (Ktistakis et al., 1995), and high endogenous levels of this enzyme have been noted in MDCK cells (Ktistakis et al., 1996). Phosphatidic acid (a product of phospholipase D action) and other negatively charged phospholipids have been implicated in the formation of coated vesicles (Ktistakis et al., 1996). Diacylglycerol may also be involved in the production of coated vesicles (Kearns et al., 1997). However, neither phosphatidic acid nor diacylglycerol is found in significant amounts in organelles at steady state, whereas SLBPA is. Because the removal of an acyl chain from bisphosphatidic acid produces SLBPA, it is also intriguing that phospholipase A2 and acyl CoA have been implicated in Golgi trafficking (Glick and Rothman, 1987; Slomiany et al., 1992). Studies are in progress to investigate the structure and biosynthesis of SLBPA, as well as its role in budding and fusion of transport vesicles.
Finally, our fractionation data indicate that Golgi networks do not always cofractionate with Golgi stacks. Because of their pleiomorphic nature, it is hard to identify these compartments and calculate the fraction of total cell membrane they represent. The close similarity between the lipid composition of the Golgi stacks obtained by subcellular fractionation and that obtained from analysis of Uukuniemi virus envelopes strongly suggests that viruses offer a useful alternative to fractionation and, for the appropriate compartment, complement and validate fractionation procedures. This is especially important for organelles or compartments for which there are a limited battery of markers.
ACKNOWLEDGEMENTS
We thank Drs. William J. Brown and Jaakko Saraste for their generous gifts of antibodies. We thank Dr. Robert Cotter of the MidAtlantic Mass Spectrometry Facility for SLBPA analysis. We also thank Dr. Ann Hubbard, Dr. Katherine Wilson, and the members of the Machamer lab and of the P01 group for their helpful advice and critical reading of the manuscript. This work was supported by the National Institutes of Health grant PO1 DK-44375.
Footnotes
1 Abbreviations used: BHK, baby hamster kidney; HPTLC, high performance TLC; IBV, infectious bronchitis virus; IC/CGN, intermediate compartment/cis Golgi network; MDCK, Madin-Darby canine kidney; pfu, plaque-forming unit; SLBPA, semilysobisphosphatidic acid; TGN, trans Golgi network; VSV, vesicular stomatitis virus; VV-IEV, intracellular enveloped virus form of vaccinia virus; VV-IMV, intracellular mature virus form of vaccinia virus.
REFERENCES
- Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Bednarek SY, Orci L, Schekman R. Traffic COPs and the formation of vesicle coats. Trends Cell Biol. 1997;6:468–473. doi: 10.1016/0962-8924(96)84943-9. [DOI] [PubMed] [Google Scholar]
- Bednarek SY, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Brotherus J, Renkonen O. Isolation and characterization of bis-phosphatidic acid and its partially deacylated derivatives from cultured BHK-cells. Chem Phys Lipids. 1974;13:11–20. doi: 10.1016/0009-3084(74)90038-3. [DOI] [PubMed] [Google Scholar]
- Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Farquhar MG. The mannose-6-phosphate receptor for lysosomal enzymes is concentrated in cis Golgi cisternae. Cell. 1984;36:295–307. doi: 10.1016/0092-8674(84)90223-x. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Farquhar MG. The distribution of 215-kilodalton mannose 6-phosphate receptors within cis (heavy) and trans (light) Golgi subfractions varies in different cell types. Proc Natl Acad Sci USA. 1987;84:9001–9005. doi: 10.1073/pnas.84.24.9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluett EB, Brown WJ. Adhesion of Golgi cisternae by proteinaceous interactions: intercisternal bridges as putative adhesive structures. J Cell Sci. 1992;103:773–784. doi: 10.1242/jcs.103.3.773. [DOI] [PubMed] [Google Scholar]
- Cluett EB, Machamer CE. The envelope of vaccinia virus reveals an unusual phospholipid in Golgi complex membranes. J Cell Sci. 1996;109:2121–2131. doi: 10.1242/jcs.109.8.2121. [DOI] [PubMed] [Google Scholar]
- Colley KJ. Golgi localization of glycosyltransferases: more questions than answers. Glycobiology. 1997;7:1–13. doi: 10.1093/glycob/7.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy WG, Rothman JE. Compartmental organization of the Golgi stack. Cell. 1985;42:13–21. doi: 10.1016/s0092-8674(85)80097-0. [DOI] [PubMed] [Google Scholar]
- Futerman AH, Steiger B, Hubbard AL, Pagano RE. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990;265:8650–8675. [PubMed] [Google Scholar]
- Glick BS, Rothman JE. Possible role for fatty acyl-coenzyme A in intracellular protein transport. Nature. 1987;326:309–312. doi: 10.1038/326309a0. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Fuller SD, Back R, Hollinshead M, Pfeffer S, Simons K. The dynamic nature of the Golgi complex. J Cell Biol. 1989;108:277–297. doi: 10.1083/jcb.108.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Rottier P. Cell biology of viruses that assemble along the biosynthetic pathway. Semin Cell Biol. 1992;3:367–381. doi: 10.1016/1043-4682(92)90022-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller G, Eibl H, Weber F. Acyl bis(monoacylglycero) phosphate, assumed to be a marker for lysosomes, is a major phospholipid of vaccinia virus. Virology. 1981;113:761–764. doi: 10.1016/0042-6822(81)90204-x. [DOI] [PubMed] [Google Scholar]
- Jantti J, Hilden P, Ronka H, Makiranta V, Keranen S, Kuismanen E. Immunocytochemical analysis of Uukuniemi virus budding compartments: role of the intermediate compartment and the Golgi stack in virus maturation. J Virol. 1997;71:1162–1172. doi: 10.1128/jvi.71.2.1162-1172.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeckel D, Karrenbauer A, Birk R, Schmidt RR, Wieland F. Sphingomyelin is synthesized in the cis Golgi. FEBS Lett. 1990;261:155–157. doi: 10.1016/0014-5793(90)80659-7. [DOI] [PubMed] [Google Scholar]
- Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA. Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature. 1997;387:101–105. doi: 10.1038/387101a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan TW, Morré DJ. Phospholipid class and fatty acid composition of Golgi apparatus isolated from rat liver and comparison with other cell fractions. Biochemistry. 1970;9:19–25. doi: 10.1021/bi00803a003. [DOI] [PubMed] [Google Scholar]
- Ktistakis NT, Brown HA, Sternweis PC, Roth MG. Phospholipase D is present on Golgi-enriched membranes and its activation by ADP ribosylation factor is sensitive to brefeldin A. Proc Natl Acad Sci USA. 1995;92:4952–4956. doi: 10.1073/pnas.92.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuismanen E, Hedman K, Saraste J, Pettersson RF. Uukuniemi virus maturation: accumulation of virus particles and viral antigens in the Golgi complex. Mol Cell Biol. 1982;2:1444–1458. doi: 10.1128/mcb.2.11.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Brake B, Banting G, Howell KE, Braghetta P, Stanley KK. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN 38) Biochem J. 1990;270:97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer CE, Rose JK. A specific transmembrane domain of a coronavirus E1 glycoprotein is required for its retention in the Golgi region. J Cell Biol. 1987;105:1205–1214. doi: 10.1083/jcb.105.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Simons K. The Golgi complex: in vitro veritas? Cell. 1992;68:829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T, Pypaert M, Hoe MH, Slusarewicz P, Berger EG, Warren G. Overlapping distribution of two glycosyltransferases in the Golgi apparatus of HeLa cells. J Cell Biol. 1993;120:5–13. doi: 10.1083/jcb.120.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Montesano R, Meda P, Malaisse-Lagae F, Brown D, Perrelet A, Vassalli P. Heterogeneous distribution of filipin-cholesterol complexes across the cisternae of the Golgi apparatus. Proc Natl Acad Sci USA. 1981;78:293–297. doi: 10.1073/pnas.78.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Perrelet A, Ravazzola M, Amherdt M, Rothman JE, Schekman R. Coatomer-rich endoplasmic reticulum. Proc Natl Acad Sci USA. 1994;91:11924–11928. doi: 10.1073/pnas.91.25.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano RE, Sepanski MA, Martin OC. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J Cell Biol. 1989;109:2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R, Petri WA, Wagner RR. Alteration of the membrane lipid composition and infectivity of vesicular stomatitis virus by growth in a Chinese hamster ovary cell sterol mutant and in lipid-supplemented baby hamster kidney clone 21 cells. J Biol Chem. 1980;255:7688–7693. [PubMed] [Google Scholar]
- Pessin JE, Glaser M. Budding of Rous sarcoma virus and vesicular stomatitis virus from localized lipid regions in the plasma membrane of chicken embryo fibroblasts. J Biol Chem. 1980;255:9044–9050. [PubMed] [Google Scholar]
- Pettersson R, Kaariainen L. The ribonucleic acids of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1973;56:608–619. doi: 10.1016/0042-6822(73)90062-7. [DOI] [PubMed] [Google Scholar]
- Powell GL, Hui S-W. Tetraoleoylpyrophosphatidic acid: a four acyl-chain lipid which forms a hexagonal II phase with high curvature. Biophys J. 1996;70:1402–1406. doi: 10.1016/S0006-3495(96)79698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkonen O, Kaariainen L, Pettersson R, Oker-Blom N. The phospholipid composition of Uukuniemi virus, a non-cubical tick-borne arbovirus. Virology. 1972;50:899–901. doi: 10.1016/0042-6822(72)90443-6. [DOI] [PubMed] [Google Scholar]
- Saraste J, Palade GE, Farquhar MG. Antibodies to rat pancreas Golgi subfractions: identification of a 58-kd cis-Golgi protein. J Cell Biol. 1987;105:2021–2029. doi: 10.1083/jcb.105.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Sodeik B, Ericsson M, Wolffe EJ, Shida H, Hiller G, Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J Virol. 1994;68:130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Clausen H, van Meer G, Hauri H-P. Localization of O-glycan initiation, sphingomyelin synthesis, and glucosylceramide synthesis in Vero cells with respect to the endoplasmic reticulum-Golgi intermediate compartment. J Biol Chem. 1994;269:4035–4041. [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Slomiany A, Grzelinska E, Kasinathan C, Yamaki K, Palecz D, Slomiany BL. Function of intracellular phospholipase A2 in vectorial transport of apoproteins from ER to Golgi. Int J Biochem. 1992;24:1397–1406. doi: 10.1016/0020-711x(92)90065-9. [DOI] [PubMed] [Google Scholar]
- Sodeik B, Doms RW, Ericsson M, Hiller G, Machamer CE, van’t Hof W, van Meer G, Moss B, Griffiths G. Assembly of vaccinia virus: the role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J Cell Biol. 1993;121:521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern DF, Burgess L, Sefton BM. Structural analysis of virion proteins of the avian coronavirus infectious bronchitis virus. J Virol. 1982;42:208–219. doi: 10.1128/jvi.42.1.208-219.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern W, Dales S. Biogenesis of vaccinia, concerning the origin of the envelope phospholipids. Virology. 1974;62:293–306. doi: 10.1016/0042-6822(74)90393-6. [DOI] [PubMed] [Google Scholar]
- Suprynowicz FA, Gerace L. A fractionated cell-free system for analysis of prophase nuclear disassembly. J Cell Biol. 1986;103:2073–2081. doi: 10.1083/jcb.103.6.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk WJ, Hilkmann H. Rapid attenuation of receptor-induced diacylglycerol and phosphatidic acid by phospholipase D-mediated transphosphatidylation, formation of bisphosphatidic acid. EMBO J. 1993;12:2655–2662. doi: 10.1002/j.1460-2075.1993.tb05926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meer G, Simons K. Viruses budding from either the apical or basolateral plasma membrane domain of MDCK cells have unique phospholipid compositions. EMBO J. 1982;1:847–852. doi: 10.1002/j.1460-2075.1982.tb01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco A, Hendricks L, Moreman KW, Tulsiani DR, Touster O, Farquhar MG. Cell type-dependent variations in the subcellular distribution of alpha-mannosidase I and II. J Cell Biol. 1993;122:39–51. doi: 10.1083/jcb.122.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]