Abstract
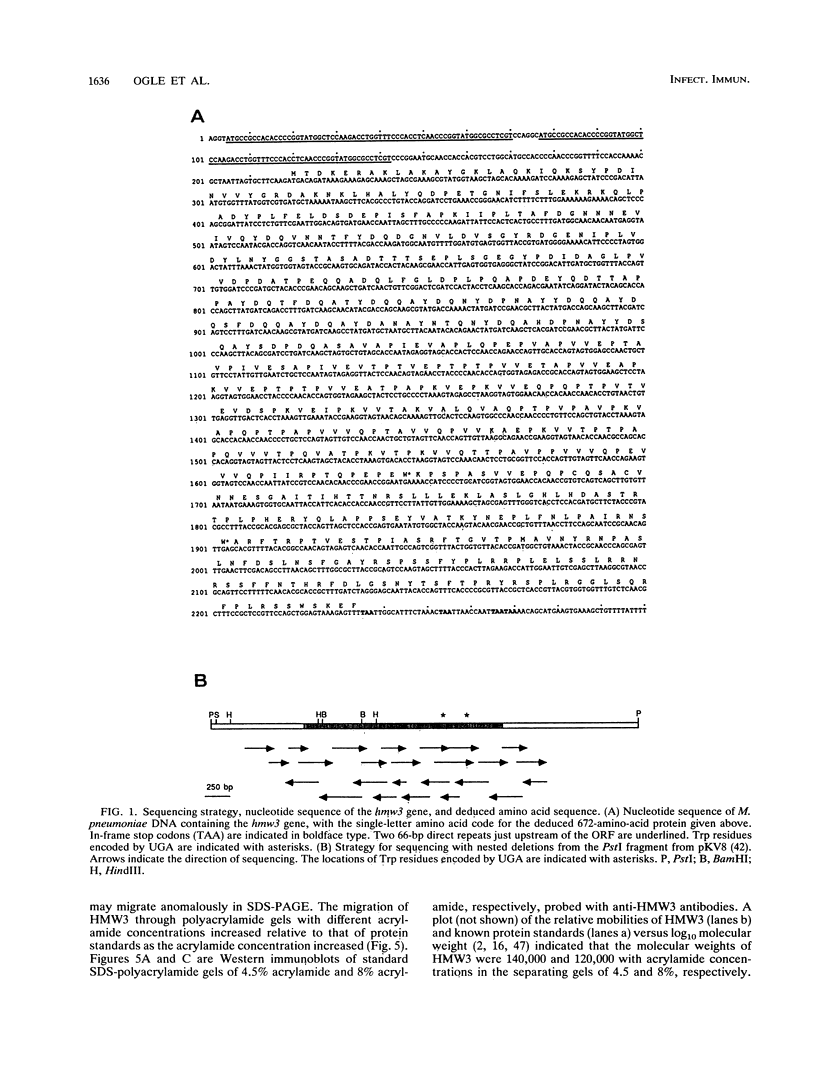
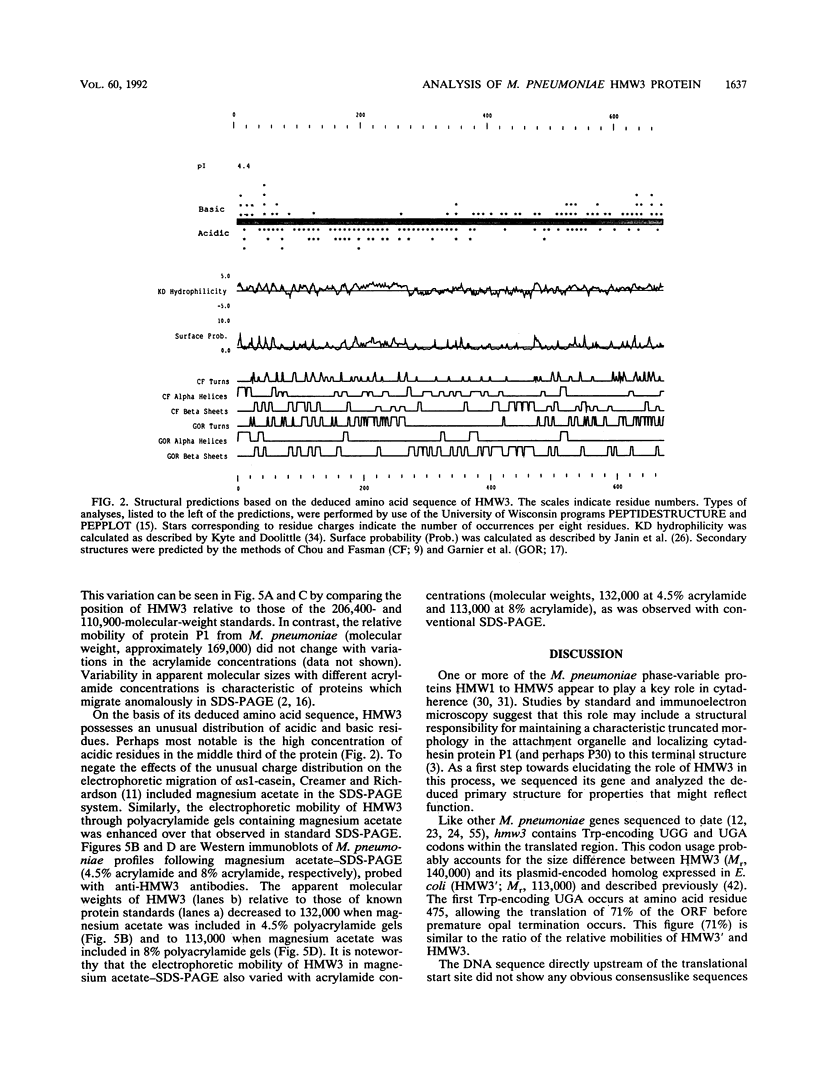
The Mycoplasma pneumoniae hmw3 gene was sequenced and its gene product was characterized with the goal of elucidating the functional role of HMW3 as an accessory component in cytadherence. A total of 2,016 bp of the hmw3 locus was sequenced, revealing an open reading frame large enough to encode a 672-amino-acid protein with a molecular weight of 73,725. No consensuslike ribosome binding or promoter sequences were identified. However, hmw3 was flanked by upstream and downstream open reading frames. Gene identity was confirmed by comparing the deduced amino acid sequence with the amino acid sequence obtained directly from N-terminal amino acid sequencing of HMW3. Analysis of the deduced amino acid sequence indicated an acidic pI (4.4), an unusual distribution of charged residues, a high degree of hydrophilicity, and a high proline content for HMW3. M. pneumoniae protein profiles obtained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) following culturing in the presence of [3H]Pro were consistent with the high Pro content predicted for HMW3 and indicated the same for cytadherence accessory protein HMW1. A discrepancy existed between the predicted size of HMW3 (Mr, 73,725) and the size of HMW3 obtained by SDS-PAGE (Mr, 140,000). Variable electrophoretic mobility in SDS-PAGE was observed at different acrylamide concentrations for HMW3, indicating that anomalous migration might account for the size discrepancy. The relative mobility of HMW3 was enhanced only slightly in the presence of magnesium acetate, suggesting that the unusual charge distribution might only be partially responsible for the anomalous migration. Secondary structure predictions were dominated by beta-sheets and nonrepetitive turns or coils, suggesting a probable extended, rigid conformation for HMW3. Finally, an unusual, highly acidic domain (residues 180 to 280) which might have particular functional significance relative to the role of HMW3 as a cytadherence accessory protein was identified.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. C. An efficient vector-primer cDNA cloning system. Methods Enzymol. 1987;154:41–64. doi: 10.1016/0076-6879(87)54069-1. [DOI] [PubMed] [Google Scholar]
- Banker G. A., Cotman C. W. Measurement of free electrophoretic mobility and retardation coefficient of protein-sodium dodecyl sulfate complexes by gel electrophoresis. A method to validate molecular weight estimates. J Biol Chem. 1972 Sep 25;247(18):5856–5861. [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Morrison-Plummer J., Drouillard D., Puleo-Scheppke B., Tryon V. V., Holt S. C. Identification of a 32-kilodalton protein of Mycoplasma pneumoniae associated with hemadsorption. Isr J Med Sci. 1987 May;23(5):474–479. [PubMed] [Google Scholar]
- Biberfeld G., Biberfeld P. Ultrastructural features of Mycoplasma pneumoniae. J Bacteriol. 1970 Jun;102(3):855–861. doi: 10.1128/jb.102.3.855-861.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A. Relationships Between Mycoplasma pneumoniae and Human Respiratory Epithelium. Infect Immun. 1971 May;3(5):694–701. doi: 10.1128/iai.3.5.694-701.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer L. K., Richardson T. Anomalous behavior of bovine alpha s1- and beta-caseins on gel electrophoresis in sodium dodecyl sulfate buffers. Arch Biochem Biophys. 1984 Nov 1;234(2):476–486. doi: 10.1016/0003-9861(84)90295-9. [DOI] [PubMed] [Google Scholar]
- Dallo S. F., Chavoya A., Baseman J. B. Characterization of the gene for a 30-kilodalton adhesion-related protein of Mycoplasma pneumoniae. Infect Immun. 1990 Dec;58(12):4163–4165. doi: 10.1128/iai.58.12.4163-4165.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A., Goldman A. Identifying nonpolar transbilayer helices in amino acid sequences of membrane proteins. Annu Rev Biophys Biophys Chem. 1986;15:321–353. doi: 10.1146/annurev.bb.15.060186.001541. [DOI] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Wilson R. M., Baseman J. B. Two-dimensional gel electrophoretic comparison of proteins from virulent and avirulent strains of Mycoplasma pneumoniae. Infect Immun. 1979 May;24(2):468–475. doi: 10.1128/iai.24.2.468-475.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Inamine J. M., Denny T. P., Loechel S., Schaper U., Huang C. H., Bott K. F., Hu P. C. Nucleotide sequence of the P1 attachment-protein gene of Mycoplasma pneumoniae. Gene. 1988 Apr 29;64(2):217–229. doi: 10.1016/0378-1119(88)90337-x. [DOI] [PubMed] [Google Scholar]
- Inamine J. M., Ho K. C., Loechel S., Hu P. C. Evidence that UGA is read as a tryptophan codon rather than as a stop codon by Mycoplasma pneumoniae, Mycoplasma genitalium, and Mycoplasma gallisepticum. J Bacteriol. 1990 Jan;172(1):504–506. doi: 10.1128/jb.172.1.504-506.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Janin J., Wodak S. Conformation of amino acid side-chains in proteins. J Mol Biol. 1978 Nov 5;125(3):357–386. doi: 10.1016/0022-2836(78)90408-4. [DOI] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Inhibition of mycoplasma pneumoniae hemadsorption and adherence to respiratory epithelium by antibodies to a membrane protein. Infect Immun. 1983 Mar;39(3):1180–1186. doi: 10.1128/iai.39.3.1180-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Baseman J. B. Mycoplasma pneumoniae proteins that selectively bind to host cells. Infect Immun. 1982 Jul;37(1):382–386. doi: 10.1128/iai.37.1.382-386.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Lee K. K. Juxtaposition of the genes encoding Mycoplasma pneumoniae cytadherence-accessory proteins HMW1 and HMW3. Gene. 1991 Oct 30;107(1):83–89. doi: 10.1016/0378-1119(91)90300-z. [DOI] [PubMed] [Google Scholar]
- Krause D. C., Leith D. K., Baseman J. B. Reacquisition of specific proteins confers virulence in Mycoplasma pneumoniae. Infect Immun. 1983 Feb;39(2):830–836. doi: 10.1128/iai.39.2.830-836.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Leith D. K., Wilson R. M., Baseman J. B. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect Immun. 1982 Mar;35(3):809–817. doi: 10.1128/iai.35.3.809-817.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K., Isemura T., Takagi T. Interaction between the alpha 1 chain of rat tail collagen and sodium dodecyl sulfate with reference to its behaviour in SDS-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1982 May 3;703(2):180–186. doi: 10.1016/0167-4838(82)90046-2. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr, Denny F. W. Characteristics of virulent, attenuated, and avirulent Mycoplasma pneumoniae strains. J Bacteriol. 1969 Nov;100(2):1037–1043. doi: 10.1128/jb.100.2.1037-1043.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur M. W., Thornton J. M. Influence of proline residues on protein conformation. J Mol Biol. 1991 Mar 20;218(2):397–412. doi: 10.1016/0022-2836(91)90721-h. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Limited N-terminal sequence analysis. Methods Enzymol. 1990;182:602–613. doi: 10.1016/0076-6879(90)82047-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Ogle K. F., Lee K. K., Krause D. C. Cloning and analysis of the gene encoding the cytadherence phase-variable protein HMW3 from Mycoplasma pneumoniae. Gene. 1991 Jan 2;97(1):69–75. doi: 10.1016/0378-1119(91)90011-y. [DOI] [PubMed] [Google Scholar]
- Powell D. A., Hu P. C., Wilson M., Collier A. M., Baseman J. B. Attachment of Mycoplasma pneumoniae to respiratory epithelium. Infect Immun. 1976 Mar;13(3):959–966. doi: 10.1128/iai.13.3.959-966.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radestock U., Bredt W. Motility of Mycoplasma pneumoniae. J Bacteriol. 1977 Mar;129(3):1495–1501. doi: 10.1128/jb.129.3.1495-1501.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard D., Chrambach A. Unified theory for gel electrophoresis and gel filtration. Proc Natl Acad Sci U S A. 1970 Apr;65(4):970–977. doi: 10.1073/pnas.65.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeslavsky O., Prescott B., Chanock R. M. Adsorption of Mycoplasma pneumoniae to neuraminic acid receptors of various cells and possible role in virulence. J Bacteriol. 1968 Sep;96(3):695–705. doi: 10.1128/jb.96.3.695-705.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M. K., Krause D. C. Disulfide-linked protein associated with Mycoplasma pneumoniae cytadherence phase variation. Infect Immun. 1990 Oct;58(10):3430–3433. doi: 10.1128/iai.58.10.3430-3433.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M. K., Krause D. C. Localization of the Mycoplasma pneumoniae cytadherence-accessory proteins HMW1 and HMW4 in the cytoskeletonlike Triton shell. J Bacteriol. 1991 Feb;173(3):1041–1050. doi: 10.1128/jb.173.3.1041-1050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Graff J. M., Albert K. A., Greengard P., Blackshear P. J. Molecular cloning, characterization, and expression of a cDNA encoding the "80- to 87-kDa" myristoylated alanine-rich C kinase substrate: a major cellular substrate for protein kinase C. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., Tryon V. V., Baseman J. B. Cloning and sequence analysis of cytadhesin P1 gene from Mycoplasma pneumoniae. Infect Immun. 1987 Dec;55(12):3023–3029. doi: 10.1128/iai.55.12.3023-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H., Modrow S., Motz M., Jameson B. A., Hermann G., Förtsch B. An integrated family of amino acid sequence analysis programs. Comput Appl Biosci. 1988 Mar;4(1):187–191. doi: 10.1093/bioinformatics/4.1.187. [DOI] [PubMed] [Google Scholar]
- Yamao F., Muto A., Kawauchi Y., Iwami M., Iwagami S., Azumi Y., Osawa S. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]