Abstract
OBJECTIVE—Islet amyloid, formed by aggregation of the β-cell peptide islet amyloid polypeptide (IAPP; amylin), is a pathological characteristic of pancreatic islets in type 2 diabetes. Toxic IAPP aggregates likely contribute to the progressive loss of β-cells in this disease. We used cultured human islets as an ex vivo model of amyloid formation to investigate whether suppression of proIAPP expression would inhibit islet amyloid formation and enhance β-cell survival and function.
RESEARCH DESIGN AND METHODS—Islets from cadaveric organ donors were transduced with a recombinant adenovirus expressing a short interfering RNA (siRNA) designed to suppress human proIAPP (Ad-hProIAPP-siRNA), cultured for 10 days, and then assessed for the presence of islet amyloid, β-cell apoptosis, and β-cell function.
RESULTS—Thioflavine S–positive amyloid deposits were clearly present after 10 days of culture. Transduction with Ad-hProIAPP-siRNA reduced proIAPP expression by 75% compared with nontransduced islets as assessed by Western blot analysis of islet lysates 4 days after transduction. siRNA-mediated inhibition of IAPP expression decreased islet amyloid area by 63% compared with nontransduced cultured islets. Cell death assessed by transferase-mediated dUTP nick-end labeling staining was decreased by 50% in transduced cultured human islets, associated with a significant increase in islet insulin content (control, 100 ± 4 vs. +Ad-siRNA, 153 ± 22%, P < 0.01) and glucose-stimulated insulin secretion (control, 222 ± 33 vs. +Ad-siRNA, 285 ± 21 percent basal, P < 0.05).
CONCLUSIONS—These findings demonstrate that inhibition of IAPP synthesis prevents amyloid formation and β-cell death in cultured human islets. Inhibitors of IAPP synthesis may have therapeutic value in type 2 diabetes.
Islet amyloid deposits are a pathological lesion of the pancreas in type 2 diabetes (1–3) formed by aggregation of islet amyloid polypeptide (IAPP; amylin) (4,5), a 37–amino acid hormone that is colocalized with insulin in β-cell granules and secreted along with insulin in response to β-cell secretagogues (6–8). Aggregates of IAPP, including small oligomeric species, are toxic to β-cells (9–14) and likely play an important role in the progressive loss of β-cells in this disease. Despite considerable study during the past decade, it is still not well understood why soluble IAPP molecules form toxic IAPP aggregates in type 2 diabetes. The presence of an amyloidogenic amino acid sequence in the human IAPP molecule (Gly-Ala-Iso-Leu-Ser [GAILS]) appears to be important but not sufficient for amyloid formation (2,3,15,16). Because production and secretion of IAPP closely mimic that of insulin, IAPP levels are elevated in conditions associated with insulin resistance and hyperinsulinemia, such as the early stages of type 2 diabetes (17). Elevated IAPP production and secretion because of increased demand for insulin along with defective trafficking and/or processing of proIAPP associated with β-cell dysfunction have been implicated as two possible factors contributing to aggregation of (pro)IAPP (i.e., proIAPP and its intermediate forms) in type 2 diabetes (2,3,16,18–23).
Studies performed using transgenic rodent models have demonstrated a strong association between β-cell expression of human IAPP and development of hyperglycemia associated with loss of β-cells (24–29). β-Cell loss in these models was attributed to the formation of toxic IAPP aggregates as either small oligomeric species or amyloid fibrils. Studies on human and nonhuman primates have also demonstrated development of islet amyloid associated with β-cell loss in type 2 diabetes (30,31). It is not clear from these studies, however, whether IAPP aggregation and amyloid formation is the cause or a consequence of β-cell dysfunction and death. In the present study, we tested the hypothesis that suppression of human IAPP expression would inhibit amyloid formation and enhance survival and/or function of human islets. We and others have found that islet amyloid forms rapidly in islets from transgenic mice with β-cell expression of human IAPP, and its formation is potentiated by elevated glucose concentrations (23,32–35). We used cultured human islets as an ex vivo model of amyloid formation to investigate whether short interfering RNA (siRNA)-mediated suppression of endogenously expressed human proIAPP will enhance β-cell survival in human islets.
RESEARCH DESIGN AND METHODS
Thioflavine S, Hoechst-33342, dithizone, BSA, aprotinin, pepstatin A, phenylmethylsulphonyl fluoride, and leupeptin were obtained from Sigma-Aldrich (Oakville, ON, Canada). Dulbecco's modified Eagle's medium (DMEM), RPMI-1640, fetal bovine serum (FBS), Lipofectamine, OptiMEM buffer, gentamicin, penicillin, and streptomycin were from Invitrogen Canada (Burlington, ON, Canada). All electrophoresis chemicals were from Bio-Rad Laboratories (Mississauga, ON, Canada).
Human islet cultures.
Human islets isolated from male and female cadaveric organ donors aged between 46 and 68 years were received from Human Islet Isolation Centers at McGill University (Montreal, Canada) and the University of Illinois (Chicago). Islets were maintained in CMRL culture medium containing BSA, 11.1 mmol/l glucose, 50 units/ml penicillin, 50 μg/ml streptomycin, and 50 μg/ml gentamicin at 37°C in a humid atmosphere of 95% air/5% CO2.
Cell lines.
COS-1 cells, a transformed monkey kidney cell line, and HEK293T cells, a human embryonic kidney cell line, were obtained from the American Type Culture Collection (Manassas, VA). COS-1 and HEK293T cells were cultured in DMEM containing 11.1 and 22 mmol/l glucose supplemented with 10% FBS, 50 units/ml penicillin, and 50 μg/ml streptomycin at 37°C in a humid atmosphere of 95% air 5% CO2. INS-1 cells, used as a control in Western blot analysis of IAPP expression in human islets, were maintained in culture as previously described (23).
Antisera and recombinant adenoviruses for expression of human IAPP.
IgG-purified antibody (RGG-7323) to IAPP was obtained from Peninsula Laboratories (Belmont, CA). Human IAPP antiserum was provided by Dr. Anne Clark (University of Oxford, Oxford, U.K.). Adenovirus expressing a human proIAPP–enhanced green fluorescent protein (EGFP) fusion chimera with green fluorescent protein (GFP), Ad-hProIAPP-EGFP, was provided by Dr. Christopher Rhodes (University of Chicago, Chicago, IL).
Generation of human proIAPP siRNA recombinant adenovirus.
The siRNA target spans from nucleotides 372 to 393 of the human IAPP mRNA (accession number NM-000415). The sense (5′-AG AGA GAG CCA CTG AAT TA-3′) and antisense (5′-TAA TTC AGT GGC TCT CTC T-3′) siRNA oligonucleotides were designed and synthesized as described previously (36) with a few modifications. Sense and antisense oligonucleotides were annealed in salt/Tris/EDTA buffer and ligated into BglII/HindIII-linearized pSuper (OligoEngine, Seattle, WA). The siRNA expression cassette was excised from the pSuper-based clone with EcoRI-HindIII and ligated into EcoRI-HindIII–linearized adenoviral shuttle vector, EH006. To create RNA interference adenovirus by homologous recombination, EH006-derived shuttle vectors were cotransfected with the adenoviral plasmid pjM17 into HEK293T cells. The Ad-hProIAPP-siRNA generated was further amplified in HEK293T cells, and cell lysates containing viral particles were purified using an adenoviral purification kit from Vivascience (Hanover, Germany). This adenovirus is predicted to express a short RNA hairpin that is further processed to the bioactive siRNA in cells. An adenovirus expressing a random siRNA (Ad-Cont-siRNA) (37) was used as a control.
Transfection and adenoviral transduction.
COS-1 cells at ∼70% confluency were transduced with AdhProIAPP-EGFP (multiplicity of infection [MOI] 5) in DMEM (22 mmol/l glucose) for 2 h followed by 4 h incubation with fresh medium to allow recovery. Cells were then transfected with 4 μg pSuper-siRNA or pSuper-siRNA-GFP (as transfection control) using Lipofectamine and OptiMEM buffer (Invitrogen) for 4 h. Cells were incubated with fresh medium for 36 h after transfection. For experiments on primary islet β-cells, islets isolated from cadaveric human donors (hand-picked, purity >90%) were cultured overnight to allow recovery from the isolation process and shipping. Purity of islets was assessed by dithizone staining before transduction. Islets were transduced with Ad-hProIAPP-siRNA (or Ad-Cont-siRNA as indicated in figure legends) overnight, washed, and incubated with CMRL medium containing 11.1 mmol/l glucose for 96 h after transduction. Culture medium was changed every 2 days.
Glucose-stimulated insulin secretion.
Human islets (50/well, three wells per condition) were preincubated (1 h) in Krebs-Ringer bicarbonate buffer (KRBB) containing 10 mmol/l HEPES (pH 7.4), 0.25% BSA, and 1.67 mmol/l glucose (KRBB-G1.67) in 48-well plates at 37°C followed by a 1-h incubation in KRBB containing either 1.67 mmol/l (basal insulin release) or 16.7 mmol/l glucose to stimulate release of insulin. Incubation media were collected gently and centrifuged (15,000g, 10 min, 4°C), and the supernatants were saved for insulin immunoassays. Human islets in each well were centrifuged, and the pellets were lysed in 100 μl lysis buffer containing 1 mol/l HCl and 0.1% BSA. Media and islet lysates were frozen at −20°C until assayed. Insulin levels were measured using a human-specific insulin ELISA kit (EZHI-14K) from Linco Research (St. Charles, MO).
Electrophoresis and immunoblotting.
Human islets were lysed in 30 μl Nonidet P-40 lysis buffer as described previously (38). Aliquots of islet protein (10 or 15 μg) were electrophoresed on a polyacrylamide gel (using Tris-Tricine buffer for IAPP) followed by immunoblot analysis for detection of IAPP using antisera from Peninsula (7323; 1:100) or A. Clark (Oxford; 1:200) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:1,000) for 1 h at room temperature. Membranes were then washed and incubated with horseradish peroxidase–conjugated anti-rabbit (or anti-mouse for GAPDH) IgG (Amersham, Baie d'Urfe, QC, Canada) diluted 1:5,000. Immunodetection was performed using an enhanced chemiluminescence detection kit (Amersham). Protein bands were analyzed by densitometry using Quantity One quantitation analysis software.
Transferase-mediated dUTP nick-end labeling, thioflavine S, and immunostaining.
To detect apoptotic cells, COS-1 cells were fixed in 4% paraformaldehyde (PFA) in 0.1 mmol/l PBS (pH 7.5) for 20 min, permeabilized with 0.5% Triton X-100 in PBS, and incubated with transferase-mediated dUTP nick-end labeling (TUNEL) reaction mixture (Roche Diagnostics, Laval, QC, Canada) for 1 h at 37°C and then stained with Hoechst-33342 for 10 min. Human islets were fixed in 4% PFA, as described above, for 40 min. For double insulin and thioflavine S staining, paraffin-embedded sections (5 μm) were blocked in 0.05 mol/l PBS plus 0.25% Triton X-100 containing 2% normal goat serum (Vector Laboratories, Burlingame, CA), rinsed, and incubated with guinea pig anti-insulin antibody (Dako, Carpinteria, CA) at a 1:100 dilution in PBS:1% BSA at 4°C overnight, followed by incubation with Texas Red–conjugated goat anti–guinea pig antibody (Jackson ImmunoResearch, West Grove, PA) for 1 h (1:100) at room temperature. The slides were rinsed and incubated in 0.5% thioflavine S solution for 2 min. For TUNEL assay, islet sections were incubated with TUNEL reaction mixture for 30 min at 37°C followed by DAPI staining (Vector) for detection of the nuclei. For double insulin and glucagon immunostaining, after incubation with anti-insulin antibody, islet sections were incubated (1 h) with goat anti–guinea pig antibody conjugated to the green fluorophore Alexa Fluor 488 (Molecular Probes, Eugene, OR). Sections were then similarly incubated with rabbit anti-glucagon antibody (Dako) and donkey anti-rabbit antibody conjugated with Texas Red (1:100) (Jackson) for 1 h. For double insulin and TUNEL staining, islet sections were incubated with TUNEL reaction mixture (Roche Diagnostics) for 1 h at 37°C. Thioflavine S–positive area in paraffin sections of cultured islets was determined as a percentage of total islet area using Image-Pro 6.2 (Media Cybernetics, Bethesda, MD). At least two different islet sections were analyzed from each culture well, and all islets in each section (∼100 islets) were included in the analysis.
Statistical analysis.
Data are expressed as means ± SE. Statistical analyses were performed using one-way ANOVA followed by Newman-Keuls or Student's t test, as appropriate. P < 0.05 was taken as significant. Data are representative of a minimum of three independent experiments performed in triplicate.
RESULTS
Suppression of adenoviral-mediated expression of human proIAPP by siRNA in COS-1 cells prevents (pro)IAPP-induced cell death.
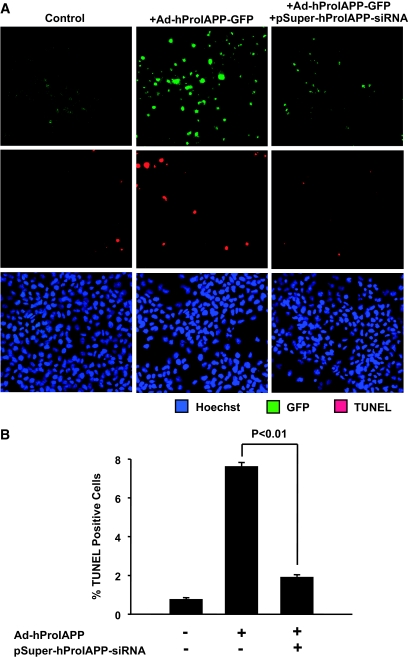
To test the efficiency of the siRNA constructs to suppress proIAPP expression, we used COS-1 cells transduced with an adenovirus encoding a human proIAPP-EGFP fusion protein (Ad-hProIAPP-EGFP) as an in vitro model for human proIAPP expression. Transduced COS-1 cells were then transfected with pSuper-hProIAPP-siRNA. Transduction efficiency with Ad-hProIAPP-EGFP, as assessed by detection of COS-1 cells expressing GFP 36 h after transduction, was ∼70%. Transfection efficiency with the siRNA construct was ∼60%, as estimated by detection of fluorescence in COS-1 cells transfected with pSuper-siRNA-GFP, a plasmid with a similar construct that expresses GFP. As expected (23,39), expression of fibrillogenic human proIAPP in COS-1 cells resulted in an increase in the number of TUNEL-positive (apoptotic) cells (Fig. 1), whereas adenoviral expression of nonamyloidogenic rat proIAPP was not toxic (data not shown). Transfection with pSuper-hProIAPP-siRNA markedly decreased the number of GFP-expressing cells, indicating effective suppression by the siRNA of the expression of the proIAPP-EGFP fusion protein in cells transduced with Ad-hProIAPP-EGFP. Interestingly, inhibition of adenoviral-mediated human proIAPP expression by transfection with pSuper-hProIAPP-siRNA was associated with a marked decrease in the number of apoptotic COS cells (Fig. 1).
FIG. 1.
Inhibition of adenoviral-mediated expression of human proIAPP in COS-1 cells by siRNA enhances cell survival. A: COS-1 cells were transduced with Ad-hProIAPP-EGFP (MOI 5; 2 h), incubated with fresh media (4 h) to allow recovery, and transfected with pSuper-hProIAPP-siRNA (4 μg; 4 h). Apoptotic cells were detected 96 h after transfection by double TUNEL (red) and Hoechst-33342 (blue) staining performed on nontransduced control cells (left), cells transduced with Ad-hProIAPP-EGFP alone (middle), or cells transduced with Ad-hProIAPP-EGFP and transfected with pSuper-hProIAPP-siRNA (right). Transduction efficiency in cells transduced with Ad-hIAPP-EGFP, estimated by the number of COS-1 cells expressing GFP 36 h after transduction, was >70%. Transfection efficiency estimated by the number of COS-1 cells expressing pSuper-siRNA-EGFP was ∼60%. Note the higher number of TUNEL-positive cells in COS-1 cells expressing human proIAPP alone (middle) compared with those expressing human proIAPP in the presence of siRNA (right). B: Quantification of TUNEL-positive COS-1 cells transduced with Ad-hProIAPP-EGFP with or without transfection with pSuper-hProIAPP-siRNA. Data are presented as means ± SE of three independent experiments performed in triplicate (P < 0.01, one-way ANOVA). (Please see http://dx.doi.org/10.2337/db08-0485 for a high-quality digital representation of this figure.)
ProIAPP gene silencing in human islets by an siRNA-expressing adenovirus.
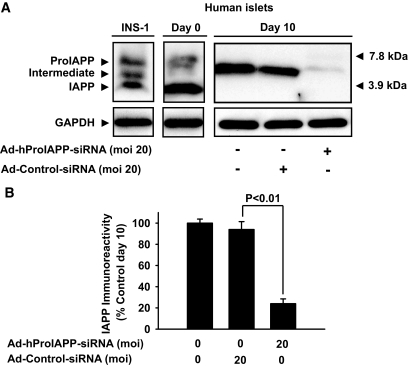
To determine whether suppression of IAPP synthesis will inhibit amyloid formation and enhance survival of β-cells, we generated an adenovirus to deliver a specific human proIAPP siRNA (Ad-hProIAPP-siRNA) into β-cells in isolated human islets. The siRNA construct that was found to provide the most effective suppression of proIAPP in COS-1 cells (Fig. 1) was chosen for generation of Ad-hProIAPP-siRNA as described in research design and methods. Isolated human islets were transduced with Ad-hProIAPP-siRNA or a control adenovirus expressing a random siRNA (Ad-Cont-siRNA) (37) overnight (16 h) and cultured in CMRL with 11.1 mmol/l glucose for up to 10 days. IAPP-immunoreactive forms were detected by Western blot 10 days after transduction. As expected, mature IAPP (∼4 kDa) was the major IAPP-immunoreactive form detected in preculture isolated human islets. Interestingly, after a 10-day culture, the major detectable (pro)IAPP form in human islets was a partially processed ∼6 kDa intermediate form (Fig. 2A), suggesting that prolonged culture in elevated glucose concentrations impairs proIAPP processing, in accordance with a previous report (20). Transduction with Ad-ProIAPP-siRNA reduced expression of human (pro)IAPP by ∼75% compared with nontransduced cultured islets or islets transduced with Ad-Cont-siRNA (Fig. 2A and B). The degree of reduction of (pro)IAPP expression induced by Ad-hProIAPP-siRNA was dependent on the viral MOI (data not shown); an MOI of 20 was found empirically to be optimal and used in subsequent experiments.
FIG. 2.
Suppression of proIAPP expression in cultured human islets by transduction with Ad-hProIAPP-siRNA. Human islets were transduced with Ad-hProIAPP-siRNA (MOI 20) or Ad-Cont-siRNA (20) and cultured in CMRL (11.1 mmol/l glucose) for 10 days. A: Western blot analysis was performed on the human islet extracts (10 μg) followed by immunoblot using human IAPP antibody. IAPP (∼4 kDa) was the predominant IAPP-immunoreactive form in preculture human islets, whereas a partially processed form of proIAPP (∼6 kDa) was the major IAPP-immunoreactive form in human islets after 10-day culture. IAPP-immunoreactive forms in INS-1 cells (30 μg) are shown as control for different (pro)IAPP molecular species (left lane). B: Densitometric analysis of immunoblots. Transduction with Ad-hProIAPP-siRNA reduced (pro)IAPP immunoreactivity ∼75%. Results are presented as the percentage of IAPP-immunoreactive forms with (pro)IAPP immunoreactivity in nontransduced cultured islets taken as 100%. Data are expressed as means ± SE of three independent experiments. *Significantly different from transduced islets (P < 0.01, ANOVA).
siRNA-mediated suppression of proIAPP expression inhibits amyloid formation and apoptosis and preserves β-cells in cultured human islets.
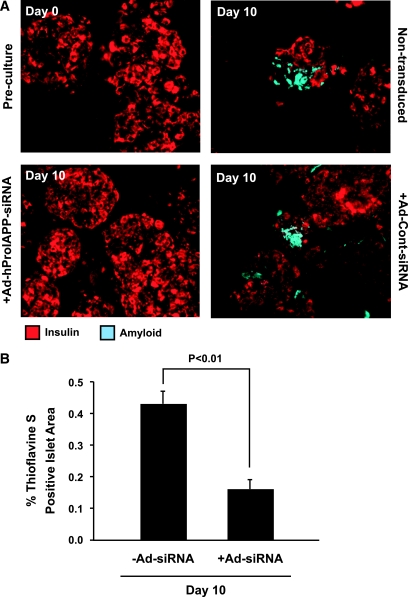
To investigate the effects of suppression of proIAPP expression on survival of cultured human islet cells, histological analysis was performed on paraffin-embedded sections of human islets transduced with Ad-hProIAPP-siRNA or Ad-Cont-siRNA or left nontransduced and cultured for 10 days. Thioflavine S staining revealed the presence of clearly detectable amyloid deposits after 10-day culture of untreated human islets (Fig. 3A, top right), similar to those observed in type 2 diabetic human pancreas, suggesting that amyloid formation occurs very rapidly in cultured human islets, as in transgenic human IAPP-expressing mouse islets (23,32–35). Islets transduced with Ad-hProIAPP-siRNA to inhibit proIAPP expression had a marked reduction in visible amyloid (Fig. 3A, bottom left), whereas those transduced with the control adenovirus (Ad-Cont-siRNA) were indistinguishable from nontransduced islets (Fig. 3A, bottom right). Quantification of amyloid area in islet cultures confirmed that adenoviral siRNA-mediated inhibition of IAPP expression reduced amyloid formation by ∼60% (P < 0.05; Fig. 3B).
FIG. 3.
Suppression of proIAPP expression in human islets inhibits amyloid formation. A: Thioflavine S (light blue) and insulin (red) double staining of paraffin-embedded human islet sections before culture and after 10-day culture with or without transduction with Ad-hProIAPP-siRNA. Amyloid deposits were readily detectable in 10-day-cultured human islets, associated with areas of decreased insulin staining. Amyloid formation was markedly reduced by siRNA-mediated suppression of proIAPP in transduced islets. B: Quantification of amyloid (thioflavine S)-positive islet areas in transduced and nontransduced cultured human islets (day 10). siRNA-mediated inhibition of human proIAPP expression resulted in a 63% decrease in islet amyloid area in transduced islets compared with nontransduced control islets (P < 0.01, Student's t test). (Please see http://dx.doi.org/10.2337/db08-0485 for a high-quality digital representation of this figure.)
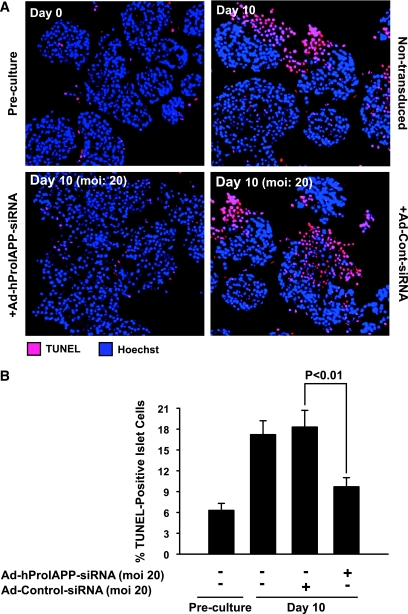
Apoptotic islet cells were detected and quantified by double TUNEL and DAPI nuclear staining. As expected, preculture human islets had low levels of TUNEL-positive cells (Fig. 4A, top left), but the number of apoptotic cells was markedly increased after a 10-day culture (Fig. 4A, top right). siRNA-mediated suppression of proIAPP expression decreased the proportion of apoptotic cells in human islets cultured for 10 days by ∼50% (Fig. 4A, bottom left, and B), whereas transduction with Ad-Cont-siRNA did not have any detectable effect on islet cell death (Fig. 4A, bottom right, and B).
FIG. 4.
Prevention of proIAPP synthesis in cultured human islets by siRNA-mediated suppression of proIAPP reduces islet cell apoptosis. A: Paraffin-embedded sections of human islets before culture and after 10-day culture with or without transduction with Ad-hProIAPP-siRNA (or Ad-Cont-siRNA) were stained for TUNEL positivity (red) and Hoechst-33342 (blue). After 10-day culture, extensive TUNEL-positive staining was observed in nontransduced cultured human islets (top right). siRNA-mediated suppression of proIAPP expression markedly reduced the number of TUNEL-positive cells in 10-day-cultured human islets (bottom left). Transduction with Ad-Cont-siRNA did not have any significant effect on the number of TUNEL-positive cells (bottom right). B: Quantification of the number of TUNEL-positive cells as a percentage of the total number of cells in transduced cultured human islets. Results are expressed as means ± SE of three independent experiments (P < 0.01, one-way ANOVA). (Please see http://dx.doi.org/10.2337/db08-0485 for a high-quality digital representation of this figure.)
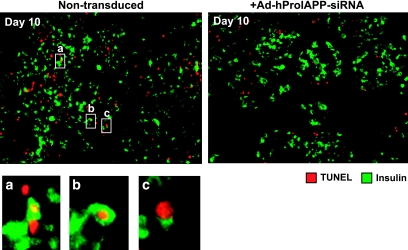
Because DAPI staining does not distinguish between β- and non–β-islet cells, we next evaluated whether β-cell apoptosis was inhibited by adenoviral siRNA-mediated suppression of IAPP expression. To this end, we double stained islet sections for insulin and TUNEL. Many insulin/TUNEL double-positive islet cells were apparent in sections of nontransduced cultured human islets (Fig. 5A, left). Although insulin/TUNEL double-positive islet cells were also observed in Ad-hProIAPP-siRNA transduced islets, these appeared lower in frequency (Fig. 5A, right). A higher magnification view of these cells revealed that they were TUNEL positive and insulin positive (Fig. 5, bottom). Collectively, these data suggest that inhibition of IAPP expression in cultured human islets decreases β-cell apoptosis.
FIG. 5.
siRNA-mediated suppression of human proIAPP expression decreases β-cell apoptosis. Double immunostaining for insulin (green) and TUNEL (red) of paraffin-embedded sections (5 μm) of human islets after 10-day culture that were either nontransduced (left) or transduced (right) with Ad-hProIAPP-siRNA (MOI 20). Double insulin/TUNEL-positive cells were observed in nontransduced and transduced human islet cultures but were more frequent in cultures transduced to express human IAPP siRNA. Higher magnification view (insets a, b, and c) shows insulin immunopositivity localized to cells with TUNEL-positive nuclei, demonstrating β-cell apoptosis. TUNEL-positive cells that are insulin negative were also frequently observed. (Please see http://dx.doi.org/10.2337/db08-0485 for a high-quality digital representation of this figure.)
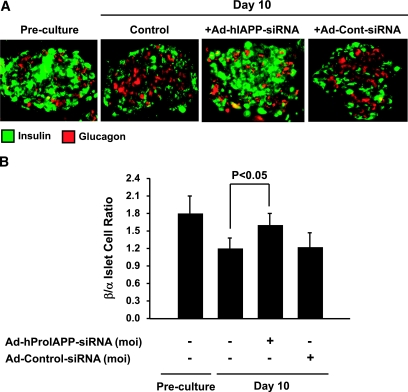
Notably, many TUNEL-positive cells in cultured human islets were found to be insulin negative (Fig. 5). Although many of these cells may be non–β-cells, we suspect that the majority of TUNEL-positive, insulin-negative cells in our islet cultures are β-cells that have lost detectable insulin staining while undergoing cell death. To evaluate this possibility, we next double immunostained, for insulin and glucagon, sections of cultured islets that had been transduced with Ad-hProIAPP-siRNA or Ad-Cont-siRNA or left untreated and quantified the ratio of β- to α-cells as a measure of β-cell–specific loss (Fig. 6). siRNA-mediated inhibition of IAPP expression was associated with a 33% increase in the ratio of β- to α-cells compared with nontransduced islets (Fig. 6). Transduction with an Ad-Cont-siRNA did not have any significant effect on the β-cell–to–α-cell ratio. These data demonstrate that inhibition of IAPP expression preserves β-cells in cultured human islets.
FIG. 6.
siRNA-mediated suppression of human IAPP expression increases β-cell–to–α-cell ratio in cultured human islets. A: Double immunostaining for insulin (green) and glucagon (red) of paraffin-embedded sections (5 μm) of human islets preculture (d 0) and after 10-day culture with or without transduction with Ad-hProIAPP-siRNA (MOI 20) or Ad-Cont-siRNA (as control for adenoviral toxicity and siRNA nonspecific effects). The number of insulin immunopositive cells and intensity of immunostaining appeared to be reduced after 10-day culture but was preserved in Ad-hProIAPP-siRNA–transduced islets. B: Quantification of insulin- and glucagon-positive cells in transduced and nontransduced cultured human islets. Results are presented as β-cell–to–α-cell ratio from at least 30 islets per condition from each human islet preparation. Note the decrease in the ratio of β- to α-cells in cultured human islets and its restoration after transduction with Ad-hProIAPP-siRNA. Transduction with Ad-Cont-siRNA did not have any significant effect on β-cell–to–α-cell ratio. Data are expressed as means ± SE of three independent experiments (P < 0.01, one-way ANOVA). (Please see http://dx.doi.org/10.2337/db08-0485 for a high-quality digital representation of this figure.)
Inhibition of human proIAPP synthesis increases insulin content and enhances β-cell function in cultured human islets.
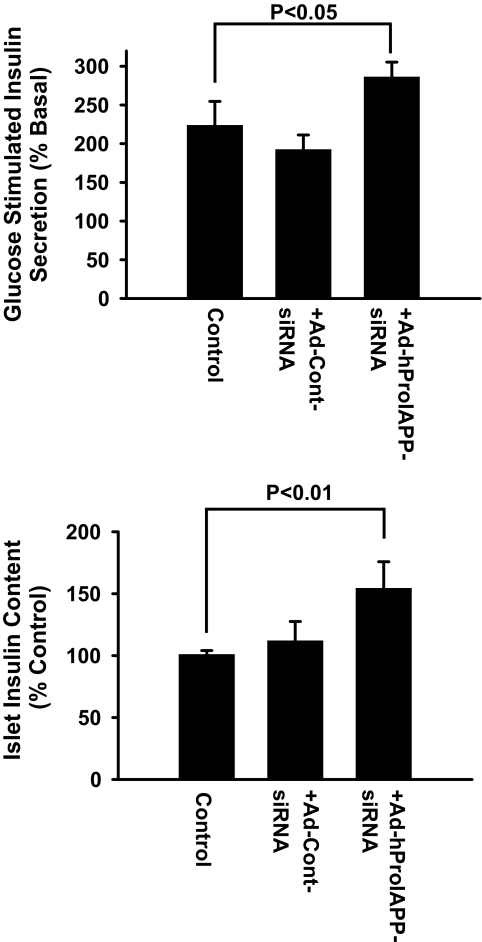
To determine whether siRNA-mediated prevention of amyloid formation and islet cell death enhances β-cell function in cultured human islets, we measured insulin content and glucose-stimulated insulin secretion (GSIS) in human islets after 10-day culture. Adenoviral siRNA-mediated inhibition of proIAPP synthesis was associated with an increase in islet insulin content and higher levels of insulin secretion after stimulation with 16.7 mmol/l glucose for 1 h (Fig. 7). Human islets transduced with Ad-Cont-siRNA had insulin content and GSIS that were similar to untreated controls (Fig. 7).
FIG. 7.
siRNA-mediated inhibition of proIAPP synthesis enhances β-cell function in cultured human islets. GSIS was assessed in nontransduced and Ad-hProIAPP-siRNA (or Ad-Cont-siRNA) transduced human islets (MOI 20) after 10-day culture in CMRL (11.1 mmol/l glucose). Islets were incubated with KRBB (1.67 mmol/l glucose) for 1 h followed by 1-h incubation in the same condition (basal release) or with KRBB containing 16.7 mmol/l glucose (stimulated insulin release). Insulin secretion (A) and islet insulin content (B) were measured by an enzyme-linked immunosorbent assay that measures mature insulin. Transduced human islets had higher insulin content and enhanced glucose response compared with nontransduced cultured islets. Results are expressed as means ± SE of three independent experiments (P < 0.01, one-way ANOVA).
DISCUSSION
Progressive loss of islet β-cell mass and function is a key defect in type 2 diabetes, ultimately leading to β-cell failure. The decrease in β-cell mass in type 2 diabetes results primarily from increased β-cell death via apoptosis (40,41), and formation of toxic IAPP aggregates is thought to be an important contributor to the progressive loss of β-cells in this disease (1,16,42). It remains unclear whether aggregation of IAPP and amyloid formation is a cause of β-cell loss or simply a marker of β-cell death and dysfunction. Here, we show, using a human islet culture model, that suppression of human IAPP expression inhibits islet amyloid formation and enhances survival and function of islet β-cells. These findings provide direct evidence to support the hypothesis that aggregation of IAPP leads to β-cell death in human islets in situ and point to inhibition of IAPP expression as a potential therapeutic target in type 2 diabetes.
Islet amyloid may take years to form in the type 2 diabetic pancreas, but recent reports have described rapid amyloid formation in islets of human IAPP transgenic mice after prolonged culture or after transplantation (23,34,43) and after transplantation of human islets into immune-deficient mice (43). Here, we show that human islets, when cultured in moderately elevated glucose concentrations, rapidly develop amyloid associated with cell death. Taken together, these findings raise the possibility that rapid amyloid formation might be a contributing factor to β-cell demise during culture or after transplant. Targeting IAPP expression might therefore have value to improve viability of human islets during pretransplant culture and after transplantation.
Although cultured human islets provide a useful model for studies on islet amyloid formation and β-cell death, this experimental model may not completely mimic the conditions in the type 2 diabetic human pancreas. One possible explanation underlying the rapid amyloid formation in cultured human islets is that clearance of proIAPP from the islet after its secretion may be impaired in isolated islets, occurring via diffusion rather than perfusion of the islet vasculature as occurs in vivo. Prolonged residence in the islet after secretion from β-cells may then allow more time for IAPP to accumulate and aggregate. A second possibility is that processing of the IAPP precursor, proIAPP, may become impaired in islets during prolonged culture, manifest as accumulation of an NH2-terminally extended proIAPP processing intermediate (see Fig. 2 and ref. 20). We and others have recently demonstrated that impaired NH2-terminal processing of proIAPP leads to fibril formation and cell death in vitro (22,23). It is possible that the culture conditions used, including high glucose (11 mmol/l), induce impaired processing of proIAPP, leading to accumulation of misfolded forms of the precursor in the ER and other intracellular compartments. Alternatively, secretion of incompletely processed proIAPP may lead to its binding to heparan sulfate proteoglycans (21) and its accumulation in the intracellular space. Whether the mechanism underlying rapid amyloid formation in cultured human islets resembles that in type 2 diabetes remains to be determined.
The proportion of TUNEL-positive cells after 10-day culture was ∼20% of the total number of islet cells and reduced to ∼10% by adenoviral expression of human IAPP siRNA. This high proportion of TUNEL-positive cells is similar to a previous report (44) and suggests a high incidence of cell death occurring in human islets during normal culture conditions. In vivo, apoptotic β-cells are rapidly cleared by macrophages, and cells are only transiently TUNEL positive, but in vitro apoptotic cells are not cleared and therefore likely remain TUNEL positive for much longer. Therefore, the number of TUNEL-positive islet cells at day 10 of culture is probably reflective of cumulative cell death over the preceding few days.
Recent studies suggest that IAPP-induced cell toxicity is mediated not by amyloid per se but primarily by smaller aggregates of IAPP, termed protofibrils or oligomers (12–14,22,25,45,46). These studies raise the important consideration that any therapeutic approach targeting IAPP and amyloid formation should not increase the proportion of these smaller toxic species. Another recently proposed mechanism suggests that growth of IAPP-derived fibrils at the cell membrane induces cytotoxicity (47). Because inhibition of IAPP expression is upstream of the earliest events in IAPP aggregation, our siRNA approach is predicted to inhibit the formation of both oligomers and amyloid fibrils. The marked improvements in cell viability that we observed point to the validity of IAPP synthesis as a target for preservation of human islet cells.
Given the potential toxicity of adenoviral infection of islet cells and the potential for nonspecific effects of siRNA expression, it is important in any such study to include appropriate controls. In this study, we also assessed amyloid formation and islet cell toxicity in cells transduced with the same MOI of adenovirus expressing a random siRNA, and we observed no differences in amyloid area, proportion of TUNEL-positive cells, insulin content, and secretion. This finding strongly suggests that the beneficial impact of adenoviral expression of human proIAPP siRNA in islets cells was due to siRNA-mediated inhibition of IAPP expression.
In summary, using cultured human islets as an ex vivo model of rapid islet amyloid formation, we showed that siRNA-mediated inhibition of human (pro)IAPP synthesis effectively prevents amyloid formation and enhances human β-cell survival and function. Assuming appropriate vehicles for delivery of siRNA to islets in vivo may become available in the future, this approach has therapeutic potential for preservation of β-cell mass in type 2 diabetes. In the meantime, targeting proIAPP expression in human islets ex vivo has potential immediate application in enhancing preculture transplant of human islets.
Acknowledgments
L.M. is the recipient of a Postdoctoral Fellowship Award from the Juvenile Diabetes Research Foundation. L.R. has received support from the Canadian Institutes of Health Research (CIHR). J.O. has received National Institutes of Health Grant U42RR023245. P.E.F. has received CIHR grants MOP-84516 and MOP-13340. P.A.H. has received Swiss National Research Foundation Grant 310000-113967/1. C.B.V. has received Canadian Diabetes Association and CIHR Grant MOP-14862 and is a Senior Scholar of the Michael Smith Foundation for Health Research (MSFHR) and an Investigator of the Child and Family Research Institute. Infrastructure support was provided by grants from the MSFHR to the Childhood Diabetes Research Unit and Centre for Human Islet Transplantation and Beta Cell Regeneration.
We thank Drs. C. Rhodes (Chicago) and L. Haataja (Los Angeles) for generation of the human proIAPP-expressing adenovirus. We gratefully acknowledge contributions of Galina Soukhatcheva, Genny Trigo-Gonzalez, Dr. Rebecca Walters, and Kate Potter to the completion of these studies.
Published ahead of print at http://diabetes.diabetesjournals.org on 11 August 2008.
L.M. is currently affiliated with the Division of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, British Columbia, Canada.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
REFERENCES
- 1.Clark A, Nilsson MR: Islet amyloid: a complication of islet dysfunction or an aetiological factor in type 2 diabetes? Diabetologia 47: 157–169, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Hull RL, Westermark GT, Westermark P, Kahn SE: Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab 89: 3629–3643, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Marzban L, Park K, Verchere CB: Islet amyloid polypeptide and type 2 diabetes. Exp Gerontol 38: 347–351, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Westermark P, Wernstedt C, Wilander E, Hayden DW, O'Brien TD, Johnson KH: Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A 84: 3881–3885, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB: Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A 84: 8628–8632, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukinius A, Wilander E, Westermark GT, Engstrom U, Westermark P: Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia 32: 240–244, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Kahn SE, D'Alessio DA, Schwartz MW, Fujimoto WY, Ensinck JW, Taborsky GJ Jr, Porte D Jr: Evidence of cosecretion of islet amyloid polypeptide and insulin by β-cells. Diabetes 39: 634–638, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Verchere CB, D'Alessio DA, Prigeon RL, Hull RL, Kahn SE: The constitutive secretory pathway is a major route for islet amyloid polypeptide secretion in neonatal but not adult rat islet cells. Diabetes 49: 1477–1484, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Lorenzo A, Razzaboni B, Weir GC, Yankner BA: Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature 368: 756–760, 1994 [DOI] [PubMed] [Google Scholar]
- 10.Saafi EL, Konarkowska B, Zhang S, Kistler J, Cooper GJ: Ultrastructural evidence that apoptosis is the mechanism by which human amylin evokes death in RINm5F pancreatic islet beta-cells. Cell Biol Int 25: 339–350, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Liu J, Dragunow M, Cooper GJ: Fibrillogenic amylin evokes islet beta-cell apoptosis through linked activation of a caspase cascade and JNK1. J Biol Chem 278: 52810–52819, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC: The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes 48: 491–498, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Green JD, Goldsbury C, Kistler J, Cooper GJ, Aebi U: Human amylin oligomer growth and fibril elongation define two distinct phases in amyloid formation. J Biol Chem 279: 12206–12212, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Konarkowska B, Aitken JF, Kistler J, Zhang S, Cooper GJ: The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. FEBS J 273: 3614–3624, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C: Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A 87: 5036–5040, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn SE, Andrikopoulos S, Verchere CB: Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes 48: 241–253, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Eriksson J, Nakazato M, Miyazato M, Shiomi K, Matsukura S, Groop L: Islet amyloid polypeptide plasma concentrations in individuals at increased risk of developing type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 35: 291–293, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Porte D Jr, Kahn SE: Hyperproinsulinemia and amyloid in NIDDM: clues to etiology of islet β-cell dysfunction? Diabetes 38: 1333–1336, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Westermark P, Engstrom U, Westermark GT, Johnson KH, Permerth J, Betsholtz C: Islet amyloid polypeptide (IAPP) and pro-IAPP immunoreactivity in human islets of Langerhans. Diabetes Res Clin Pract 7: 219–226, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Hou X, Ling Z, Quartier E, Foriers A, Schuit F, Pipeleers D, Van Schravendijk C: Prolonged exposure of pancreatic beta cells to raised glucose concentrations results in increased cellular content of islet amyloid polypeptide precursors. Diabetologia 42: 188–194, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Park K, Verchere CB: Identification of a heparin binding domain in the N-terminal cleavage site of pro-islet amyloid polypeptide: implications for islet amyloid formation. J Biol Chem 276: 16611–16616, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Paulsson JF, Westermark GT: Aberrant processing of human proislet amyloid polypeptide results in increased amyloid formation. Diabetes 54: 2117–2125, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Marzban L, Rhodes CJ, Steiner DF, Haataja L, Halban PA, Verchere CB: Impaired NH2-terminal processing of human proislet amyloid polypeptide by the prohormone convertase PC2 leads to amyloid formation and cell death. Diabetes 55: 2192–2201, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Verchere CB, D'Alessio DA, Palmiter RD, Weir GC, Bonner-Weir S, Baskin DG, Kahn SE: Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci U S A 93: 3492–3496, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler AE, Janson J, Soeller WC, Butler PC: Increased β-cell apoptosis prevents adaptive increase in β-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52: 2304–2314, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC: Diabetes due to a progressive defect in β-cell mass in rats transgenic for human islet amyloid polypeptide (HIP rat): a new model for type 2 diabetes. Diabetes 53: 1509–1516, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Matveyenko AV, Butler PC: β-Cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes 55: 2106–2114, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Zraika S, Hull RL, Udayasankar J, Clark A, Utzschneider KM, Tong J, Gerchman F, Kahn SE: Identification of the amyloid-degrading enzyme neprilysin in mouse islets and potential role in islet amyloidogenesis. Diabetes 56: 304–310, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Janson J, Soeller WC, Roche PC, Nelson RT, Torchia AJ, Kreutter DK, Butler PC: Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A 93: 7283–7288, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJ, Holman RR, Turner RC: Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res 9: 151–159, 1988 [PubMed] [Google Scholar]
- 31.de Koning EJP, Bodkin NL, Hansen BC, Clark A: Diabetes mellitus in Macaca mulatta monkey is characterised by islet amyloidogenesis and reduction in beta-cell population. Diabetologia 36: 378–384, 1993 [DOI] [PubMed] [Google Scholar]
- 32.de Koning EJ, Morris ER, Hofhuis FM, Posthuma G, Hoppener JW, Morris JF, Capel PJ, Clark A, Verbeek JS: Intra- and extracellular amyloid fibrils are formed in cultured pancreatic islets of transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A 91: 8467–8471, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henson MS, Buman BL, Jordan K, Rahrmann EP, Hardy RM, Johnson KH, O'Brien TD: An in vitro model of early islet amyloid polypeptide (IAPP) fibrillogenesis using human IAPP-transgenic mouse islets. Amyloid 13: 250–259, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Hull RL, Zraika S, Udayasankar J, Kisilevsky R, Szarek WA, Wight TN, Kahn SE: Inhibition of glycosaminoglycan synthesis and protein glycosylation with WAS-406 and azaserine result in reduced islet amyloid formation in vitro. Am J Physiol Cell Physiol 293: C1586–C1593, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zraika S, Hull RL, Udayasankar J, Utzschneider KM, Tong J, Gerchman F, Kahn SE: Glucose- and time-dependence of islet amyloid formation in vitro. Biochem Biophys Res Commun 354: 234–239, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bain JR, Schisler JC, Takeuchi K, Newgard CB, Becker TC: An adenovirus vector for efficient RNA interference-mediated suppression of target genes in insulinoma cells and pancreatic islets of langerhans. Diabetes 53: 2190–2194, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Schisler JC, Jensen PB, Taylor DG, Becker TC, Knop FK, Takekawa S, German M, Weir GC, Lu D, Mirmira RG, Newgard CB: The Nkx6.1 homeodomain transcription factor suppresses glucagon expression and regulates glucose-stimulated insulin secretion in islet beta cells. Proc Natl Acad Sci U S A 102: 7297–7302, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marzban L, Trigo-Gonzalez G, Zhu X, Rhodes CJ, Halban PA, Steiner DF, Verchere CB: Role of β-cell prohormone convertase (PC)1/3 in processing of pro-islet amyloid polypeptide. Diabetes 53: 141–148, 2004 [DOI] [PubMed] [Google Scholar]
- 39.O'Brien TD, Butler PC, Kreutter DK, Kane LA, Eberhardt NL: Human islet amyloid polypeptide expression in COS-1 cells: a model of intracellular amyloidogenesis. Am J Pathol 147: 609–616, 1995 [PMC free article] [PubMed] [Google Scholar]
- 40.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Ahren B: Type 2 diabetes, insulin secretion and beta-cell mass. Curr Mol Med 5: 275–286, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Hoppener JW, Ahren B, Lips CJ: Islet amyloid and type 2 diabetes mellitus. N Engl J Med 343: 411–419, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Westermark G, Westermark P, Eizirik DL, Hellerstrom C, Fox N, Steiner DF, Andersson A: Differences in amyloid deposition in islets of transgenic mice expressing human islet amyloid polypeptide versus human islets implanted into nude mice. Metabolism 48: 448–454, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L: Cell loss in isolated human islets occurs by apoptosis. Pancreas 20: 270–276, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Anguiano M, Nowak RJ, Lansbury PT Jr: Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry 41: 11338–11343, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG: Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem 280: 17294–17300, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Engel MF, Khemtemourian L, Kleijer CC, Meeldijk HJ, Jacobs J, Verkleij AJ, de Kruijff B, Killian JA, Hoppener JW: Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proc Natl Acad Sci U S A 105: 6033–6038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]