Abstract
OBJECTIVE—This study was designed to determine whether the transcription factor FoxM1 was required for regeneration of β-cell mass via proliferation and/or neogenesis in the adult after 60% partial pancreatectomy (PPx).
RESEARCH DESIGN AND METHODS—Adult mice with a pancreas-wide deletion of Foxm1 (Foxm1flox/flox;Pdx1-Cre [FoxM1Δpanc]) and their control littermates (Foxm1flox/flox) were subjected to PPx or a sham operation, after which islet expression of Foxm1 and several target genes, β-cell mass, proliferation, β-cell size, islet size, islet density, and neurogenin-3 expression were analyzed.
RESULTS—In control mice, PPx stimulated β-cell proliferation and neogenesis and upregulated Foxm1 and several of its known targets (Plk1, Cenp-a, Birc5/Survivin, and Ccnb1) in islets. Within 1 week post-PPx, control mice underwent significant regeneration of β-cell mass, and average islet size within the regenerating lobe was similar to that after a sham operation. However, FoxM1Δpanc mice exhibited specific impairments in β-cell mass regeneration and islet growth after PPx, with reduced proliferation of α- and β-cells but no impairments in acinar or ductal cell proliferation. Interestingly, FoxM1 was not required for proliferation of β-cells within small endocrine cell clusters located in the regenerating portion of the pancreas but was specifically required for proliferation of β-cells within larger islets. Additionally, FoxM1 was not required for β-cell neogenesis following PPx.
CONCLUSIONS—Our results indicate that FoxM1 is partially required for increased β-cell proliferation, but not β-cell neogenesis, stimulated by PPx. Furthermore, FoxM1 seems to be dispensable for proliferation of β-cells following neogenesis but is required for proliferation of preexisting β-cells.
As type 1 and type 2 diabetes result from absolute or relative deficiencies in β-cell number, respectively, understanding how β-cell number is determined and can be manipulated may lead to new therapeutic options. While transplantation of cadaveric islets remains promising, additional sources of islets or β-cells are required to make this treatment more widely available (1). Diabetic patients would also benefit from in vivo manipulation of their β-cell population. β-Cells proliferate slowly after birth (2), but increased proliferation can be stimulated under various conditions, including obesity, pregnancy, and growth factor overexpression (rev. in 3). In the adult mouse, β-cell replication is the primary mechanism by which new β-cells are formed (4,5). However, a recent study (6) in mice has conclusively shown that new β-cells are generated from facultative adult progenitor cells (neogenesis) in response to pancreatic injury, in a manner reminiscent of embryonic endocrine cell differentiation. Furthermore, patients with type 1 diabetes exhibit evidence for continued β-cell production in the face of immune destruction (7,8), providing support for the ability of human β-cells to regenerate.
We previously showed that the forkhead box transcription factor FoxM1 regulates postnatal β-cell proliferation (9). In other tissues, FoxM1 transactivates genes that coordinate the G1/S and G2/M transitions, karyokinesis, and cytokinesis (10,11). For example, the FoxM1 targets Cdc25A and Cdc25B (cell division cycle 25 homolog A and B, respectively) dephosphorylate and activate cyclin-dependent kinase 2 (Cdk2) and Cdk1, respectively (12). Cdk1 activity also depends on its binding to CcnB1 (cyclin B1), another FoxM1 target (12,13). Additional targets include S-phase kinase-associated protein 2 (Skp2) and Cdk subunit 1 (Cks1), components of the SCF (Skp1-Cullin1-F box) ubiquitin ligase complex that mediates degradation of the Cdk inhibitors p21Cip1, p27Kip1, and p57Kip2 (14). FoxM1 also directly activates transcription of polo-like kinase 1 (Plk1) (15), aurora B kinase, centromere protein A (Cenp-A), Cenp-B, and survivin (Birc5) (14). Plk1 is necessary for centrosome duplication and, with aurora B kinase, mediates attachment of the microtubule spindles to kinetochores. Survivin regulates aurora B kinase centromere localization, while Cenp-A and -B are involved in kinetochore assembly.
Although all proliferating cells express FoxM1, certain cell types are more profoundly affected than others by Foxm1 deletion. Foxm1−/− mice die during late embryogenesis due to hepatic and cardiac defects (16). Additionally, pancreas-wide Foxm1 deletion (Foxm1flox/flox;Pdx15.5kb-Cre [FoxM1Δpanc]) does not affect embryonic pancreas or β-cell development but does impair postnatal β-cell mass expansion via reduced β-cell proliferation, resulting in progressive diabetes in male mice (9). These outcomes mirror those reported for widespread deletion of other cell cycle regulators (Cdk4 and cyclin D), with endocrine cell–specific effects causing diabetes (17–19). Female FoxM1Δpanc mice remain glucose tolerant despite significantly reduced β-cell mass and thus are the focus of this study, allowing for analysis of β-cell proliferation and regeneration without the confounding effects of hyperglycemia.
To determine whether FoxM1 is required for β-cell regeneration, we performed 60% partial pancreatectomy (PPx) on adult female FoxM1Δpanc and control (Foxm1flox/flox) littermates. Here, we show that Foxm1 and some of its transcriptional targets were upregulated within islets following PPx during the period of peak regeneration and that endocrine cell proliferation and regeneration of β-cell mass following PPx were specifically impaired in FoxM1Δpanc mice. Furthermore, we present evidence that loss of FoxM1 did not impair islet neogenesis but did impair islet growth after PPx.
RESEARCH DESIGN AND METHODS
Foxm1flox/flox mice on a mixed background (129SvJ, C57BL/6) were described previously (12). Briefly, loxP sequences flank exons 4–7, yielding a null allele after Cre-mediated recombination. Pdx15.5kb-Cre mice (on a mixed ICR, CBA, and C57BL/6 background) were generously provided by Guoqiang Gu (Vanderbilt University) (20) and express Cre recombinase throughout the Pdx1 domain, including the entire pancreatic epithelium, by embryonic day (e) 10.5 (21,22). Foxm1 and Cre were genotyped as described (9,20). Rosa26-FoxM1 transgenic (Tg) mice (on a mixed FVB/N, C57BL/6 background) were previously described (23) and genotyped (as in 24). For embryonic analyses, the morning of vaginal plug was considered e0.5. All mice received food and drink ad libitum and were on a 12-h light-dark cycle. All mouse studies were performed in accordance with the Vanderbilt Institutional Animal Care and Use Committee guidelines under the supervision of the Division of Animal Care.
Sixty percent PPx and intraperitoneal glucose tolerance test.
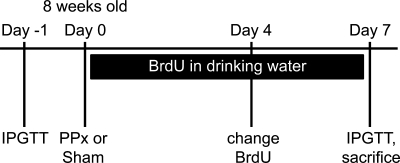
Eight-week-old female mice were anesthetized using isoflurane. A midline abdominal incision allowed exteriorization of the splenic lobe of the pancreas (between the gastroduodenal junction and the spleen). Sixty percent PPx was performed by gently denuding the pancreatic tissue from the splenic lobe using cotton-tipped swabs soaked in 0.9% saline, leaving the main pancreatic duct and splenic artery intact. For sham operations, the splenic lobe was isolated using cotton-tipped swabs and gently rubbed with a moistened gloved finger for 1 min. Abdominal muscles were sutured using 5-0 vicryl (Ethicon), and the skin was stapled using 9.0-mm staples (Reflex Skin Closure Systems). A total of 0.1 mg/kg buprenorphine-HCl in normal saline was injected subcutaneously for analgesia, and 0.8 mg/ml bromo-deoxyuridine (BrdU) (Sigma-Aldrich) was administered in drinking water in a light-safe bottle and was replaced on the 4th day. Mice were killed 1 week postoperation by cervical dislocation, and the splenic and duodenal lobes of the pancreas were harvested and analyzed separately. One day before and 7 days after the operation, intraperitoneal glucose tolerance tests (IPGTTs) were performed as described previously (9) (see Fig. 1 for experimental timeline).
FIG. 1.
Experimental timeline for 60% PPx. Either PPx or a sham operation was performed on 8-week-old female mice, after which BrdU was administered in the drinking water for 1 week. IPGTT was performed 1 day before and 7 days following the operation, and the mice were killed on day 7.
RNA isolation and real-time quantitative RT-PCR.
RNA from whole pancreas or freshly isolated islets was extracted and DNase treated using the RNAqueous and Turbo DNA-free kits (Ambion). RNA was assessed using the ND-1000 Spectrophotometer (NanoDrop) and the 2100 Electrophoresis Bioanalyzer (Agilent). cDNA was generated from 20 ng RNA using the Superscript III First-Strand Synthesis System for RT-PCR (Invitrogen). Real-time PCR was performed using the iQ5 Real-Time PCR Detection System (Bio-Rad) with iQ Real-Time SYBR Green PCR Supermix (Bio-Rad) and the primer sets listed in online appendix Table S1 (available at http://dx.doi.org/10.2337/db08-0878). Results were quantitated using the ΔΔCt method.
Tissue preparation and histology.
Pancreatic tissue was fixed in 4% paraformaldehyde for 2–4 h at 4°C, processed for paraffin embedding, and sectioned at 5 μm. Primary antibodies (guinea pig anti-insulin or anti-glucagon [both 1:1,000; Linco], rabbit anti-cytokeratin [1:1,000; DakoCytomation], rat anti-BrdU [1:400; Accurate Chemical and Scientific], rabbit anti–phospho-histone H3 [1:250; Upstate Cell Signaling Solutions], and mouse anti–neurogenin-3 [Ngn3] [1:100; Developmental Studies Hybridoma Bank, University of Iowa]) were incubated overnight at 4°C. Secondary antibodies (peroxidase-conjugated donkey anti–guinea pig [1:250; Jackson Laboratories] and Cy2- or Cy3-conjugated donkey anti–guinea pig, anti-rabbit, or anti-rat [all 1:500; Jackson Laboratories]) were incubated for 1 h at room temperature. Detection of cytokeratin required pretreatment with 20 μg/ml proteinase-K for 5 min at room temperature, and detection of Ngn3 required microwave antigen retrieval with 10 mmol/l Tris-EGTA, pH 9.0, and tyramide signal amplification with TSA Kit no. 2 (Invitrogen).
β-Cell mass analysis.
Sections 250 μm apart (5–15 sections per sample) were incubated with anti-insulin primary antibody followed by peroxidase-conjugated secondary antibody, visualized using a DAB Peroxidase Substrate Kit (Vector Laboratories), and counterstained with eosin. Digital images were created using a Nikon Coolscan 9000 and NikonScan version 4.0.2. Total pancreatic and insulin+ areas of each section were measured using MetaMorph version 6.1 (Molecular Devices). β-Cell mass equaled the ratio of total insulin+ area to total pancreatic area of all sections, multiplied by tissue wet weight.
Proliferation analyses.
Sections 250–750 μm apart (three to eight sections per sample) were labeled with anti-insulin and anti-BrdU primary antibodies. Nuclei were labeled with 1.5 μg/ml 4′,6′-diamidino-2-phenylindole (DAPI) (Molecular Probes) in mounting media. All insulin+ cells on each section were imaged at ×400 magnification on an Olympus BX41 microscope with a digital camera using Magnafire (Optronics). Percent proliferating β-cells equaled the number of insulin/BrdU double-positive cells divided by the total number of insulin+ cells. α-Cell proliferation was measured similarly, using anti-glucagon labeling. Ductal cells were identified by cytokeratin labeling on sections 250–750 μm apart, and images were captured at ×200 magnification. Acinar cells were identified by morphological characteristics on three separate ×200 images from sections 750 μm apart. BrdU labeling was previously described (9). Phospho-histone H3 labeling of sections 125 μm apart (seven to nine sections per sample) required microwave antigen retrieval in 10 mmol/l sodium citrate, pH 6.5, followed by 0.2% Triton-X-100 in PBS.
Islet, β-cell, and nuclear size analyses.
Using images collected for β-cell proliferation, the cross-sectional area of each insulin+ cell grouping was circumscribed and measured using MetaMorph. Average cross-sectional β-cell size per islet equaled the total area of the islet divided by the number of β-cell nuclei within it. β-Cell nuclear size was measured by circumscribing the DAPI+ cross-sectional area within insulin+ cells.
Statistical analyses.
Data were analyzed by unpaired t test or two-way ANOVA with Bonferroni's posttests, as appropriate, using GraphPad Prism version 5.01. P < 0.05 was considered significant. Specific analyses performed and sample sizes are included in the figure legends.
RESULTS
Sixty percent PPx upregulated Foxm1 and select targets within islets while maintaining normoglycemia.
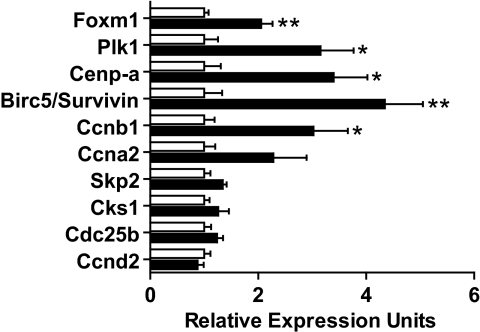
FoxM1 mRNA and protein are relatively low in adult islets (data not shown and 9), but it was unknown whether upregulation occurs upon stimulation of endocrine cell proliferation. Indeed, Foxm1 expression was upregulated twofold in control islets 6 days following PPx versus sham (Fig. 2), suggesting that FoxM1 plays a role in the regenerative response of endocrine cells. Importantly, Foxm1 upregulation corresponded with the peak of β-cell proliferation following PPx (25).
FIG. 2.
Expression levels of Foxm1 mRNA and some of its targets were upregulated following 60% PPx. Real-time quantitative RT-PCR was performed on RNA extracted from isolated islets 6 days following either PPx (▪) or sham (□). Error bars represent SE of the mean. Unpaired t tests were used to measure significance. *P < 0.05; **P < 0.01. n = 4–6 per group.
Additionally, we observed significant upregulation of known FoxM1 targets, including Plk1, Cenp-a, Birc5/survivin, and Ccnb1 (Fig. 2). Other targets, including Skp2, Cks1, and Cdc25b, displayed a trend of upregulation following PPx versus sham but did not reach significance. A similar trend was observed for Ccna2 but not for Ccnd2, both of which have been linked to β-cell proliferation (17,18,26), but neither of which is a known FoxM1 target.
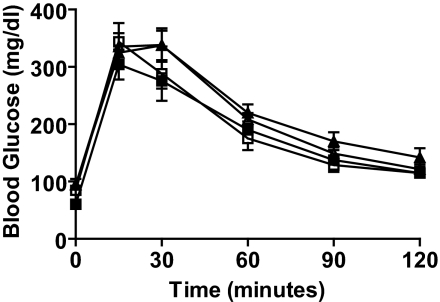
To determine whether FoxM1 is necessary for endocrine cell regeneration following pancreatic injury, FoxM1Δpanc and control female littermates underwent PPx or sham operation at 8 weeks of age. Previous reports showed that 60% PPx does not induce hyperglycemia (25), unlike 90% PPx (27). IPGTTs performed 7 days following 60% PPx or sham confirmed that control and FoxM1Δpanc female mice remained glucose tolerant (Fig. 3).
FIG. 3.
Glucose tolerance was maintained following 60% PPx. No significant differences in blood glucose levels were observed in either control or FoxM1Δpanc mice following PPx compared with sham at any time point during IPGTT. Error bars represent SE. Two-way ANOVA with Bonferroni's posttests was used to measure significance. n = 5–7 per group. □, control sham day 7; ▪, FoxM1Δpanc sham day 7; ▵, control PPx day 7; ▴, FoxM1Δpanc PPx day 7.
Regeneration of β-cell mass within the splenic lobe was specifically impaired in FoxM1Δpanc mice.
Similar to FoxM1Δpanc male mice (9), FoxM1Δpanc female mice also exhibited ∼50% reduced β-cell mass at 9 weeks of age compared with littermate controls (data not shown), although FoxM1Δpanc female mice remained normoglycemic. This ∼50% reduction in β-cell mass was evident in the splenic, but not duodenal, lobe and was unchanged after a sham operation (Fig. 4B and C). Total pancreatic weight and weight of the individual lobes were not significantly different between control and FoxM1Δpanc mice (Fig. 4A) (data not shown), indicating a specific defect in the endocrine compartment of the pancreas. Consistent with previous findings (28), a significant majority of β-cell mass was located within the splenic versus duodenal lobe of control mice after a sham operation (P < 0.01).
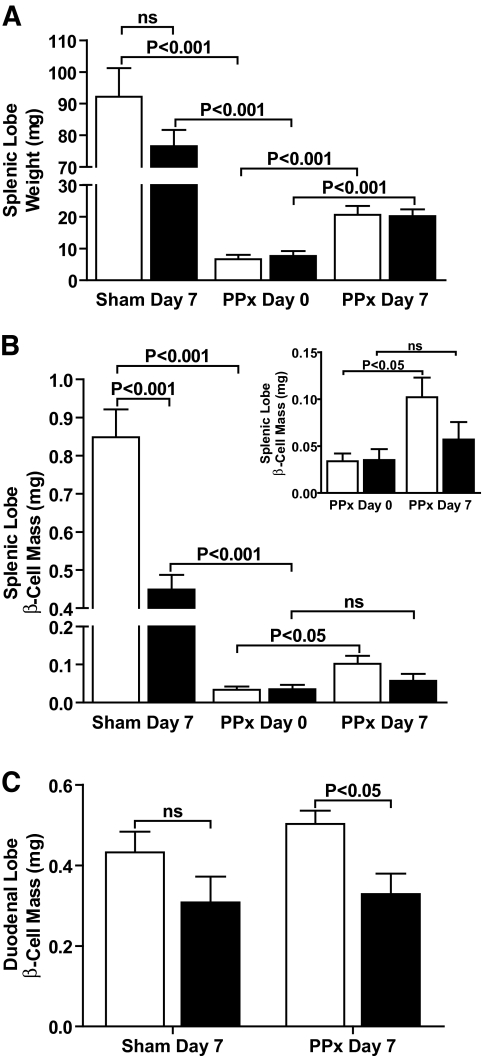
FIG. 4.
Regeneration of β-cell mass, but not gross pancreatic tissue, was impaired in FoxM1Δpanc mice. A: Wet weight of the pancreatic splenic lobe was measured immediately following (day 0) or 7 days after PPx or sham. The majority of tissue was removed by PPx, and a moderate amount of regeneration occurred within 7 days in both control (□) and FoxM1Δpanc (▪) mice. B: β-Cell mass, which takes into account tissue wet weight, was significantly reduced within the splenic lobe of FoxM1Δpanc pancreata after a sham operation, and regeneration of β-cell mass was not observed in FoxM1Δpanc mice following PPx. C: β-Cell mass within the duodenal lobe of FoxM1Δpanc pancreata was only significantly reduced in comparison with control pancreata following PPx. Error bars represent SE. Two-way ANOVA with Bonferroni's posttests, comparing sham day 7 versus PPx day 0 and PPx day 0 versus PPx day 7, was used to measure significance. n = 4–6 per group. ns, not significant.
PPx eliminated the majority of splenic lobe tissue, and within 7 days a significant and equivalent amount of tissue regenerated in both FoxM1Δpanc and control mice (Fig. 4A) (online appendix Fig. S1A–D), suggesting that overall pancreas regeneration during this time period was not impaired by Foxm1 deletion. β-Cell mass regeneration within the splenic lobe, however, was impaired in FoxM1Δpanc mice, as β-cell mass in these mice did not significantly increase within 7 days following PPx, in contrast to control littermates (Fig. 4B).
β-Cell proliferation was reduced in FoxM1Δpanc mice after 60% PPx.
Because β-cells are replenished primarily by self-replication (4), and FoxM1 is important for postnatal β-cell proliferation (9), it seemed likely that reduced β-cell proliferation contributed to impaired β-cell mass regeneration in FoxM1Δpanc mice. Indeed, PPx dramatically stimulated β-cell proliferation in both pancreatic lobes of control mice, and these effects were significantly blunted in FoxM1Δpanc mice (Fig. 5A–F). Moreover, β-cell proliferation within the duodenal lobe of FoxM1Δpanc mice did not significantly increase after PPx.
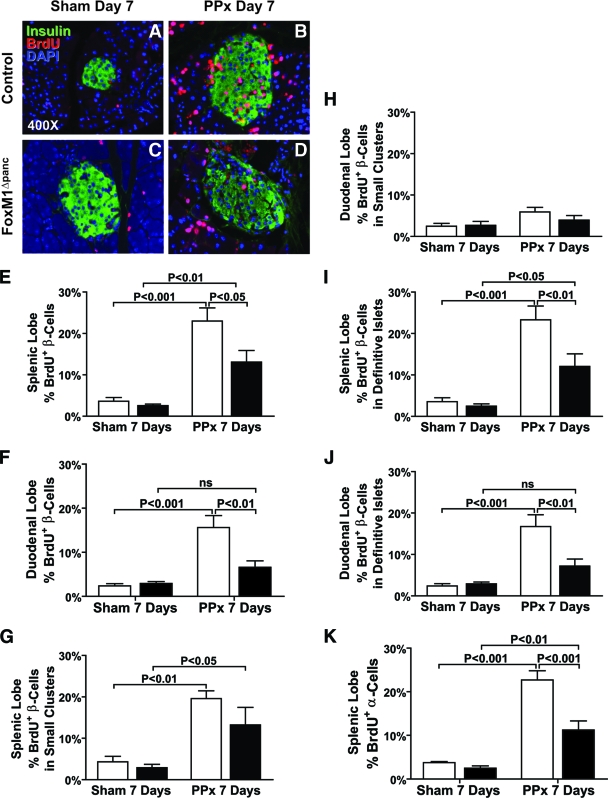
FIG. 5.
Proliferation of endocrine cells was impaired in FoxM1Δpanc mice following 60% PPx. BrdU incorporation (red) over 7 days was used as a marker of cell proliferation. Double labeling with insulin (green) revealed low, basal levels of β-cell proliferation within the splenic lobes following a sham operation in both control (A) and FoxM1Δpanc (C) mice. PPx enhanced BrdU incorporation into all pancreatic cell types within the splenic lobe of control mice (B), but incorporation into endocrine cells was reduced in FoxM1Δpanc mice (D). β-Cell proliferation was quantified for both the splenic (E) and duodenal (F) pancreatic lobes. β-Cell proliferation within small endocrine cell clusters (eight or less β-cells) after PPx versus sham was enhanced in the splenic lobes of FoxM1Δpanc and control mice (G) but was not altered in the duodenal lobes (H). β-Cell proliferation within definitive islets (nine or more β-cells) after PPx was enhanced in the splenic lobe of control mice, and this effect was partially inhibited in FoxM1Δpanc mice (I). PPx also significantly stimulated β-cell proliferation within definitive islets in the duodenal lobe of control, but not FoxM1Δpanc, mice (J). α-Cell proliferation within the splenic lobe of FoxM1Δpanc pancreata (K) was also impaired in comparison with control pancreata following PPx. Error bars represent SE. Two-way ANOVA with Bonferroni's posttests was used to measure significance. n = 4–5 per group for β-cell proliferation, n = 3–4 per group for α-cell proliferation. ns, not significant. E–K: □, control; ▪, FoxM1Δpanc. (Please see http://dx.doi.org/10.2337/db08-0878 for a high-quality digital representation of this figure.)
β-Cell proliferation in the splenic lobe of FoxM1Δpanc mice did increase significantly following PPx versus sham, despite Foxm1 deletion (Fig. 5E), due to increased proliferation within small endocrine cell clusters (eight or fewer insulin+ cells) and definitive islets (nine or more insulin+ cells) (Fig. 5G and I). However, β-cell proliferation within definitive islets was significantly reduced in FoxM1Δpanc versus control mice (Fig. 5I). Because there was no significant impairment in β-cell proliferation in small endocrine cell clusters in FoxM1Δpanc mice, we hypothesized that these clusters represented newly formed β-cells that did not require FoxM1 for proliferation. This hypothesis was supported by the finding that embryonic β-cell proliferation did not require FoxM1 (online appendix Fig. S2A and B). Additionally, proliferation of β-cells within small endocrine cell clusters after PPx was specifically stimulated in the regenerating splenic (Fig. 5G), but not duodenal (Fig. 5H), lobe. We hypothesize that just as perinatal β-cells can proliferate in the absence of FoxM1, newly differentiated β-cells in the adult can undergo several rounds of FoxM1-independent replication before switching to FoxM1-dependent proliferation.
PPx stimulated β-cell proliferation within definitive islets of control mice to the same extent (sevenfold) in both the splenic and duodenal lobes versus sham (Fig. 5I and J). Proliferation in the splenic lobe of FoxM1Δpanc mice increased only fivefold, while the increase within the duodenal lobe did not reach significance, suggesting that proliferation of preexisting β-cells was impaired in the absence of FoxM1. The increased BrdU incorporation observed within definitive islets in the splenic lobe of FoxM1Δpanc mice 7 days after PPx versus sham (Fig. 5I) may represent proliferation that occurred at an earlier stage of new islet development. These results are summarized in Table 1. α-Cells similarly required FoxM1 for PPx-stimulated proliferation (Fig. 5K). In contrast, acinar and ductal cell proliferation was not impaired in FoxM1Δpanc mice (online appendix Fig. S3A and B), revealing that FoxM1 is specifically required for endocrine cell proliferation.
TABLE 1.
Summary of β-cell proliferation results for control and FoxM1Δpanc mice after sham versus PPx
| β-Cell proliferation in clusters | β-Cell proliferation in islets | |
|---|---|---|
| Control PPx versus sham | ||
| Splenic lobe | ↑ | ↑ |
| Duodenal lobe | ⇆ | ↑ |
| FoxM1Δpanc PPx versus sham | ||
| Splenic lobe | ↑ | ↑ |
| Duodenal lobe | ⇆ | ⇆ |
| FoxM1Δpanc sham versus control sham | ||
| Splenic lobe | ⇆ | ⇆ |
| Duodenal lobe | ⇆ | ⇆ |
| FoxM1Δpanc PPx versus control PPx | ||
| Splenic lobe | ⇆ | ↓ |
| Duodenal lobe | ⇆ | ↓ |
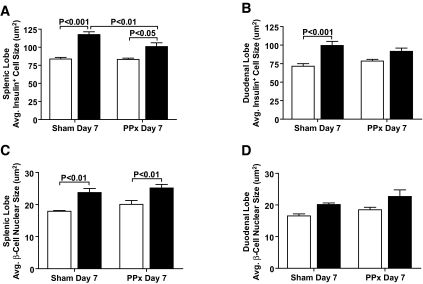
FoxM1Δpanc mice exhibited increased β-cell and nucleus size.
Sham-operated FoxM1Δpanc mice exhibited larger average β-cell size in both pancreatic lobes (Fig. 6A and B) versus control littermates, due in part to increased β-cell nucleus size (Fig. 6C and D). However, hypertrophy also contributed, likely as a compensatory measure due to reduced β-cell number. PPx did not alter β-cell size in control mice. However, average β-cell size was significantly reduced specifically within the splenic lobe of FoxM1Δpanc mice after PPx, likely due to new β-cell formation.
FIG. 6.
FoxM1Δpanc mice exhibited increased β-cell and nucleus size. After a sham operation, FoxM1Δpanc mice exhibited increased β-cell size in both the splenic (A) and duodenal (B) pancreatic lobes. Increased β-cell size was also observed after PPx in the splenic lobe of FoxM1Δpanc mice versus controls but was significantly reduced compared with after a sham operation. The average β-cell size in control pancreata was unchanged by PPx. FoxM1Δpanc mice also exhibited increased β-cell nucleus size in the splenic (C) but not duodenal (D) lobe, which was unchanged by PPx. Error bars represent SE. Two-way ANOVA with Bonferroni's posttests was used to measure significance. n = 4–5 per group. □, control; ▪, FoxM1Δpanc.
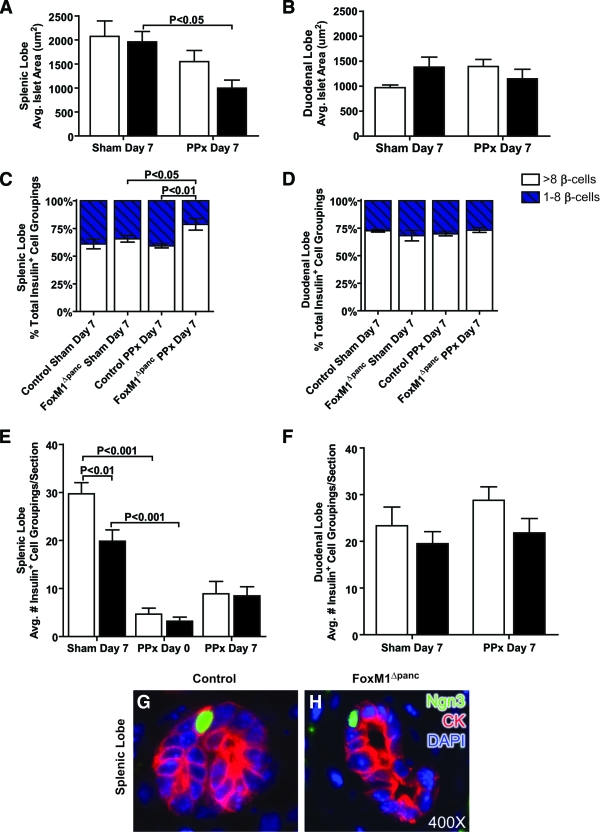
Islet size in FoxM1Δpanc mice was reduced following 60% PPx.
Although PPx reduced total β-cell number within the splenic lobe, islets present within this lobe 7 days after PPx were of the same average size as after the sham operation in control mice (Fig. 7A). However, FoxM1Δpanc islets were significantly smaller 7 days after PPx compared with sham. Similarly, the average islet size within the duodenal lobe of control mice trended toward an increase following PPx versus sham, but no expansion was observed in FoxM1Δpanc mice (Fig. 7B). These data suggest that Foxm1 deletion impairs islet growth after PPx. Of note, islets within the splenic lobe of control mice after sham operation averaged two times larger than islets within the duodenal lobe (P < 0.05), which may be due to a greater proportion of small endocrine cell clusters versus definitive islets normally found in the duodenal versus splenic lobe (P < 0.05) (Fig. 7C and D). Interestingly, after PPx FoxM1Δpanc mice exhibited an increased proportion of small endocrine cell clusters versus definitive islets within their splenic lobe in comparison with control mice (Fig. 7C), which likely contributed to their reduced average islet size. No differences were observed in the proportion of small endocrine cell clusters between control and FoxM1Δpanc mice after sham operation or within the duodenal lobe under any condition (Fig. 7D).
FIG. 7.
FoxM1Δpanc mice exhibited impaired islet growth but no impairment in islet neogenesis after 60% PPx. There was no significant difference in average islet size, measured as the cross-sectional area of each insulin+ cell grouping, between control (□) and FoxM1Δpanc (▪) mice after a sham operation or PPx in either the splenic (A) or duodenal (B) pancreatic lobes. Following PPx, the average islet size in the splenic lobe of control pancreata was similar to that observed in sham-operated mice, but FoxM1Δpanc islets were significantly reduced in size. Within the duodenal lobe, control mice trended toward increased average islet size after PPx, but no increase was observed in FoxM1Δpanc mice. FoxM1Δpanc mice specifically exhibited an increased proportion of small insulin+ cell clusters (one to eight β-cells) within the splenic lobe after PPx (C); no differences were observed within the duodenal lobe (D). ▪, more than eight β-cells; □, one to eight β-cells. The number of insulin+ cell groupings per section was reduced within the splenic lobe of FoxM1Δpanc mice compared with control littermates after a sham operation (E), but at day 0 and day 7 following PPx, the number of insulin+ cell groupings per section was equivalent between FoxM1Δpanc and control mice. Seven days after PPx, there was a trend in both groups of mice to show increased numbers of insulin+ cell groupings per section within the splenic lobe, but no differences between any groups of mice were observed in the duodenal lobe (F). Ngn3 expression was detected only after PPx within cytokeratin+ (CK+) ductal epithelial cells located within foci of regeneration in the splenic lobes of both control (G) and FoxM1Δpanc (H) mice. Error bars represent SE. Two-way ANOVA with Bonferroni's posttests was used to measure significance. n = 4–5 per group. (Please see http://dx.doi.org/10.2337/db08-0878 for a high-quality digital representation of this figure.)
β-Cell neogenesis following 60% PPx was not impaired in FoxM1Δpanc mice.
The average number of insulin+ cell groupings in the splenic lobe of both FoxM1Δpanc and control mice trended toward an increase 7 days after PPx versus day 0 (Fig. 7E), while no differences were observed in the duodenal lobes of either genotype (Fig. 7F). Insulin+ cells were observed within foci of regeneration in the splenic lobes of both groups of mice (online appendix Fig. 1B and D), suggesting that generation of new islets, or neogenesis, occured specifically in the splenic lobe after PPx and was not impaired in FoxM1Δpanc mice. These findings were supported by observation of Ngn3, a marker of endocrine progenitor cells (29), in ductal epithelial cells in both control (Fig. 7G) and FoxM1Δpanc (Fig. 7H) mice following PPx. Importantly, Ngn3 expression was only observed in ductules within the regenerating portion of the splenic lobe but not within differentiated pancreas tissue. No difference in the number of Ngn3+ ductal epithelial cells was detected between control and FoxM1Δpanc mice following PPx.
Foxm1 overexpression did not enhance β-cell mass regeneration following 60% PPx.
To determine whether Foxm1 overexpression affects pancreas and β-cell regeneration following PPx, we utilized Rosa26-FoxM1 Tg mice that constitutively and ubiquitously overexpress human FoxM1 (23). Despite FoxM1 overexpression within islets (online appendix Fig. S4A), Tg mice exhibited no significant differences in glucose tolerance (online appendix Fig. S4B) or β-cell mass following PPx (online appendix Fig. S4C) compared with wild-type littermates. Furthermore, we observed no differences in pancreatic tissue weight (online appendix Fig. S4D), average islet size (online appendix Fig. S4E), or islet density (online appendix Fig. S4F) between Tg and wild-type mice in either lobe.
DISCUSSION
FoxM1 is highly expressed within pancreatic endocrine cells during embryogenesis and is downregulated with age (9), corresponding with reduced β-cell proliferation. We previously showed that mice with Foxm1 deleted throughout the pancreatic epithelium (FoxM1Δpanc) exhibit impaired growth and maintenance of postnatal β-cell mass secondary to reduced postnatal β-cell proliferation, associated with increased nuclear-localized p27Kip1 and premature cellular senescence (9). Several of FoxM1's direct transcriptional targets inhibit p27Kip1 by promoting its ubiquitination and subsequent degradation (Skp2, Cks1) (14) or its nuclear export (kinase-interacting stathmim) (30). Here, we show that FoxM1Δpanc mice have impaired β-cell mass regeneration following PPx due to reduced β-cell proliferation but that neogenesis proceeds normally.
Sixty percent PPx is a normoglycemic pancreatic injury model that stimulates regeneration by differentiation and proliferation and hyperplasia of preexisting tissue. We found that Foxm1 and some of its targets were upregulated within islets following PPx during a period of enhanced α- and β-cell proliferation, and we hypothesized that FoxM1 was required for injury-stimulated endocrine cell proliferation. Indeed, we found that FoxM1Δpanc mice exhibited impaired α- and β-cell proliferation and impaired β-cell mass regeneration following PPx. PPx stimulated β-cell proliferation in both the splenic (regenerating) and duodenal (expanding) lobes of control mice but not in the duodenal lobe of FoxM1Δpanc mice. Thus, hyperplasia of preexisting β-cells after injury requires FoxM1.
Interestingly, PPx-stimulated β-cell proliferation was blunted, but not completely inhibited within the splenic lobe of FoxM1Δpanc mice, due at least in part to impaired proliferation in definitive islets, but not small endocrine cell clusters, in the absence of FoxM1. These data suggest that another pathway(s), which may mimic that utilized during embryonic pancreas development, compensates for loss of FoxM1 during regeneration, as FoxM1 was also not required for embryonic β-cell proliferation. Thus, preexisting β-cells seemingly exhibit a stronger requirement for FoxM1 than new β-cells formed by neogenesis. Although it is currently unclear which factors are required for β-cell proliferation during embryogenesis and following islet neogenesis, our studies support the hypothesis that neogenesis in the adult is similar to embryonic β-cell development, both with regard to reexpression of Ngn3 and initial FoxM1-independent proliferation.
Because BrdU was administered over 7 days, we may have underestimated the effects of Foxm1 deletion on endocrine cell proliferation. Previous studies have shown that Foxm1−/− cells can undergo multiple rounds of DNA synthesis without karyo- or cytokinesis (endoreduplication), resulting in polyploid nuclei (16). We found evidence of polyploid β-cells and exocrine cells in FoxM1Δpanc mice. These cells would have been counted as proliferating cells in our analyses but would not have actually resulted in increased cell number. This phenomenon could partially explain the increased BrdU incorporation in β-cells without significant β-cell mass regeneration within the splenic lobe of FoxM1Δpanc mice after PPx compared with sham, although it most likely would not be restricted to one lobe.
Additionally, incomplete Foxm1 recombination could have resulted in mosaic expression, allowing some β-cells to continue proliferating. However, we think that this is unlikely because we previously showed that Pdx15.5kb-Cre recombined the Rosa26-LacZ reporter allele within all endodermally derived pancreatic cells and yielded a significant reduction in FoxM1 protein by e15.5 (9). We also found that Foxm1 transcripts were significantly reduced in FoxM1Δpanc neonatal pancreas (online appendix Fig. S5A) and even more dramatically in adult islets (online appendix Fig. S5B). These tissues contain nonendodermally derived mesenchymal, endothelial, and neuronal cells, which would not undergo Foxm1 recombination and thus likely account for remaining transcript expression.
Proliferation of α- and β-cells, but not acinar or ductal cells, was reduced in FoxM1Δpanc mice versus control littermates following PPx, suggesting that FoxM1 is required specifically within endocrine cells. These data concur with other studies (17–19,31) showing that endocrine cells are particularly susceptible to perturbations in cell cycle regulation. The current study utilized quantitative RT-PCR on islet RNA to assess changes in Foxm1 expression following PPx. Unfortunately, antibodies to detect FoxM1 protein in mouse tissue do not currently exist, precluding precise identification of the cell population(s) in which FoxM1 expression or localization changes with time and after PPx. Such studies will allow for better understanding of cell type–specific requirements for FoxM1 and may help to explain the differing effects of FoxM1 absence that we observe between endocrine and exocrine cells following PPx. However, our expression analyses identified several potentially important regulators of pancreatic endocrine cell proliferation and regeneration that are themselves regulated by FoxM1 (Plk1, Cenp-a, Birc5/survivin, and Ccnb1).
We also presented evidence of β-cell neogenesis following 60% PPx, including Ngn3+ duct cells, insulin+ cells within regenerating tissue, and increased islet density. These findings were equivalent between control and FoxM1Δpanc mice, revealing that β-cell neogenesis does not require FoxM1. Peshavaria et al. (25) showed increased small endocrine cell clusters by 2 days post-PPx, which normalized by 7 days. Our data are consistent with this finding, as control mice at 7 days post-PPx exhibited a normal proportion of small endocrine cell clusters. However, FoxM1Δpanc mice had an increased proportion of small endocrine cell clusters at 7 days post-PPx, indicating that formation of these clusters is not impaired in the absence of FoxM1, but significant growth is. These data support our hypothesis that initial proliferation of newly differentiated β-cells does not require FoxM1, but these cells later switch to a FoxM1-dependent state. Unfortunately, current technology precludes real-time monitoring of β-cell proliferation and islet growth. However, this study highlights the importance of β-cell proliferation as a mechanism of β-cell mass regeneration, as our data show that neogenesis, without adequate proliferation, is not sufficient to regenerate a significant amount of β-cell mass after 60% PPx.
As β-cell regeneration after partial duct ligation mimics embryonic β-cell differentiation with Ngn3 reexpression (6), it is not surprising that similar processes occur following 60% PPx, although perhaps to a lesser extent. Previous studies (5,32) asserting the lack of reactivation of the embryonic program, and thus neogenesis, following PPx were performed in a manner that likely precluded true regeneration, with surgical excision of the splenic lobe and pancreatic duct, while other studies (4) did not analyze the splenic lobe specifically, possibly missing the contribution of neogenesis and regeneration to new β-cell formation. The current study removed tissue from the splenic lobe, leaving the main pancreatic duct intact. This lobe was analyzed separately, and only specific foci of regeneration exhibited evidence of neogenesis. Thus, this study does not contradict but adds to the current knowledge regarding the potential for pancreas regeneration and β-cell neogenesis.
FOXM1 overexpression did not affect β-cell regeneration following PPx, suggesting that FoxM1 is not sufficient to induce β-cell proliferation, emphasizing that the β-cell cell cycle is under tight negative regulatory control, even after injury. As reviewed (33), β-cells express all of the cell cycle “brakes” studied thus far but only some “accelerators.” Thus, it may be more difficult than initially anticipated to stimulate β-cell proliferation in vivo or in vitro as a therapeutic option for diabetic patients. Because FoxM1 positively regulates cell cycle accelerators and negatively regulates cell cycle brakes, it is of particular interest. However, better understanding of posttranslational and inhibitory regulators is necessary to successfully manipulate FoxM1 activity in β-cells.
Supplementary Material
Acknowledgments
Islets were isolated by Zhongyi Chen and Anastasia Golovin of the Vanderbilt Islet Procurement and Analysis Core, supported by the Diabetes Research and Training Center (P60 DK20593). RNA quality was assessed by the Vanderbilt Microarray Shared Resource, supported by the Vanderbilt Ingram Cancer Center (P30 CA68485), the Vanderbilt Digestive Disease Center (P30 DK58404), and the Vanderbilt Vision Center (P30 EY08126). A.A.M. is supported by an American Diabetes Association Medical Scholars Training Award (7-07 MS-01) and by the Vanderbilt Medical Scientist Training Program (T32 GM007347). M.G. is supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK071052).
We thank Elizabeth Tweedie Ables, Michelle Guney, and Renuka Menon for critical reading of the manuscript and helpful suggestions. We also thank Young Ah Oh, Christine Pope, Xue Feng, Usa G. Kopsombut, Andre Boustani, and Jacob Kaufman for their technical assistance.
This article is dedicated to R.H.C., who passed away during the course of this study.
Published ahead of print at http://diabetes.diabetesjournals.org on 26 August 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Shapiro AMJ, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems J-A, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DER, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JRT: International Trial of the Edmonton Protocol for Islet Transplantation. N Engl J Med 355: 1318–1330, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Finegood DT, Scaglia L, Bonner-Weir S: Dynamics of β-cell mass in the growing rat pancreas: estimation with a simple mathematical model. Diabetes 44: 249–256, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Ackermann AM, Gannon M: Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol 38: 193–206, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429: 41–46, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA: Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 12: 817–826, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van De Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H: Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132: 197–207, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Meier J, Bhushan A, Butler A, Rizza R, Butler P: Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 48: 2221–2228, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Meier J, Lin J, Butler A, Galasso R, Martinez D, Butler P: Direct evidence of attempted beta cell regeneration in an 89-year-old patient with recent-onset type 1 diabetes. Diabetologia 49: 1838–1844, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Ackermann AM, Gusarova GA, Lowe D, Feng X, Kopsombut UG, Costa RH, Gannon M: The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Mol Endocrinol 20: 1853–1866, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Costa RH: FoxM1 dances with mitosis. Nat Cell Biol 7: 108–110, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Laoukili J, Stahl M, Medema RH: FoxM1: At the crossroads of ageing and cancer. Biochim Biophys Acta 1775: 92–102, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Kiyokawa H, Dennewitz MB, Costa RH: The forkhead box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci U S A 99: 16881–16886, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung TWC, Lin SSW, Tsang ACC, Tong CSW, Ching JCY, Leung WY, Gimlich R, Wong GG, Yao KM: Over-expression of FoxM1 stimulates cyclin B1 expression. FEBS Lett 507: 59–66, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Wang I, Chen Y, Hughes D, Petrovic V, Major M, Park H, Tan Y, Ackerson T, Costa R: Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol 25: 10875–10894, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laoukili J, Kooistra MRH, Bras A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH: FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol 7: 126–136, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Krupczak-Hollis K, Wang X, Kalinichenko VV, Gusarova GA, Wang I-C, Dennewitz MB, Yoder HM, Kiyokawa H, Kaestner KH, Costa RH: The mouse forkhead box m1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Dev Biol 276: 74–88, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Georgia S, Bhushan A: Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest 114: 963–968, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF: Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol 25: 3752–3762, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M: Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat Genet 22: 44–52, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Gu G, Dubauskaite J, Melton DA: Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129: 2447–2457, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Gannon M, Gamer LW, Wright CV: Regulatory regions driving developmental and tissue-specific expression of the essential pancreatic gene pdx1. Dev Biol 238: 185–201, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Stoffers DA, Heller RS, Miller CP, Habener JF: Developmental expression of the homeodomain protein IDX-1 in mice transgenic for an IDX-1 promoter/lacZ transcriptional reporter. Endocrinology 140: 5374–5381, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, Yoder HM, Costa RH: Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury. J Biol Chem 278: 37888–37894, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Ye H, Holterman AX, Yoo KW, Franks RR, Costa RH: Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Mol Cell Biol 19: 8570–8580, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peshavaria M, Larmie BL, Lausier J, Satish B, Habibovic A, Roskens V, Larock K, Everill B, Leahy JL, Jetton TL: Regulation of pancreatic β-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes 55: 3289–3298, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Inada A, Weir GC, Bonner-Weir S: Induced ICER I-gamma down-regulates cyclin A expression and cell proliferation in insulin-producing beta cells. Biochem Biophys Res Commun 329: 925–929, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S: The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes 48: 507–513, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Spooner BS, Walther BT, Rutter WJ: The development of the dorsal and ventral mammalian pancreas in vivo and in vitro. J Cell Biol 47: 235–246, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gradwohl G, Dierich A, LeMeur M, Guillemot F: neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A 97: 1607–1611, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrovic V, Costa RH, Lau LF, Raychaudhuri P, Tyner AL: FoxM1 regulates growth factor-induced expression of kinase-interacting stathmin (KIS) to promote cell cycle progression. J Biol Chem 283: 453–460, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Mettus RV, Rane SG: Characterization of the abnormal pancreatic development, reduced growth and infertility in Cdk4 mutant mice. Oncogene 22: 8413–8421, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Lee CS, De Leon DD, Kaestner KH, Stoffers DA: Regeneration of pancreatic islets after partial pancreatectomy in mice does not involve the reactivation of neurogenin-3. Diabetes 55: 269–272, 2006 [PubMed] [Google Scholar]
- 33.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada R, Stewart AF: Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev 27: 356–370, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.