Abstract
OBJECTIVE—YKL-40 is produced by macrophages, and plasma YKL-40 is elevated in patients with diseases characterized by inflammation. In the present study, YKL-40 was examined in relation to obesity, inflammation, and type 2 diabetes.
RESEARCH DESIGN AND METHODS—Plasma YKL-40 and adipose tissue YKL-40 mRNA levels were investigated in 199 subjects who were divided into four groups depending on the presence or absence of type 2 diabetes and obesity. In addition, plasma YKL-40 was examined in healthy subjects during a hyperglycemic clamp, in which the plasma glucose level was kept at 15 mmol/l for 3 h, and during a hyperinsulinemic-euglycemic clamp.
RESULTS—Patients with type 2 diabetes had higher plasma YKL-40 (76.7 vs. 45.1 ng/ml, P = 0.0001) but not higher expression in adipose tissue YKL-40 mRNA (1.20 vs. 0.98, P = 0.2) compared with subjects with a normal glucose tolerance. Within the groups with normal glucose tolerance and type 2 diabetes, obesity subgroups showed no difference with respect to either plasma YKL-40 or adipose tissue YKL-40 mRNA levels. Multivariate regression analysis showed that plasma YKL-40 was associated with fasting plasma glucose (β = 0.5, P = 0.0014) and plasma interleukin (IL)-6 (β = 0.2, P = 0.0303). Plasma YKL-40 was not related to parameters of obesity. There were no changes in plasma YKL-40 in healthy subjects during either hyperglycemic or hyperinsulinemic-euglycemic clamps.
CONCLUSIONS—Plasma YKL-40 was identified as an obesity-independent marker of type 2 diabetes related to fasting plasma glucose and plasma IL-6 levels.
YKL-40 (chitinase-3-like-1 [CHI3L1], human cartilage glycoprotein-39), is a heparin-, chitin-, and collagen-binding lectin produced by immunologically active cells such as macrophages (1) and neutrophils (2). YKL-40 is a member of the mammalian chitinase-like proteins and is a phylogenetically highly conserved serum protein (1,3–5). Other cells shown to produce YKL-40 are vascular smooth muscle and endothelia cells (6–8), arthritic chondrocytes (3), cancer cells (9), and embryonic and fetal cells (10). The exact functions of YKL-40 are unknown. Currently, YKL-40 is known to stimulate growth of fibroblast cells (11), activate the AKT and phosphoinositide-3 kinase signaling pathway, exert antiapoptosis (12), and function in angiogenesis (7) and may take part in the innate immune response (13). High plasma concentrations of YKL-40 are found in patients with diseases characterized by inflammation or increased tissue remodeling or with cancer (1,9).
Adipose tissue is recognized as a source of inflammation (14–16). A high BMI is associated with increased levels of proinflammatory cytokines, and obesity is characterized as a state of chronic systemic low-grade inflammation (17). Studies demonstrate an accumulation of activated macrophages and other immune active cells in adipose tissue from obese subjects (17,18) as possible sources of inflammatory cytokines, determining a link between obesity, low-grade inflammation, and insulin resistance, and both obesity and low-grade inflammation have been linked with the development of insulin resistance and type 2 diabetes (19).
One previous study (20) has shown an elevation of serum YKL-40 in type 2 diabetes. In the present study, using plasma and adipose tissue biopsy material from 103 healthy control subjects and 96 patients with type 2 diabetes with a wide range of BMI, we studied the possible relationship between plasma YKL-40 and adipose tissue expression of YKL-40 on the one hand and obesity, insulin resistance, and inflammation on the other.
We further measured the macrophage marker CD68 in adipose tissue. We hypothesized that macrophages in the adipose tissue might secrete YKL-40 and that plasma YKL-40 would represent macrophage infiltration in adipose tissue and serve as a marker of insulin resistance. In order to obtain further information about the regulation of systemic YKL-40, we examined plasma YKL-40 during hyperglycemic and hyperinsulinemic-euglycemic conditions.
RESEARCH DESIGN AND METHODS
Cohort study.
Using a cross-sectional, case-control design, the participants in this study were divided into four distinct groups according to BMI (<30 or ≥30 kg/m2) and according to normal glucose tolerance and the diagnosis of type 2 diabetes. To verify correct diagnosis, an oral glucose tolerance test was performed and the World Health Organization diagnostic criteria for diabetes were used. Participants were carefully screened, and exclusion criteria were treatment with insulin, recent or ongoing infection, history of malignant disease, or treatment with anti-inflammatory drugs. Subjects and protocol have been previously described (21,22). Participants (n = 199) were given both oral and written information about the experimental procedures before giving their written informed consent.
Subjects.
Participants reported to the laboratory between 8 and 10 a.m. after an overnight fast. Medication was paused for 24 h and oral antidiabetes medication for 1 week before the examination day. A general health examination was performed; blood samples were drawn from an antecubital vein, adipose tissue biopsy was obtained, an oral glucose tolerance test and a fitness test were performed (cardiorespiratory fitness was measured by the Åstrand-Rhyming indirect test of maximal oxygen uptake [Vo2max]) (23), and subjects were scanned on a dual-energy X-ray absorptiometry whole-body scanner, as previously described (21,22).
Blood analysis.
Plasma concentrations of YKL-40 were determined in duplicate by a commercial enzyme-linked immunosorbent assay (Quidel, San Diego, CA) (24). The recovery is 102%, detection limit 20 μg/l, intra-assay coefficient of variation < 5.0%, and interassay coefficient of variation <6.3%. Plasma concentrations of tumor necrosis factor (TNF)-α (intra- and interassay coefficient of variation 8.8 and 16.7%, respectively) and interleukin (IL)-6 (intra- and interassay coefficient of variation 6.9 and 9.6%, repectively) were measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). Plasma C-reactive protein (intra- and interassay coefficient of variation 2.8 and 4.6%, respectively) was measured at the Department of Clinical Biochemistry, Rigshospitalet, Copenhagen, Denmark, using Tina-quant CRPLX (Roche Diagnostics, Mannheim, Germany). Other measurements have been previously described (21,22).
Adipose tissue YKL-40 mRNA and CD68 mRNA.
Adipose tissue biopsies were obtained from abdominal subcutaneous adipose tissue, as previously described (22). Real-time PCR was performed using predeveloped TaqMan assays (Applied Biosystems, Foster City, CA) for YKL-40 (Hs00542562_m1), CD68 (Hs00154355_m1), and endogenous control glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Hs99999905_m1). The mRNA content of both targets and GAPDH were calculated from the cycle threshold values using the standard curve method, and relative expression of YKL-40 and CD68 were determined after normalization to GAPDH. Not all tissue samples resulted in a sufficient amount of cDNA for all analysis, explaining the difference in sample size, as indicated in Fig. 1.
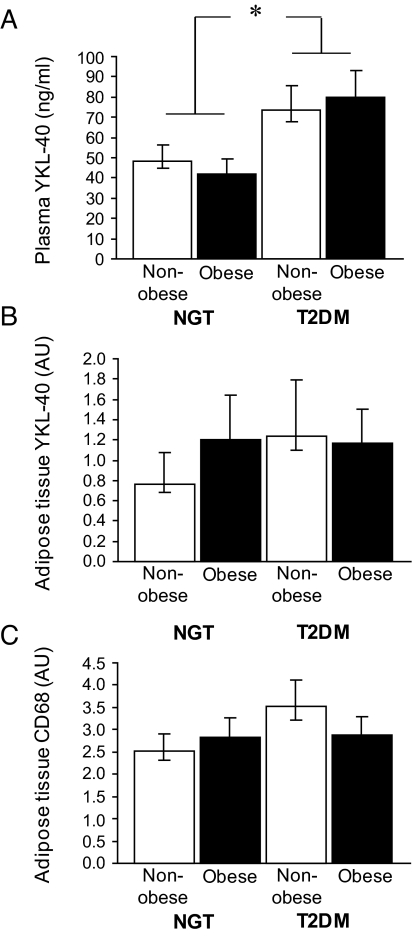
FIG. 1.
A: Plasma concentrations of YKL-40 in the four groups (n = 196): normal glucose tolerance (NGT)/nonobese, NGT/obese, type 2 diabetes (T2DM)/nonobese, and T2DM/obese. B: YKL-40 mRNA/GAPDH mRNA expression level in adipose tissue in the four groups (n = 159). C: CD68 mRNA/GAPDH mRNA expression level in adipose tissue in the four groups (n = 154). Data are presented as geometric means ± SE. Difference between glycemia group (NGT vs. T2DM), *P < 0.001.
Clamp study.
Seven healthy men (mean age 26.7 years [range 23–34]; mean BMI 23.7 kg/m2 [21.2–27.7]) were included, following provision of oral and written informed consent. Before the study, the subjects underwent a clinical examination as previously described (21). All subjects underwent two separate trials at least 1 month apart. One trial comprised a steady-state hyperglycemic clamp (blood glucose clamped at 15 mmol/l) and the other a hyperinsulinemic-euglycemic clamp (blood glucose clamped at 5 mmol/l) in combination with an insulin infusion of 80 mU · min−1 · m−2.
Ethics.
The studies conformed to the Helsinki Declaration and were approved by the ethics committee of Copenhagen and Frederiksberg, Denmark (KF 01-141/04 and 01-257245).
Statistics.
Plasma levels of homeostasis model assessment, version 2 (HOMA2), YKL-40, adipose tissue YKL-40 mRNA, and CD68 mRNA were log10 transformed to approximate normal distribution. Differences between glycemia and obesity groups were tested with a two-way ANOVA (PROC GLM). Multiple regression analysis (PROC REG) was performed to identify whether the level of BMI, parameters of inflammation, and type 2 diabetes (explanatory variable) could explain the variation in plasma YKL-40 and adipose tissue YKL-40 mRNA expression (dependent variable). Using a two-way ANOVA (PROC MIXED), the effect of time and group was tested in the clamp study. Normality of the residuals was assessed graphically. P < 0.05 was considered significant. All analyses were performed with SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
Subject characteristics.
The cohort has previously been described (21). Characteristics of the four main groups included in the present study are shown in Table 1. Plasma YKL-40 within these four groups is shown in Fig. 1A. Plasma YKL-40 was increased in type 2 diabetic patients compared with subjects with normal glucose tolerance, independently of obesity (P < 0.0001). The expression of YKL-40 mRNA and CD68 mRNA in adipose tissue was not different with regard to either glycemia group or obesity (Fig. 1B and C). No interaction between obesity and diabetes were found in plasma or mRNA analyses.
TABLE 1.
Subject characteristics
| Normal glucose tolerance
|
Type 2 diabetes
|
|||
|---|---|---|---|---|
| Nonobese | Obese | Nonobese | Obese | |
| n (male/female) | 62 (42/20) | 41 (28/13) | 50 (38/12) | 46 (34/12) |
| Age (years) | 56 ± 2 | 48 ± 2† | 58 ± 2 | 58 ± 1 |
| BMI (kg/m2) | 25.7 ± 0.4 | 36.7 ± 0.7‡ | 26.6 ± 0.3 | 35.5 ± 0.7‡ |
| HOMA2 | 0.66 (0.60–0.70) | 1.28 (1.09–1.38)‡ | 1.22 (1.01–1.32) | 2.27 (1.97–2.42)‡§ |
Data are means ± SE for continuous variables and geometric means (limits for SE of geometric means), unless otherwise indicated. General characteristics of the study population divided into four groups on the basis of obesity and diagnosis of type 2 diabetes. Normal glucose tolerance/nonobese, normal glucose tolerance/obese, type 2 diabetes/nonobese, and type 2 diabetes/obese. Difference between obesity groups within each glycemia group,
P < 0.01;
P < 0.001. Difference between glycemia group (normal glucose tolerance versus type 2 diabetes),
P < 0.001. For age and BMI, there was an interaction between glycemia group and obesity.
YKL-40 and type 2 diabetes
Plasma YKL-40.
Univariate and multivariate regression analyses with parameters of obesity, type 2 diabetes, and inflammation as the explanatory variables and plasma YKL-40 as the dependent variable, stratified or not according to normal glucose tolerance/type 2 diabetes, are shown in Table 2. In the multivariate analysis, we adjusted for age, sex, fitness, and either plasma TNF-α or fasting plasma glucose since these parameters were highly associated with YKL-40 in the univariate analysis. No interactions were found between glycemia group and the explanatory variables, indicating that the slopes between YKL-40 and the explanatory variables did not differ between subgroups with normal glucose tolerance and those with type 2 diabetes. Therefore, here we focus on the nonstratified analyses.
TABLE 2.
Plasma YKL-40
| Covariate | Normal glucose tolerance
|
Type 2 diabetes
|
Normal glucose tolerance and type 2 diabetes
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate
|
Multivariate
|
Univariate
|
Multivariate
|
Univariate
|
Multivariate
|
||||||||||
| β (95% CI) | R2 | P | β (95% CI) | P | β (95% CI) | R2 | P | β (95% CI) | P | β (95% CI) | R2 | P | β (95% CI) | P | |
| BMI | −0.0 (−0.0 to 0.0) | — | NS | — | — | −0.0 (−0.0 to 0.0) | — | NS | — | — | −0.0 (−0.0 to 0.0) | — | NS | — | — |
| Glucose (0 h) | 0.3 (−1.3 to 2.0) | — | NS | — | — | 0.5 (0.1–0.9) | 0.06 | * | 0.4 (−0.1 to 0.8) | NS | 0.7 (0.4–1.0) | 0.11 | ‡ | 0.5 (0.2–0.8) | † |
| Insulin (0 h) | −0.0 (−0.2 to 0.2) | — | NS | — | — | 0.1 (−0.1 to 0.3) | — | NS | — | — | 0.2 (0.0–0.3) | 0.02 | * | 0.1 (−0.1 to 0.3) | NS |
| HOMA2 | −0.0 (−0.2 to 0.2) | — | NS | — | — | 0.2 (−0.0 to 0.4) | 0.03 | 0.0774 | 0.2 (−0.0 to 0.4) | NS | 0.2 (0.1–0.3) | 0.04 | † | 0.1 (−0.0 to 0.3) | 0.0545 |
| A1C | −2.5 (−5.6 to 0.7) | — | NS | — | — | 0.2 (−0.6 to 1.0) | — | NS | — | — | 0.9 (0.3–1.5) | 0.04 | † | 0.4 (−0.2 to 1.0) | NS |
| IL-6 | 0.0 (−0.2 to 0.2) | — | NS | — | — | 0.3 (0.1–0.5) | 0.07 | * | 0.3 (0.1–0.5) | * | 0.2 (0.0–0.3) | 0.03 | * | 0.2 (0.0–0.3) | * |
| TNF-α | 0.2 (−0.5 to 1.0) | — | NS | — | — | 0.8 (0.2–1.3) | 0.08 | † | 0.4 (−0.2 to 1.0) | NS | 0.8 (0.3–1.2) | 0.06 | ‡ | 0.2 (−0.2 to 0.7) | NS |
| C-reactive protein | 0.0 (−0.2 to 0.2) | — | NS | — | — | 0.1 (−0.1 to 0.3) | — | NS | — | — | 0.1 (−0.0 to 0.2) | — | NS | — | — |
Univariate and multivariate regression analyses with parameters for type 2 diabetes, inflammation, and BMI as predictors of plasma YKL-40 and adipose tissue YKL-40 mRNA expression. Both YKL-40 and all covariate measurements (except BMI) were log10 transformed, and a 1-unit increase, hence, signifies a 10-fold increase. Multivariate analyses were adjusted for age (years), sex (male/female), fitness [log(Vo2 · kg−1 · fat free mass−1)], and either plasma TNF-α (glucose [0 h], insulin [0 h], HOMA2, and A1C), or fasting plasma glucose (IL-6, TNF-α, and C-reactive protein).
P < 0.05;
P < 0.01;
P < 0.001. NS, not significant.
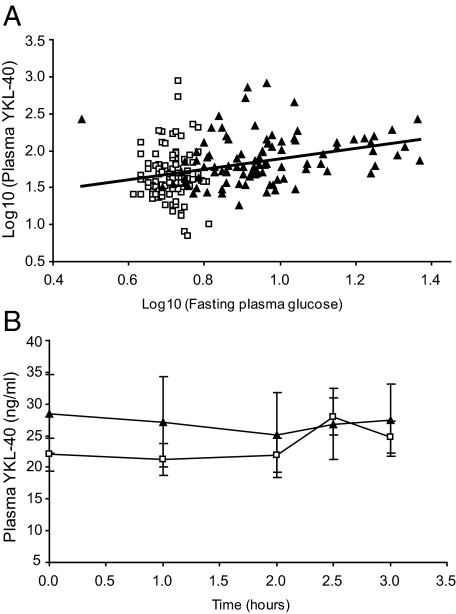
In univariate analysis, plasma YKL-40 was positively associated with fasting plasma glucose (Fig. 2A), fasting plasma insulin, HOMA2, A1C, plasma IL-6, and plasma TNF-α. After adjusting for age, sex, fitness level, and either TNF-α or fasting plasma glucose, plasma YKL-40 was positively associated with fasting plasma glucose (P = 0.0014) and plasma IL-6 (P = 0.0303). There was a tendency toward a positive association between plasma YKL-40 and HOMA2 (P = 0.0545). No association with parameters of obesity was found. Age and fitness level showed separate associations with plasma YKL-40 (β = 0.01, P = 0.0001 and β = −1.0, P = 0.0001, respectively). No interactions between the various covariates and these confounders were found.
FIG. 2.
A: Association between fasting plasma glucose and plasma YKL-40 levels in subjects with normal glucose tolerance (n = 101) (□) and type 2 diabetes patients (n = 95) (▴). Logarithmic data are presented. R2 = 0.11. B: Changes in plasma YKL-40 in healthy subjects during two different clamp conditions. ▴, plasma YKL-40 during hyperglycemic clamp (n = 7). □, plasma YKL-40 during hyperinsulinemic-euglycemic clamp (n = 7). Data are presented as means ± SE.
YKL-40 mRNA.
Univariate and multivariate regression analyses between YKL-40 mRNA in adipose tissue and explanatory variables stratified or not into groups with normal glucose tolerance and groups with type 2 diabetes are presented in Table 3. As for plasma YKL-40, no interaction was found between YKL-40 mRNA and explanatory variables. In the nonstratified univariate analysis, there was an association between adipose tissue YKL-40 mRNA and plasma YKL-40 (P = 0.0134), but this was not present after adjustments. There was no association between adipose tissue CD68 mRNA and plasma YKL-40 (P = 0.75).
TABLE 3.
Adipose tissue YKL-40 mRNA
| Covariate | Normal glucose tolerance
|
Type 2 diabetes
|
Normal glucose tolerance and type 2 diabetes
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate
|
Multivariate
|
Univariate
|
Multivariate
|
Univariate
|
Multivariate
|
||||||||||
| β (95% CI) | R2 | P | β (95% CI) | P | β (95% CI) | R2 | P | β (95% CI) | P | β (95% CI) | R2 | P | β (95% CI) | P | |
| BMI | 0.0 (−0.0 to 0.0) | 0.05 | 0.0603 | 0.0 (−0.0 to 0.0) | NS | −0.0 (−0.0 to 0.0) | — | NS | — | — | 0.0 (−0.0 to 0.0) | — | NS | — | — |
| Glucose (0 h) | −0.0 (−3.0 to 3.0) | — | NS | — | — | 0.7 (−0.0 to 1.4) | 0.05 | 0.0569 | 0.5 (−0.2 to 1.2) | NS | 0.5 (0.1–1.0) | 0.03 | * | 0.4 (−0.1 to 1.0) | NS |
| Insulin (0 h) | 0.4 (0.1–0.8) | 0.06 | * | 0.5 (0.0–1.0) | * | 0.4 (0.0–0.7) | 0.06 | * | 0.4 (0.0–0.7) | * | 0.4 (0.2–0.7) | 0.07 | † | 0.5 (0.2–0.7) | ‡ |
| HOMA2 | 0.4 (0.0–0.8) | 0.06 | * | 0.5 (0.0–1.0) | * | 0.4 (0.1–0.7) | 0.08 | * | 0.4 (0.1–0.7) | NS | 0.4 (0.2–0.7) | 0.08 | † | 0.5 (0.2–0.7) | ‡ |
| A1C | −1.2 (−8.0 to 5.5) | — | NS | — | — | 1.3 (0.0–2.5) | 0.05 | * | 1.0 (−0.3 to 2.2) | — | 1.2 (0.2–2.1) | 0.04 | * | 1.0 (−0.1 to 2.0) | 0.0645 |
| IL-6 | 0.2 (−0.2 to 0.5) | — | NS | — | — | 0.0 (−0.4 to 0.4) | — | NS | — | — | 0.1 (−0.1 to 0.4) | — | NS | — | — |
| TNF-α | −0.5 (−1.8 to 0.9) | — | NS | — | — | 1.0 (0.1–1.9) | 0.06 | * | 0.9 (−0.0 to 1.9) | 0.0622 | 0.6 (−0.1 to 1.3) | — | NS | — | — |
| C-reactive protein | 0.3 (0.0–0.6) | 0.06 | * | 0.3 (−0.1 to 0.6) | NS | 0.0 (−0.3 to 0.3) | — | NS | — | — | 0.2 (−0.0 to 0.4) | 0.02 | 0.0755 | 0.2 (−0.1 to 0.4) | NS |
Univariate and multivariate regression analyses with parameters for type 2 diabetes, inflammation, and BMI as predictors of plasma YKL-40 and adipose tissue YKL-40 mRNA expression. Both YKL-40 and all covariate measurements (except BMI) were log10 transformed, and a 1-unit increase, hence, signifies a 10-fold increase. Multivariate analyses were adjusted for age (years), sex (male/female), fitness [log(Vo2 · kg−1 · fat free mass−1)], and either plasma TNF-α (glucose [0 h], insulin [0 h], HOMA2, and A1C), or fasting plasma glucose (IL-6, TNF-α, and C-reactive protein).
P < 0.05;
P < 0.01;
P < 0.001. NS, not significant.
Adipose tissue YKL-40 mRNA showed positive associations with fasting plasma glucose, fasting plasma insulin, HOMA2, and A1C and a tendency with plasma C-reactive protein (P = 0.0755). In multiple regression analysis, positive associations were found with fasting plasma insulin (P = 0.0018) and HOMA2 (P = 0.0011). There was a tendency to a positive association between YKL-40 mRNA and A1C (P = 0.0645). No association was found between adipose tissue YKL-40 mRNA expression and parameters of inflammation or parameters of obesity.
YKL-40 during clamp.
In healthy subjects, 3 h of hyperglycemic clamp conditions or hyperinsulinemic-euglycemic clamp conditions did not change plasma YKL-40 (Fig. 2B).
DISCUSSION
In the present study, we demonstrated that patients with type 2 diabetes have elevated plasma YKL-40 compared with healthy control subjects. In multivariate regression analysis adjusted for age, sex, fitness, and either plasma TNF-α or fasting plasma glucose, we found significant associations between plasma YKL-40 and fasting plasma glucose and plasma IL-6 but no associations with parameters of obesity.
Plasma IL-6 and obesity (BMI) showed a strong positive association (R2 = 0.2, β = 7.9, P = 0.0001). In addition, we found a relationship between plasma IL-6 and plasma YKL-40 but no association between YKL-40 and markers of obesity. Ongoing studies in our laboratory show that acute elevation of plasma IL-6 (by intravenous infusion) increase plasma YKL-40 in humans. At first glance, it seems paradoxical that plasma YKL-40 is not related to obesity. However, as is evident from Table 2A, plasma IL-6 can only explain 3% of the variation in plasma YKL-40, indicating that other factors may be more important for the production/release of YKL-40. It is possible that acute changes in plasma IL-6, as found in infections, can exert acute changes in plasma YKL-40, whereas in type 2 diabetes, characterized by low-grade inflammation, IL-6 may not be the most important regulator of YKL-40.
The positive correlation between subcutaneous adipose tissue YKL-40 mRNA and plasma YKL-40 support the idea that adipose tissue contributes to circulating YKL-40. Given that YKL-40 is produced by macrophages, it is surprising that CD68 mRNA expression in adipose tissue did not correlate with plasma YKL-40. However, YKL-40 may only be produced by a subgroup of macrophages (CD14+ and CD16+) in adipose tissue, as seen in other diseases (1), in contrast with CD68, which is produced by all monocytes and macrophages. Studying adipose tissue biopsies, we were not able to distinguish between the roles of macrophages and, for example, endothelial cells or smooth muscle cells with regard to the production of YKL-40. It cannot be excluded that adipose tissue is a source of YKL-40 production, though the production of YKL-40 may be attributed to different cells. Furthermore, visceral adipose tissue is more inflamed than subcutaneous adipose tissue and is a possible source of plasma YKL-40, but our study design did not allow for the illumination of the role of visceral fat.
The precise role of YKL-40 remains elusive, but our findings suggest that YKL-40 might be involved in metabolism. However, here we demonstrate that YKL-40 levels do not fluctuate with acute changes in plasma glucose or plasma insulin. Studies are needed to determine the role of adipocytes, macrophages, smooth muscle, and endothelial cells as sources of YKL-40 in adipose tissue and to clarify whether YKL-40 is directly involved in the pathophysiology of type 2 diabetes or may be a more general marker of inflammation. In conclusion, we identify plasma YKL-40 as an obesity-independent marker of type 2 diabetes that is positively associated with fasting plasma glucose and plasma IL-6 level.
Acknowledgments
The Centre of Inflammation and Metabolism is supported by a grant from the Danish National Research Foundation (no. 02-512-55). The study was further supported by grants from the Danish Medical Research Council (no. 22-01-009), the Commission of the European Communities (contract no. LSHM-CT-2004-005272 EXGENESIS), the Copenhagen Hospital Corporation, the University of Copenhagen, and the Overlæge Johan Boserup and Lise Boserups Fond. These granting agencies are all public or nonprofit organizations supporting science in general. Quidel (San Diego, CA) provided the study with the YKL-40 ELISA kits. Quidel played no role whatsoever in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, and approval of the manuscript.
Ruth Rousing and Hanne Villumsen (the Centre of Inflammation and Metabolism, Department of Infectious Diseases and Copenhagen Muscle Research Center, Rigshospitalet, Faculty of Health Sciences, University of Copenhagen, Denmark) and Tonni Løve Hansen and Debbie Nadelmann (Herlev Hospital, University of Copenhagen, Denmark) are acknowledged for their excellent technical assistance. The patients and healthy subjects are thanked for their willingness to participate.
Published ahead of print at http://diabetes.diabetesjournals.org on 23 July 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Johansen JS: Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull 53: 172–209, 2006 [PubMed] [Google Scholar]
- 2.Volck B, Price PA, Johansen JS, Sorensen O, Benfield TL, Nielsen HJ, Calafat J, Borregaard N: YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc Assoc Am Physicians 110: 351–360, 1998 [PubMed] [Google Scholar]
- 3.Hakala BE, White C, Recklies AD: Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem 268: 25803–25810, 1993 [PubMed] [Google Scholar]
- 4.Rehli M, Krause SW, Andreesen R: Molecular characterization of the gene for human cartilage gp-39 (CHI3L1), a member of the chitinase protein family and marker for late stages of macrophage differentiation. Genomics 43: 221–225, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Bussink AP, Speijer D, Aerts JM, Boot RG: Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics 177: 959–970, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shackelton LM, Mann DM, Millis AJ: Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. J Biol Chem 270: 13076–13083, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJ: Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res 250: 168–173, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa KC, Millis AJ: gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Exp Cell Res 287: 79–87, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA: Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev 15: 194–202, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Johansen JS, Hoyer PE, Larsen LA, Price PA, Mollgard K: YKL-40 protein expression in the early developing human musculoskeletal system. J Histochem Cytochem 55: 1213–1228, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Recklies AD, White C, Ling H: The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J 365: 119–126, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling H, Recklies AD: The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. Biochem J 380: 651–659, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickey BF: Exoskeletons and exhalation. N Engl J Med 357: 2082–2084, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM: Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95: 2409–2415, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW: Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 82: 4196–4200, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Lee YH, Pratley RE: The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep 5: 70–75, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A: Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 49: 744–747, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sell H, etze-Schroeder D, Eckel J: The adipocyte-myocyte axis in insulin resistance. Trends Endocrinol Metab 17: 416–422, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Rathcke CN, Johansen JS, Vestergaard H: YKL-40, a biomarker of inflammation, is elevated in patients with type 2 diabetes and is related to insulin resistance. Inflamm Res 55: 53–59, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AM, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H, Pedersen BK: Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 50: 431–438, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Plomgaard P, Nielsen AR, Fischer CP, Mortensen OH, Broholm C, Penkowa M, Krogh-Madsen R, Erikstrup C, Lindegaard B, Petersen AM, Taudorf S, Pedersen BK: Associations between insulin resistance and TNF-alpha in plasma, skeletal muscle and adipose tissue in humans with and without type 2 diabetes. Diabetologia 50: 2562–2571, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Strand PO, Ryhming I: A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. J Appl Physiol 7: 218–221, 1954 [DOI] [PubMed] [Google Scholar]
- 24.Harvey S, Weisman M, O'Dell J, Scott T, Krusemeier M, Visor J, Swindlehurst C: Chondrex: new marker of joint disease. Clin Chem 44: 509–516, 1998 [PubMed] [Google Scholar]