Abstract
OBJECTIVE—Long-standing type 1 diabetes is associated with deficits on neurocognitive testing that suggest central white matter dysfunction. This study investigated whether diffusion tensor imaging (DTI), a type of magnetic resonance imaging that measures white matter integrity quantitatively, could identify white matter microstructural deficits in patients with long-standing type 1 diabetes and whether these differences would be associated with deficits found by neurocognitive tests.
RESEARCH DESIGN AND METHODS—Twenty-five subjects with type 1 diabetes for at least 15 years and 25 age- and sex-matched control subjects completed DTI on a 3.0 Tesla scanner and a battery of neurocognitive tests. Fractional anisotropy was calculated for the major white matter tracts of the brain.
RESULTS—Diabetic subjects had significantly lower mean fractional anisotropy than control subjects in the posterior corona radiata and the optic radiation (P < 0.002). In type 1 diabetic subjects, reduced fractional anisotropy correlated with poorer performance on the copy portion of the Rey-Osterreith Complex Figure Drawing Test and the Grooved Peg Board Test, both of which are believed to assess white matter function. Reduced fractional anisotropy also correlated with duration of diabetes and increased A1C. A history of severe hypoglycemia did not correlate with fractional anisotropy.
CONCLUSIONS—DTI can detect white matter microstructural deficits in subjects with long-standing type 1 diabetes. These deficits correlate with poorer performance on selected neurocognitive tests of white matter function.
Type 1 diabetes is a complex metabolic disease that can have devastating effects on multiple organ systems. Although complications involving the kidneys, nerves, and eyes have long been recognized, the effect of diabetes on cognition has not been as clearly understood. Patients with type 1 diabetes have been found to have deficits on standard neurocognitive tests of information processing (1–4), psychomotor efficiency (1,2), motor speed (3,5–7), visuoconstruction (4,8), attention (4), somatosensory examination, motor strength (7), and executive function (9). Based on the type of cognitive deficits observed in patients with diabetes, there has been speculation that abnormalities in white matter may be at least partly responsible for cognitive dysfunction, particularly in patients with type 1 diabetes (2). Supporting this hypothesis, several studies have found gross morphological changes (10) and reduced white matter volume as measured by voxel-based morphometry (4) in patients with type 1 diabetes. However, prior studies have not looked specifically at the microstructural aspects of white matter integrity.
Over the last decade, a new type of magnetic resonance imaging (MRI) called diffusion tensor imaging (DTI) has been developed that is uniquely suited to assess white matter microstructure. DTI measures the magnitude and directionality of water diffusion in tissues, which may permit identification of tissue injury before it has progressed to the point of detection by more conventional imaging techniques. Without barriers, water molecules move uniformly in all directions, a phenomenon referred to as isotropic diffusion. In the presence of barriers, such as cell membranes, fibers, and myelin, the diffusion rate is greater in one direction, which is termed anisotropic diffusion. Fractional anisotropy provides a quantitative measure of the degree of diffusion anisotropy. Fractional anisotropy is high in regularly organized and structured white matter, such as the corpus callosum, and is lower in less organized tissues, such as gray matter (11,12). Previous DTI studies have shown that reduced fractional anisotropy correlates to cognitive dysfunction in a variety of conditions, including schizophrenia (13,14), depression (15), chronic alcohol use (16), Alzheimer's disease (17,18), and chronic cocaine use (19). In addition, a decrease in brain fractional anisotropy correlated negatively with viral load in patients with known HIV infection (20). This is of particular interest because patients living with HIV have many of the cognitive impairments that patients with type 1 diabetes exhibit, including decreased attention and speed of information processing (11).
To determine whether abnormalities in white matter microstructure might underlie the cognitive deficiencies identified in patients with longstanding type 1 diabetes, we used DTI to obtain quantitative data about white matter structural integrity in this population. We hypothesized that patients with long-standing type 1 diabetes will have decreased fractional anisotropy when compared with matched controls. To determine whether any identified reductions in fractional anisotropy correlated with abnormalities in neurocognitive function, subjects also completed a battery of standardized neurocognitive tests to assess performance on tasks believed to be supported by white matter regions. This analysis was done to test the hypothesis that fractional anisotropy will correlate with neurocognitive test performance in patients with type 1 diabetes, specifically with tests that depend on white matter integrity.
RESEARCH DESIGN AND METHODS
Twenty-five type 1 diabetic subjects were recruited from the Endocrine Clinics at the University of Minnesota Medical Center–Fairview and through institutional review board–approved fliers distributed around the University of Minnesota campus. To ensure that the subjects resembled those included in previous studies assessing cognitive function in diabetes (3,21), only subjects with a diabetes duration of 15 years or more were eligible for participation. Exclusion criteria included a history of or current evidence of any substance abuse disorder other than tobacco or caffeine dependence; severe psychiatric disorder, including major depressive disorder; seizure disorder (not related to hypoglycemia); transient ischemic attack; stroke; head injury; other diseases of the central nervous system; chemotherapy; and any condition that precluded the performance of MRI (weight >300 lbs [subjects of this size cannot fit into the magnetic resonance instrument], presence of metal implants or shrapnel, claustrophobia, etc.). Education history was also assessed. Twenty-five healthy volunteers without diabetes and free of the exclusion criteria were recruited from the University of Minnesota community to match the subjects with respect to age and sex (Table 1).
TABLE 1.
Characteristics of subjects
| Subjects with type 1 diabetes | Control subjects | |
|---|---|---|
| n | 25 | 25 |
| Age | 45.1 ± 10.5 | 45.6 ± 10.8 |
| Sex (F/M) | 17/8 | 17/8 |
| Education (years) | 16.7 ± 1.9 | 16.1 ± 2.3 |
| Duration of diabetes (years) | 30.3 ± 10.8 | NA |
| A1C (%) | 7.4 ± 1.0 | NA |
| Blood glucose before MRI (mmol/l [mg/dl]) | 9.3 ± 3.6 (168 ± 64) | NA |
| Blood glucose before neurocognitive testing (mmol/l [mg/dl]) | 8.4 ± 2.7 (152 ± 49) | NA |
Data are means ± SD. NA, not applicable.
All subjects reported to the General Clinical Research Center at the University of Minnesota for neurocognitive testing after breakfast or lunch. On a separate day but within 2 weeks of the neurocognitive testing, subjects reported to the Center for Magnetic Resonance Research at the University of Minnesota for DTI, again after either breakfast or lunch. Diabetic subjects were instructed to manage their condition in their usual manner. Upon arrival, diabetic subjects performed fingerstick blood glucose testing to ensure that blood glucose was between 5.5–13.9 mmol/l (100–250 mg/dl). If blood glucose was outside of this glycemic range, appropriate therapy was administered by the investigator to bring them into target within 1 h or the study was rescheduled. A1C was also measured in diabetic subjects using a blood sample obtained on the day of neurocognitive testing.
Neurocognitive testing.
The neurocognitive testing battery included the Wechsler Abbreviated Scale of Intelligence (WASI) and tests of psychomotor speed and executive function that have been found to be impaired in subjects with white matter disease (22,23). These tests included the Paced Auditory Serial Addition Test (PASAT), the Digit Vigilance Test (DVT), Trails A and B, the Benton Judgment of Line Orientation Test (JLO), the Rey-Osterreith Complex Figure Drawing Test (Rey-O), the Grooved Pegboard Test, and the Conners’ Continuous Performance Test–Second Edition (CPT-II). Each testing session lasted 2–3 h. All tests were administered and scored by trained personal and interpreted by one of the authors (F.S.A.). Comparison of diabetic subjects and control subjects was based on the Rey-O (copy and delayed recall), the JLO, the Grooved Pegboard Test (dominant and nondominant hands), the DVT (total time needed to complete), the WASI (full-scale IQ score), and the PASAT (with numbers being separated by 2.4, 2.0, 1.6, and 1.2 s). The System Checklist-90-R was also given as a psychological screen.
MRI collection.
DTI was performed by positioning the field of view to cover the entire cerebrum. Acquisition parameters for the axially acquired, dual spin echo, single shot, echo planar, diffusion-weighted sequence were repetition time 10,500 ms, echo time 98 ms, matrix 128 × 128, field of view 256 mm, slice thickness 2 mm, 64 contiguous slices, 2 averages, and a b value 1,000. A volume without diffusion weighting and 12 volumes with noncollinear diffusion directions were acquired. A B0 fieldmap sequence with identical voxel parameters to the DTI scan was acquired after the DTI sequence. A three-dimensional T1 Magnetization Prepared Rapid Gradient Echo sequence with repetition time 2,530 ms, echo time 3.63 ms, inversion time 1,100 ms, isotropic voxel 1 mm, field of view 256 mm, and matrix 256 × 256 was also acquired to provide anatomical information.
DTI data processing.
Diffusion-weighted images were processed using tools from the FMRIB Software Library (FSL) (24). Eddy current-correction of the individual diffusion-weighted echo-planar images was performed using an affine transformation (25), and magnetic field susceptibility effects were corrected using the B0 fieldmap image. The diffusion tensor was calculated using the nondiffusion and 12 diffusion images, and fractional anisotropy maps were created using eq. 1 below, where FA is fractional anisotrophy and λ1, λ2, and λ3 represent values along the three major axes of diffusion (26).
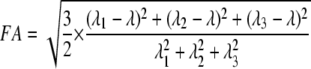 |
1 |
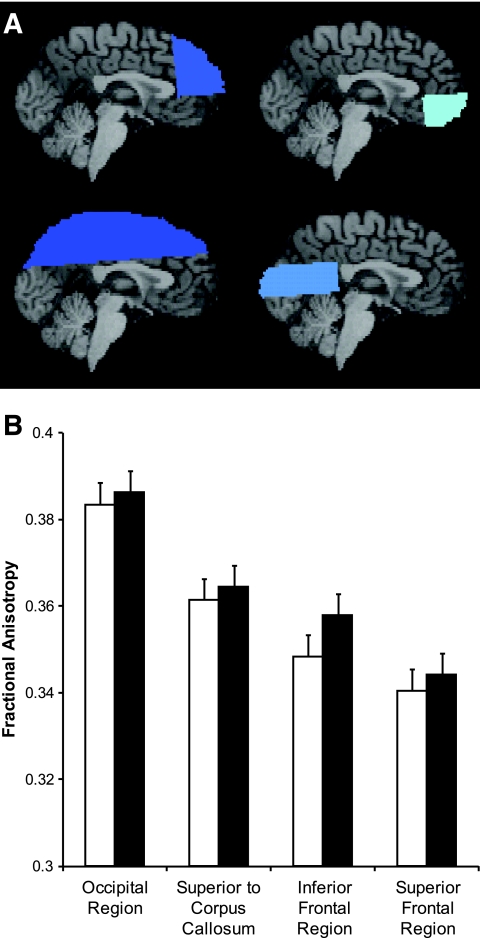
The mean fractional anisotropy was computed for the white matter of four brain regions defined by anatomical landmarks that include the genu and splenium of the corpus callosum and the anterior and posterior commisures (Fig. 1A). The Tract-Based Spatial Statistics (TBSS) analysis program bundled with FSL (27) was used to determine average fractional anisotropy values at the center of several tracts of interest. TBSS generated a fractional anisotropy skeleton of the major white matter tracts of the brain for each subject. The fractional anisotropy map from the control subject with a brain size at the median for the controls was used to align the fractional anisotropy maps from the other control subjects and diabetic subjects. Once the fractional anisotropy maps were aligned, a common white matter skeleton was generated and then further segmented into anatomically defined tracts using the primary eigenvector information for the target subject's DTI data based on atlas information from Mori et al. (28). These tracts were selected on the basis of relative size and ease of differentiation from surrounding tracts. The resulting tracts from this procedure included bilateral forceps minor, cingulum bundle, medial corona radiata, superior longitudinal fasciculus, and optic radiation (Fig. 2A). Additionally, six regions of interest (genu, rostral body, anterior midbody, posterior midbody, isthmus, and splenium; Fig. 2A) in the corpus callosum were generated using standardized subdivisions (29).
FIG. 1.
Fractional anisotropy in major brain regions. A: Superior frontal region, inferior frontal region, occipital region, and region superior to corpus callosum (going clockwise starting at top left). There is ∼15% overlap between the frontal regions and the region superior to the corpus callosum. B: Fractional anisotropy in major brain regions; there was no significant difference in any of the major regions. □, type 1 diabetic subjects; ▪, control subjects. Data are means ± SE.
FIG. 2.
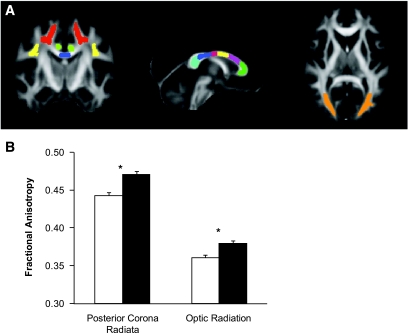
Fractional anisotropy of specific white matter tracts. A: Left-coronal view: corona radiata* (red), superior longitudinal fasciculus (yellow), corpus callosum isthmus (blue), and cingulum (green). Middle-saggital view: genu (green), rostral body (purple), anterior midbody (yellow), posterior midbody (red), isthmus (blue), and splenium (teal) of corpus callosum. Right-axial view: optic radiation* (orange). *P < 0.002 between diabetic subjects and controls. B: Fractional anisotropy in posterior corona radiata and optic radiation. □, type 1 diabetic subjects; ▪, control subjects. *P < 0.002 between diabetic subjects and controls. Data are means ± SE. (Please see http://dx.doi.org/10.2337/db08-0724 for a high-quality digital representation of this image.)
Comparison of diabetic subjects and control subjects was done with three separate groups of neural regions: 1) superior frontal region, inferior frontal region, occipital region, and region superior to corpus callosum (Fig. 1A); 2) optic radiation, splenium of the corpus callosum, and posterior corona radiata (Fig. 2A); and 3) forceps minor, nonsplenium subdivisions of the corpus callosum (genu, rostral body, anterior midbody, posterior midbody, and isthmus), right cingulum, left cingulum, right superior longitudinal fasciculus, left superior longitudinal fasciculus, and medial corona radiata (Fig. 2A).
Statistics.
Neurocognitive test scores were compared between diabetic subjects and control subjects using t tests as an initial screening. Tests thought to be associated with white matter function were compared simultaneously in a general linear mixed model (SAS Proc Mixed) with a random intercept for each subject-pair and an unstructured covariance matrix for the repeated test scores within subjects. The same model was used with the three groups of brain regions to compare fractional anisotropy measurements between diabetic subjects and control subjects. Results are reported as mean ± SE. Spearman correlation is reported as a measure of association. All computations were performed in SAS version 9.1.3 (SAS Institute, Cary, NC). Throughout this statistical analysis, all results were corrected for multiple comparisons.
RESULTS
Demographic data.
Clinical and demographic characteristics of the 25 type 1 diabetic subjects and the 25 age- and sex-matched healthy controls are summarized in Table 1. The diabetic subjects and controls were similar in all of the demographic measures collected. Three diabetic subjects reported a history of retinopathy, two reported a history of gastroparesis, and three reported histories of both retinopathy and neuropathy. All but one subject who reported retinopathy had intervention (laser therapy or vitrectomy). No diabetic subjects reported a history of nephropathy. Fifteen of the 25 diabetic subjects reported a history of severe hypoglycemia, defined as having seizures, loss of consciousness, or needing another person's help to treat the symptoms of low blood glucose.
Neurocognitive testing data.
No tests included in our battery distinguished type 1 diabetic subjects from control subjects. However, diabetic subjects appeared to have a tendency to perform more poorly on the Rey-O, a test believed to be a measure of white matter function that requires a high degree of visuospatial orientation and planning. This test is scored based on the number of correctly copied elements, and the diabetic subjects had a mean score of 31 ± 0.6, and the controls scored 33 ± 0.6 (P = 0.063).
DTI data.
In the four large regions of analysis (inferior frontal, superior frontal, superior to the corpus callosum, and occipital regions with ∼15% overlap between the frontal regions and the region superior to the corpus callosum), fractional anisotropy was lower but not significantly in type 1 diabetic subjects (Fig. 1). In more specific major white matter tracts, diabetic subjects had significantly lower fractional anisotropy than control subjects in both the posterior corona radiata (0.443 ± 0.004 vs. 0.471 ± 0.004, P < 0.0001) and the optic radiation (0.361 ± 0.004 vs. 0.380 ± 0.004, P = 0.0017) (Fig. 2). Type 1 diabetic subjects also tended to have lower fractional anisotropy than control subjects in the splenium (0.806 ± 0.006 vs. 0.820 ± 0.006, P = 0.0947) and the posterior body of the corpus callosum (0.805 ± 0.007 vs. 0.821 ± 0.007, P = 0.1397).
Correlation of DTI and neurocognitive testing data.
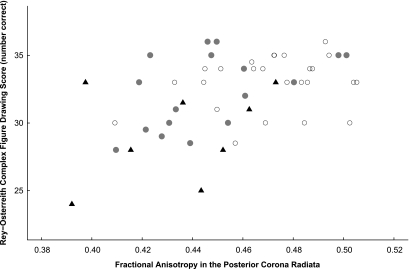
In diabetic subjects, there was a significant correlation between performance on the copying portion of the Rey-O and fractional anisotropy in the posterior corona radiata, with poorer scores associated with reduced fractional anisotropy (Table 2). These subjects also demonstrated a significant association between longer time required to complete the Grooved Pegboard Test, using both the nondominant hand and dominant hand, and reduced fractional anisotropy in the optic radiation, posterior corona radiata, and splenium of the corpus callosum (Table 2). Control subjects demonstrated a similar correlation between performance on the Grooved Pegboard Test and fractional anisotropy in the optic radiation (dominant hand only) and, unlike diabetic subjects, the fractional anisotropy in the posterior body of the corpus callosum (both hands) (Table 2). For all subjects, correlation between Rey-O and fractional anisotropy in the posterior corona radiata is shown in Fig. 3. To assess the effects of microvascular complications, the group with diabetes was divided into those with (n = 8) and without (n = 17) such complications; both subgroups had significantly lower fractional anisotropy in the posterior corona radiata than controls, and those with microvascular complications also had significantly lower Rey-O scores (Table 3).
TABLE 2.
Spearman correlation coefficients between fractional anisotropy and neurocognitive measurements (scores) in type 1 diabetic subjects and control subjects
| Optic radiation
|
Posterior corona radiata
|
Splenium corpus callosum
|
Posterior body of corpus callosum
|
|||||
|---|---|---|---|---|---|---|---|---|
| Type 1 diabetes | Control | Type 1 diabetes | Control | Type 1 diabetes | Control | Type 1 diabetes | Control | |
| Rey-O copy | 0.10 | −0.10 | 0.46* | 0.16 | 0.06 | 0.06 | −0.07 | 0.02 |
| Grooved Peg Board, nondominant | −0.66* | −0.42* | −0.72* | −0.04 | −0.56* | −0.20 | −0.32 | −0.60* |
| Grooved Peg Board, dominant | −0.40* | −0.39 | −0.70* | 0.09 | −0.48* | −0.05 | −0.24 | −0.53* |
Significantly different from zero (P < 0.05).
FIG. 3.
Rey-O copy score versus fractional anisotropy in the posterior corona radiata. Overall Spearman correlation = 0.43 (P = 0.0018). ○, Control subjects;  , diabetic subjects without microvascular complications; ▴, diabetic subjects with microvascular complications.
, diabetic subjects without microvascular complications; ▴, diabetic subjects with microvascular complications.
TABLE 3.
Comparison of fractional anisotropy in diabetic subjects with microvascular complications, diabetic subjects without microvascular complications, and control subjects
| Diabetic subjects with microvascular complications | Diabetic subjects with no microvascular complications | Control subjects | F test P value | |
|---|---|---|---|---|
| Posterior corona radiata | 0.4350 ± 0.0097a | 0.4470 ± 0.0059a | 0.4710 ± 0.0042b | 0.0003 |
| Rey-O | 30 ± 1.3a | 32 ± 0.8ab | 33 ± 0.6b | 0.0696 |
Data are means ± SE. Comparisons are within rows: means that do not share letters are significantly different (P < 0.05).
Correlation of DTI with characteristics of diabetic subjects.
Age, duration of diabetes, and A1C were negatively correlated with reduced fractional anisotropy of the optic radiation and posterior corona radiata, whereas duration of diabetes was also negatively correlated with reduced fractional anisotropy of the splenium of the corpus callosum (Table 4). No significant correlations were identified between severe hypoglycemia or microvascular complications and reduced fractional anisotropy in any of the major white matter tracts.
TABLE 4.
Spearman correlation coefficients of age, duration of diabetes, and A1C with fractional anisotropy
| Optic radiation | Posterior corona radiata | Splenium | |
|---|---|---|---|
| Age of type 1 diabetic subjects | −0.58* | −0.76* | −0.32 |
| Duration of diabetes | −0.60* | −0.61* | −0.46* |
| A1C | −0.47* | −0.46* | −0.38 |
Significantly different from zero (P < 0.05).
DISCUSSION
In this investigation, we found that white matter integrity, as measured by fractional anisotropy using DTI, was lower in several white matter tracts, including the posterior corona radiata and optic radiation, in patients with long-standing type 1 diabetes compared with age- and sex-matched controls. We also observed there to be a significant correlation between reduced fractional anisotropy in white matter tracts and reduced performance on neurocognitive tests thought to assess white matter function, including the Rey-O copy and the Grooved Pegboard Test. Together, these results demonstrate for the first time that microstructural abnormalities can be identified in the brains of subjects with long-standing type 1 diabetes that may underlie the cognitive dysfunction identified in this population. This work may open the door for the development of a novel biomarker for cognitive dysfunction that can be used to better understand the cerebral complications of diabetes. If such a biomarker can be developed, future study could focus on identifying interventions that can prevent the appearance or slow the progression of white matter microstructural changes in patients with diabetes.
Over the last 10 years, investigators have used a variety of imaging techniques, including MRI, single photon emission computed tomography (30,31), and positron emission tomography (32), to determine whether structural abnormalities could be identified in the brains of patients with type 1 diabetes. Although Biessels et al. (10) observed high signal lesions in cerebral white matter (or leukoaraiosis) in these patients, most studies have not confirmed this finding (1,33,34). Others have found increased cerebral spinal fluid and cerebral global atrophy (35,36), stable hippocampal and amygdala volumes (36), and decreased cerebral gray matter density (37,38) in type 1 diabetic subjects. Some of these structural abnormalities have corresponded to age of diabetes onset (35), A1C levels, hypoglycemia (37), and the presence of retinopathy (38). Recently, Wessels et al. (4) applied MRI voxel-based morphometry to measure white matter volumes in patients with type 1 diabetes and found subjects with proliferative retinopathy to have significantly smaller white matter volumes than diabetic subjects who were free of proliferative retinopathy and control subjects without diabetes. In the Wessels et al. study, reduced white matter volume correlated with worse performances on tests for attention, speed of information processing, and executive function. Whether reduced white matter volumes correlates with a reduction in fractional anisotropy is uncertain, but in our study, we did not find a relationship between the presence of retinopathy and either reduced fractional anisotropy or mild cognitive impairment. Future investigation should focus on this point because an association with retinopathy suggests that a common mechanism may be responsible for the development of both retinopathy and changes in white matter structure.
Previous studies that were designed to assess neurocognitive dysfunction in patients with type 1 diabetes identified abnormalities with information processing (1–4), psychomotor efficiency (1,2), motor speed (3,5–7), visuoconstruction (4,8), attention (4), somatosensory examination, motor strength (7), and executive function (9). Generally, these cognitive domains, which require the integration of several different tasks, are thought to be associated with the integrity of the white matter tracts that connect gray matter regions. In selecting our neurocognitive battery, we focused on measures like the PASAT, the DVT, the Grooved Pegboard Test, and the Trails A and B, which have previously identified differences in “psychomotor efficiency” between subjects with and without diabetes (2). We also selected measures that assessed other white matter function domains (e.g., processing speed, attention, and visual-spatial processing), such as the JLO, the Trails A and B, the PASAT, the Rey-O, the Grooved Pegboard Test, the CPT-II, and the DVT. Importantly, our battery also included the WASI, a test that screens for intellectual ability, as a control because most studies in type 1 diabetic subjects show no deficits in general intelligence (1–3). Our results demonstrated that no differences could be identified between type 1 diabetic subjects and control subjects on the WASI or the majority of the other tests performed. Most likely, this is secondary to our relatively small sample size, and with a larger number, perhaps we would have found significance in such tests as the Rey-O Copy and the Grooved Peg Board Test. Nevertheless, there were significant associations between these neurocognitive tests and reduced fractional anisotropy in patients with type 1 diabetes.
In this investigation, we used DTI to evaluate white matter microstructure. Although this form of imaging is known to measure the magnitude and directionality of water diffusion in tissues, what specific biochemical or morphological abnormality underlies the change in diffusion remains unknown. Like diabetes (39), many of the diseases that have similar changes in fractional anisotropy are associated with increases in oxidative stress, including chronic alcoholism (40), depression (41), Alzheimer's disease (42), chronic cocaine use (43), schizophrenia (44), and HIV infection (45). Based on this, it is tempting to think that oxidative stress could affect the integrity of myelinated fibers in vivo.
There were several limitations to this study. Historical information about the diabetic subjects was limited to self-report and a single A1C value. We did not confirm the presence or absence of retinopathy in our subjects, and if we had, we may have found an association between white matter structure/function and retinopathy as did Wessels et al. (4,38). We did identify a significant relationship between A1C and fractional anisotropy, but an examination of the relationship between a measure of long-term glycemia and changes in white matter microstructure would provide greater understanding of the role of chronic hyperglycemia in the development of reduced fractional anisotropy. Similarly, if a detailed history of hypoglycemia events and an assessment of hypoglycemia unawareness had been obtained, we may have been able to identify a link between hypoglycemia and structural changes as Musen et al. (37) did using MRI. Reduced fractional anisotropy was correlated with duration of diabetes; however, it was also correlated with increasing age. Future studies hopefully will be needed to confirm whether the duration of diabetes correlates with fractional anisotropy independent of age.
In summary, we found that DTI reveals white matter microstructure deficits (reduced fractional anisotropy) in patients with longstanding type 1 diabetes compared with controls. In addition, white matter microstructure deficits seen in type 1 diabetic subjects correlate with impaired performance on neurocognitive tests that are thought to be associated with white matter function. Based on these findings, prospective, longitudinal studies are needed to both better understand the natural evolution of cognitive dysfunction in type 1 diabetes and to establish DTI as an ideal methodology to follow cognitive dysfunction in diabetes. If further studies confirm our findings correlating diabetes, white matter microstructural changes, and neurocognitive dysfunction, future work will determine what factors will modify the progression of this diabetic complication (i.e., reducing A1C levels, novel pharmacological interventions, etc.).
Acknowledgments
This work has received Grant M01-RR-00400 from the NCRR, a component of the NIH; NIH Grant R01-MH-060662; and funding from the Pennock Family Diabetes Research Fund.
We thank Danielle Seim for her contribution to the project.
Published ahead of print at http://diabetes.diabetesjournals.org on 11 August 2008.
The opinions expressed in this article are those of the authors and do not necessarily reflect the official views of the National Center for Research Resources (NCRR) or the National Institutes of Health (NIH).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Brands AM, Kessels RP, Hoogma RP, Henselmans JM, van der Beek Boter JW, Kappelle LJ, de Haan EH, Biessels GJ: Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes 55: 1800–1806, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Ryan CM, Geckle MO, Orchard TJ: Cognitive efficiency declines over time in adults with type 1 diabetes: effects of micro- and macrovascular complications. Diabetologia 46: 940–948, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Ryan CM, Williams TM, Finegold DN, Orchard TJ: Cognitive dysfunction in adults with type 1 (insulin-dependent) diabetes mellitus of long duration: effects of recurrent hypoglycaemia and other chronic complications. Diabetologia 36: 329–334, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Wessels AM, Rombouts SA, Remijnse PL, Boom Y, Scheltens P, Barkhof F, Heine RJ, Snoek FJ: Cognitive performance in type 1 diabetes patients is associated with cerebral white matter volume. Diabetologia 20: 1763–1769, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Hershey T, Bhargava N, Sadler M, White NH, Craft S: Conventional versus intensive diabetes therapy in children with type 1 diabetes: effects on memory and motor speed. Diabetes Care 22: 1318–1324, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Ryan CM: Neurobehavioral complications of type I diabetes: examination of possible risk factors. Diabetes Care 11: 86–93, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Skenazy JA, Bigler ED: Neuropsychological findings in diabetes mellitus. J Clin Psychol 40: 246–258, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Northam EA, Anderson PJ, Werther GA, Warne GL, Adler RG, Andrewes D: Neuropsychological complications of IDDM in children 2 years after disease onset. Diabetes Care 21: 379–384, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA: Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care 24: 1541–1546, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Biessels GJ, Kappelle AC, Bravenboer B, Erkelens DW, Gispen WH: Cerebral function in diabetes mellitus. Diabetologia 37: 643–650, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP: Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry 51: 890–895, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Wozniak JR, Lim KO: Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci Biobehav Rev 30: 762–774, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoptman MJ, Ardekani BA, Butler PD, Nierenberg J, Javitt DC, Lim KO: DTI and impulsivity in schizophrenia: a first voxelwise correlational analysis. Neuroreport 15: 2467–2470, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A: Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry 56: 367–374, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO: Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry 159: 1929–1932, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M: Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med 44: 259–268, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Chen SQ, Kang Z, Hu XQ, Hu B, Zou Y: Diffusion tensor imaging of the brain in patients with Alzheimer's disease and cerebrovascular lesions. J Zhejiang Univ Sci B 8: 242–247, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg SO: White matter damage in Alzheimer disease and mild cognitive impairment: assessment with diffusion-tensor MR imaging and parallel imaging techniques. Radiology 243: 483–492, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA: Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology 30: 610–617, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W: Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. Am J Neuroradiol 22: 277–283, 2001 [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J: Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 356: 1842–1852, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.deGroot JC, DeLeeuw F, Oudkerk M, van Gijn J, Hofman A, Jolles J, Breteler MM: Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Ann Neurol 47: 145–151, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Desmond DW: Cognition and white matter lesions. Cerebrovasc Dis 13 (Suppl. 2): 53–57, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM: Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 (Suppl. 1): S208–S219, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Haselgrove JC, Moore JR: Correction for distortion of echo-planar images used to calculate the apparent diffusion coefficient. Magn Reson Med 36: 960–964, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Basser PJ, Pierpaoli C: Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111: 209–219, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, Behrens TE: Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc 2: 499–503, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM: MRI Atlas of Human White Matter. Amsterdam, Elsevier, 2005
- 29.Witelson SF: Hand and sex differences in the isthmus and genu of the human corpus callosum: a postmortem morphological study. Brain 112: 799–835, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Keymeulen B, de Metz K, Cluydts R, Bossuyt A, Somers G: Technetium-99m hexamethylpropylene amine oxime single-photon emission tomography of regional cerebral blood flow in insulin-dependent diabetes. Eur J Nucl Med 23: 163–168, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Sabri O, Hellwig D, Schreckenberger M, Schneider R, Kaiser HJ, Wagenknecht G, Mull M, Buell U: Influence of diabetes mellitus on regional cerebral glucose metabolism and regional cerebral blood flow. Nucl Med Commun 21: 19–29, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Chabriat H, Sachon C, Levasseur M, Grimaldi A, Pappata S, Rougemont D, Masure MC, De Recondo A, Samson Y: Brain metabolism after recurrent insulin induced hypoglycaemic episodes: a PET study. J Neurol Neurosurg Psychiatry 57: 1360–1365, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinger K, Jacobson AM, Musen G, Lyoo IK, Ryan CM, Jimerson DC, Renshaw PF: The effects of type 1 diabetes on cerebral white matter. Diabetologia 51: 417–425, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Yousem DM, Tasman WS, Grossman RI: Proliferative retinopathy: absence of white matter lesions at MR imaging. Radiology 179: 229–230, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Ferguson SC, Blane A, Wardlaw J, Frier BM, Perros P, McCrimmon RJ, Deary IJ: Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care 28: 1431–1437, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Lobnig BM, Kromeke O, Optenhostert-Porstt C, Wolf OT: Hippocampal volume and cognitive performance in long-standing type 1 diabetic patients without macrovascular complications. Diabet Med 23: 32–39, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM: Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 55: 326–333, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Wessels AM, Simsek S, Remijnse PL, Veltman DJ, Biessels GJ, Barkhof F, Scheltens P, Snoek FJ, Heine RJ, Rombouts SA: Voxel-based morphometry demonstrates reduced grey matter density on brain MRI in patients with diabetic retinopathy. Diabetologia 49: 2474–2480, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Brownlee M: The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Kashem MA, James G, Harper C, Wilce P, Matsumoto I: Differential protein expression in the corpus callosum (splenium) of human alcoholics: a proteomics study. Neurochem Int 50: 450–459, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R: Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep 8: 365–370, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Casadesus G, Moreira PI, Nunomura A, Siedlak SL, Bligh-Glover W, Balraj E, Petot G, Smith MA, Perry G: Indices of metabolic dysfunction and oxidative stress. Neurochem Res 32: 717–722, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Poon HF, Abdullah L, Mullan MA, Mullan MJ, Crawford FC: Cocaine-induced oxidative stress precedes cell death in human neuronal progenitor cells. Neurochem Int 50: 69–73, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Sarandol A, Kirli S, Akkaya C, Altin A, Demirci M, Sarandol E: Oxidative-antioxidative systems and their relation with serum S100 B levels in patients with schizophrenia: effects of short term antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry 31: 1164–1169, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Steiner J, Haughey N, Li W, Venkatesan A, Anderson C, Reid R, Malpica T, Pocernich C, Butterfield DA, Nath A: Oxidative stress and therapeutic approaches in HIV dementia. Antioxid Redox Signal 8: 2089–2100, 2006 [DOI] [PubMed] [Google Scholar]