Abstract
Endurance exercise training is accompanied by physiological changes that improve muscle function and performance. Several studies have demonstrated that markers of mitochondrial capacity are elevated, however, these studies tend to be performed ex vivo under conditions that yield maximal enzyme activities or in vivo but monitoring the response to exercise. Therefore, it is unclear whether basal mitochondrial metabolism is affected by exercise training. To explore whether resting muscle metabolism was altered in trained individuals in vivo, two independent parameters of metabolic function—tricarboxylic acid (TCA) cycle flux (VTCA), and ATP synthesis (VATP)—were assessed noninvasively by using magnetic resonance spectroscopy in a cohort of young endurance trained subjects (n = 7) and a group of matched sedentary subjects (n = 8). VTCA was 54% higher in the muscle of endurance trained compared with sedentary subjects (91.7 ± 7.6 vs. 59.6 ± 4.9 nmol/g/min, P < 0.01); however, VATP was not different between the trained and sedentary subjects (5.98 ± 0.43 vs. 6.35 ± 0.70 μmol/g/min, P = 0.67). The ratio VATP/VTCA (an estimate of mitochondrial coupling) was also significantly reduced in trained subjects (P < 0.04). These data demonstrate that basal mitochondrial substrate oxidation is increased in the muscle of endurance trained individuals yet energy production is unaltered, leading to an uncoupling of oxidative phosphorylation at rest. Increased mitochondrial uncoupling may represent another mechanism by which exercise training enhances muscle insulin sensitivity via increased fatty acid oxidation in the resting state.
Keywords: ATP synthesis, exercise, magnetic resonance spectroscopy, mitochondria, TCA cycle
Endurance exercise training is accompanied by a number of physiological adaptations that improve muscle function and exercise performance (1). In particular, trained muscle exhibits remodeling toward a more oxidative phenotype (2, 3) that involves changes from the ultrastructural to the subcellular levels. These modifications include an increase in muscle capillary density (3), expanded intramyocellular storage of glycogen (4, 5), and lipid (6) and enhanced insulin responsiveness (7). However, one of the most profound effects of endurance training is the stimulation of mitochondrial biogenesis with an increase in mitochondrial number that is apparent after relatively few weeks of training (8, 9) and is likely mediated by the chronic activation of AMPK (10, 11).
The metabolic response of the muscle to endurance training has been estimated by measuring markers of oxidative capacity with increases in the activities of enzymes involved in mitochondrial metabolism and fat utilization catalogued by numerous studies (5, 12, 14), suggesting that the maximal capacity of the mitochondria, in general, and fat oxidation, in particular, may be increased. These data have been substantiated by studies of mitochondria isolated from trained muscle that demonstrate that elevated mitochondrial enzyme activities are accompanied by enhanced respiratory capacity (9, 15, 16), fatty-acid oxidation (13, 17), and ATP production (18). However, one limitation of these studies is that they were performed in vitro or ex vivo under which conditions enable full activation of enzymes and thus represent measurements of maximal capacity rather than function.
Other studies have used indirect calorimetry to estimate whole-body oxidation rates either at rest or during exercise. The data from studies assessing resting metabolic rate are equivocal with no clear effect of endurance training on energy expenditure (19, 20), whereas endurance training consistently appears to improve fatty-acid oxidation in exercising muscle (21, 22). 31P magnetic resonance spectroscopy (MRS) has also been used to monitor the recovery of phosphocreatine (PCr) after muscle contraction with increased rates of recovery observed in trained individuals (23, 24), suggesting elevated oxidative capacity. However, calorimetry techniques are constrained by the extrapolation of whole-body oxygen consumption data to muscle metabolism and studies performed during exercise give no insight into basal mitochondrial function.
Therefore, despite a considerable body of work investigating the response of muscle metabolism to endurance training, it is unclear whether resting metabolism, including mitochondrial function, is altered in vivo. This is particularly relevant because a reduction in basal muscle metabolism has been associated with the development of insulin resistance in both the elderly (25) and offspring of type 2 diabetic patients (26, 27). Endurance exercise training may represent an effective treatment not only to improve insulin responsiveness in these individuals (28, 29) but also to elevate resting muscle metabolism.
The aim of this study was to investigate the effect of endurance training on basal muscle metabolism in vivo using two independent MRS techniques (25–27). The rate of flux through the tri-carboxylic acid (TCA) cycle, a direct measure of mitochondrial oxidative function, was determined by using 13C-MRS to monitor the oxidation of intravenously infused [2-13C]acetate. The unidirectional rate of muscle ATP synthesis, an assessment of overall energy production, was estimated during the same session by using 31P saturation-transfer MRS. These data provide insight into the effects that endurance training may have on resting mitochondrial function in vivo.
Results
Subject Characteristics.
Endurance trained and sedentary subjects were matched for age, height, weight, and body-mass index (BMI) (Table 1). The endurance trained group undertook significantly more exercise than the sedentary group as assessed by the exercise index of the activity questionnaire (5.37 ± 0.26 vs. 2.24 ± 0.16, P < 0.0001). Consistent with these data, the peak oxygen consumption (VO2 peak) in the trained group (60.9 ± 4.6 ml/kg/min) was increased well above the normal sedentary range (30–40 ml/kg/min).
Table 1.
Characteristics of endurance trained and sedentary subject groups
| Sedentary | Trained | |
|---|---|---|
| n | 8 | 7 |
| Age, yr | 26.8 ± 3.0 | 26.1 ± 1.6 |
| Height, m | 1.80 ± 0.02 | 1.81 ± 0.03 |
| Weight, kg | 74.8 ± 2.8 | 78.4 ± 3.9 |
| BMI, kg/m2 | 23.0 ± 1.03 | 24.0 ± 1.3 |
| Activity Index | 2.24 ± 0.16 | 5.37 ± 0.26* |
BMI, body-mass index.
*P < 0.0001.
Muscle Substrate Oxidation – TCA Cycle Flux.
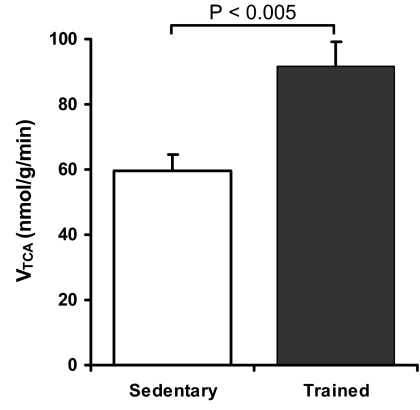
Rates of substrate oxidation via the TCA cycle (VTCA) were 54% higher in the muscle of endurance trained individuals compared with sedentary control subjects (91.7 ± 7.6 vs. 59.6 ± 4.9 nmol/g/min, P < 0.005, Fig. 1). There were no differences in the steady-state concentration or enrichment of plasma acetate between the groups, or in the maximal enrichment of the muscle C2 glutamate pool (Table 2). The proportion of acetate oxidized via the TCA cycle (VAC/VTCA) was also equivalent between groups, indicating that entry of acetate into the myocyte was not limiting (Table 2). There was a trend for a decreased layer of subcutaneous fat between the MR probe and the gastrocnemius muscles of the calf in the trained subjects (3.0 ± 0.5 mm vs. 4.25 ± 0.43 mm, P = 0.09), and the total muscle cross-sectional area (CSA) detectable during the 13C-MRS experiment was 18% larger in the endurance trained group (19.6 ± 0.5 vs. 16.7 ± 0.6 cm2, P < 0.005) because of a significant increase in the MR-detectable CSA of the gastrocnemius (12.9 ± 0.9 vs. 7.7 ± 1.1 cm2, P < 0.005, Table 3). However, VTCA was not correlated with any parameter of muscle CSA within either subject group or for all subjects combined. Whole-body energy expenditure, respiratory quotient, and fat and glucose oxidation, estimated from indirect calorimetry performed during the MR session, were not different between the trained and sedentary groups (Table 3). There was no correlation between VTCA and any parameter of whole-body metabolism.
Fig. 1.
Muscle TCA cycle flux [nmol/g(muscle)/min] for sedentary (white bar, n = 8) and endurance trained (black bar, n = 7) subjects.
Table 2.
Muscle and plasma metabolite enrichment data and metabolic fluxes obtained during the TCA cycle flux experiment
| Sedentary | Trained | |
|---|---|---|
| Plasma [acetate], mM | 0.63 ± 0.07 | 0.65 ± 0.03 |
| Plasma [2-13C]acetate APE, % | 85.4 ± 1.1 | 86.4 ± 1.0 |
| Muscle C2-glutamate APE, % | 1.31 ± 0.32 | 1.39 ± 0.18 |
| Muscle C4-glutamate APE†, % | 2.62 ± 0.64 | 2.80 ± 0.36 |
| VTCA, nmol/g (muscle) per min | 59.6 ± 4.9 | 91.7 ± 7.6* |
| VAC/VTCA, % | 3.6 ± 0.9 | 3.4 ± 0.4 |
APE, atom percent excess.
*P < 0.05.
†Calculated from C2-glutamate assuming 5% anaplerosis.
Table 3.
Parameters of whole-body energy expenditure, assessed by indirect calorimetry and the cross-sectional areas of muscles contributing to the MR signal in endurance trained (n = 7) and sedentary subjects (n = 8)
| Sedentary | Trained | |
|---|---|---|
| Energy expenditure, kcal/kg/24 hr | 23.7 ± 0.8 | 24.2 ± 1.2 |
| Respiratory quotient† | 0.78 ± 0.02 | 0.77 ± 0.02 |
| Fat oxidation†, mg/kg per min | 4.88 ± 0.79 | 4.89 ± 0.72 |
| Glucose Oxidation†, mg/kg per min | 0.46 ± 0.20 | 0.47 ± 0.12 |
| Total muscle CSA, cm2 | 16.7 ± 0.6 | 19.6 ± 0.5* |
| CSA soleus, cm2 | 9.0 ± 1.0 | 6.7 ± 1.0 |
| CSA gastrocnemius, cm2 | 7.7 ± 1.1 | 12.9 ± 0.9* |
CSA, cross sectional area.
*P < 0.005.
†n = 7.
Muscle ATP Synthesis.
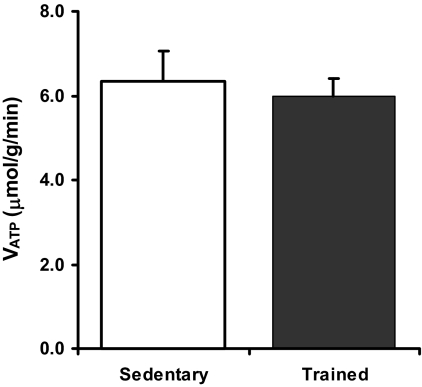
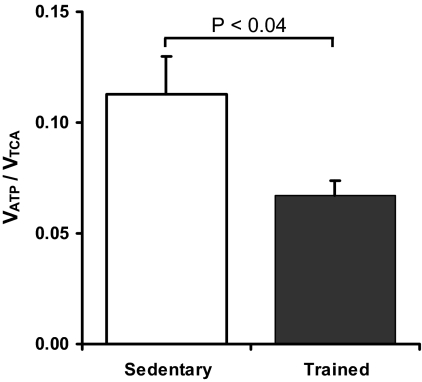
There was no difference in the resting rate of muscle ATP synthesis (VATP) between the endurance trained and sedentary groups (Fig. 2), with similar values for both the pseudo first order rate constant for Pi exchange (k′) and the intracellular inorganic phosphate (Pi) concentration (Table 4). The ratio of VATP/VTCA was significantly decreased in the endurance trained subjects (0.067 ± 0.007 vs. 0.113 ± 0.017, P < 0.05, Fig. 3). Muscle 31P metabolite concentrations and ratios were unchanged in the endurance trained group compared with sedentary subjects, and there was no difference in the concentration of free ADP ([ADP]free) estimated from muscle 31P metabolite concentrations and the intracellular pH (Table 4).
Fig. 2.
Muscle ATP synthesis rates [μmol/g(muscle)/min] for sedentary (white bar, n = 8) and endurance trained (black bar, n = 7) subjects.
Table 4.
Muscle 31P metabolite concentrations and ATP synthesis rate (VATP) in endurance trained and sedentary subjects
| Sedentary | Trained | |
|---|---|---|
| VATP, μmol/g per min | 6.35 ± 0.70 | 5.98 ± 0.43 |
| k′, min−1 | 0.043 ± 0.006 | 0.040 ± 0.003 |
| [Pi], μmol/g | 2.67 ± 0.24 | 2.49 ± 0.15 |
| [PCr], μmol/g | 32.2 ± 1.5 | 29.9 ± 1.4 |
| pH | 7.08 ± 0.01 | 7.06 ± 0.01 |
| [ADP]free, nmol/g | 10.5 ± 0.2 | 10.1 ± 0.3 |
Fig. 3.
The ratio of muscle ATP synthesis/TCA cycle flux (VATP/VTCA), an estimate of the efficiency of muscle energy production, for sedentary (white bar, n = 8) and endurance trained (black bar, n = 7) subjects.
Discussion
In this study, we found that resting substrate oxidation, calculated by monitoring the rate of incorporation of [2-13C]acetate into [4-13C]glutamate using 13C-MRS, was significantly increased in the muscle of endurance trained male individuals compared with healthy age, height, weight, and sex-matched sedentary subjects. However, the basal rate of muscle ATP synthesis, measured by 31P-saturation-transfer MRS, was not different between the endurance trained and sedentary subjects, suggesting that there is an uncoupling of oxidation from energy production in endurance-trained muscle at rest. These MRS-based techniques for measuring metabolic fluxes are independent of the mass of tissue that contributes to the MR signal; therefore, the observed increase in TCA cycle flux rate and decrease in VATP/VTCA are unaffected by the larger muscle mass of subjects in the trained group.
Our observation of increased basal TCA cycle flux in the muscle of endurance trained individuals is consistent with prior studies that have reported increases in mitochondrial number and oxidative enzyme content in trained muscle, accompanied by an enhanced capacity for respiration, fatty-acid oxidation, and ATP production under maximal conditions, and faster recovery of PCr after a muscle contraction (5, 8, 9, 12–18, 21–24). However, using in vivo MRS techniques to investigate the muscle under basal conditions, we have shown that resting muscle oxidation is augmented as well as maximal capacity.
Similar relationships have also been observed in isolated muscle preparations. Resting oxygen consumption (VO2) was severalfold higher in the highly oxidative soleus muscle compared with the predominantly glycolytic gracilis muscle group in cat, and this was correlated with the mitochondrial density of the muscles (30). Furthermore, chronic low frequency electrical stimulation of the gracilis muscle, designed to mimic exercise training, caused an increase in mitochondrial density accompanied by enhanced VO2. Six weeks of endurance training in humans caused in increase in VO2 in permeabilized vastus-lateralis fibers (31), whereas four weeks of treadmill running in rats increased maximal oxygen consumption in skinned soleus fibers but not gastrocnemius (15).
These data also provide an explanation for the inconsistent results of studies which used indirect calorimetry to investigate the effects of endurance training on resting metabolic rate (19, 20). Although substrate oxidation was elevated in the muscle of trained subjects in this study, we observed no significant effect on energy expenditure measured simultaneously by indirect calorimetry, demonstrating that measurements of whole-body energy expenditure cannot accurately predict significant changes in muscle TCA cycle activity.
Despite increased basal rates of oxidation via the TCA cycle, resting rates of ATP synthesis were unchanged in the muscle of endurance trained individuals, resulting in a significant decrease in the ratio of VATP/VTCA. Although this ratio is not directly equivalent to the traditional parameter of mitochondrial coupling, the P:O ratio (the amount of ATP produced per oxygen atom consumed), it does reflect an estimate of the efficiency between energy production and oxidative metabolism, and suggests that this relationship is less efficient at rest in trained muscle. Endurance training alters the relative protein composition of the mitochondria (32) which may affect the efficiency of multiple steps in the oxidative phosphorylation pathway. Loss of mitochondrial efficiency—“uncoupling”—may occur via proton (H+) leak across the inner mitochondrial membrane (IMM), redox “slip” of the electron transport chain (ETC), and modulated stoichiometry of the ATP synthase (33). Substrate selection and mitochondrial respiratory control may also play a role in dictating the efficiency of energy transduction.
Uncoupling protein 3 (UCP3) is a putative uncoupler of mitochondrial oxidative phosphorylation, mediating H+ leak across the IMM (34). Acute bouts of exercise have been demonstrated to transiently induce the expression of UCP3 in both rodents and humans (34, 35). However, UCP3 expression is lower in oxidative type I muscle fibers (36, 37) than the more glycolytic type II fibers, and expression and protein content appear to be down-regulated in endurance trained muscle (16, 37), making it an unlikely candidate for the decreased efficiency observed in these studies. However, the adenine nucleotide translocase (ANT) has been shown to be increased in endurance trained muscle to a greater extent than other mitochondrial proteins (16). This protein is fundamental to viable mitochondrial function and its increased expression in trained muscle is likely necessary to cope with the high ATP production during exercise. ATP/ADP exchange via the ANT incurs an energetic cost because nucleotide transport is driven by the mitochondrial membrane potential and the ANT may facilitate fatty-acid induced uncoupling (38) or basal mitochondrial H+ leak (33) proportional to its content, but not activity (39).
The efficiency of oxidative phosphorylation is also dependent on substrate selection with different proportions of reducing equivalents (NADH and FADH2) generated by the full oxidation of glucose (glycolysis plus TCA cycle) compared with fatty-acids (β-oxidation plus TCA cycle) such that the P:O ratio of fatty acid oxidation is lower and thus less efficient that of glucose. The 31P-saturation-transfer technique measures ATP synthesized from all metabolic sources, and the methodology used in the present study does not permit the relative contributions of fatty-acids or glucose to TCA cycle flux to be estimated. However, modulation of substrate selection is unlikely to have caused the observed differences in efficiency because: (i) the contribution of glucose to total oxidation in resting muscle is approximately 30% (unpublished data), and ii) to generate an equivalent amount of ATP, fatty-acid oxidation requires fewer “turns” of the TCA cycle. Entry of substrates into the TCA cycle via anaplerotic pathways may similarly modulate the relative production of reducing equivalents. Given that there was no difference in the enrichment of the muscle C2-glutamate pool between the subject groups, effects of anaplerosis were unlikely to be a factor in this study.
Mitochondrial efficiency exhibits tissue-specificity (40); therefore, it is plausible that muscle fiber type may influence efficiency. Mitochondria isolated from oxidative and glycolytic muscles (41, 42) exhibited no difference in the maximally stimulated (State III) P:O ratio, suggesting that fiber-type per se does not influence mitochondrial efficiency. In the current study, there was also no correlation between VATP/VTCA and any parameter of muscle CSA, diminishing the likelihood that the observed decrease in efficiency was influenced by muscle composition.
Contrasting effects of endurance training have been demonstrated on the different subcellular populations of mitochondria, and it is possible that functional efficiency may vary between mitochondrial subtypes. Subsarcolemmal (SS) mitochondria, located at the cell membrane, are more responsive to training than intermyofibrillar (IMF) mitochondria and undergo greater proliferation, increases in enzyme content and fatty-acid oxidation (17, 43, 44). However, absolute oxidative enzyme activities and respiration rates are higher in IMF mitochondria (43–45) and there is no evidence for a difference in P:O between mitochondrial populations.
The free intracellular concentration of ADP ([ADP]free) has been postulated as a regulator of mitochondrial function (46) and endurance training may modulate the respiratory control of mitochondrial function by ADP. The effects of training on mitochondrial sensitivity to ADP have proved inconclusive, with opposing effects observed in human (31) and rat muscle fibers (15). Similarly, comparisons of oxidative and glycolytic muscle groups have demonstrated either no change (41) or an increase in ADP sensitivity with oxidative phenotype (15). It is pertinent to note that studies using skinned muscle or isolated mitochondria preparations perform measurements of mitochondrial function under conditions where ADP concentrations are an order of magnitude higher than those typically encountered in resting (5–10 μM) or exercising (50–100 μM) muscle and exhibit a dependence on substrate provision (42, 45) and the presence of Cr/PCr (15), so may not accurately represent the metabolic state encountered in vivo, particularly in resting skeletal muscle. In this study, resting [ADP]free, estimated from basal 31P MR spectra, was not different between the trained and sedentary groups, consistent with the similar rates of ATP synthesis but suggesting that [ADP]free does not regulate TCA cycle flux.
Under resting conditions, when [ADP]free is low, mitochondrial function will become dominated by the contribution of State IV respiration – oxygen consumption in the absence of ADP, and this parameter may give greater insight into the efficiency of resting energy production. State IV respiration is higher in IMF than SS mitochondria (44, 45) suggesting decreased efficiency at low [ADP]free despite increased State III (maximal) respiration. The preferential proliferation of SS mitochondria at endurance training suggests that an increase in efficiency might be expected, however, endurance training may selectively increase the State IV respiration of SS mitochondria (43) and impair their efficiency, perhaps indicative of remodeling toward an IMF mitochondrial phenotype. Accurate interpretations of basal respiration in isolated mitochondrial are again complicated by the influence of available substrate on functional parameters. In addition, effects of compartmentation of metabolites, in particular [ADP]free, cannot be excluded which could give rise to subcellular modulation of Km(ADP) and mitochondrial fluxes.
The observation that mitochondrial P:O is proportional to respiration rate (41) promotes the idea of the following unifying hypothesis on the effects of training on mitochondrial function and efficiency. Endurance training stimulates mitochondrial biogenesis resulting in an increase in mitochondrial volume, with a particular increase in the subsarcolemmal compartment. ATP demand in resting muscle is unchanged; therefore, ATP synthesis per unit of mitochondrial volume is decreased. Energy production is less efficient with a lower respiration rate per unit of mitochondria as total oxygen consumption becomes dominated by basal uncoupling mechanisms, potentially exacerbated by increased H+ leak because of the increased content of the ANT. Respiratory control by ADP is limited because of the low intracellular [ADP]free. The decreased efficiency of energy production causes a concomitant rise in basal TCA cycle flux and resting oxygen consumption. The elevation of resting muscle TCA cycle flux in trained individuals may also provide an advantage at the onset of exercise by permitting a more rapid response to an increased demand for ATP.
In summary, we have demonstrated that the basal rate of substrate oxidation via the TCA cycle is increased in the muscle of endurance trained individuals compared with matched sedentary control subjects. In contrast, muscle energy production, measured as the rate of ATP synthesis, is unaltered in endurance trained individuals indicating a decrease in the efficiency of mitochondrial energy coupling. Studies have demonstrated protective effects of muscle specific overexpression of UCP1 (47) and UCP3 (48) from fat-induced insulin resistance in skeletal muscle; therefore, these data suggest that increased mitochondrial uncoupling may represent an additional mechanism by which endurance training protects against insulin resistance.
Materials and Methods
Human Subjects.
This study was approved by the Yale Human Investigation Committee and was conducted in accordance with the declaration of Helsinki. Written, informed consent was obtained from each subject after an explanation of the purpose, nature, and complications of the protocol. Subjects were recruited from the local community and were prescreened to be in excellent health, lean, nonsmoking, and taking no medications. All individuals completed an activity index questionnaire (49) and a cohort of young endurance-trained subjects (exercise index >3.8, n = 7) were selected along with a group of age-height-weight-matched sedentary control subjects (exercise index <2.9, n = 8). Only male subjects were recruited because gender has been demonstrated to influence oxidation rates (50). All trained individuals participated in running or running-based sports for >4 h per week, and their status was confirmed several days before the MRS studies by an incremental treadmill-based VO2-peak test using a modified Bruce protocol (51).
Study Protocol.
For 72 h preceding the MRS studies, subjects consumed a weight-maintenance diet and were instructed not to perform any exercise other than normal walking. The evening before the MRS studies, subjects were admitted to the Yale-New Haven Hospital General Clinical Research Center and fasted overnight, with free access to drinking water. After an overnight 12-h fast, subjects were transported to the Yale Magnetic Resonance Research Center via wheelchair. All experiments were performed using a 2.1 T whole-body MR magnet (Magnex Instruments) interfaced to a Bruker Avance console (Bruker Biospin).
Muscle TCA Cycle Flux – 13C-MRS.
The muscles of the right calf were positioned within a custom-built MR probe assembly over a 9-cm diameter 13C surface coil with twin, orthogonal 13-cm 1H quadrature coils for imaging, shimming, and decoupling. After tuning, transverse, gradient-echo, scout images of the calf were acquired to ensure correct positioning and to define a volume for localized shimming by using the FASTMAP procedure (52); typical 1H line widths within the volume of interest were 12 Hz. 13C MR spectra were acquired by using a nonlocalized sequence with NOE, WALTZ16 decoupling, and a repetition time (TR) of 1.4 seconds (26). Signals from overlapping lipid resonances were suppressed by T1-selective nulling after an adiabatic inversion pulse. Temporal resolution was 10 min, corresponding to 424 averages. Spectra were acquired for 20 min before and during a 120-min infusion of 99% enriched [2-13C]acetate (350 mmol/liter sodium salt) at a rate of 3.0 mg/kg/min. Plasma samples were obtained at 10-min intervals throughout the study for the measurement of plasma acetate concentration and fractional enrichment by gas chromatography/mass spectrometry (26).
To determine the time course of 13C incorporation into C4-glutamate during the [2-13C]acetate infusion, difference spectra for each time-point were obtained by subtracting averaged reference spectra and the increment in the C4-glutamate peak was determined by integration (± 0.5 ppm). Absolute enrichment of the C2-glutamate peak at the end of infusion was determined by integration (± 0.5 ppm) relative to the natural abundance enrichment (1.1%) of the reference spectra; maximal enrichment at C4-glutamate was calculated from that of C2-glutamate (assuming 5% dilution of the C2 pool because of anaplerosis). VTCA for each individual was determined by computer modeling by using CWave software (see below).
Muscle ATP Synthesis – 31P Saturation-Transfer MRS.
After a short break, the subject was repositioned in the magnet with the right calf muscles positioned over an MR probe consisting of a 9-cm diameter 31P surface coil and a concentric 13-cm diameter 1H surface coil. After imaging and shimming, VATP was measured in each subject by using the 31P saturation-transfer experiment to determine the rate of phosphate exchange between Pi and ATP. Irradiating the γATP peak causes a decrease in the Pi signal that is equivalent to the pseudo-first-order rate constant of the exchange reaction (k′) multiplied by the longitudinal relaxation time (T1) of Pi (Eq. 1). VATP is equal to the rate constant multiplied by the concentration of Pi (Eq. 2).
 |
Nonlocalized 31P MR spectra were acquired with frequency-selective saturation of the γATP peak or with saturation at a downfield frequency equidistant from Pi. The T1 relaxation time of Pi during γATP saturation was estimated by using a modified inversion-recovery sequence with γATP saturation during the TR and the inversion delay. Concentrations of 31P metabolites were determined from control spectra assuming a constant muscle ATP concentration of 5.5 mmol/kg. Intracellular pH was calculated from the chemical shift (δ) of the Pi peak relative to the PCr peak (53).
[ADP]free was determined from the 31P spectra according to the creatine-kinase equilibrium (54), with the equilibrium constant (Keq) corrected for the effects of pH (55) and assuming that 15% of total creatine is unphosphorylated at rest (56).
CWave Modeling.
VTCA was determined by modeling the incorporation of 13C label from plasma [2-13C]acetate into the muscle [4-13C]glutamate pool [supporting information (SI) Fig. S1], using CWave software (26). The CWave model consists of isotopic mass balance equations that describe the metabolic fate of the plasma [2-13C]acetate (see SI Text). Upon entry into the myocyte, [2-13C]acetate is converted (VAC) into [2-13C]acetylCoA which enters the TCA cycle by condensing with oxaloacetate to form [4-13C]citrate. Entry of unlabeled substrates into the TCA cycle via acetyl CoA or anaplerosis was incorporated into the model as a separate reaction (VPDH+FA). The position of the 13C label is conserved through the initial steps of the TCA cycle, labeling α-ketoglutarate at the C4 position. Glutamate and α-ketoglutarate are in rapid exchange and equilibration results in the formation of [4-13C]glutamate. As the TCA cycle progresses, the 13C label becomes scrambled between the C2 and C3 positions because of the symmetry of the succinate molecule; a second turn of the TCA cycle yields [2-13C] and [3-13C]glutamate. CWave determines VTCA as the rate of total carbon flow from acetyl CoA to α-ketoglutarate by using a nonlinear least-squares algorithm to fit the curve of C4-glutamate enrichment. In this model, VTCA is equal to the sum of (VAC+VPDH+FA) but is independent of the absolute fractional enrichment at C4-glutamate (VAC/VPDH+FA). The intracellular concentration of glutamate was measured by muscle biopsy and was assumed to be 2.41 mmol/liter (25) and unchanged by endurance training (57). The ratio of natural abundance C2-glutamate/C2-creatine was the same between the groups and there was no significant difference in the fractional enrichment at C2-glutamate at the end of the [2-13C]acetate infusion, confirming that the intracellular concentration of glutamate was unchanged in endurance trained individuals. The rate of exchange between α-ketoglutarate and glutamate (VX) was determined in prior studies (25) and was fixed at 150 nmol/g(muscle)/min that is significantly faster than the TCA cycle flux.
Muscle Cross-sectional Area.
The relative contributions of the soleus and gastrocnemius muscles to the observed MR signal were estimated from MR images obtained during the study. The MR-detectable CSA of each muscle group was measured in the image slice that corresponded with the center of the MR probe.
Indirect Calorimetry.
Whole-body energy expenditure was measured during the MR studies by the ventilated hood technique by using a Deltatrac Metabolic Monitor. Nonprotein respiratory quotients assuming 100% oxidation of fat or carbohydrates were 0.707 and 1.00, respectively.
Statistical Analysis.
All data are expressed as mean ± SE. Statistical analyses were performed by using InStat3 software (Graphpad Software). Statistically significant differences between trained and sedentary subjects were detected by using an unpaired 2-tailed student's t test. A P value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We thank the subjects for their participation in this study, Dr. James Dziura for statistical assistance, and Yanna Kosover, Anthony Romanelli, Mikhail Smolgovsky, and the staff of the Yale Center for Clinical Investigation for technical assistance with the studies. This work was supported by National Institutes of Health Grants R01 NS-037527 (to D.L.R.), K02 AA-13430 (to G.F.M.), R01 AG-23686 (to K.F.P.), M01 RR-00125, R01 DK-49230 (to G.I.S.), and P01 DK-68229 (to G.I.S.), The Keck Foundation, and a Distinguished Clinical Scientist Award from the American Diabetes Association (to G.I.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808889105/DCSupplemental.
References
- 1.Fitts RH. Effects of regular exercise training on skeletal muscle contractile function. Am J Phys Med Rehabil. 2003;82:320–331. doi: 10.1097/01.PHM.0000059336.40487.9C. [DOI] [PubMed] [Google Scholar]
- 2.Bergh U, et al. Maximal oxygen uptake and muscle fiber types in trained and untrained humans. Med Sci Sports. 1978;10:151–154. [PubMed] [Google Scholar]
- 3.Coggan AR, et al. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72:1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- 4.Holloszy JO, Booth FW. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- 5.Gollnick PD, et al. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973;34:107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- 6.Hoppeler H, et al. Endurance training in humans: Aerobic capacity and structure of skeletal muscle. J Appl Physiol. 1985;59:320–327. doi: 10.1152/jappl.1985.59.2.320. [DOI] [PubMed] [Google Scholar]
- 7.Ebeling P, et al. Mechanism of enhanced insulin sensitivity in athletes. Increased blood flow, muscle glucose transport protein (GLUT-4) concentration, and glycogen synthase activity. J Clin Invest. 1993;92:1623–1631. doi: 10.1172/JCI116747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hood DA. Invited Review: Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol. 2001;90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- 9.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 10.Bergeron R, et al. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol Endocrinol Metab. 1999;276:E938–E944. doi: 10.1152/ajpendo.1999.276.5.E938. [DOI] [PubMed] [Google Scholar]
- 11.Zong H, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 13.Mole PA, Oscai LB, Holloszy JO. Adaptation of muscle to exercise. Increase in levels of palmityl Coa synthetase, carnitine palmityltransferase, and palmityl Coa dehydrogenase, and in the capacity to oxidize fatty acids. J Clin Invest. 1971;50:2323–2330. doi: 10.1172/JCI106730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiens B, et al. Membrane associated fatty acid binding protein (FABPpm) in human skeletal muscle is increased by endurance training. Biochem Biophys Res Commun. 1997;231:463–465. doi: 10.1006/bbrc.1997.6118. [DOI] [PubMed] [Google Scholar]
- 15.Burelle Y, Hochachka PW. Endurance training induces muscle-specific changes in mitochondrial function in skinned muscle fibers. J Appl Physiol. 2002;92:2429–2438. doi: 10.1152/japplphysiol.01024.2001. [DOI] [PubMed] [Google Scholar]
- 16.Fernstrom M, Tonkonogi M, Sahlin K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J Physiol. 2004;554:755–763. doi: 10.1113/jphysiol.2003.055202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koves TR, et al. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol. 2005;288:C1074–C1082. doi: 10.1152/ajpcell.00391.2004. [DOI] [PubMed] [Google Scholar]
- 18.Wibom R, et al. Adaptation of mitochondrial ATP production in human skeletal muscle to endurance training and detraining. J Appl Physiol. 1992;73:2004–2010. doi: 10.1152/jappl.1992.73.5.2004. [DOI] [PubMed] [Google Scholar]
- 19.Poehlman ET, Melby CL, Badylak SF, Calles J. Aerobic fitness and resting energy expenditure in young adult males. Metabolism. 1989;38:85–90. doi: 10.1016/0026-0495(89)90185-6. [DOI] [PubMed] [Google Scholar]
- 20.Bullough RC, Gillette CA, Harris MA, Melby CL. Interaction of acute changes in exercise energy expenditure and energy intake on resting metabolic rate. Am J Clin Nutr. 1995;61:473–481. doi: 10.1093/ajcn/61.3.473. [DOI] [PubMed] [Google Scholar]
- 21.Martin WH, 3rd, et al. Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am J Physiol. 1993;265:E708–E714. doi: 10.1152/ajpendo.1993.265.5.E708. [DOI] [PubMed] [Google Scholar]
- 22.Coggan AR, et al. Fat metabolism during high-intensity exercise in endurance-trained and untrained men. Metabolism. 2000;49:122–128. doi: 10.1016/s0026-0495(00)90963-6. [DOI] [PubMed] [Google Scholar]
- 23.McCully KK, et al. Wrist flexor muscles of elite rowers measured with magnetic resonance spectroscopy. J Appl Physiol. 1989;67:926–932. doi: 10.1152/jappl.1989.67.3.926. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi M, et al. Control of the rate of phosphocreatine resynthesis after exercise in trained and untrained human quadriceps muscles. Eur J Appl Physiol Occup Physiol. 1995;71:396–404. doi: 10.1007/BF00635872. [DOI] [PubMed] [Google Scholar]
- 25.Petersen KF, et al. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Befroy DE, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–1381. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen KF, et al. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perseghin G, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 29.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes. 2003;52:2191–2197. doi: 10.2337/diabetes.52.9.2191. [DOI] [PubMed] [Google Scholar]
- 30.Hoppeler H, Hudlicka O, Uhlmann E. Relationship between mitochondria and oxygen consumption in isolated cat muscles. J Physiol. 1987;385:661–675. doi: 10.1113/jphysiol.1987.sp016513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh B, Tonkonogi M, Sahlin K. Effect of endurance training on oxidative and antioxidative function in human permeabilized muscle fibres. Pflugers Arch. 2001;442:420–425. doi: 10.1007/s004240100538. [DOI] [PubMed] [Google Scholar]
- 32.Holloszy JO, Oscai LB, Don IJ, Mole PA. Mitochondrial citric acid cycle and related enzymes: Adaptive response to exercise. Biochem Biophys Res Commun. 1970;40:1368–1373. doi: 10.1016/0006-291x(70)90017-3. [DOI] [PubMed] [Google Scholar]
- 33.Brand MD. The efficiency and plasticity of mitochondrial energy transduction. Biochem Soc Trans. 2005;33:897–904. doi: 10.1042/BST0330897. [DOI] [PubMed] [Google Scholar]
- 34.Schrauwen P, Saris WH, Hesselink MK. An alternative function for human uncoupling protein 3: Protection of mitochondria against accumulation of nonesterified fatty acids inside the mitochondrial matrix. FASEB J. 2001;15:2497–2502. doi: 10.1096/fj.01-0400hyp. [DOI] [PubMed] [Google Scholar]
- 35.Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- 36.Hesselink MK, et al. Protein expression of UCP3 differs between human type 1, type 2a, and type 2b fibers. FASEB J. 2001;15:1071–1073. [PubMed] [Google Scholar]
- 37.Russell AP, et al. UCP3 protein expression is lower in type I, IIa and IIx muscle fiber types of endurance-trained compared to untrained subjects. Pflugers Arch. 2003;445:563–569. doi: 10.1007/s00424-002-0943-5. [DOI] [PubMed] [Google Scholar]
- 38.Simonyan RA, Skulachev VP. Thermoregulatory uncoupling in heart muscle mitochondria: Involvement of the ATP/ADP antiporter and uncoupling protein. FEBS Lett. 1998;436:81–84. doi: 10.1016/s0014-5793(98)01106-5. [DOI] [PubMed] [Google Scholar]
- 39.Brand MD, et al. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J. 2005;392:353–362. doi: 10.1042/BJ20050890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cocco T, Pacelli C, Sgobbo P, Villani G. Control of OXPHOS efficiency by complex I in brain mitochondria. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.08.002. in press. [DOI] [PubMed] [Google Scholar]
- 41.Mogensen M, Sahlin K. Mitochondrial efficiency in rat skeletal muscle: Influence of respiration rate, substrate and muscle type. Acta Physiol Scand. 2005;185:229–236. doi: 10.1111/j.1365-201X.2005.01488.x. [DOI] [PubMed] [Google Scholar]
- 42.Pande SV, Blanchaer MC. Carbohydrate and fat in energy metabolism of red and white muscle. Am J Physiol. 1971;220:549–553. doi: 10.1152/ajplegacy.1971.220.2.549. [DOI] [PubMed] [Google Scholar]
- 43.Krieger DA, Tate CA, McMillin-Wood J, Booth FW. Populations of rat skeletal muscle mitochondria after exercise and immobilization. J Appl Physiol. 1980;48:23–28. doi: 10.1152/jappl.1980.48.1.23. [DOI] [PubMed] [Google Scholar]
- 44.Cogswell AM, Stevens RJ, Hood DA. Properties of skeletal muscle mitochondria isolated from subsarcolemmal and intermyofibrillar regions. Am J Physiol. 1993;264:C383–C389. doi: 10.1152/ajpcell.1993.264.2.C383. [DOI] [PubMed] [Google Scholar]
- 45.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 46.Kemp GJ, Taylor DJ, Radda GK. Control of phosphocreatine resynthesis during recovery from exercise in human skeletal muscle. NMR Biomed. 1993;6:66–72. doi: 10.1002/nbm.1940060111. [DOI] [PubMed] [Google Scholar]
- 47.Gates AC, et al. Respiratory uncoupling in skeletal muscle delays death and diminishes age-related disease. Cell Metab. 2007;6:497–505. doi: 10.1016/j.cmet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Choi CS, et al. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest. 2007;117:1995–2003. doi: 10.1172/JCI13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 50.Watt MJ, Heigenhauser GJ, Spriet LL. Intramuscular triacylglycerol utilization in human skeletal muscle during exercise: Is there a controversy? J Appl Physiol. 2002;93:1185–1195. doi: 10.1152/japplphysiol.00197.2002. [DOI] [PubMed] [Google Scholar]
- 51.Krssak M, et al. Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: A 13C and 1H nuclear magnetic resonance spectroscopy study. J Clin Endocrinol Metab. 2000;85:748–754. doi: 10.1210/jcem.85.2.6354. [DOI] [PubMed] [Google Scholar]
- 52.Shen J, Rycyna RE, Rothman DL. Improvements on an in vivo automatic shimming method [FASTERMAP] Magn Reson Med. 1997;38:834–839. doi: 10.1002/mrm.1910380521. [DOI] [PubMed] [Google Scholar]
- 53.Taylor DJ, et al. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983;1:77–94. [PubMed] [Google Scholar]
- 54.Lawson JW, Veech RL. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979;254:6528–6537. [PubMed] [Google Scholar]
- 55.Golding EM, Teague WE, Jr, Dobson GP. Adjustment of K′ to varying pH and pMg for the creatine kinase, adenylate kinase and ATP hydrolysis equilibria permitting quantitative bioenergetic assessment. J Exp Biol. 1995;198:1775–1782. doi: 10.1242/jeb.198.8.1775. [DOI] [PubMed] [Google Scholar]
- 56.Boska M. ATP production rates as a function of force level in the human gastrocnemius/soleus using 31P MRS. Magn Reson Med. 1994;32:1–10. doi: 10.1002/mrm.1910320102. [DOI] [PubMed] [Google Scholar]
- 57.Dawson KD, et al. Short-term training attenuates muscle TCA cycle expansion during exercise in women. J Appl Physiol. 2003;95:999–1004. doi: 10.1152/japplphysiol.01118.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.