Abstract
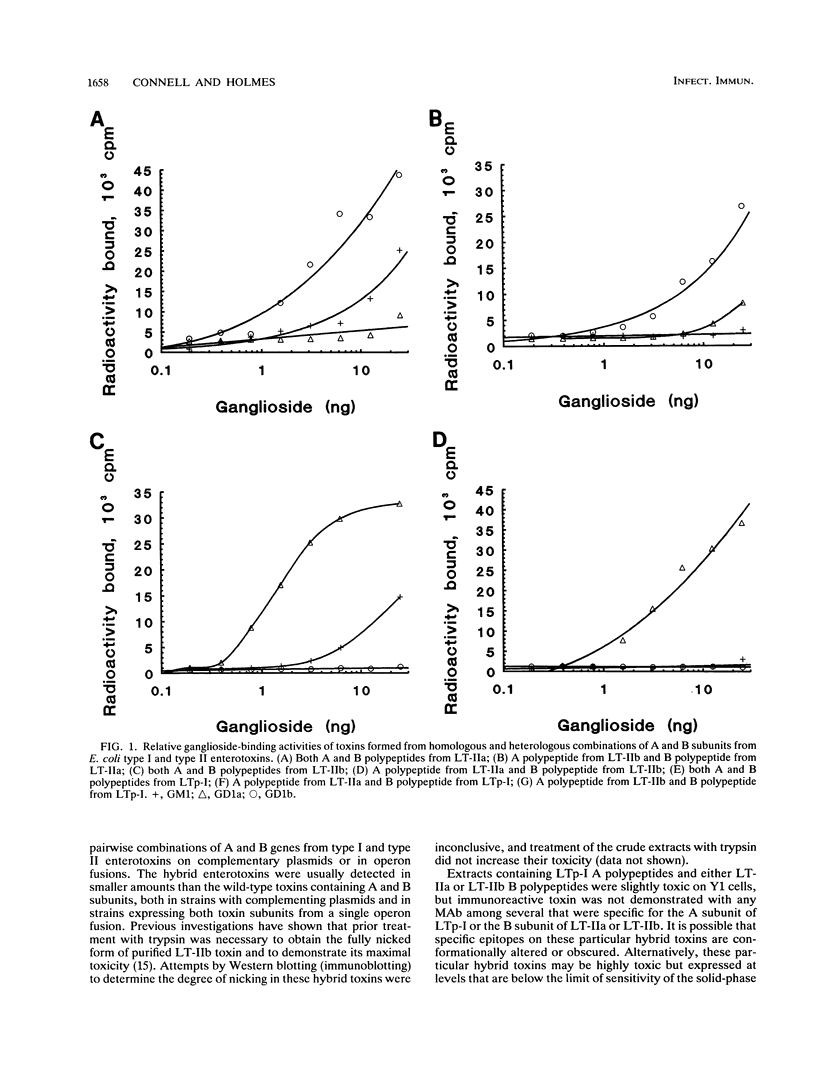
The genes encoding the individual A and B polypeptides of the type I enterotoxin LTp-I and type II enterotoxins LT-IIa and LT-IIb were cloned and tested for complementation in Escherichia coli. Each gene encoding an A polypeptide was cloned into pACYC184, and each gene encoding a B polypeptide was cloned into the compatible plasmid Bluescript KS+. In addition, operon fusions representing all combinations of A and B genes were constructed in Bluescript KS+. Extracts from strains of E. coli expressing each combination of A and B genes, either from compatible plasmids or from operon fusions, were tested for immunoreactive holotoxin by radioimmunoassays and for toxicity by Y1 adrenal cell assays. Biologically active holotoxin was detected in each case, but the toxicity of extracts containing the hybrid toxins was usually less than that of extracts containing the wild-type holotoxins. The ganglioside-binding activity of each holotoxin was tested, and in each case, the B polypeptide determined the ganglioside-binding specificity. The A and B polypeptides of the type II heat-labile enterotoxins were also shown to form holotoxin in vitro without exposure to denaturing conditions, in contrast to the polypeptides of the type I enterotoxins that failed to form holotoxin in vitro under comparable conditions. These findings suggest that type I and type II enterotoxins have conserved structural features that permit their A and B polypeptides to form hybrid holotoxins, although the B polypeptides of the type I and type II enterotoxins have very little amino acid sequence homology.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belisle B. W., Twiddy E. M., Holmes R. K. Characterization of monoclonal antibodies to heat-labile enterotoxin encoded by a plasmid from a clinical isolate of Escherichia coli. Infect Immun. 1984 Mar;43(3):1027–1032. doi: 10.1128/iai.43.3.1027-1032.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S. Conformity between heat-labile toxin genes from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1983 May;40(2):647–652. doi: 10.1128/iai.40.2.647-652.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature. 1980 Dec 4;288(5790):499–501. doi: 10.1038/288499a0. [DOI] [PubMed] [Google Scholar]
- Dallas W. S., Gill D. M., Falkow S. Cistrons encoding Escherichia coli heat-labile toxin. J Bacteriol. 1979 Sep;139(3):850–858. doi: 10.1128/jb.139.3.850-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Burks M. F., Zupan A., Dallas W. S., Jacob C. O., Ludwig D. S. Antigenic determinants of the cholera/coli family of enterotoxins. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S490–S502. doi: 10.1093/clinids/9.supplement_5.s490. [DOI] [PubMed] [Google Scholar]
- Francis M. L., Moss J., Fitz T. A., Mond J. J. cAMP-independent effects of cholera toxin on B cell activation. I. A possible role for cell surface ganglioside GM1 in B cell activation. J Immunol. 1990 Nov 15;145(10):3162–3169. [PubMed] [Google Scholar]
- Fukuta S., Magnani J. L., Twiddy E. M., Holmes R. K., Ginsburg V. Comparison of the carbohydrate-binding specificities of cholera toxin and Escherichia coli heat-labile enterotoxins LTh-I, LT-IIa, and LT-IIb. Infect Immun. 1988 Jul;56(7):1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Clements J. D., Robertson D. C., Finkelstein R. A. Subunit number and arrangement in Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Sep;33(3):677–682. doi: 10.1128/iai.33.3.677-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S. L., Finkelstein R. A., Critchley D. R. Characterization of the receptor for cholera toxin and Escherichia coli heat-labile toxin in rabbit intestinal brush borders. Biochem J. 1986 Sep 1;238(2):313–322. doi: 10.1042/bj2380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth B. E., Pickett C. L., Twiddy E. M., Holmes R. K., Gomes T. A., Lima A. A., Guerrant R. L., Franco B. D., Trabulsi L. R. Production of type II heat-labile enterotoxin by Escherichia coli isolated from food and human feces. Infect Immun. 1986 Nov;54(2):587–589. doi: 10.1128/iai.54.2.587-589.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth B. E., Twiddy E. M., Trabulsi L. R., Holmes R. K. Variation in chemical properties and antigenic determinants among type II heat-labile enterotoxins of Escherichia coli. Infect Immun. 1986 Nov;54(2):529–536. doi: 10.1128/iai.54.2.529-536.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. J., Holmgren J., Johansson S., Sanchez J., Hirst T. R. Coordinated assembly of multisubunit proteins: oligomerization of bacterial enterotoxins in vivo and in vitro. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7109–7113. doi: 10.1073/pnas.85.19.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Holmgren J. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7418–7422. doi: 10.1073/pnas.84.21.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst T. R., Sanchez J., Kaper J. B., Hardy S. J., Holmgren J. Mechanism of toxin secretion by Vibrio cholerae investigated in strains harboring plasmids that encode heat-labile enterotoxins of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7752–7756. doi: 10.1073/pnas.81.24.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Twiddy E. M. Characterization of monoclonal antibodies that react with unique and cross-reacting determinants of cholera enterotoxin and its subunits. Infect Immun. 1983 Dec;42(3):914–923. doi: 10.1128/iai.42.3.914-923.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Twiddy E. M., Pickett C. L. Purification and characterization of type II heat-labile enterotoxin of Escherichia coli. Infect Immun. 1986 Sep;53(3):464–473. doi: 10.1128/iai.53.3.464-473.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Tsuji T., Takeda Y., Miwatani T. Immunological nonidentity of heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1981 Nov;34(2):337–340. doi: 10.1128/iai.34.2.337-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling M. G., Holmes R. K. Analysis of structure and function of the B subunit of cholera toxin by the use of site-directed mutagenesis. Mol Microbiol. 1991 Jul;5(7):1755–1767. doi: 10.1111/j.1365-2958.1991.tb01925.x. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Chang P. P., Tsai S. C., Adamik R., Price S. R., Kunz B. C., Moss J., Twiddy E. M., Holmes R. K. Activation of Escherichia coli heat-labile enterotoxins by native and recombinant adenosine diphosphate-ribosylation factors, 20-kD guanine nucleotide-binding proteins. J Clin Invest. 1991 May;87(5):1780–1786. doi: 10.1172/JCI115197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J., Vinal A. C., Dallas W. S. Nucleotide sequence comparison between heat-labile toxin B-subunit cistrons from Escherichia coli of human and porcine origin. Infect Immun. 1985 Apr;48(1):73–77. doi: 10.1128/iai.48.1.73-77.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman H. A., Galen J. E., Kaper J. B. Vibrio cholerae enterotoxin genes: nucleotide sequence analysis of DNA encoding ADP-ribosyltransferase. J Bacteriol. 1984 Sep;159(3):1086–1089. doi: 10.1128/jb.159.3.1086-1089.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell M. M., Hibberd M., Field A. M., Chart H., Rowe B. Characterization of a new putative colonization factor (CS17) from a human enterotoxigenic Escherichia coli of serotype O114:H21 which produces only heat-labile enterotoxin. J Infect Dis. 1990 Feb;161(2):343–347. doi: 10.1093/infdis/161.2.343. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakula A. A., Sauer R. T. Genetic analysis of protein stability and function. Annu Rev Genet. 1989;23:289–310. doi: 10.1146/annurev.ge.23.120189.001445. [DOI] [PubMed] [Google Scholar]
- Peterson J. W., Ochoa L. G. Role of prostaglandins and cAMP in the secretory effects of cholera toxin. Science. 1989 Aug 25;245(4920):857–859. doi: 10.1126/science.2549637. [DOI] [PubMed] [Google Scholar]
- Pickett C. L., Twiddy E. M., Belisle B. W., Holmes R. K. Cloning of genes that encode a new heat-labile enterotoxin of Escherichia coli. J Bacteriol. 1986 Feb;165(2):348–352. doi: 10.1128/jb.165.2.348-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. L., Twiddy E. M., Coker C., Holmes R. K. Cloning, nucleotide sequence, and hybridization studies of the type IIb heat-labile enterotoxin gene of Escherichia coli. J Bacteriol. 1989 Sep;171(9):4945–4952. doi: 10.1128/jb.171.9.4945-4952.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. L., Weinstein D. L., Holmes R. K. Genetics of type IIa heat-labile enterotoxin of Escherichia coli: operon fusions, nucleotide sequence, and hybridization studies. J Bacteriol. 1987 Nov;169(11):5180–5187. doi: 10.1128/jb.169.11.5180-5187.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seriwatana J., Echeverria P., Taylor D. N., Rasrinaul L., Brown J. E., Peiris J. S., Clayton C. L. Type II heat-labile enterotoxin-producing Escherichia coli isolated from animals and humans. Infect Immun. 1988 May;56(5):1158–1161. doi: 10.1128/iai.56.5.1158-1161.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixma T. K., Pronk S. E., Kalk K. H., Wartna E. S., van Zanten B. A., Witholt B., Hol W. G. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991 May 30;351(6325):371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Honda T., Taga S., Miwatani T. In vitro formation of hybrid toxins between subunits of Escherichia coli heat-labile enterotoxin and those of cholera enterotoxin. Infect Immun. 1981 Nov;34(2):341–346. doi: 10.1128/iai.34.2.341-346.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Nakazawa T., Miyata T., Kaji A., Yokota T. Evolution and structure of two ADP-ribosylation enterotoxins, Escherichia coli heat-labile toxin and cholera toxin. FEBS Lett. 1984 Apr 24;169(2):241–246. doi: 10.1016/0014-5793(84)80326-9. [DOI] [PubMed] [Google Scholar]


