Abstract
Purpose
To evaluate the prognostic roles of metastasectomy and an established risk stratification system for patients experiencing a disease recurrence following nephrectomy for non-metastatic renal cell carcinoma (RCC).
Methods
A retrospective analysis was performed on 129 patients with localized RCC treated by partial or radical nephrectomy and subsequently diagnosed with disease recurrence. At the time of recurrence, a previously validated risk score based on Karnofsky performance status, interval from nephrectomy, and serum hemoglobin, calcium, and lactate dehydrogenase levels was used to categorize patients as favorable, intermediate, or poor-risk. Survival from recurrence was assessed based on risk categorization and metastasectomy
Results
Median time from nephrectomy to recurrence was 16 months. Median and two-year survival rates were strongly associated with the risk score (favorable-risk: 73 months and 81%; intermediate-risk: 28 months and 54%; poor-risk: 6 months and 11%; log-rank<0.001). Metastasectomy was performed in 44 patients (34%) and found to be of clinical benefit across the various risk categories (interaction analysis, p=0.8). On multivariate analysis, a better risk category (p<0.001) and undergoing a metastasectomy (p<0.001) were each independently associated with a more favorable survival and when combined provided six different risk categories with an estimated two-year survival ranging from 0 – 93%.
Conclusions
The clinical course for patients with an RCC recurrence following nephrectomy can be variable and is independently impacted by an objectively obtained risk score and whether the patient undergoes a metastasectomy.
Keywords: renal cell carcinoma, disease recurrence, nephrectomy, surveillance, prognosis, metastasectomy
INTRODUCTION
For patients with localized or locally advanced renal cell carcinoma (RCC), the risk of recurrence following partial or radical nephrectomy largely depends on tumor size, histology, stage, grade, completeness of resection, presence of symptoms, and patient performance status1–5. Contingent upon the varying distribution of these factors, five-year rates of recurrence range from 15 – 27%3, 4.
Largely based on retrospective analyses, several surveillance protocols have been proposed, tailoring the follow-up scheme to the estimated individual risk of disease recurrence.4, 6–8. The premise of each follow-up strategy is to allow prompt diagnosis and treatment of relapse at an early, solitary, and low-volume stage. Most physicians and patients intuitively presuppose that timely intervention with surgery, radiation or systemic agents are capable of providing a meaningful, and potentially curative, benefit; however, this paradigm has never been appropriately compared to the initiation of treatment when the tumor burden is more substantive.
At the time of local or systemic disease recurrence, the dilemma for clinicians and patients is deciding whether treatment is beneficial compared to observation alone and, if so, by what modality. Approximately one-quarter of patients with recurrent RCC are deemed suitable candidates for resection of measurable disease.9 For these patients, the five-year survival rates following surgery are 30 – 50% and prognosis has been associated with the extent and location of metastases, prior disease-free interval, and ability to attain a complete resection10–13.
Our group has recently shown that a simply attained risk score, which may reflect biologic aggressiveness (time to recurrence), tumor burden (LDH), hematopoietic suppression or skeletal involvement (serum hemoglobin and calcium) and the impact of disease on patient function (Karnofsky performance status), provides a powerful risk-stratification tool for patients with disease recurrence following nephrectomy 9. Based on risk-score and irrespective of whether their recurrent disease was resected, patients can be categorized into favorable, intermediate, or poor-risk groups with corresponding median survival times of 72, 25, and 6 months. We therefore hypothesized that the improved survival previously noted in patients undergoing metastasectomy may simply reflect a selection bias of patients at low risk for progression rather than a true surgical benefit. In this context we investigated the impact of metastasectomy on survival in patients with recurrent disease following nephrectomy using our previously validated risk stratification tool to control for individual risk of disease progression.
METHODS
Study cohort
Following Institutional Review Board approval, we queried our departmental renal tumor database to identify 167 patients undergoing partial or radical nephrectomy for clinically localized disease from January 1989 to June 2007 who subsequently developed a local or systemic recurrence. All patients were staged pre-operatively with an abdominal and pelvic computed tomography (CT) scan, chest imaging (x-ray or CT), serum comprehensive metabolic panel, and, if indicated by symptoms or laboratory values, bone scan or imaging of the brain.
Patients with bilateral renal masses at the time of presentation (n=7), von Hippel-Lindau disease (n=1), history of contralateral RCC prior to recurrence (n=3), or incomplete clinical (n=11) or follow-up (n=16) data were excluded, leaving 129 patients available for evaluation.
Follow-up and prognostic scoring system
Surveillance strategies after nephrectomy were at the discretion of the treating physician but generally consisted of a history and physical examination, serum chemistries, and chest imaging every 3–6 months during the first two years, every 6 months during years 3–5, and annually thereafter. Renal, abdominal, and pelvic imaging, either by ultrasound or CT scan were generally performed semi-annually. A disease recurrence was defined as radiographic evidence of disease on CT, MRI, or bone scan. Equivocal radiographic findings were assessed by follow-up imaging or biopsy and, when deemed appropriate, classified as a disease recurrence. Local disease recurrence was defined as tumor relapse in a prior nephrectomy bed.
At the time of recurrence, a five-point prognostic scoring system for patients with advanced RCC was applied9, 14. One point was assigned for each adverse parameter met, up to a maximum of five points, and consisted of: 1) time from nephrectomy to recurrence < 12 months, 2) serum hemoglobin less than age-specific lower limit of normal (male: < 13 g/dl, female: < 11.5 g/dl), 3) serum calcium > 10 mg/dl after correction for serum albumin, 4) Karnofsky performance status < 80%, and 5) serum LDH > 300 U/L. Each patient was then assigned to a risk category based on our previous work: favorable-risk (0 points), intermediate-risk (1–2 points), or poor-risk (3–5 points)9.
Treatment at the time of disease recurrence was at the discretion of the physician and patient. Surgical metastasectomy was generally offered if disease sites were felt to be amenable to a complete resection, particularly if they were solitary or confined to lung only, and based on a patient’s overall medical condition and ability to tolerate surgery.
Statistics
One-way ANOVA was used to compare time to recurrence based on RCC histology and pathologic stage. Survival analyses were performed using the Kaplan-Meier method. Time to event was coded as the interval from disease recurrence to last known follow-up or death. Disease-specific and overall survivals were equivalent, as all deaths in our cohort were attributed to progressive metastatic RCC. A Cox proportional hazards regression analysis using risk score and metastasectomy status was performed to evaluate predictors of overall survival. A p-value < 0.05 was considered significant.
RESULTS
Of the 129 patients with a disease recurrence following surgery for RCC, 87 (67%) were male, 123 (95%) underwent a prior radical nephrectomy, 103 (80%) had clear cell histology, and 77 (60%) exhibited pathologically advanced features (pT3 or pT4) (Table 1).
Table 1.
Clinical and Pathologic Characteristics at Time of Nephrectomy
| Number of patients | Percent | |
|---|---|---|
| Number of patients | 129 | |
| Gender | ||
| Male | 87 | 67% |
| Female | 42 | 33% |
| Median age (IQR) at nephrectomy (years) | 61.7 (52.7, 70.8) | |
| Type of surgery | ||
| Radical nephrectomy | 123 | 95% |
| Partial nephrectomy | 6 | 5% |
| Pathologic stage | ||
| T1 | 27 | 21% |
| T2 | 25 | 19% |
| T3 | 67 | 52% |
| T4 | 10 | 8% |
| Histologic subtype | ||
| Clear cell | 103 | 80% |
| Chromophobe | 7 | 5% |
| Papillary | 6 | 5% |
| Multiple | 4 | 3% |
| Collecting duct | 2 | 2% |
| Unclassified | 7 | 5% |
Median (IQR) time from nephrectomy to recurrence was 16.0 (4.5, 32.8) months with 82 (64%) and 115 (89%) within two and five years, respectively. The lengthiest interval to recurrence was 136 months. Pathologic stage was associated with mean (SD) time to recurrence [pT1: 38 (32) months, pT2: 39 (35) months, pT3: 19 (23) months, pT4: 9 (13) months; p=0.001] whereas RCC histology was not [chromophobe: 30 (11) months, clear cell: 27 (31) months, papillary: 17 (23) months, other: 15 (18) months; p=0.4].
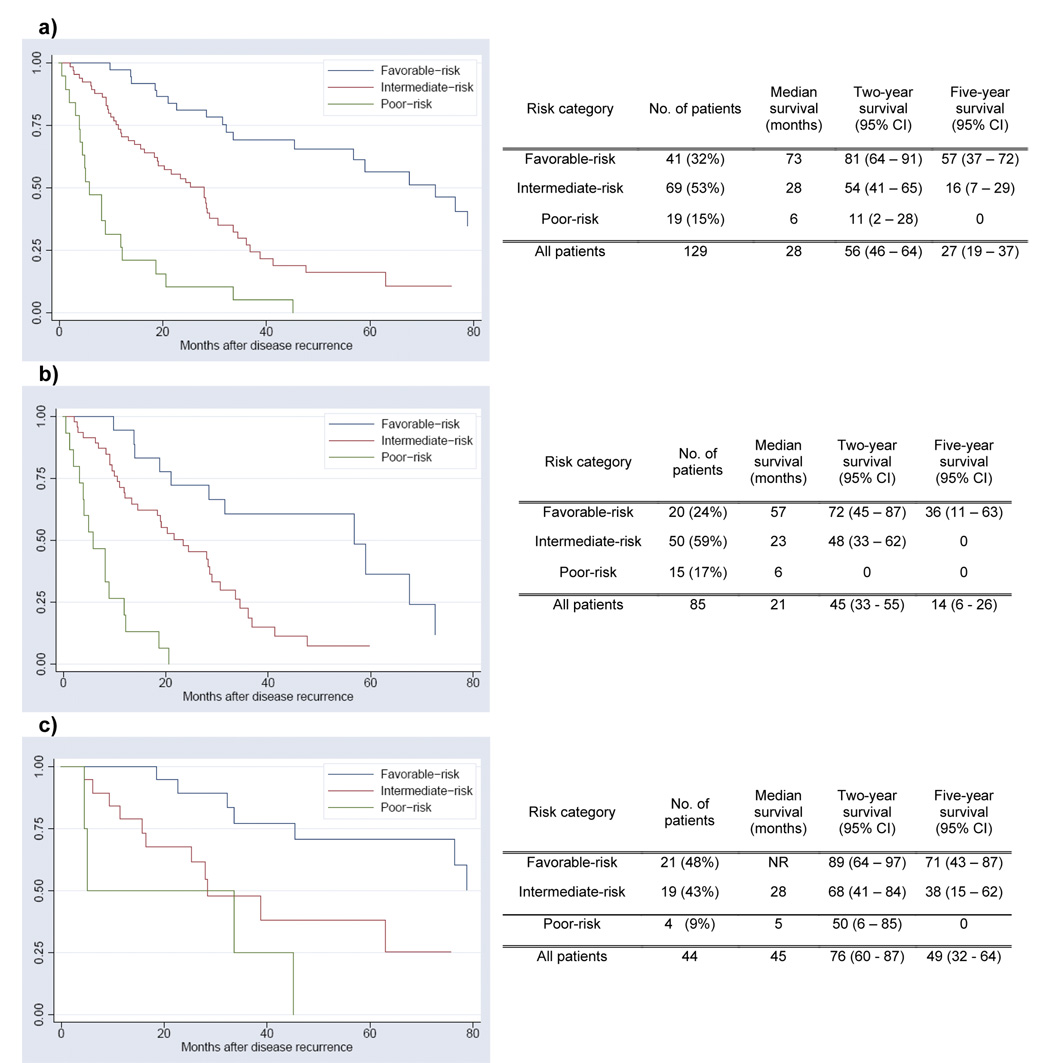
Median survivals from recurrence for all patients and for those alive at the conclusion of the study were 28 and 30 months, respectively. Of the five factors utilized for risk stratification, 23 (18%) patients met criteria for poor performance status (KPS<80%), 8 (6%) for elevated LDH, 22 (17%) for elevated calcium, 43 (33%) for decreased hemoglobin, and 57 (44%) for interval to recurrence < 12 months (Table 2). After determining a risk score for each patient, 41 (32%) were classified as favorable-risk, 69 (53%) intermediate-risk, and 19 (15%) poor-risk (Figure 1a). Median survival rates and the two-year and five-year survival probabilities were strongly associated with the risk category (favorable-risk: 73 months, 81%, 57%; intermediate-risk: 28 months, 54%, 16%; poor-risk: 6 months, 11%, 0%, respectively).
Table 2.
Characteristics at Time of Disease Recurrence
| Number of patients | Percent | |
|---|---|---|
| Year of recurrence | ||
| 1990–1995 | 22 | 17% |
| 1996–2001 | 64 | 50% |
| 2002–2007 | 43 | 33% |
| Site of recurrence* | ||
| Lung | 82 | 64% |
| Bone | 21 | 16% |
| Lymph node-regional | 14 | 11% |
| Liver | 13 | 10% |
| Local recurrence | 13 | 10% |
| Brain | 8 | 6% |
| Lymph node – distant | 5 | 4% |
| Other# | 14 | 11% |
| Karnofsky performance status | ||
| ≤60% | 8 | 6% |
| 70% | 15 | 12% |
| 80% | 33 | 25% |
| 90% | 46 | 36% |
| 100% | 27 | 21% |
| LDH > 1.5x upper limit of normal | ||
| Yes | 8 | 6% |
| No | 121 | 94% |
| Corrected calcium > 10 mg/dl | ||
| Yes | 22 | 17% |
| No | 107 | 83% |
| Hemoglobin (female<11.5 g/dl, male<13.0 g/dl) | ||
| Yes | 43 | 33% |
| No | 66 | 67% |
| Time to recurrence < 12 months | ||
| Yes | 57 | 44% |
| No | 72 | 56% |
Includes patients with more than one site of recurrence
Includes pancreas (5), contralateral adrenal gland (4), thyroid (2) and one each for vaginal, spleen, and ethmoid
Figure 1.
Survival From Time of Disease Recurrence Based on Risk Category: a) all patients (n=129); log-rank<0.001, b) no metastasectomy (n=85); log-rank<0.001, c) metastasectomy (n=44); log-rank=0.003
Lung was the most common site of recurrence (82 patients: 64%). Representative of the diverse metastatic potential of RCC, twelve different organs were sites of relapse (Table 2). Metastasectomies were performed in 44 (34%) patients and on 11 different organs (Table 3). Metastasectomy intent was curative in 40 (91%) patients. All 4 patients undergoing a palliative metastasectomy [9%; brain (2), femur, and spinal] died of disease (4, 11, 23, and 39 months after recurrence). Patients designated as favorable-risk were more likely to undergo metastasectomy (51%) compared to intermediate (28%) or poor-risk (21%) (p=0.02), highlighting the variability of the surgical selection process.
Table 3.
Sites of Metastasectomy
| Site of Metastasectomy | Number of patients |
|---|---|
| Lung | 19 |
| Distant lymph nodes | 4 |
| Regional lymph nodes | 3 |
| Brain | 3 |
| Bone | 3 |
| Liver | 3 |
| Local recurrence | 3 |
| Adrenal | 2 |
| Ethmoid | 1 |
| Pancreas | 1 |
| Spleen | 1 |
| Thyroid | 1 |
The risk strata provided meaningful prognostic information regardless of whether patients underwent a metastasectomy (Figures 1a–c, all log-rank = 0.003). To test the hypothesis that metastasectomy has an effect on survival only among a particular risk strata, we looked for an interaction between metastasectomy and risk group. An interaction between two variables exists if the effect of one variable depends on the level of the other variable. If the interaction term between metastasectomy and risk group (favorable vs intermediate vs poor risk) is statistically significant, then the change in survival associated with metastasectomy is affected by the patient’s risk characteristics. The interaction term between metastasectomy and risk group was not statistically significant (p=0.8) and therefore we have no evidence that metastasectomy is of benefit only to patients of a particular risk category.
On multivariate Cox regression analysis predicting death from disease, not undergoing a metastasectomy (HR=2.7; 95% CI: 1.6 – 4.5; p<0.001) and a higher risk category (intermediate vs. favorable risk: HR=3.0, 95% CI: 1.7 – 5.3, p<0.001; poor vs. favorable risk: HR=12.4, 95% CI: 6.0 – 25.5, p<0.001) were both independently associated with decreased survival (Table 4). Not undergoing a metastasectomy independently predicts for adverse survival; the impact of being intermediate versus favorable risk is similar to not having a metastasectomy and being poor risk versus favorable risk portends a worse outcome compared to not having a metastasectomy.
Table 4.
Multivariate Cox Regression: Overall Survival from Time of Recurrence
| HR | 95% CI | p-value | |
|---|---|---|---|
| Metastasectomy | |||
| Yes | REF | REF | <0.001 |
| No | 2.7 | 1.6 – 4.5 | |
| Risk score | |||
| Favorable | REF | REF | <0.001 |
| Intermediate | 3.0 | 1.7 – 5.3 | |
| Poor | 12.4 | 6.0 – 25.5 | |
HR: hazard ratio
CI: confidence interval
REF: reference
DISCUSSION
For the 15% of patients diagnosed with an RCC recurrence within five years of nephrectomy (ranging from 5% to 27% based on tumor size), management options typically include newly available targeted agents, systemic immunotherapy, surgical resection or observation. Tyrosine kinase inhibitors (sorafenib and sunitinib) and mTOR inhibitors (temsirolimus and RAD-001) prolong time to progression versus standard therapy but durable complete responses have not been reported with these agents alone. Immunotherapies such as interleukin-2 and interferon-α induce durable complete responses in approximately 5%15 of patients but are less frequently used since the introduction of newer, less-toxic agents.
Largely based on small retrospective reports showing five-year disease-free survival rates of 30 – 50% following metastasectomy10–13, patients with resectable disease at the time of recurrence are typically considered candidates for potentially curative surgery. However, the relatively favorable outcomes compared to systemic agents alone are at least partially a consequence of patient selection and the variable growth kinetics of individual metastatic renal cancers9.
There are two main findings of our study. First, patients undergoing metastasectomy for RCC recurrence following nephrectomy appear to experience a survival benefit. Secondly, our objective risk stratification at the time of recurrence is a valuable tool containing powerful prognostic information that should aid patient counseling and decision making.
Corroborating another study primarily evaluating patients with metastatic disease at diagnosis16, we found metastasectomy to be independently and strongly associated with an improved survival outcome. Our data, along with the findings of earlier studies from Memorial Sloan-Kettering11, Mayo Clinic12 17, and Martin Luther University10 showing the five-year disease-free survival of 30 – 50% following metastasectomy, supports the notion of offering metastasectomy to individuals with surgically resectable disease. Our patient cohort was largely offered metastasectomy with the objective of achieving a disease-free status. Intriguingly, even when the likelihood of complete disease resection is low, metastasectomy appears to maintain its clinical benefit as Vogl et al showed that when the indication was commonly for pain control, prevention of pathologic fractures, or brain metastases (74%) and rarely achieved a disease-free status (21%), metastasectomy independently predicted for an improvement in survival16.
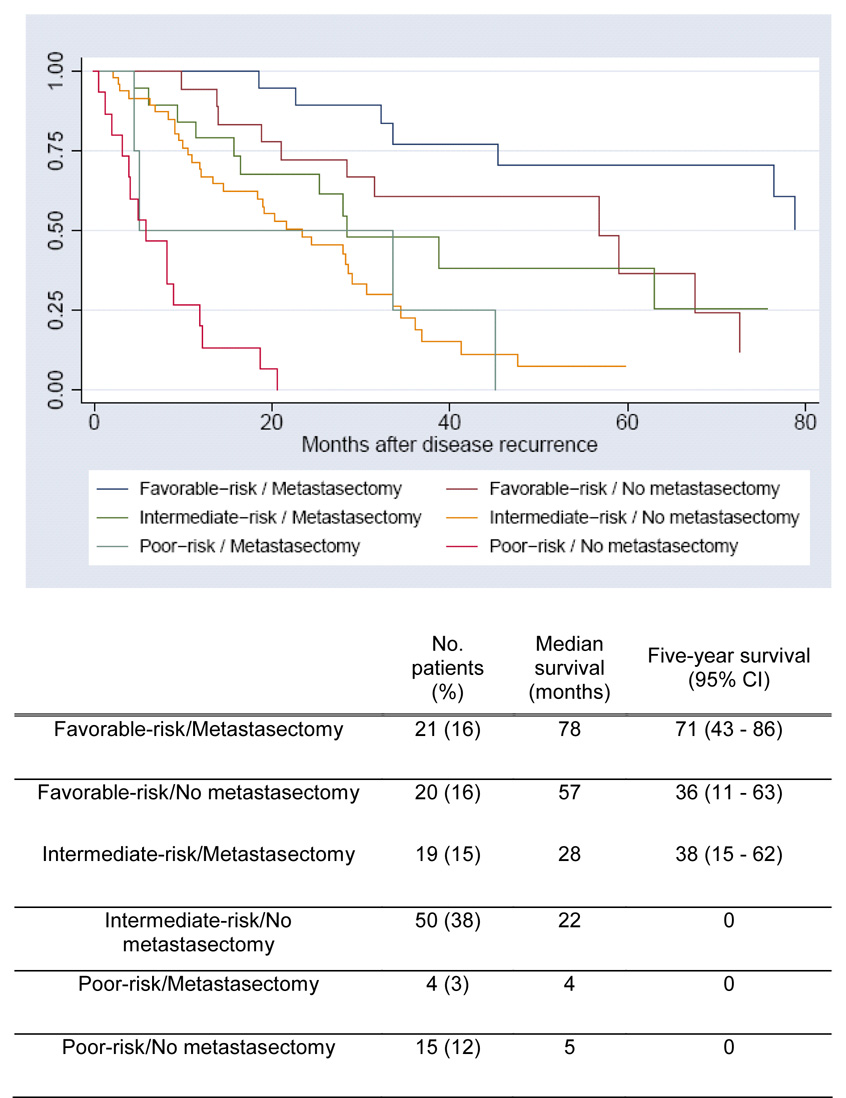
The objective risk stratification score is independently associated with outcome and in conjunction with the presence or absence of metastasectomy provides more specific prognostic information (estimated two-year survival range: 0 – 93%; Figure 2). Components of this five-point scoring system are valuable in the recurrent9, newly-diagnosed metastatic14, and treatment-refractory metastatic18 settings, incorporated into the National Comprehensive Cancer Network Kidney Cancer Guidelines, and used for balanced randomization in prospective investigational trials19.
Figure 2.
Overall Survival Based on Metastasectomy Status and Risk Score
Whether to proceed with metastasectomy depends on multiple factors: sites and number of metastases, resectability, surgical expertise, patient compliance and general medical condition. Since there are very few absolute indications for surgery, the decision regarding metastasectomy is often empiric. Our data suggests that in appropriately selected patients it appears to be associated with a survival benefit.
Though not our primary study intent, other interesting observations can be extracted. Relative to all patients in our database undergoing nephrectomy for localized disease, those experiencing a recurrence were expectedly more likely to have had a radical nephrectomy (95% vs 70%), pathologic T3-4 disease (60% vs 25%), and clear cell histology (80% vs 61%). Further, time from nephrectomy to recurrence can be quite protracted (11% greater than 5 years) with advanced pathologic features being associated with a shorter time to recurrence.
Limitations of our study should be considered when interpreting the results. The bona fide role of surgery at the time of RCC recurrence can only be definitively understood through prospective evaluation with a standardized treatment protocol, particularly since a selection bias in recommending metastasectomy may exist. Although the original development of the risk score considered a multitude of variables, it was by no means constructed to be definitive, as there are most certainly other parameters that serve as surrogates of outcome. However, the risk score as presented benefits from its simplicity, ease in collecting the required information, validation, and familiarity to many oncologists. Our study cohort may be too small to recognize the differential impact of metastasectomy by risk group when evaluated in the interaction analysis. Future studies with more patients are required to confirm and more strongly establish these risk groups. The variables comprising the risk score, although proven and validated, do not represent every known predictive factor and others may correlate with outcome. In the original study establishing the five variables comprising the risk score, a multitude of laboratory values and metastases-specific data (site, number, etc) were not independently associated with survival and therefore not included in the risk score14. While histologic subtype and grade from the original nephrectomy were not part of that analysis, in our cohort they were not associated with outcome following recurrence (data not shown).
CONCLUSIONS
For patients with an RCC recurrence following nephrectomy for localized disease, the clinical course from the time of recurrence can be highly variable and ranges from cure to a rapid death. Prognosis can be estimated based on an objective risk categorization score and whether the metastasis is resected. For select patients, regardless of risk score, metastasectomy appears to be associated with a survival benefit.
Acknowledgments
SEE is funded through a National Institute of Health Ruth Kirchstein National Research Service Award (T32-CA82088-06)
Glossary
- LDH
lactate dehydrogenase
- RCC
renal cell carcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no potential conflicts of interest
REFERENCES
- 1.Cindolo L, Patard JJ, Chiodini P, Schips L, Ficarra V, Tostain J, et al. Comparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy: a multicenter European study. Cancer. 2005;104:1362. doi: 10.1002/cncr.21331. [DOI] [PubMed] [Google Scholar]
- 2.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Leibovich BC, et al. A multifactorial postoperative surveillance model for patients with surgically treated clear cell renal cell carcinoma. J Urol. 2003;170:2225. doi: 10.1097/01.ju.0000095541.10333.a7. [DOI] [PubMed] [Google Scholar]
- 3.Kattan MW, Reuter V, Motzer RJ, Katz J, Russo P. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63. [PubMed] [Google Scholar]
- 4.Lam JS, Shvarts O, Leppert JT, Pantuck AJ, Figlin RA, Belldegrun AS. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol. 2005;174:466. doi: 10.1097/01.ju.0000165572.38887.da. [DOI] [PubMed] [Google Scholar]
- 5.Lee CT, Katz J, Fearn PA, Russo P. Mode of presentation of renal cell carcinoma provides prognostic information. Urol Oncol. 2002;7:135. doi: 10.1016/s1078-1439(01)00185-5. [DOI] [PubMed] [Google Scholar]
- 6.Levy DA, Slaton JW, Swanson DA, Dinney CP. Stage specific guidelines for surveillance after radical nephrectomy for local renal cell carcinoma. J Urol. 1998;159:1163. [PubMed] [Google Scholar]
- 7.Sandock DS, Seftel AD, Resnick MI. A new protocol for the followup of renal cell carcinoma based on pathological stage. J Urol. 1995;154:28. [PubMed] [Google Scholar]
- 8.Stephenson AJ, Chetner MP, Rourke K, Gleave ME, Signaevsky M, Palmer B, et al. Guidelines for the surveillance of localized renal cell carcinoma based on the patterns of relapse after nephrectomy. J Urol. 2004;172:58. doi: 10.1097/01.ju.0000132126.85812.7d. [DOI] [PubMed] [Google Scholar]
- 9.Eggener SE, Yossepowitch O, Pettus JA, Snyder ME, Motzer RJ, Russo P. Renal cell carcinoma recurrence after nephrectomy for localized disease: predicting survival from time of recurrence. J Clin Oncol. 2006;24:3101. doi: 10.1200/JCO.2005.04.8280. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann HS, Neef H, Krohe K, Andreev P, Silber RE. Prognostic factors and survival after pulmonary resection of metastatic renal cell carcinoma. Eur Urol. 2005;48:77. doi: 10.1016/j.eururo.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Kavolius JP, Mastorakos DP, Pavlovich C, Russo P, Burt ME, Brady MS. Resection of metastatic renal cell carcinoma. J Clin Oncol. 1998;16:2261. doi: 10.1200/JCO.1998.16.6.2261. [DOI] [PubMed] [Google Scholar]
- 12.Kierney PC, van Heerden JA, Segura JW, Weaver AL. Surgeon's role in the management of solitary renal cell carcinoma metastases occurring subsequent to initial curative nephrectomy: an institutional review. Ann Surg Oncol. 1994;1:345. doi: 10.1007/BF02303572. [DOI] [PubMed] [Google Scholar]
- 13.Master VA, Gottschalk AR, Kane C, Carroll PR. Management of isolated renal fossa recurrence following radical nephrectomy. J Urol. 2005;174:473. doi: 10.1097/01.ju.0000165574.62188.d0. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 15.McDermott DF. Update on the application of interleukin-2 in the treatment of renal cell carcinoma. Clin Cancer Res. 2007;13:716s. doi: 10.1158/1078-0432.CCR-06-1872. [DOI] [PubMed] [Google Scholar]
- 16.Vogl UM, Zehetgruber H, Dominkus M, Hejna M, Zielinski CC, Haitel A, et al. Prognostic factors in metastatic renal cell carcinoma: metastasectomy as independent prognostic variable. Br J Cancer. 2006;95:691. doi: 10.1038/sj.bjc.6603327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leibovich B, Cheville J, Lohse C, Zincke H, Frank I, Kwon E, et al. A scorig algorithm to predict survival for patients with metastatic clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Journal of Urology. 2005;174(5):1759. doi: 10.1097/01.ju.0000177487.64651.3a. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 19.Escudier B, Koralewski P, Pluzanska E, Ravaud A, Bracarda S, Szczylik C, et al. A randomized, controlled, double-blind phase III study (AVOREN) of bevacizumab/interferon-α2a vs placebo/interferon- α2a as first-line therapy in metastatic renal cell carcinoma. Presented at the ASCO Annual Meeting Proceedings; Chicago. 2007. [Google Scholar]