Abstract
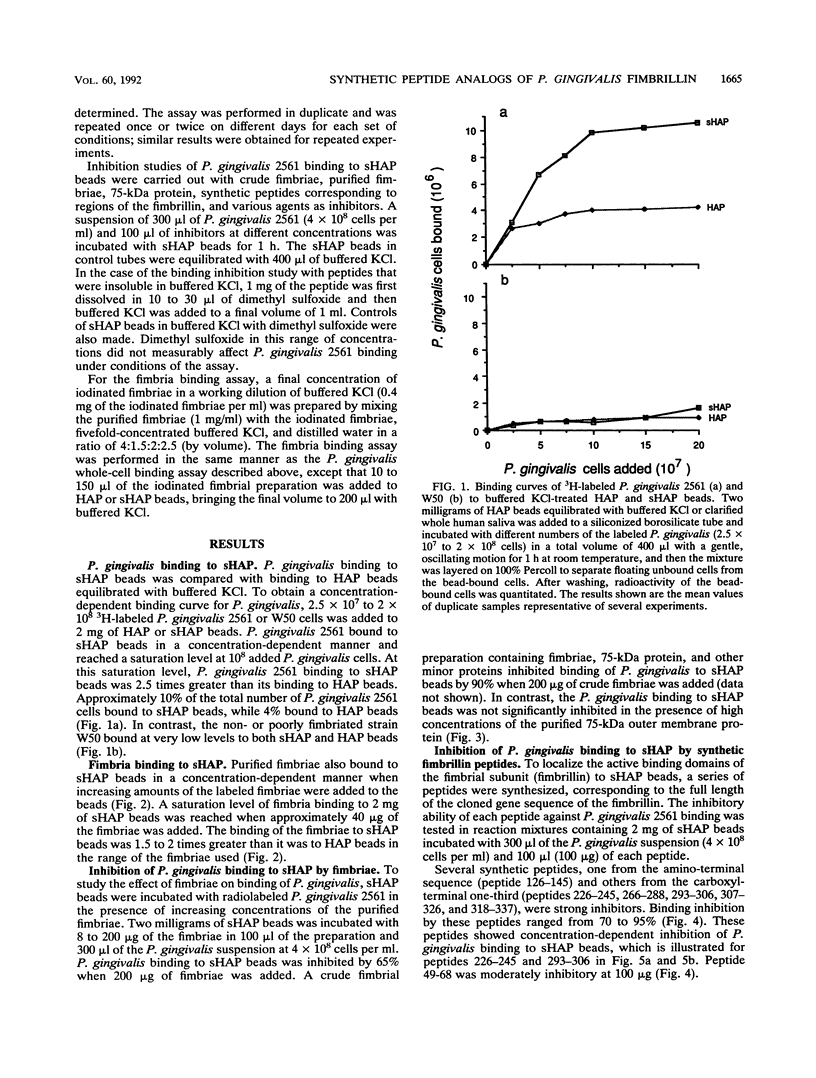
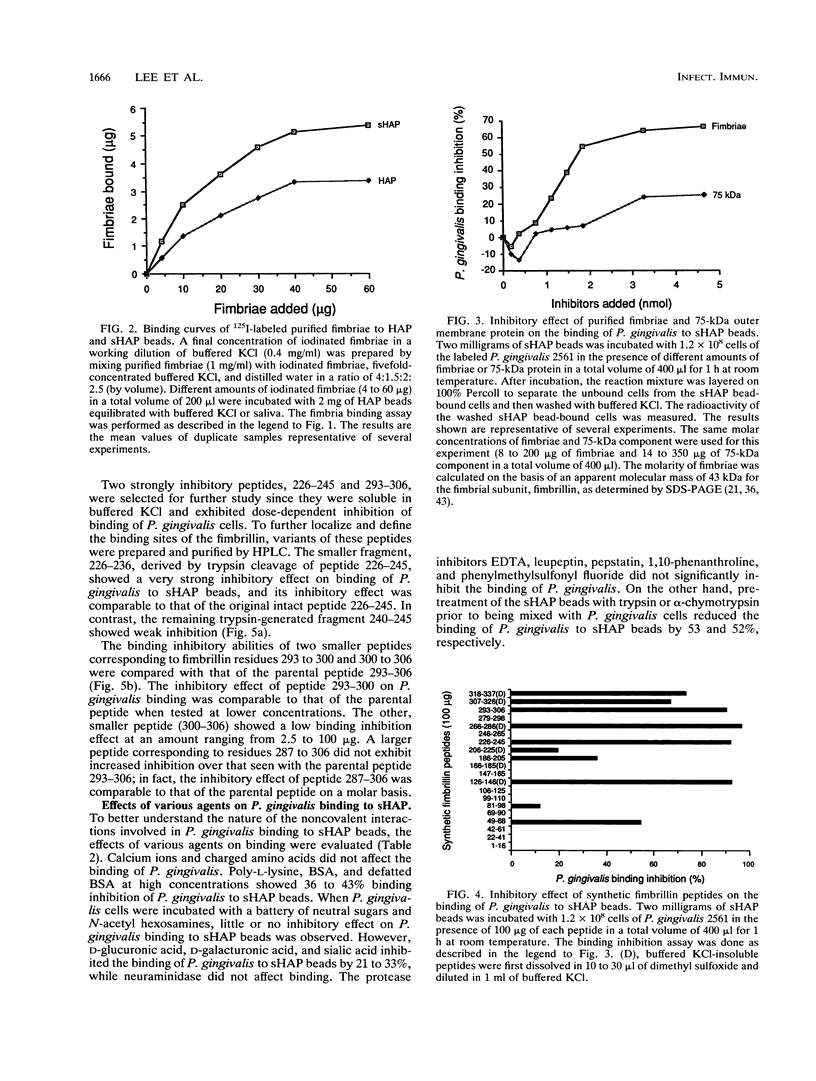
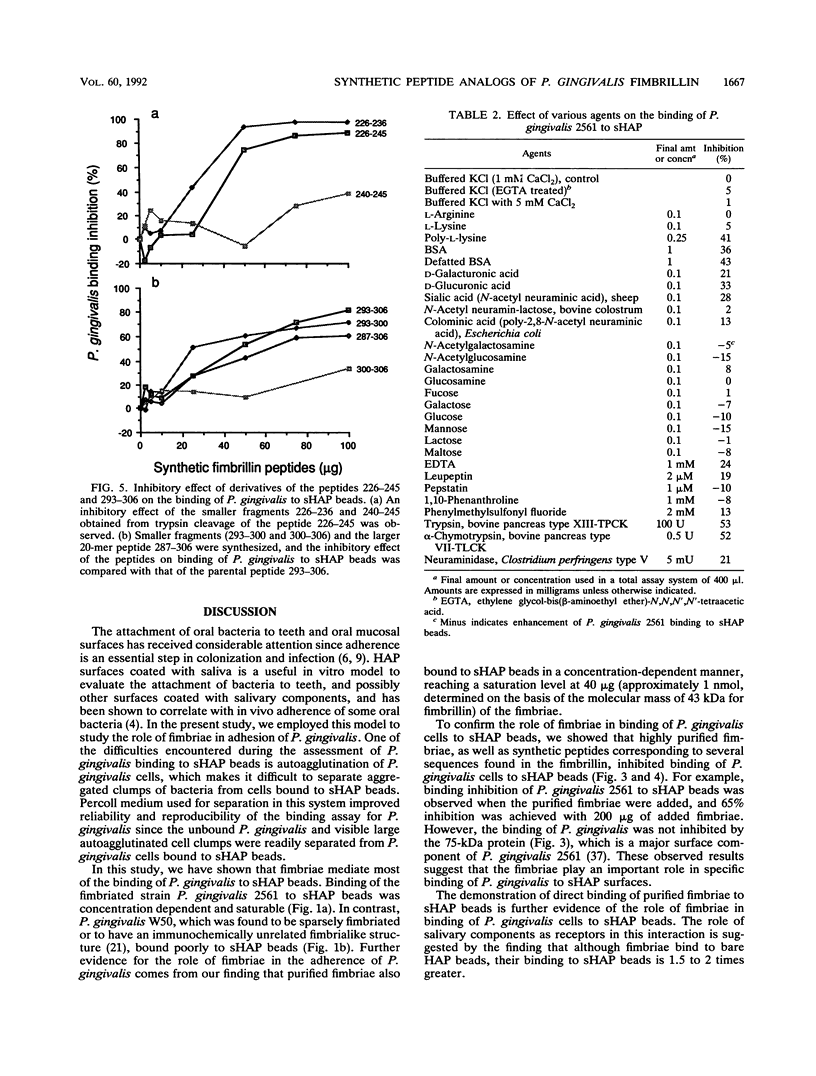
Fimbriae are important in the adherence of many bacterial species to the surfaces they eventually colonize. Porphyromonas (Bacteroides) gingivalis fimbriae appear to mediate adherence to oral epithelial cells and the pellicle-coated tooth surface. The role and contribution of fimbriae in the binding of P. gingivalis to hydroxyapatite (HAP) coated with saliva as a model for the pellicle-coated tooth surface were investigated. 3H-labeled P. gingivalis or the radioiodinated purified fimbriae were incubated with 2 mg of HAP beads coated with whole human saliva (sHAP) and layered on 100% Percoll to separate unbound from sHAP-bound components. The radioactivity of the washed beads was a measure of the bound components. The binding of P. gingivalis 2561 (381) cells and that of purified fimbriae were concentration dependent and saturable at approximately 10(8) cells and 40 micrograms of fimbriae added, respectively. The addition of fimbriae inhibited binding of P. gingivalis to sHAP beads by 65%, while the 75-kDa protein, which is another major surface component of P. gingivalis 2561, did not show significant inhibition, suggesting that the fimbriae are important in adherence. Encapsulated and sparsely fimbriated P. gingivalis W50 did not bind to sHAP beads. On the basis of the predicted sequence of the fimbrillin, a structural subunit of fimbriae, a series of peptides were synthesized and used to localize the active fimbrillin domains involved in P. gingivalis adherence to sHAP beads. Peptides from the carboxyl-terminal one-third of the fimbrillin strongly inhibited P. gingivalis binding to sHAP beads. Active residues within the sequence of inhibitory peptide 226-245 (peptide containing residues 226 to 245) and peptide 293-306 were identified by using smaller fragments prepared either by trypsin cleavage of the peptide 226-245 or by synthesis of smaller segments of peptide 293-306. Hemagglutinin activity, lectinlike binding, and ionic interaction did not seem to be involved in this binding since lysine, arginine, carbohydrates, and calcium ions failed to affect the binding of P. gingivalis. The observation that poly-L-lysine, bovine serum albumin, and defatted bovine serum albumin, even at high concentrations, only partially blocked the binding of P. gingivalis indicates that hydrophobic interactions are not the major forces involved in P. gingivalis binding to sHAP beads. Protease inhibitors such as EDTA, leupeptin, pepstatin, 1,10-phenanthroline, and phenylmethylsulfonyl fluoride did not interfere with the binding of P. gingivalis. However, the binding of P. gingivalis to trypsin- or chymotrypsin-pretreated sHAP beads was reduced.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd J., McBride B. C. Fractionation of hemagglutinating and bacterial binding adhesins of Bacteroides gingivalis. Infect Immun. 1984 Aug;45(2):403–409. doi: 10.1128/iai.45.2.403-409.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs W. C., 3rd, Gibbons R. J. Selective modulation of bacterial attachment to oral epithelial cells by enzyme activities associated with poor oral hygiene. J Periodontal Res. 1990 May;25(3):172–178. doi: 10.1111/j.1600-0765.1990.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Cimasoni G., Song M., McBride B. C. Effect of crevicular fluid and lysosomal enzymes on the adherence of streptococci and bacteroides to hydroxyapatite. Infect Immun. 1987 Jun;55(6):1484–1489. doi: 10.1128/iai.55.6.1484-1489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. P., Kubiniec M. A., Yoshimura F., Genco R. J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988 Apr;170(4):1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose A. C., Karush F. Induction of polyclonal and monoclonal antibody responses to cholera toxin by the synthetic peptide approach. Mol Immunol. 1988 Mar;25(3):223–230. doi: 10.1016/0161-5890(88)90013-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Adherent interactions which may affect microbial ecology in the mouth. J Dent Res. 1984 Mar;63(3):378–385. doi: 10.1177/00220345840630030401. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989 May;68(5):750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Comparative hydrophobicities of oral bacteria and their adherence to salivary pellicles. Infect Immun. 1983 Sep;41(3):1190–1196. doi: 10.1128/iai.41.3.1190-1196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulbourne P. A., Ellen R. P. Evidence that Porphyromonas (Bacteroides) gingivalis fimbriae function in adhesion to Actinomyces viscosus. J Bacteriol. 1991 Sep;173(17):5266–5274. doi: 10.1128/jb.173.17.5266-5274.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect Immun. 1987 Jan;55(1):111–117. doi: 10.1128/iai.55.1.111-117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hanazawa S., Hirose K., Ohmori Y., Amano S., Kitano S. Bacteroides gingivalis fimbriae stimulate production of thymocyte-activating factor by human gingival fibroblasts. Infect Immun. 1988 Jan;56(1):272–274. doi: 10.1128/iai.56.1.272-274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanazawa S., Murakami Y., Hirose K., Amano S., Ohmori Y., Higuchi H., Kitano S. Bacteroides (Porphyromonas) gingivalis fimbriae activate mouse peritoneal macrophages and induce gene expression and production of interleukin-1. Infect Immun. 1991 Jun;59(6):1972–1977. doi: 10.1128/iai.59.6.1972-1977.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai H., Isogai E., Yoshimura F., Suzuki T., Kagota W., Takano K. Specific inhibition of adherence of an oral strain of Bacteroides gingivalis 381 to epithelial cells by monoclonal antibodies against the bacterial fimbriae. Arch Oral Biol. 1988;33(7):479–485. doi: 10.1016/0003-9969(88)90028-3. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Molecular mapping of human band 3 anion transport regions using synthetic peptides. FASEB J. 1991 Jan;5(1):109–115. doi: 10.1096/fasebj.5.1.1991578. [DOI] [PubMed] [Google Scholar]
- König W., Geiger R. Eine neue Methode zur Synthese von Peptiden: Aktivierung der Carboxylgruppe mit Dicyclohexycarbodiimid unter Zusatz von 1-Hydroxy-benzotriazolen. Chem Ber. 1970;103(3):788–798. doi: 10.1002/cber.19701030319. [DOI] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J Clin Microbiol. 1982 Jan;15(1):97–102. doi: 10.1128/jcm.15.1.97-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. Y., Sojar H. T., Bedi G. S., Genco R. J. Porphyromonas (Bacteroides) gingivalis fimbrillin: size, amino-terminal sequence, and antigenic heterogeneity. Infect Immun. 1991 Jan;59(1):383–389. doi: 10.1128/iai.59.1.383-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ellen R. P., Hoover C. I., Felton J. R. Association of proteases of Porphyromonas (Bacteroides) gingivalis with its adhesion to Actinomyces viscosus. J Dent Res. 1991 Feb;70(2):82–86. doi: 10.1177/00220345910700021501. [DOI] [PubMed] [Google Scholar]
- Londoño J. A., Gras-Masse H., Dubeaux C., Tartar A., Druilhe P. Secondary structure and immunogenicity of hybrid synthetic peptides derived from two Plasmodium falciparum pre-erythrocytic antigens. J Immunol. 1990 Sep 1;145(5):1557–1563. [PubMed] [Google Scholar]
- Magnusson K. E., Davies J., Grundström T., Kihlström E., Normark S. Surface charge and hydrophobicity of Salmonella, E. coli, Gonococci in relation to their tendency to associate with animal cells. Scand J Infect Dis Suppl. 1980;Suppl 24:135–140. [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Gibbons R. J. Attachment of Bacteroides gingivalis to collagenous substrata. J Dent Res. 1988 Aug;67(8):1075–1080. doi: 10.1177/00220345880670080301. [DOI] [PubMed] [Google Scholar]
- Nesbitt W. E., Doyle R. J., Taylor K. G. Hydrophobic interactions and the adherence of Streptococcus sanguis to hydroxylapatite. Infect Immun. 1982 Nov;38(2):637–644. doi: 10.1128/iai.38.2.637-644.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikata M., Yoshimura F., Nodasaka Y. Possibility of Bacteroides gingivalis hemagglutinin possessing protease activity revealed by inhibition studies. Microbiol Immunol. 1989;33(1):75–80. doi: 10.1111/j.1348-0421.1989.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Okuda K., Yamamoto A., Naito Y., Takazoe I., Slots J., Genco R. J. Purification and properties of hemagglutinin from culture supernatant of Bacteroides gingivalis. Infect Immun. 1986 Dec;54(3):659–665. doi: 10.1128/iai.54.3.659-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peros W. J., Etherden I., Gibbons R. J., Skobe Z. Alteration of fimbriation and cell hydrophobicity by sublethal concentrations of tetracycline. J Periodontal Res. 1985 Jan;20(1):24–30. doi: 10.1111/j.1600-0765.1985.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Raja R. H., Raucci G., Hook M. Peptide analogs to a fibronectin receptor inhibit attachment of Staphylococcus aureus to fibronectin-containing substrates. Infect Immun. 1990 Aug;58(8):2593–2598. doi: 10.1128/iai.58.8.2593-2598.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Buivids I. A., Ellen R. P. Adhesion of Actinomyces viscosus to Porphyromonas (Bacteroides) gingivalis-coated hexadecane droplets. J Bacteriol. 1991 Apr;173(8):2581–2589. doi: 10.1128/jb.173.8.2581-2589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., Ellen R. P., Grove D. A. Bacteroides gingivalis-Actinomyces viscosus cohesive interactions as measured by a quantitative binding assay. Infect Immun. 1987 Oct;55(10):2391–2397. doi: 10.1128/iai.55.10.2391-2397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Enzymatic characterization of some oral and nonoral gram-negative bacteria with the API ZYM system. J Clin Microbiol. 1981 Sep;14(3):288–294. doi: 10.1128/jcm.14.3.288-294.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojar H. T., Lee J. Y., Bedi G. S., Cho M. I., Genco R. J. Purification, characterization and immunolocalization of fimbrial protein from Porphyromonas (bacteroides) gingivalis. Biochem Biophys Res Commun. 1991 Mar 15;175(2):713–719. doi: 10.1016/0006-291x(91)91624-l. [DOI] [PubMed] [Google Scholar]
- Sojar H. T., Lee J. Y., Bedi G. S., Cho M. I., Genco R. J. Purification, characterization, and localization of a major membrane protein antigen from Porphyromonas (bacteroides) gingivalis. Biochem Int. 1991 Oct;25(3):437–446. [PubMed] [Google Scholar]
- Stinson M. W., Safulko K., Levine M. J. Adherence of Porphyromonas (Bacteroides) gingivalis to Streptococcus sanguis in vitro. Infect Immun. 1991 Jan;59(1):102–108. doi: 10.1128/iai.59.1.102-108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vordermeier H. M., Hoffmann P., Gombert F. O., Jung G., Bessler W. G. Synthetic peptide segments from the Escherichia coli porin OmpF constitute leukocyte activators. Infect Immun. 1990 Aug;58(8):2719–2724. doi: 10.1128/iai.58.8.2719-2724.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



