Abstract
Objectives
While the classification of cancer has traditionally focused on gross and microscopic characteristics of the tumor, overall health of a patient can impact survival. Since patients with renal cell carcinoma (RCC) often have other medical conditions, we explored the impact of preexisting medical disease on survival following radical and partial nephrectomy.
Methods
Between January 1995 and August 2003, comorbidity status of 697 nonmetastatic RCC patients who underwent radical or partial nephrectomy was prospectively coded using the Adult Comorbidity Evaluation-27. Histopathologic review of all slides was performed according to the 2004 World Health Organization scheme. Other variables analyzed include age, gender, ethnicity, pathologic stage, Fuhrman grade, and tumor size. The effect of these factors on overall survival (OS) was analyzed using Cox Proportional Hazards Regression.
Results
The median follow-up was 32.2 months for survivors and 36.5 months for all patients. OS rate at 1, 3, and 5 years was 92.0% (641 patients), 75.3% (525 patients) and 52.7% (367 patients). Univariate analyses demonstrated that age, comorbidity, tumor size, Fuhrman grade, and pathologic stage were significant predictors of OS. Multivariate analysis revealed that age (HR 1.42, 95% CI 1.10–1.82, p=0.0067), comorbidity (HR 1.37, 95% CI 1.16–1.63, p=0.0002), pathologic stage (HR 1.97, 95% CI 1.60–2.41, p<0.0001) and grade (HR 1.83, 95% CI 1.28–2.59, p=0.0008) predicted OS.
Conclusions
This study demonstrates that comorbidity is an independent prognostic factor for OS in RCC patients. Capturing comorbidity information using validated instruments can improve the preoperative evaluation of patients by providing more accurate prognostic information.
INTRODUCTION
Renal cell carcinoma (RCC) is currently the 7th most common tumor and the 10th most common cause of cancer death in the United States with an estimated 51,190 new cases and 12,890 deaths in 2007.1 The age-adjusted incidence of RCC has increased 52% since 1983.2 Unfortunately, the cancer-specific mortality and overall mortality have also increased over the same period despite increased use of curative surgery.
In assessing prognosis, physicians and national cancer organizations focus on standard tumor characteristics, since these have been proven to predict risk of recurrence, progression, metastasis, and death.3–5 New prognostic tools focus on markers of angiogenesis, cell cycle dysregulation, immune function, and apoptosis.4,6–8 These new biomarkers can be integrated with the standard clinical model to produce a more accurate prognostic model,9 but none has entered routine clinical practice.
Comorbidity is defined as any coexisting disease or condition that may impact on diagnosis, treatment, and prognosis for an index disease under study.10 While the potential for cancer patients’ overall health to impact outcome is universally accepted, standard and valid methods for capturing comorbidity in cancer registries have not been adopted.11 The prognostic impact of comorbidity has been demonstrated in various cancers, including colon, lung, and prostate cancer.12–14 Not surprisingly, comorbidity has greater impact in indolent cancers, rather than aggressive tumors.15
The goal of this study was to evaluate the impact of comorbidity on overall survival (OS) in patients with localized RCC treated surgically. A validated comorbidity instrument specifically designed for cancer patients was used.
MATERIAL AND METHODS
Study Design
This was a retrospective analysis of data collected prospectively by tumor registrars at the time of cancer diagnosis.
Study Population
Clinical and pathologic data of all patients treated by partial or radical nephrectomy for RCC at Barnes-Jewish Hospital (BJH)/Siteman Cancer Center from January 1995 to August 2003 was reviewed. Patients with metastasis at diagnosis, patients without pathologic specimens to re-review, and patients who underwent nephrectomy for benign disease but were found to have incidental RCC were excluded. The final study population consisted of 697 RCC patients treated with radical or partial nephrectomy with curative intent.
Data Collection
BJH Oncology Data Services is an American College of Surgeons Committee on Cancer (CoC)-approved registry. Patient and tumor variables are extracted prospectively by certified tumor registrars for all patients diagnosed or first treated at BJH within six months of diagnosis. Follow-up information, including survival status, is routinely obtained from treating physicians and national death indices.
Comorbidity Data
Comorbidity was captured with Adult Comorbidity Evaluation-27 (ACE-27), a 27-item comorbidity instrument validated on adult oncology patients.11 Comorbidity information was obtained by review of medical records. Comorbidity was defined as pre-existing medical conditions present at time of cancer diagnosis, including previous or synchronous cancers. In the ACE-27 system, specific diseases are graded into one of three levels of severity (None, Mild, Moderate, or Severe) according to level of individual organ decompensation and prognostic importance. Following classification of individual diseases, an overall comorbidity score (None, Mild, Moderate, or Severe) is assigned based on the highest-ranked single ailment, or as Severe in the case of two or more moderate organ decompensations in different organ systems. The ACE-27 on-line calculator can be viewed at: http://oto.wustl.edu/clinepi/calc.html.
Data Classification
Time of cancer diagnosis was defined as “zero-time” for each patient. Clinical information was based on physician reports. Anatomic extent of tumor was classified using the 2002 American Joint Commission on Cancer (AJCC) staging system. Staging data included clinical and pathologic classification for T, N, and M. Pathologic stage and clinical stage were derived from the six total TNM data elements using the AJCC staging system. Sections and slides from all surgical specimens were re-reviewed, and Fuhrman histologic grading and WHO 2004 histologic type assigned by a single pathologist (MS).
Data Analysis
Statistical analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC). Variables with p<0.1 on univariate analyses were included in multivariate (Cox Proportional hazard) analyses. Variables with p<0.05 on multivariate analysis remained in the final model. Performance of prognostic models with and without comorbidity was evaluated based on measures of discrimination and overall accuracy. We quantified discrimination using the c-statistic as a measure of concordance. To measure whether the addition of comorbidity was statistically significant, bootstrapped estimates of the c-statistics in each model were compared using a student’s t-test. Model performance was also assessed using likelihood ratio chi-square statistics. The likelihood ratio chi-square of the more complex model was compared to the model without comorbidity, to determine whether addition of comorbidity fits the data significantly better.
RESULTS
Median age of the population was 61 years, and 521 patients (75%) had at least one comorbid condition. Median follow-up was 32.2 months for survivors and 36.5 months for all patients. Overall survival at 1, 3, and 5 years was 92.0%, 75.3% and 52.7% respectively.
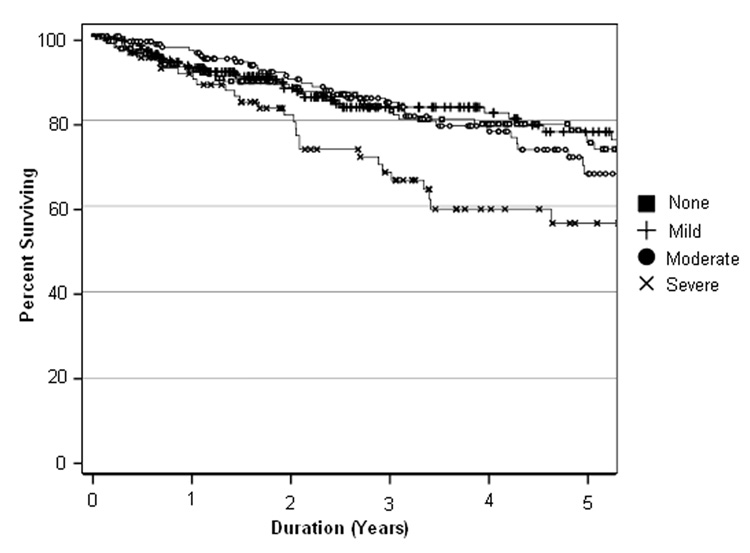
Relationship between severity of comorbidity and OS is illustrated with a Kaplan-Meier survival curve (Figure 1). As can be seen, there is a clear prognostic gradient for patients with Severe comorbidity. The impact of Moderate and Mild comorbidity is less clear, although the overall relationship of severity of comorbidity and survival is significant (Log-Rank chi-square 12.84; p=0.005).
Figure 1.
Five-year Overall Survival as a Function of Comorbidity Severity Log Rank Chi-Square=12.84 (p=0.005)
Relationship of patient-specific and tumor-specific variables to OS, as expressed by unadjusted hazards ratio, is shown in Table 1. On univariate analysis, age, comorbidity, tumor size, Fuhrman grade, and pathologic stage predicted OS. Multivariate Cox proportional hazards analysis revealed that age (HR 1.42; 95% CI 1.10–1.82; p=0.0067), comorbidity (HR 1.37; 95% CI 1.16–1.63; p=0.0002), stage (HR 1.97; 95% CI 1.60–2.41; p<0.0001), and grade (HR 1.83; 95% CI 1.28–2.59; p=0.0008) remained significant (Table 2).
Table 1.
Relationship Between Demographic, Clinical, and Tumor Features and Overall Survival.
| Variables | N (%) | Unadjusted HR* (95% CI^) | p value |
|---|---|---|---|
| Sex | |||
| Female | 258 (37%) | 1.0 (ref) | - |
| Male | 439 (63%) | 1.244 (0.877–1.764) | 0.220 |
| Ethnicity | |||
| White | 583 (84%) | 1.0 (ref) | - |
| Black | 106 (15%) | 0.921 (0.584–1.450) | 0.722 |
| Others | 8 (1%) | # | |
| Age | |||
| ≤65 years old | 135 (19%) | 1.0 (ref) | - |
| 66–75 years old | 271 (39%) | 0.822 (0.485–1.394) | 0.467 |
| ≥76 years old | 291 (42%) | 1.726 (1.079–2.760) | 0.023 |
| Comorbidity Severity | |||
| None | 176 (25%) | 1.0 (ref) | - |
| Mild | 256 (37%) | 1.012 (0.644–1.592) | 0.957 |
| Moderate | 172 (25%) | 1.157 (0.726–1.844) | 0.541 |
| Severe | 93 (13%) | 2.119 (1.305–3.443) | 0.002 |
| Tumor Size | |||
| <4cm | 364 (52%) | 1.0 (ref) | - |
| 4–7 cm | 166 (24%) | 1.696 (1.113–1.585) | <.0001 |
| >7 cm | 167 (24%) | 2.265 (1.550–3.310) | <0.001 |
| AJCC Pathologic | |||
| Stage I | 410 (59%) | 1.0 (ref) | - |
| Stage II | 199 (29%) | 1.575 (1.063–2.335) | 0.024 |
| Stage III | 73 (10%) | 3.070 (1.925–4.895) | <0.001 |
| Stage IV | 15 (2%) | 11.705 (6.192–22.124) | <0.001 |
| Grade | |||
| Low (I,II) | 497 (71%) | 1.0 (ref) | - |
| High (III,IV) | 200 (29%) | 2.065 (1.474–2.893) | <0.001 |
| Histology | |||
| MLC | 15 (2%) | 1.0 (ref) | - |
| Chromophobe | 26 (4%) | 2.020 (0.210–19.418) | 0.543 |
| Papillary | 110(16%) | 3.859 (0.522–28.527) | 0.186 |
| Clear Cell | 504 (72%) | 3.750 (0.523–26.875) | 0.188 |
| Oncocytoma | 22 (3%) | 3.331 (0.401–27.681) | 0.265 |
| UNCL | 10 (1%) | 4.143 (0.495–45.787) | 0.219 |
| Collecting Duct | 10 (1%) | 7.735 (0.803–74.485) | 0.077 |
Hazard ratio (HR)
Confidence Interval (CI)
No patients died in this ethnicity
Table 2.
Multivariate Analysis of Prognostic Factors Related to Overall Survival
| Variable | No. | Adjusted HR* | 95% CI^ | p value |
|---|---|---|---|---|
| Age | 697 | 1.42 | 1.10 to 1.82 | 0.0067 |
| ≤65 years | 135 | 1.0 | (ref) | - |
| 66–75 years | 271 | 0.678 | (0.394–1.165) | 0.159 |
| ≥76 years | 291 | 1.671 | (1.010–2.763)) | 0.045 |
| Comorbidity Severity | 697 | 1.37 | 1.16 to 1.63 | 0.0002 |
| None | 176 | 1.0 | (ref) | - |
| Mild | 256 | 1.191 | (0.736–1.927) | 0.477 |
| Moderate | 172 | 1.432 | (0.866–2.370) | 0.162 |
| Severe | 93 | 2.929 | (1.749–4.904) | <0.0001 |
| Pathologic Stage | 697 | 1.97 | 1.60 to 2.41 | 0.0001 |
| Stage I | 410 | 1.0 | (ref) | - |
| Stage II | 199 | 1.675 | (1.127–2.492) | 0.011 |
| Stage III | 73 | 2.795 | (1.727–4.524) | <0.0001 |
| Stage IV | 15 | 14.341 | (7.139–28.808) | <0.0001 |
| Grade | 697 | 1.83 | 1.28 to 2.59 | 0.0008 |
| Low (I,II) | 497 | 1.0 | (ref) | - |
| High (III,IV) | 200 | 1.822 | (1.275–2.604) | 0.001 |
Hazard Ratio (HR)
Confidence Interval (CI)
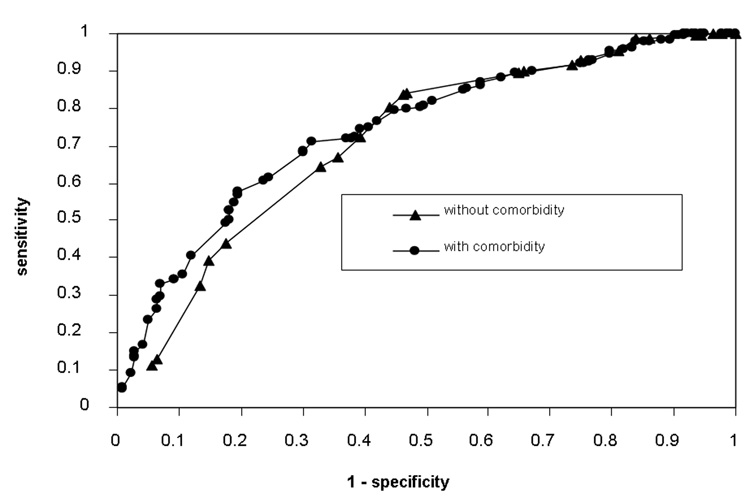
The model without comorbidity was sufficiently able to discriminate between those patients who lived and those who did not, but addition of comorbidity significantly increased discriminative ability (c=0.735, 0.731–0.738 vs. c=0.769, 0.766–0.772, p<0.0001). Comparison of likelihood ratio chi-square values demonstrated similar results. Test of difference in models also revealed a statistically significant improvement in model fit from the addition of comorbidity (χ2=90.52 vs. χ2=112.04, p<0.0001). Figure 2 shows Receiver-Operating-Characteristics (ROC) curves for discriminative ability of the model with and without comorbidity. As can be seen, the model with comorbidity is superior to the model without, particularly at lower false positive rates (1-specificity).
Figure 2.
ROC Curves for the Discriminative Ability of Predictive Model with and without Comorbidity.
COMMENT
In this study, we examined the effect of comorbidity on OS and found that comorbidity in addition to age, stage, and grade predicted OS for patients receiving radical or partial nephrectomy for RCC. While surgeons assess comorbidity in deciding if a patient can tolerate surgery and/or if a patient will benefit from surgery, this information is not formally integrated into assessment of survival after surgery. Additionally, no validated instrument is used to assess the global effect of comorbidity. ACE-27 is a validated comorbidity instrument for adult oncology patients. While many of the individual comorbid conditions, such as recent myocardial infarction, included in the ACE-27 would preclude surgery, there are situations where a patient would be assigned the severe overall comorbidity score and still be considered a surgical candidate. For example, the ACE-27 would classify a patient with a remote history of a heart attack and a body mass index of greater than 38 as having severe comorbidity. In our study, global assessment of comorbidity using a validated instrument was a strong predictor of survival in patients believed to be good surgical candidates. This demonstrates that comorbidity is a potentially valid adjunct to tumor-specific variables in determining patient outcome.
Our conclusion is supported by the findings by Berndt and colleagues16 who studied outcomes in RCC patients and noted worse OS for African Americans compared to Caucasians. This disparity remained even after controlling for demographic and cancer prognostic factors, but substantially decreased after further adjustment for comorbidity and nephrectomy treatment. This is consistent with our findings; in our population, which is a nephrectomy cohort, we did not find a significant impact of ethnicity on OS after controlling for comorbidity. The study by Berndt and colleagues as well as our study highlight the importance of assessing comorbidity whenever outcome analyses are performed.
Our study builds on prior work. Zisman et. al. integrated the Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) with stage and grade for evaluating treatment success for 161 non-metastatic RCC patients treated with partial or radical nephrectomy.17 They demonstrated that ECOG PS, predicted OS, while stage and grade did not. In a follow-up study, Zisman developed a model to calculate the personalized survival for RCC patients. For nonmetastatic subjects, Fuhrman's grade and ECOG PS were the only significant variables in multivariate analysis. The authors claim that this tool could allow a physician to better forecast the need for adjuvant therapy.18
Han et al, in a multicenter international collaborative study, also assessed the effect of ECOG PS, in addition to other prognostic factors, on disease-specific survival following surgical treatment of RCC. They found that ECOG PS predicted disease-specific survival along with stage and grade.19
Gettman et. al. studied the effect of comorbidity in patients with renal vein or IVC thrombus, using the Charlson Comorbidity Index (CCI).20 They retrospectively studied 303 patients who underwent surgical resection for RCC and found that CCI was not an effective predictor of cause-specific survival compared to the standard tumor-specific parameters of AJCC stage, grade, and tumor size. Our results may differ because our cohort consists of all pathologic stages, not just patients with vascular extension.
Our study has several strengths. First, it is the largest to date examining the relationship between comorbidity and OS in RCC patients. Second, it improves on the previous studies by collecting comorbidity information prospectively at the time of diagnosis and initial treatment. Finally, it uses an instrument specifically designed for, and validated on oncology patients.11 It is important to note that while ACE-27 was designed specifically to assess comorbidity in adult oncology patients, and has been shown in our study to be of prognostic value in RCC patients, a prospective comparison of the different instruments (for example ACE-27 and ECOG PS) will need to be performed by future studies to determine the relative value of each instrument for RCC patients.
In this study we also found that increasing age was associated with decreasing OS. Given that older patients have shorter life expectancy, this is not surprising. Importantly, however, age and comorbidity were independently associated with OS, and therefore age cannot be used as a proxy for comorbidity.
While it is widely believed that histology impacts OS, we did not find an association between histologic subtype, as defined by WHO 2004 histologic classification scheme, and OS. Consistent with our findings, an international multi-center retrospective analysis of 4063 patients demonstrated that histologic subtype had no association with OS.21 An explanation could be that histology may affect disease-specific survival but patient-specific factors, such as age and comorbidity blunt the effect of histology on OS.
Our study did not examine the association between comorbidity and disease-specific survival because disease-specific data are not available. However, we believe that OS is a more critical endpoint in understanding the effect of prognostic variables, including comorbidity, on outcome. Disease-specific survival for RCC has improved dramatically over the past few decades, while mortality from this disease continues to increase.22 Disease-specific survival, therefore, may not provide an accurate assessment of the effect of treatment or prognostic variables. Ultimately, what the patient cares about is his longevity, not what leads to his demise.
The differences in patient survival in any cohort are multi-factorial, related to both tumor and patient factors. We are not suggesting that a surgeon should disregard the tumor-specific factors, but instead, recognize that comorbid conditions are important prognostic factors and should be included in tumor registries, and considered in treatment planning.
CONCLUSIONS
Severe comorbidity is a significant, independent predictor of OS in patients undergoing radical or partial nephrectomy for RCC. While our results demonstrate that OS is dependent on patient-specific variables (comorbidity and age), tumor-specific variables (pathologic stage and grade) continue to be important factors predicting outcome. Capturing comorbidity information with a validated instrument can improve the preoperative evaluation of patients by providing more accurate prognostic information.
Acknowledgments
Source of Research Support: D Berger, None; A. Vlahiotis, None; M. Radwan, None; I. Megwalu, Washington University Summer Research Grant; M.F. Serrano, None; P.A. Humphrey, None; J.F. Piccirillo, RO1 CA104797-01; A.S. Kibel, R01 CA112028-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The contents of this article are original. It has not been published elsewhere; the abstract was presented at the American Urological Association Annual Meeting 2006. None of the authors has a conflict of interest associated with this manuscript.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 3.Frank I, Blute ML, Cheville JC, et al. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217–2220. doi: 10.1097/01.ju.0000095475.12515.5e. [DOI] [PubMed] [Google Scholar]
- 4.Sorbellini M, Kattan MW, Snyder ME, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173:48–51. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- 5.Thrasher JB, Paulson DF. Prognostic factors in renal cancer. Urol Clin North Am. 1993;20:247–262. [PubMed] [Google Scholar]
- 6.Weiss RH, Borowsky AD, Seligson D, et al. p21 is a prognostic marker for renal cell carcinoma: implications for novel therapeutic approaches. J Urol. 2007;177:63–69. doi: 10.1016/j.juro.2006.08.073. [DOI] [PubMed] [Google Scholar]
- 7.Kramer BA, Gao X, Davis M, et al. Prognostic significance of ploidy, MIB-1 proliferation marker, and p53 in renal cell carcinoma. J Am Coll Surg. 2005;201:565–570. doi: 10.1016/j.jamcollsurg.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 9.Kim HL, Seligson D, Liu X, et al. Using tumor markers to predict the survival of patients with metastatic renal cell carcinoma. J Urol. 2005;173:1496–1501. doi: 10.1097/01.ju.0000154351.37249.f0. [DOI] [PubMed] [Google Scholar]
- 10.Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis. 1970;23:455–469. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- 11.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. Jama. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 12.Tammemagi CM, Neslund-Dudas C, Simoff M, et al. In lung cancer patients, age, race-ethnicity, gender and smoking predict adverse comorbidity, which in turn predicts treatment and survival. J Clin Epidemiol. 2004;57:597–609. doi: 10.1016/j.jclinepi.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Post PN, Hansen BE, Kil PJ, et al. The independent prognostic value of comorbidity among men aged < 75 years with localized prostate cancer: a population-based study. BJU Int. 2001;87:821–826. doi: 10.1046/j.1464-410x.2001.02189.x. [DOI] [PubMed] [Google Scholar]
- 14.Yancik R, Wesley MN, Ries LA, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998;82:2123–2134. [PubMed] [Google Scholar]
- 15.Read WL, Tierney RM, Page NC, et al. Differential prognostic impact of comorbidity. J Clin Oncol. 2004;22:3099–3103. doi: 10.1200/JCO.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 16.Berndt SI, Carter HB, Schoenberg MP, et al. Disparities in treatment and outcome for renal cell cancer among older black and white patients. J Clin Oncol. 2007;25:3589–3595. doi: 10.1200/JCO.2006.10.0156. [DOI] [PubMed] [Google Scholar]
- 17.Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19:1649–1657. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 18.Zisman A, Pantuck AJ, Dorey F, et al. Mathematical model to predict individual survival for patients with renal cell carcinoma. J Clin Oncol. 2002;20:1368–1374. doi: 10.1200/JCO.2002.20.5.1368. [DOI] [PubMed] [Google Scholar]
- 19.Han KR, Bleumer I, Pantuck AJ, et al. Validation of an integrated staging system toward improved prognostication of patients with localized renal cell carcinoma in an international population. J Urol. 2003;170:2221–2224. doi: 10.1097/01.ju.0000096049.64863.a1. [DOI] [PubMed] [Google Scholar]
- 20.Gettman MT, Boelter CW, Cheville JC, et al. Charlson co-morbidity index as a predictor of outcome after surgery for renal cell carcinoma with renal vein, vena cava or right atrium extension. J Urol. 2003;169:1282–1286. doi: 10.1097/01.ju.0000049093.03392.cc. [DOI] [PubMed] [Google Scholar]
- 21.Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763–2771. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 22.Parsons JK, Schoenberg MS, Carter HB. Incidental renal tumors: casting doubt on the efficacy of early intervention. Urology. 2001;57:1013–1015. doi: 10.1016/s0090-4295(01)00991-8. [DOI] [PubMed] [Google Scholar]