Abstract
Male mice (mus musculus) of a mixed B6/129 background were used to establish food demand functions in a closed economy. The mice lived continuously in operant chambers and worked for 20-mg nutritionally complete food pellets. First, a series of incrementing fixed ratio (FR) costs per pellet were imposed, and the results showed that demand declined as unit price increased. The number of meals taken per day was dependent on the temporal criterion used to define a meal, but the number of meals did not change across the FR series. Next, a series of incrementing progressive ratio (PR) schedules were used, and a meal was defined by a programmed schedule reset interval. Total food intake declined slightly, and the mean meal size also decreased, across the series. Lastly, a nose poke response requirement was imposed as the procurement cost to activate a lever press device for food; under these conditions the meal number changed dramatically as the procurement cost was increased, whereas total intake declined only modestly. These data show in mice that large changes in unit price or consummatory cost have relatively small effects on demand and meal patterns, but small amounts of foraging (procurement) cost have very large effects.
Keywords: Food intake, meal patterns, consummatory cost, procurement cost, lever press, nose poke, mice
INTRODUCTION
Demand functions describe the way in which price or effort affects the demand, in this case food consumption, for a commodity. Demand functions that have been reported for rats (e.g., Bauman, 1991; Hursh, 1984; Hursh, Raslear, Shurtleff, Bauman, & Simmons, 1988), as well as other species, all have manipulated the unit price of food pellets. These studies have used open or closed economies; operationally, these often translate into short sessions or continuous access, respectively. Demand studies focus on the amount consumed, but few have studied the patterns of eating under these conditions. Under continuous access conditions, total intake is distributed in discrete meals of variable sizes (Smith, 2004). Collier and his colleagues (Collier, 2005; Collier, Hirsch, & Hamlin, 1972; Collier, Johnson, Hill, & Kaufman, 1986; Collier, Johnson, & Mathis, 2002; Collier & Rovee-Collier, 1981) have shown that manipulation of consummatory cost (equivalent to unit cost) does not produce large changes in the size and number of meals taken per day. In contrast, they found that very modest increases in effort that emulate travel to a food patch (procurement cost) led to large declines in number of feeding episodes and an inverse increase in average meal size, while total intake was relatively conserved. The impact of cost on meal parameters and demand thus depends on when that effort is imposed relative to food delivery.
FR schedules have been the most commonly used to study demand functions, yet PR schedules may be more useful as a measure of motivation or response strength (Hursh & Silberberg, 2008). Under FR schedules the unit cost of food is not under the subject's control. In contrast, under the type of resetting PR that we will use here, the subject can minimize its average cost for food by taking many small meals (Johnson, Triblehorn, & Collier, 1993). In a previous study (Vaughan et al., 2005), we used a single step size PR and examined 3- and 20-min reset times to define a meal; the 3-min reset lead to many small or fragmented meals, so we deemed 20 min more suitable. This temporal criterion has been validated in mouse free feeding studies (Chi & Powley, 2003). However, there is a considerable literature on how criterion choice can affect meal parameters (e.g., Clifton, 2000; Demaria-Pesce & Nicolaidis, 1998; Zorrilla et al, 2005), so it is appropriate for us to examine the effect of criteria within the present protocols.
Mice have become a species of choice for contemporary studies in physiology and behavioral neuroscience, largely because of their potential for genetic applications. The identification of several genes involved in obesity have generated considerable interest in studies of food intake in mice, yet the number of published works that have examined meal parameters is still quite small and the results are often inconsistent (see Vaughan & Rowland, 2003), in part because mice present some challenges. For example, meal studies in rats often have used open jars of food on a scale, but mice often dig the food out and live in the jar. Methodologies in which discrete small food items are presented sequentially, such as in a standard operant test protocol, are particularly appealing because they minimize food spoiling behavior. A few such studies have appeared in the recent literature (e.g., Chi & Powley, 2003; Donovan, Paulino & Raybould, 2007; Fox & Byerly, 2004; Tabarin et al., 2007) but all have used a low and constant unit cost, so do not address economic considerations. We have previously reported results from operant protocols in normal and genetically obese mice (Vaughan, Moore, Haskell-Luevano, & Rowland, 2006; Vaughan & Rowland, 2003), but those studies did not examine systematic variation in cost parameters. The present studies determine the effects of systematic variation in consummatory or procurement costs on intake and meal parameters in a wild type strain of mice living in a closed economy.
METHOD
Subjects
The subjects were 6 male mice (mus musculus) from a mixed 129/B6 background, initially about 9 mo of age. They were housed individually in operant chambers during the test phases, and in polycarbonate standard cages between study phases. The vivarium was maintained at 23±2°C with lights on from 0700-1900. Food (Purina 5001 pellets) and tap water were available ad libitum in the standard cages. Nutritionally complete 20-mg mouse pellets (Purina Test Diet 5TUM) and water was available at all times inside the chambers. The study was run in two contiguous parts, the first using 2 mice and the second using 4 mice.
Apparatus
Four modular mouse operant chambers (21.6 × 17.8 × 12.7 cm; Med Associates, St. Albans, VT), computer-controlled using Med-PC software, were available. Each chamber was equipped, in the middle of one side, with a food receptacle into which pellets could be delivered from a noiseless pellet dispenser. A mouse-appropriate lever manipulandum, with a cue light above, was situated to the left of the food receptacle about 2 cm above the floor. In one phase of study, a mouse nosepoke manipulandum also was present to the right of the food receptacle and about 1 cm above the floor. A sipper tube of tap water was available through a hole in the wall opposite the pellet receptacle. Each cage was contained within a ventilated sound-attenuating cubicle and was illuminated indirectly by a 15-W incandescent bulb controlled on the same 12:12 light cycle as the room. During experimental phases mice lived in the chambers for 23.5 h/day. They were weighed and placed in a holding cage without food for a short service period (at about 1000) each day.
Procedure
Mice were tested in ratio schedules of consummatory or unit cost in which each pellet was delivered contingent upon completion of a programmed number of lever press responses. The sequence of schedules and the number of days on each are shown in Table 1. After initial training at fixed ratio (FR) 1, all mice received an ascending FR 1,10,30,50, then after a return via FR1 or 5, to a progressive ratio (PR) series 1,2 and 3. In PR schedules, the cost of successive pellets within a meal was incremented by the value of the PR (p) so that the cost of the (i+1)th pellet in a sequence, Ci+1 = 1 + p × i. Thus, for PR2, the actual successive costs within a meal were 1,3,5,7, etc. In all PR series, a meal was declared as finished when 20 min elapsed since the last pellet delivery, and the ratio was programmed to reset to the initial value of 1. Larger meals translate into greater mean unit cost per pellet: for PR = p, the mean price per pellet within a meal of n pellets = 1+ (n−1)p/2, thus, the price increases linearly with n and with a slope of p/2. In this analysis, n is equivalent to the break point of the PR within each meal. The FR values were adopted based on literature in rats (eg Bauman 1991; Hursh 1984), but with the caveat that weight losses in excess of 15% are not allowed by our IACUC. Thus, the range studied was not designed to produce a large drop in demand. The PR step values and reset interval were adopted based on our previous work (Vaughan, Moore, Haskell-Luevano, & Rowland, 2006).
TABLE 1.
NUMBER OF DAYS ON EACH SCHEDULE OF REINFORCEMENT
| Schedule | Mice 1-2 | Mice 3-6 |
|---|---|---|
| FR1 | 16 | 15 |
| FR10 | 8 | 13 |
| FR30 | 9 | 8 |
| FR50 | 7 | 12 |
| FR5 | x | 5 |
| FR1 | 15 | x |
| PR1 | 13 | 12 |
| PR2 | 11 | 6 |
| PR3 | 13 | 4 |
| FR1 | 8 | x |
| NP | X | 32 |
| NP10LP5 | X | 9 |
| NP30LP5 | X | 13 |
| NP50LP5 | X | 12 |
| NP100LP5 | x | 8 |
| Total days: | 100 | 149 |
Note: FR=fixed ratio; PR=progressive ratio; NP=nosepoke; LP=lever press; x=not tested.
Lever presses and pellet deliveries were recorded every 60 s throughout the 23.5 h sessions by Med-PC software. Total number of pellets consumed (pellets acquired minus pellets spilled into the tray beneath the cage) and lever presses emitted were recorded, and the daily average cost per pellet was computed. The number of meals per day was determined using a 20-min criterion: when 20 min elapsed between successive pellet deliveries, these were considered separate meals (eg Chi & Powley, 2003). For the last 4 mice, 10- and 30-min criteria were applied to the same data set.
In the final studies in mice #3-6 (Table 1) a nosepoke device was also present. Initially, the nosepoke was substituted for the lever to thoroughly accustom the mice to the new operant. Thereafter, both nosepoke and lever devices were present. Completion of a ratio (initially FR10) on the nose poke device activated the lever for food delivery, signaled to the mouse by illumination of a cue light above the lever. Consummatory cost per food pellet was held constant at 5 lever presses. Once the consummatory lever was activated, mice could eat as much as they wished with the limitation that whenever 20 min elapsed without a pellet delivery the meal was declared ended and the lever cue light was extinguished. The mouse then had to complete another procurement ratio on the nosepoke device in order to activate the food lever.
Data analysis
Individual data were averaged for the last 3 days of stable responding. This was usually the last 3 days on a given ratio (Table 1) unless equipment malfunction occurred during this time; an additional requirement was <15% daily variation and no systematic trend across the selected days. (Normally, all of the days on a given schedule were relatively constant, and 3 days was chosen to be compatible with the shorted dwell times at a given ratio). These individual 3 day means for each schedule are presented in the graphs. These data were also used for statistical analysis by one-way analysis of variance (ANOVA) and t-tests with significance levels set at 0.05.
RESULTS
Fixed-Ratio Conditions
At FR 1, several mice spilled very large numbers of pellets that became spoiled in the pan beneath the cage and so could not be reliably estimated. Thus, FR1 data are shown only for those mice (n=3) in which spillage could be counted accurately. At the higher ratios, the intakes of these 3 mice were similar to others in the group, so only their FR1 data were excluded. Spillage was quite small (<5% of total pellets taken) at FR10 and above.
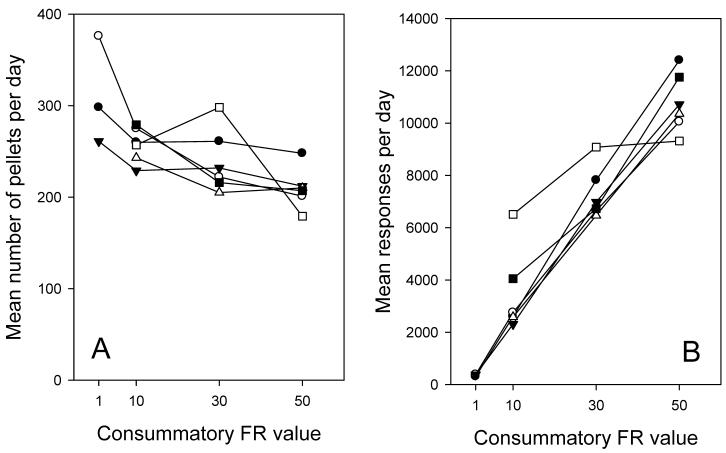
The number of pellets taken per day declined significantly as a function of increasing unit price per pellet, F(3,20) = 7.25 P< .01, from a median of 310 pellets (6g/day) at FR1 to 210 pellets (4 g/day) at FR50 (Figure 1A). Each mouse consumed less at FR 50 than FR 10, although the steepness of that decline varied between individuals. Slight weight loss (up to 10%) occurred at the highest ratio, so higher costs were not attempted. Since the unit cost increased 50-fold across this range of FRs, the total lever presses increased correspondingly (Figure 1B). The mean numbers of lever presses emitted per pellet eaten (the actual unit cost) were 1.17 at FR 1, 13.2 at FR10, 30.5 at FR 30, and 51.5 at FR 50.
FIGURE 1.
Panel A shows average number of 20-mg pellets consumed daily (the demand function) by individual mice at a fixed ratio consummatory price per pellet of 1,10,30 and 50. Panel B shows the corresponding mean number of lever presses emitted per day; the symbols in the two graphs are the same for each individual. (Data for 3 mice are omitted at FR1 due to excessive spillage).
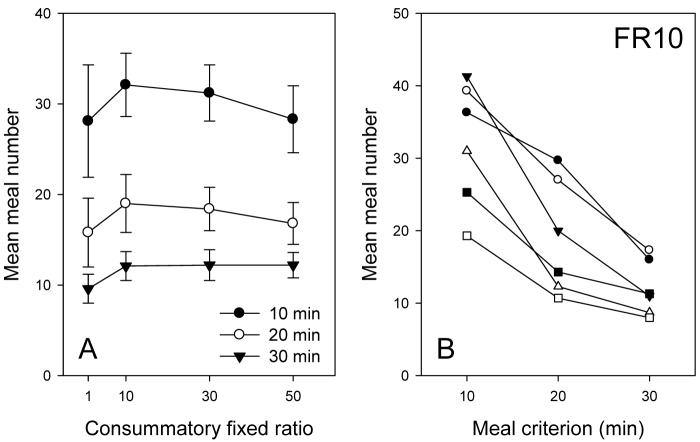
Despite the change in total intake, the number of meals taken per day using the 20-min criterion remained constant, F(4,23) = 0.18, across this range of FRs (Figure 2A). Since the total food intake declined by about 30% across this range (Figure 1A) it follows that the mean meal size was also reduced by about 20%. The data were also analyzed using criteria of 10, 20 or 30 min to define a separate meal. The results are shown in Figure 2B and ANOVA showed that meal number was highly sensitive to criterion, F(3,11) = 133.7, P<.001. Also, while the changes within each individual were similar, it is noteworthy that the numbers of meals taken show a two-fold individual variability at each criterion.
FIGURE 2.
Panel A shows the mean (±standard error) numbers of meals taken per day for four mice using three different meal-defining criteria as a function of the FR price per pellet. Panel B shows corresponding individual meal numbers as a function of meal criterion for FR10 only (the other FRs were similar).
Progressive-Ratio Conditions
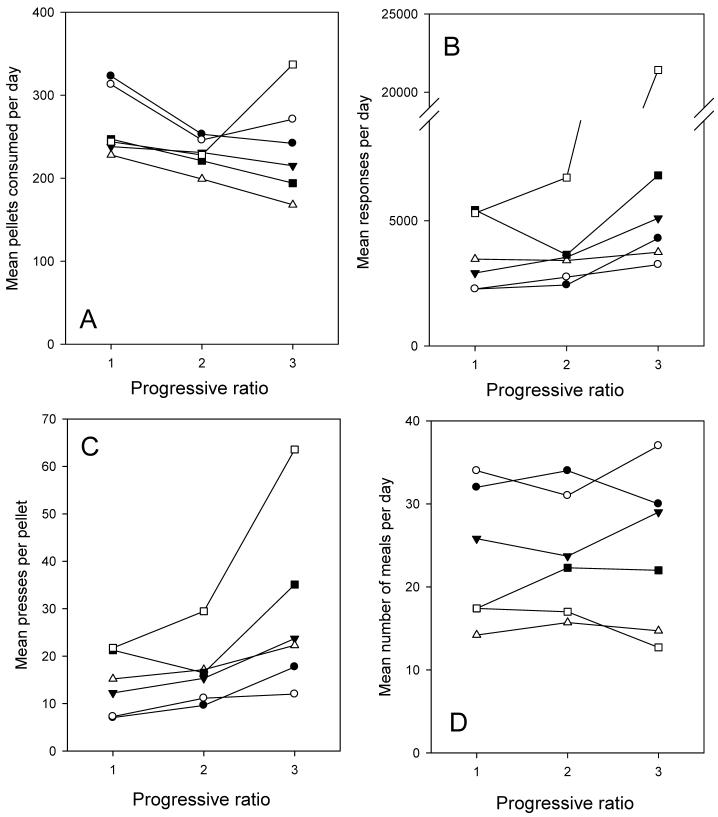
The demand function for mice under the three PR schedules is shown in Figure 3A. Mouse #6 (open square symbol) spilled excessively at PR3 and, with this datum excluded, food demand decreased significantly, F(2,10) = 7.76, P<0.02, by 19%, across the series. Figure 3B shows the corresponding mean number of lever presses, which did not change significantly (note that mouse 6 is again atypical). Figure 3C shows the derived mean number of presses per pellet (actual unit cost) and Figure 3D the mean number of meals per day, which did not change significantly, F(2,10)= 0.32, across the PR series. Since food demand decreased but meal number remained constant as PR increased, it follows that meal size decreased (by 30%) across this series (P<0.02, data not shown).
FIGURE 3.
Panel A shows the mean number of food pellets consumed daily by six individual mice studied under three progressive ratio conditions. Panel B shows the corresponding mean number of responses per day for each mouse, panel C the mean presses emitted per pellet (total daily presses/pellets consumed), and panel D the mean number of meals per day. The symbols for each mouse are the same across panels.
Interestingly, while day-to-day variability in meal parameters within a subject was low on the PR, like in the FR there was considerable variability between subjects (Figure 3D). This variability in meal number and size on a PR means that the actual mean price paid per pellet varied between individuals (Figure 3C). Animals that take few but large meals pay a higher mean price than those taking many small meals.
Procurement Cost Conditions
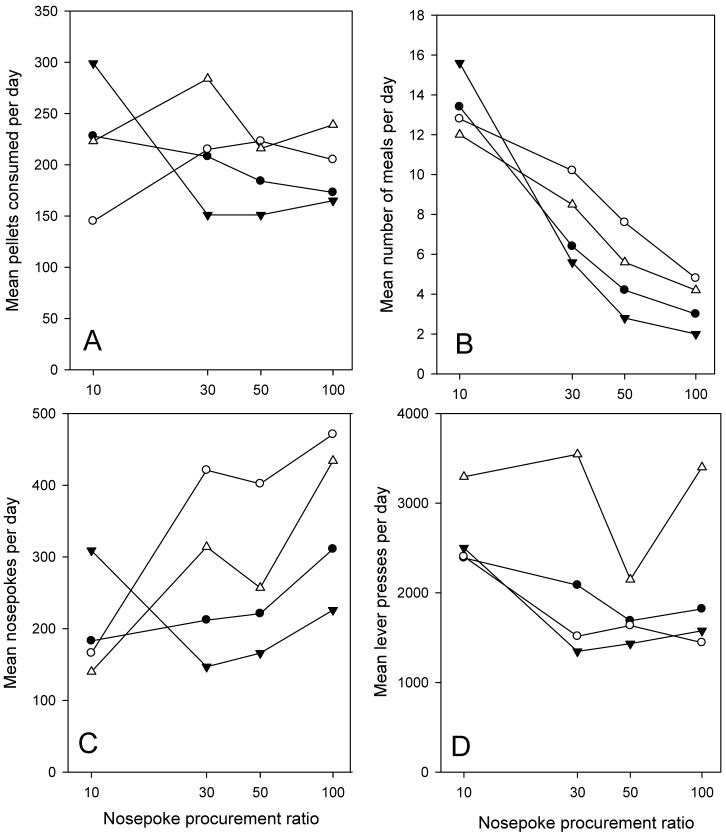
The results of the conditions in which completion of a nose poke ratio activated the consummatory food lever are shown in Figure 4. Whereas total pellets consumed per day (panel A), and thus total the consummatory cost (panel D), changed little as the nose poke requirement was increased (F tests not significant) the number of nose pokes increased by about 2-fold in 3 out of the 4 mice (panel C), F(4,19) = 4.81, P<0.02. This increase is far less than the concomitant 10-fold increase in the nose poke procurement cost. Instead, mice took progressively fewer meals (panel B), decreasing 3-4 fold across the range, F(3,12) = 24.55, P<0.001 (meal number and size data are not available for the lowest procurement ratio). Mean meal size was reciprocally increased in relation to total intake (data not shown). Individual variability in number of meals is evident, although at the lowest procurement cost this variability is less than in the foregoing FR or PR studies.
FIGURE 4.
Mean data from 4 mice emitting nose poke responses to procure access to (activate) a consumption lever on which food was available at FR5. As the nose poke requirement increased from 10 to 100, the total number of pellets consumed (panel A) showed no consistent trend to change, nor did the total lever presses (panel D). However, total meals per day (panel B) showed a marked decline and, as a result, the total nose pokes emitted per day (panel C) increased only modestly. Symbols in all four panels are the same for a given subject.
DISCUSSION
Using closed economies and FR schedules of food procurement, several authors have established demand functions in rats (Bauman, 1981; Hursh, 1984; Hursh et al, 1988; Hursh & Silberberg, 2008) and noted that these functions are essentially economic in nature. The shape of that demand function defines the elasticity of demand. Since food is essential to survival, or to use the recent terminology of Hursh & Silberberg (2008) we can say that food has a high “essential value”, it would be expected that the demand functions for a single nutritionally complete food would be fairly inelastic as demand approaches the minimum needed for survival. Thus, using a lever press operant, Bauman (1991) reported that demand decreased only slightly up to about FR100 in rats; at higher ratios, demand showed greater individual differences. Similar data were found by Collier et al (1986) and Hursh et al. (1988). Bauman (1991) showed that an imposed delay equivalent to the time for FR produced similar effects, suggesting that time rather than effort was the currency of demand, a conclusion also reached by Collier et al. (2002).
The results of the FR phase of the present study in mice are consistent with these general observations. Food intake declined by about 20% across a 5-fold increase in unit FR cost. Although demand was higher at even lower unit costs, these were associated with excessive spillage of food, as has been noted before (Vaughan & Rowland, 2003). At the other end of the demand function, we did not increase FR further because this may have led to weight loss in excess of that allowed by animal welfare norms, although such low levels of weight may not be unusual in wild animals. This study established that a unit price of 50 presses per pellet, and 10,000 presses daily, is quite easily tolerated by a standard mouse strain. We did not conduct a fine-grained enough analysis to examine inter-response intervals or run lengths, but it is certainly reasonable to assume that an FR100 imposed an inter-pellet interval of at least 1-2 min, and so meals which averaged ∼15 pellets would have been extended in duration at high compared with low FRs. Nonetheless, the meal number and size were unaffected under these conditions by this forced slowing of local eating rate.
We should first ask, what are the limits of this analysis? In the hypothetical limit in which the meal is consumed instantaneously, and with no circadian rhythmicity, then with a 20-min reset the maximum number of meals per 23.5 hr would be 70. At 70 meals per day and a food demand of 210 pellets, mean meal size would be 3 pellets, which would require a total of 6 or 12 presses per meal at PR 1 and 3, respectively, and corresponding total presses per day of 420 and 840. The actual number of presses emitted per day was several fold higher than this (Figure 3B). The mean number of meals taken per day (Figure 3D) ranged from 12 to 37, all values that are below the theoretical maximum but show great individual variation. Alternatively, if assumptions are made about local response rate and pellet handling/consumption time, then the actual duration of a meal will increase substantially with its size, and the net time eating per day is likely to be longer with a large meal/small number strategy compared with a small meal/large number strategy. More detailed analysis of this question will require a closer temporal acquisition of data than was attempted in the present study (but see Collier & Rovee-Collier, 1981, for a detailed consideration of this issue and accompanying changes in response rate in rats).
Nonetheless, comparison of the PR and FR results is informative. The mean number of meals per day under the FR (using the 20-min meal criterion) was 19 (Fig 2A), with a mean price per pellet of up to 50. This price imposes a significant inter-pellet interval to complete the FR, probably of the order of 1 s per response. Under the PR, with the exception of mouse #6 at PR 3, the total number of presses emitted per day was less than half that emitted on the FR 50, so the mice should not have been exhibiting a physical strain. Additionally, with the same individual an exception, the average price per pellet under PR was well below 50, and so the inter-pellet intervals and thus the meal durations should not have been very long compared with those at FR 50. Under the PR schedules and relative to the FR mean of 19 meals (20 min criterion), only some of the mice took smaller and more frequent meals, demonstrating a partial shift in meal parameters resulting in reduced mean price per pellet.
Under the PR, mean price per pellet is partially under the subject's control. In contrast, in a pure procurement cost protocol (Collier, Hirsch, & Hamlin, 1972) almost all of the cost is under the subject's control, and animals show a very clear strategy of optimal foraging by reducing meal number as procurement costs rise (Collier, 2005). We have shown a similar effect in mice in which a lever press procurement cost was coupled with a small consummatory cost (FR 5 or 10) on a second lever (Vaughan & Rowland, 2003). The purpose of our final set of conditions was to extend this to a situation using different devices for the procurement and consummatory phases. Under these conditions, the food demand decreased by about 20% across a 20-fold increase in the procurement cost (Fig 4D) but that the actual number of nose pokes emitted per day increased by only about 4-fold, from about 80 to 360, because the meal number decreased by about 5-fold. The number of consummatory presses (FR5) remained relatively constant (ca. 2,000/day), and low compared with those emitted in the first study. Nose poke responses often are considered more natural, and possibly easier, than lever presses (e.g., Tabarin et al., 2007) and so it is difficult to argue that a few hundred nose pokes (Fig 4A) constitute a significant amount of effort compared with a few thousand lever presses (Fig 4C). Nonetheless, the imposition of this procurement cost had dramatic effects on meal size and number (Fig 3B). This demonstrates that cost is not a unitary dimension. Others have considered time as important a cost as physical effort (Bauman, 1991; Collier, Johnson & Mathis, 2002). However, to emit 100 nose pokes presumably would take little more time than 100 lever presses, and so the time contribution of the procurement time to the overall time consuming must be less than 10% with the present set of parameters, so neither time nor effort are sufficient explanations for the elective change in temporal organization of behavior.
In other studies in our laboratory (Atalayer & Rowland, in preparation), we have directly compared nose poke and lever press responses in mice using various consummatory schedules. While the data are generally similar for the two responses, overall food intake was consistently 5-10% higher in nose pokers. Tabarin et al (2007) also used a nose poke response (FR1) to deliver individual pellets to a trough, and reported ∼26 meals per day in B6/129 mice, a wild type mixed strain similar to that used in the present study. In contrast, using wild type mice of different strains but free delivery of pellets to a trough, other investigators consistently report 8-16 meals per day (Chi & Powley, 2003; Fox & Byerly, 2004; Donovan et al, 2007). Thus, meal size under free feeding or consummatory cost conditions in mice seems to be easily influenced by these types of variables, and as shown in the present study there may be substantial individual variability. In contrast, when a relatively small procurement cost is imposed, large and relatively uniform changes in meal patterns are observed (Figure 3B).
The present study has allowed a detailed account of meal taking behavior of a few individuals. One aspect that is striking in the data is the extreme orderliness of effects within an individual but the relatively high difference between individuals. Studies of meal patterns have rarely commented on these individual differences, but instead have been more interested in differences between treatment groups (eg genotypes, drugs). The operant approach seems to be well suited for future consideration of individual variability.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bauman R. An experimental analysis of the cost of food in a closed economy. Journal of the Experimental Analysis of Behavior. 1991;56:33–50. doi: 10.1901/jeab.1991.56-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi MM, Powley TL. C-Kit mutant mouse behavioral phenotype: altered meal patterns and CCK sensitivity but normal daily food intake and body weight. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2003;285:R1170–R1183. doi: 10.1152/ajpregu.00015.2003. [DOI] [PubMed] [Google Scholar]
- Clifton PG. Meal patterning in rodents: psychopharmacological and neuro-anatomical studies. Neuroscience and Biobehavioral Reviews. 2000;24:213–222. doi: 10.1016/s0149-7634(99)00074-3. [DOI] [PubMed] [Google Scholar]
- Collier G. A functional analysis of feeding. Advances in the Study of Behavior. 2005;35:63–103. [Google Scholar]
- Colllier G, Hirsch E, Hamlin P. The ecological determinants of reinforcement in the rat. Physiology and Behavior. 1972;9:705–716. doi: 10.1016/0031-9384(72)90038-8. [DOI] [PubMed] [Google Scholar]
- Collier GH, Johnson DF, Hill WL, Kaufman LW. The economics of the law of effect. Journal of the Experimental Analysis of Behavior. 1986;46:113. doi: 10.1901/jeab.1986.46-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier G, Johnson DF, Mathis C. The currency of procurement cost. Journal of the Experimental Analysis of Behavior. 2002;78:31–61. doi: 10.1901/jeab.2002.78-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier GH, Rovee-Collier CK. A comparative analysis of optimal foraging behavior: laboratory simulations. In: Kamil AC, Sargent TD, editors. Foraging behavior: ecological, ethological, and psychological approaches. Garland STPM Press; New York: 1981. pp. 39–76. [Google Scholar]
- Demaria-Pesce VH, Nicolaidis S. Mathematical determination of feeding patterns and its consequences on correlational studies. Physiology and Behavior. 1998;65:157–170. doi: 10.1016/s0031-9384(98)00159-0. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Paulino G, Raybould HE. CCK1 receptor is essential for normal meal patterning in mice fed high fat diet. Physiology and Behavior. 2007;92:969–974. doi: 10.1016/j.physbeh.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EA, Byerly MS. A mechanism underlying mature-onset obesity: evidence from the hyperhpagic phenotype of brain-derived neurotrophic factor mutants. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2004;286:R994–R1004. doi: 10.1152/ajpregu.00727.2003. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics. Journal of the Experimental Analysis of Behavior. 1984;42:435–452. doi: 10.1901/jeab.1984.42-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Raslear TG, Shurtleff D, Bauman R, Simmons L. A cost-benefit analysis of demand for food. Journal of the Experimental Analysis of Behavior. 1988;50:419–440. doi: 10.1901/jeab.1988.50-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychological Review. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Johnson DF, Triblehorn J, Collier G. The effect of patch depletion on meal patterns in rats. Animal Behaviour. 1993;46:55–62. [Google Scholar]
- Petersen S, McCarthy JC. Correlated changes in feeding behavior on selection for large and small body size in mice. Behavior Genetics. 1981;11:57–64. doi: 10.1007/BF01065828. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Vaughan CH, Mathes CM, Mitra A. Feeding behavior, obesity, and neuroeconomics. Physiology and Behavior. 2008;93:97–109. doi: 10.1016/j.physbeh.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP. The controls of eating: a shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–820. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- Tabarin A, Diz-Chaves Y, Consoli D, Monsaingeon M, Bale TL, Culler MD, Datta R, Drago F, Vale WW, Koob GF, Zorrilla EP, Contarino A. Role of the corticotrophin-releasing factor receptor type 2 in the control of food intake in mice: a meal pattern analysis. European Journal of Neuroscience. 2007;26:2303–2314. doi: 10.1111/j.1460-9568.2007.05856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CH, Rowland NE. Meal patterns of lean and leptin-deficient obese mice in a simulated foraging environment. Physiology and Behavior. 2003;79:75–79. doi: 10.1016/s0031-9384(03)00094-5. [DOI] [PubMed] [Google Scholar]
- Vaughan CH, Moore MC, Haskell-Luevano C, Rowland NE. Meal patterns and foraging in melanocortin receptor knockout mice. Physiology and Behavior. 2005;84:129–33. doi: 10.1016/j.physbeh.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Vaughan CH, Moore MC, Haskell-Luevano C, Rowland NE. Food motivated behavior of melanocortin-4 receptor knockout mice under a progressive ratio schedule. Peptides. 2006;27:2829–35. doi: 10.1016/j.peptides.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. American Journal of Physiology Regulatory Integrative Comparative Physiology. 2005;288:R1450–1467. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]