Abstract
Historically, most studies of vertebrate central pattern generators (CPGs) have focused on mechanisms for locomotion and respiration. Here, we highlight new results for ectothermic vertebrates, namely teleost fish and amphibians, showing how androgenic steroids can influence the temporal patterning of CPGs for social vocalization. Investigations of vocalizing teleosts show how androgens can rapidly (within minutes) modulate the neurophysiological output of the vocal CPG (fictive vocalizations that mimic the temporal properties of natural vocalizations) inclusive of their divergent actions between species, as well as intraspecific differences between male reproductive morphs. Studies of anuran amphibians (frogs) demonstrate that long-term steroid treatments (wks) can masculinize the fictive vocalizations of females, inclusive of its sensitivity to rapid modulation by serotonin. Given the conserved organization of vocal control systems across vertebrate groups, the vocal CPGs of fish and amphibians provide tractable models for identifying androgen-dependent events that are fundamental to the mechanisms of vocal motor patterning. These basic mechanisms can also inform our understanding of the more complex CPGs for vocalization, and social behaviors in general, that have evolved among birds and mammals.
Keywords: CPG, Vocalization, Vocal plasticity, Androgens, Steroids, Neuromodulation
Introduction
The following descriptors provide a focus for this essay on androgen modulation of vocal motor systems: “central pattern generator” (CPG), “neuromodulation”, “sexually dimorphic, hormone-sensitive behavior”. The phrase “CPG” is most commonly associated with the spatiotemporal patterning of action potentials by neurons that orchestrate feeding, locomotion and heartbeat rate among invertebrates (Getting, 1989; Marder and Calabrese, 1996; Marder and Bucher, 2001), and locomotion and respiration among vertebrates (Grillner, 2003; Feldman and Del Negro, 2006). Central pattern generators, whether in a vertebrate or invertebrate preparation, have also served as models for “neuromodulation” to show the actions of a wide spectrum of non-steroidal neurochemicals on intrinsic membrane properties and the strength of synaptic interactions (Getting, 1989; Marder and Calabrese, 1996; Marder and Bucher, 2001). Lastly, “sexually dimorphic, hormonal-sensitive behavior” is a phrase often used in the context of reproduction, courtship and aggression, and how steroid hormones influence these behaviors. In this context, we are reminded of steroid effects on the neuronal substrates controlling these behaviors and where steroid receptors are located in the central nervous system (CNS) (De Vries, 2004; Kelley and Brenowitz, 2002; Morris et al., 2004).
Here, we consider recent evidence showing that the vocal behaviors and motor systems of teleost fish and anuran amphibians in particular can serve as important models for investigating the androgen-dependent neuromodulation of CPGs for sexually dimorphic social behavior among all vertebrate groups. While there is a long history of investigating androgen effects on the morphology of vocal motor systems, androgen effects on neurophysiological mechanisms remain relatively unexplored. Vocal systems are especially amenable for neurophysiological studies for several reasons. First, the behaviors of interest, vocalizations, are often repetitive and stereotyped with a predictable temporal pattern. Second, vocal motor patterning is established by a discrete set of CNS nuclei (Fig. 1). Third, like other CPG models (Marder and Bucher, 2001), isolation of either all or part of the CNS containing the vocal motor system shows that these regions are both necessary and sufficient to elicit “fictive” motor patterns/vocalizations that would produce natural calls if the sound generating muscles were active. Fourth, and especially pertinent to this special issue of Hormones and Behavior, the suite of neurochemicals that influence the activity patterns of vocal CPGs include androgenic steroids.
Fig. 1.
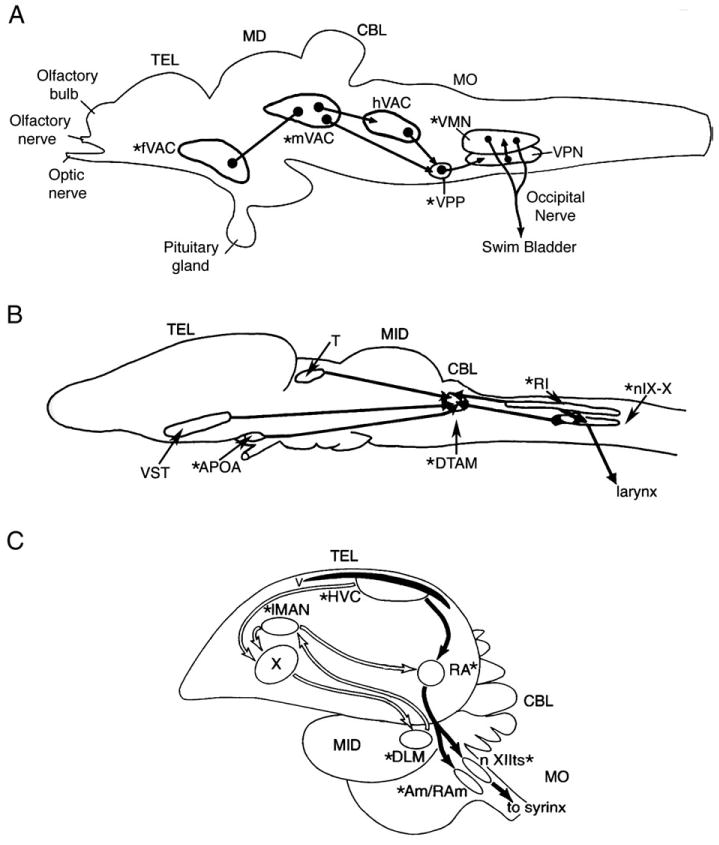
Line drawings of sagittal views of the brain and spinal cord of a teleost, amphibian and a songbird show the major nuclei of the vocal control system and the positions of androgen receptors (asterisk). (A) Teleosts (adapted from Goodson and Bass, 2002; Bass and McKibben, 2003). The vocal swim bladder muscles of batrachoidids are innervated by a vocal motor nucleus (VMN) via occipital nerve roots that are comparable to the hypoglossal nerve of tetrapods (Bass and Baker, 1997). The VMN receives input from an adjacent column of pacemaker neurons (VPN) that receives input from a more rostral prepacemaker nucleus (VPP). The VPP-VPN-VMN circuit is referred to as the vocal CPG. Nuclei within forebrain (f), midbrain (m) and hindbrain (h) vocal-acoustic centers (VAC) form part of a descending vocal system that includes links with the auditory system (see Bass et al., 2000; Goodson and Bass, 2002 for details). In particular, the mVAC includes the midbrain's periaqueductal gray that directly links forebrain vocal sites in the anterior hypothalamus to the vocal CPG. (B) Amphibians (adapted from Wetzel et al., 1985 and Kelley, 1986 with kind permission of Wiley-Liss, Inc. a subsidiary of John Wiley & Sons, Inc. and Springer Science and Business Media): Frogs have laryngeal muscles innervated by the vagus nerve by motor nucleus IX–X that receives input from the adjacent intermediate reticular formation (RI) and the dorsal tegmental area of the medulla (DTAM). DTAM receives afferents from the anterior preoptic area (APOA) and the ventral striatum (VST). DTAM together with IX–X/RI form a vocal CPG (see Rhodes et al., 2007; Zornik and Kelley, 2007). Not shown are the positions of glottal motoneurons that are involved in respiratory mechanisms because their circuitry is less well known, but see Zornik and Kelley (2007). (C) Birds (adapted from Schlinger and Brenowitz, 2002; see Reiner et al., 2004 for nomenclature). The syrinx is innervated via the hypoglossal nerve by the tracheosyringeal division of the hypoglossal motor nucleus (XIIts). Respiratory-related musculature is innervated by nucleus ambiguous (Am). XIIts receives direct input from the telencephalic nucleus robustus archistriatum (RA). Nucleus retroambigualis (RAm) is directly adjacent to Am and integrates vocal and respiratory activity (see Sturdy et al., 2003). Both XIIts and Am receive input from the midbrain's dorsal medial nucleus (not shown but see Wild, 2004). A series of other nuclei form a forebrain vocal network (see Wild, 2004). This includes the high vocal center (HVC), the robust nucleus of the arcopallium (RA), lateral area X of the striatum (X), the lateral portion of the magnocellular nucleus of the anterior neostriatum (IMAN) and the medial portion of the dorsolateral nucleus of the thalamus (DLM). Studies of songbirds and hummingbirds show androgen target sites in forebrain nuclei dedicated to vocal function (Gahr, 2001; Ball et al., 2002; Schlinger and Brenowitz, 2002). Batrachoidids (Fine et al., 1996; Forlano et al., 2005b) also have androgen receptors in the supracommisural nucleus of area ventralis in the telencephalon (part of fVAC), a vocally active site (Fine and Perrini, 1994; Goodson and Bass, 2002) that is a possible homologue of the basal amygdala of mammals (Northcutt, 1995). Other abbreviations: CBL, cerebellum; MID, midbrain, MO, medulla oblongata; TEL, telencephalon.
Vocal control systems, CPGs and androgens
Among vertebrates, vocal control systems include peripheral sound producing organs and CNS nuclei that are dedicated to performing one behavioral function, namely vocalization. Over the course of the past three decades, neuroanatomical studies have delineated a central vocal motor network comprising discrete populations of neurons at forebrain, midbrain and hindbrain-spinal levels among teleost fish (Goodson and Bass, 2002), frogs (Wetzel et al., 1985; Zornik and Kelley, 2007), birds(Wild, 2004), and mammals (Jürgens, 2002) (Fig. 1).
Studies of vocal motor systems in teleost fish and anurans during the 1960s and 1970s provided the foundations for ongoing studies of the androgen-sensitive, neurophysiological mechanisms of vocal CPGs (Pappas and Bennett, 1966; Demski and Gerald, 1972; Schmidt, 1976). During the 1970s, several reports mapped vocal pathways and sites of steroid binding in several species of songbirds, and essentially launched three decades of investigations into steroid influences on the neural mechanisms of birdsong (Zigmond et al., 1973; Arnold et al., 1976; Nottebohm and Arnold, 1976; Nottebohm et al., 1976). While recent analyses have approached the songbird vocal system mainly from a CPG perspective (e.g., Sturdy et al., 2003; Ashmore et al., 2005; Leonardo and Fee, 2005; Solis and Perkel, 2005), studies of androgen influences on the electroresponsive properties of neurons within the avian song system are just beginning (e.g., White et al., 1999; Park et al., 2005; Meitzen et al., 2007). Investigations dating from the 1970s also began to map a vocal control system among mammals (Jürgens, 2002; Jürgens and Hage, 2006). However, to our knowledge, there are no available studies of steroid influences on the neurophysiological mechanisms of vocal patterning among mammals (but see Jürgens, 2002 for studies of other potential neuromodulators).
Most of this review will focus on teleost fish and frogs given the long history of neurophysiological studies of vocal CPGs (see above) and, as reviewed here, the development of fictive calling preparations in these two groups, including investigations of androgen-sensitive mechanisms.
Vocal communication signals
All vertebrate groups include species that use vocalizations to mediate the dynamics of ongoing social behaviors (Bradbury and Vehrencamp, 1998). The term ‘vocal’ is appropriate here, as the major groups of vocal vertebrates (frogs, birds, fish, mammals) share common embryonic origins for both the motoneurons and skeletal muscles involved in sound production, i.e., vocalization (Bass, 1989; Bass et al., 1994, 2005a; Bass and Baker, 1997). Two broad categories of acoustic communication signals that are also observed in fictive preparations (see later sections) are advertisement and agonistic calls that are mainly related to, respectively, mating and territoriality (inclusive of aggressive interactions). While vocal signals can be characterized in the spectral and temporal domains (Bradbury and Vehrencamp, 1998), it is their temporal features (e.g., rate, duration and fundamental frequency) that can be largely shaped by CPGs. As Capranica (1992) observed: “the time varying acoustic waveform is the physical signal that is actually produced by the temporally patterned action of the motor system under ongoing control of the central nervous system. The generation of that temporal waveshape is the signal that the nervous system controls.” Temporal parameters are also those most relevant to studies of auditory encoding in both ectotherms and endotherms (e.g., see Bass et al., 2005b references therein) and to the recent studies of androgen modulation of vocal CPGs that are reviewed here.
Fictive vocalizations predict the temporal patterns of social vocalizations
In this section, we first provide background information on vocal CPGs in teleost fish and frogs. The next two sections will then consider neuroanatomical studies of androgen target sites in vocal nuclei that provide a setting for the subsequent discussion of androgen actions on the activity patterns of vocal CPGs.
Teleost fish
The vocalizing abilities of teleosts have long been well known (Fish and Mowbray, 1970; Tavolga, 1971; also see Ladich et al., 2006 for recent reviews). While neuroanatomical studies have identified a vocal (sonic) motor nucleus across a number of distantly related teleosts (Bass and Baker, 1991; Ladich and Bass, 2005; Onuki and Somiya, 2007), neurophysiological studies of a vocal CPG have mainly been completed for batrachoidid fish that includes several genera commonly referred to as toadfish and midshipman fish (Nelson, 1994; see Bass and Baker, 1991 for preliminary data on vocal CPGs in other species). We provide an extended discussion of batrachoidids for three main reasons. The first is the relative simplicity of the vocal CPG of batrachoidids that controls a single set of muscles, whereas vocalization in most other vertebrates involves multiple sets of vocal and respiratory muscles (Bass, 1989; Bass and Baker, 1997). Second, the studies of batrachoidids illustrate how the oscillatory-like activity of individual premotor and motor neurons is translated into the temporal attributes of vocalizations such as fundamental frequency and duration (Bass and Baker, 1990). The vocal CPG of batrachoidids is proposed as a model for the basic premotor timing/ synchronization unit in the brainstem of other, more complex vocal control networks (Bass and Baker, 1997). Third, several studies now show that the vocal CPGs of midshipman and toadfish are acutely modulated by androgens.
Sound production among batrachoidid fish depends upon the contraction of one pair of muscles attached to the walls of the gas-filled swim bladder (Skoglund, 1961; Cohen and Winn, 1967). Early studies provided concurrent recordings of vocalizations and electromyograms and/or action potentials from the swim bladder muscles of Opsanus tau and the plainfin midshipman Porichthys notatus to show that the simultaneous contraction of both muscles is matched 1:1 with each sound pulse (Skoglund, 1961; Cohen and Winn, 1967). Neurophysiological analyses of the CNS of batrachoidids have roots in intracellular recording studies of electrotonic coupling in the vocal motor system of the oyster toadfish O. tau (Pappas and Bennett, 1966), and brain stimulation studies of the Gulf toadfish Opsanus beta that mapped vocally active sites throughout the CNS (Demski and Gerald, 1972; see Fine and Perini, 1994 and Kittelberger et al., 2006 for overviews of subsequent studies).
The most complete studies of a vocal motor network are for the plainfin midshipman fish, P. notatus. Midshipman produce two main classes of vocalizations, advertisement and agonistic calls that are especially different in duration (Fig. 2A; Brantley and Bass, 1994; Bass et al., 1999). In midshipman, and batrachoidids in general, each vocal muscle is innervated by an ipsilateral vocal motor nucleus that extends along the midline at caudal hindbrain-rostral spinal cord levels (VMN, Figs. 1A, 2B). The combined firing of motoneurons on each side of the midline is reflected in a single, spike-like potential of a motor volley that can be readily recorded intracranially from ventral occipital nerve roots (Fig. 2D). (The occipital roots are considered homologues of hypoglossal nerve roots in tetrapods; see Bass and Baker, 1997 for extended discussion). Premotor neurons (VPN, Figs. 2B, C) form a column along the ventrolateral margin of each motor nucleus and generate a single pacemakerlike action potential just prior to the firing of each motoneuron (Fig. 2D; Bass and Baker, 1990). Hence, the activity pattern of the vocal pacemaker-motoneuron circuit establishes the 1:1 relationship between the firing rate and duration of the motor volley, the rate and duration of vocal muscle contraction, and the fundamental frequency/pulse repetition rate and duration of natural calls. The direct translation of the temporal firing pattern of the occipital nerve's motor volley into the temporal features of the natural call is the basis for designating it a fictive vocalization, which can be elicited in an in vivo neurophysiological preparation that is treated to prevent muscle contractions.
Fig. 2.
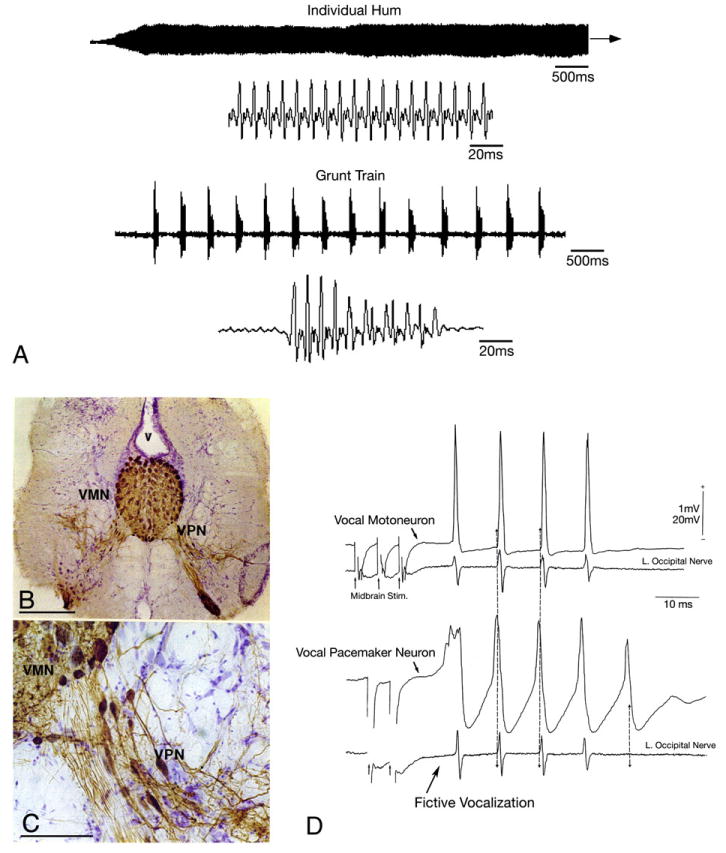
Vocal behaviors and central pattern generator (CPG) in the plainfin midshipman fish, Porichthys notatus. (A) Oscillograms of a long duration advertisement hum and a train of brief duration agonistic grunts on two time scales (adapted from Bass et al. 1999 and 2005a,b with kind permission of Springer Science and Business Media). (B, C) Vocal CPG morphology (adapted from Bass et al., 1996). Following application of biocytin to the cut end of the vocal nerve that innervates a single vocal muscle, both the midline vocal motor nucleus (VMN) and the adjacent column of pacemaker neurons (VPN) are labeled bilaterally (v, fourth ventricle). Bar scale represents 300 μm in ‘A’ and 75 μm in ‘B’. (D) Vocal CPG physiology (adapted from Bass and Baker, 1990). Intracellular records from anatomically-identified vocal motor and pacemaker neurons following midbrain stimulation (each trace is average of four sweeps). Both intracellular (top) and intracranial (bottom; left vocal occipital nerve) records are shown. Small arrows at the beginning of the lower traces indicate onset of midbrain electrical microstimulation. The highly synchronous nerve discharges were aligned (vertical lines) to show relative timing between pacemaker and motoneuron firing. Time scale and direction of polarity for all records are indicated.
Bilateral coupling of the vocal pacemaker-motoneuron circuit is provided by pacemaker neuron axons that innervate both motor nuclei, and by a more rostral prepacemaker nucleus (VPP, previously referred to as a ventral medullary nucleus; Fig. 1A) that links the paired pacemaker columns (Bass and Baker, 1990; Bass et al., 1994; Goodson and Bass, 2002). It is the extensive coupling of the pacemaker-motoneuron columns that leads to the simultaneous contraction of both swim bladder muscles and the production of one sound pulse in Porichthys and Opsanus (Bass and Baker, 1990, 1991). Surgical isolation of the CNS region containing theprepacemaker–pacemaker-motoneuron circuit confirms that this region alone can generate a rhythmic vocal motor volley (Weiser et al., 1986; Remage-Healey and Bass, 2004, 2006b). As such, the prepacemaker–pacemaker-motoneuron circuit is designated the “vocal CPG”.
Neuroanatomical and neurophysiological investigations have further delineated an expansive network of vocal control nuclei at forebrain, midbrain and rostral hindbrain levels (Fig. 1A) that are coupled to the auditory system (Bass et al., 1994, 2000; Goodson and Bass, 2002). The midbrain vocal-auditory center includes the periaqueductal gray (PAG) that links forebrain vocal sites (fVAC, Fig. 1A) to the vocal CPG at hindbrain-spinal levels (Goodson and Bass, 2002; Kittelberger et al., 2006). Functional similarities between the vocal control systems of batrachoidids and tetrapods are highlighted by the most recent single neuron recording study showing the essential role played by the PAG in call initiation (Kittelberger et al., 2006). Perhaps somewhat remarkably, the neurophysiological properties of PAG neurons closely resemble those of mammals, including primates (see Kittelberger et al., 2006 and discussion therein).
To our knowledge, the above studies in midshipman fish (Bass and Baker, 1990) provided the first intracellular recording and staining study of individual, anatomically-identified neurons within a brainstem vocal CPG. Essential to the subsequent development of midshipman fish as a model system for behavioral neuroendocrinology, the intracellular recording studies also identified intersexual and intrasexual (male) differences in the morpho-physiological traits of individual vocal pacemaker and motor neurons. Midshipman have two male reproductive morphs referred to as types I and II that diverge in a large suite of behavioral, somatic, endocrine and vocal motor traits (Bass, 1992,1996). The two male morphs exhibit alternative reproductive tactics (Brantley and Bass, 1994; Lee and Bass, 2006). Type I males build nests in the intertidal zone under rocky shelters from where they acoustically court females with a long duration (minutes — > 1 h) advertisement call known as a hum (see Fig. 2A). Type II males are on average about 50% smaller in body size than type I males, although the gonad weight/ body weight ratio of type II males is about 9 fold greater than that of type I males (Bass and Marchaterre, 1989; Brantley and Bass, 1994). Type II males neither build nests nor court females, but instead either sneak or satellite spawn to steal fertilizations from type I males. Type II males, like females, are only known to infrequently produce grunts in non-spawning contexts; type I males produce long trains of grunts (Fig. 2A; Brantley and Bass, 1994). Type II males essentially trade an expanded vocal motor system, used by type I males in female courtship, for an earlier sexual maturity (Bass, 1996).
As with vocal behavior, the vocal motor traits of type I males differ from those of type II males and females that are similar to each other. The latter range from the production of advertisement hums by type I males alone, to the aerobic capacity and ultrastructure of the vocal muscle, the dimensions of vocal neurons (somata, dendrites, axons, neuromuscular junctions) and modulation of the vocal CPG by neuropeptides (reviews: Bass, 1992; Walsh et al., 1995; also see Goodson and Bass, 2000). Recordings from individual motor and pacemaker neurons in type II males and females during the production of fictive grunts also show that they have a firing rate similar to each other, but significantly less than that of type I males (Bass and Baker, 1990, 1991). The morph differences for fictive grunts parallel morph differences in the pulse repetition rate of natural grunts (Brantley and Bass, 1994; M. Marchaterre and A. Bass, unpublished observations). Underwater playbacks support the hypothesis that variation in call pulse repetition rate could contribute to reproductive morph recognition (see Bass and McKibben, 2003). Fictive grunt duration is significantly greater in type I males compared to type II males and females (Fig. 3), consistent with the type I male's ability to generate long duration advertisement hums. Sound duration also figures prominently into behavioral recognition mechanisms (Bass and McKibben, 2003). More recent studies have reported alternative, size-dependent tactics among type I males (Lee and Bass, 2006), but the neurobiological correlates of such divergence among type I males has yet to be investigated. As reviewed below, neurophysiological studies of androgen modulation have now revealed distinct properties of the vocal CPG in each of the adult reproductive morphs.
Fig. 3.
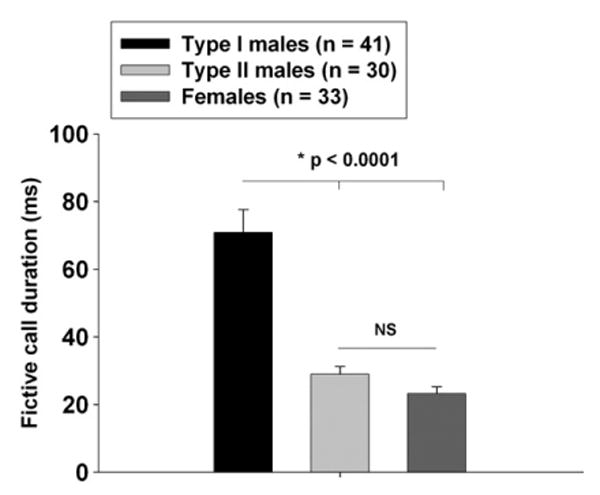
Fictive call duration among midshipman fish. Shown are histogram plots of the average baseline duration (prior to any hormone treatment)+S.E.M. of fictive calls. Type I males have significantly longer duration calls than both type II males and females (One-way ANOVA, F=29.86; df=2, 101; p<0.0001). Post-hoc tests show that mean duration was significantly longer in type I males (mean=70.85 ms; SE=6.83; n=41) vs. type II males (mean=28.96 ms; SE=2.30; n=30; p<0.0001 compared to type I males) and females (mean=23.21 ms; SE=2.03; n=33; p<0.0001 compared to type I males). There were no significant differences between type II males and females (p=0.44). (L. Remage-Healey and A. H. Bass, unpublished results).
Anuran amphibians
For terrestrial amphibians (frogs and toads), like other terrestrial vertebrates, vocalization depends upon its coordination with respiration (Gans, 1974; Bass, 1989; Nowicki et al., 1992). Schmidt mapped out a “mating calling pattern generator” in Northern leopard frogs (Lithobates pipiens; formerly Rana pipiens, see Frost, 2006), mainly by using a combination of brain transections with local electrical microstimulation and evoked potential recordings in an isolated brain preparation from males (e.g., see Schmidt, 1976, 1992). Schmidt's work led to a model that included two “semi-independent” pattern generators, a pretrigeminal nucleus positioned at isthmal levels and “the classical respiration generator” at the level of motor nucleus IX–X in the caudal medulla (Schmidt, 1992; see Vasilokos et al., 2004 for recent studies of Rana catesbiana showing two respiratory-related CPG regions in the anterior and caudal medulla).
Extensive neuroanatomical studies by Kelley and colleagues (Wetzel et al., 1985; Zornik and Kelley, 2007) identified a central calling circuit for the fully aquatic frog Xenopus laevis that does not depend upon respiration for calling (Yager, 1992). The Xenopus map closely matches the neurophysiological one for Lithobates depicted by Schmidt (1992). The most recent studies of Xenopus have further investigated the connectivity of the dorsal tegmental area of the medulla (DTAM, homologue of pretrigeminal nucleus of Lithobates) and of vocal (laryngeal) and respiratory (glottal) motoneurons within the motor nucleus IX–X (Zornik and Kelley, 2007). An initial neurophysiological study in Xenopus identified sex differences in the active and passive membrane properties of laryngeal motoneurons (Yamaguchi et al., 2003), while a more recent one describes a fictive call preparation for isolated brains (Rhodes et al., 2007). Like vocal fish, a fictive call composed of a volley of compound action potentials can be recorded from nerve roots, in this case carrying the axons of laryngeal motoneurons, that is predictive of the temporal pattern of natural calls (Fig. 4A). An additional highlight of this work is the demonstration that bath application of serotonin can rapidly evoke fictive calls from male and female brains that resemble, respectively, male advertisement calls and female release calls (male brains also generate release-like calls) (Fig. 4A). Male brains require a briefer exposure to serotonin to induce fictive calls. Serotonin also evokes fictive bursts from respiratory-related glottal motoneurons, but these do not overlap with the fictive calls (see Fig. 4A also see Schmidt, 1992 for L. pipiens). Besides showing that the vocal CPG is sexually differentiated in Xenopus, Rhodes et al. (2007) use a combination of brain transections and brief electrical microstimulation to show that the brain region containing DTAM is necessary for the induction of fictive calls by serotonin. The new results for fully aquatic Xenopus, together with the earlier ones for terrestrial frogs (Schmidt, 1992), support the general hypothesis that both DTAM-pretrigeminal nucleus and the more caudal region around and inclusive of motor nucleus IX–X are essential components of a vocal CPG in anuran amphibians (see Rhodes et al., 2007 and Zornik and Kelley, 2007 for further discussion).
Fig. 4.
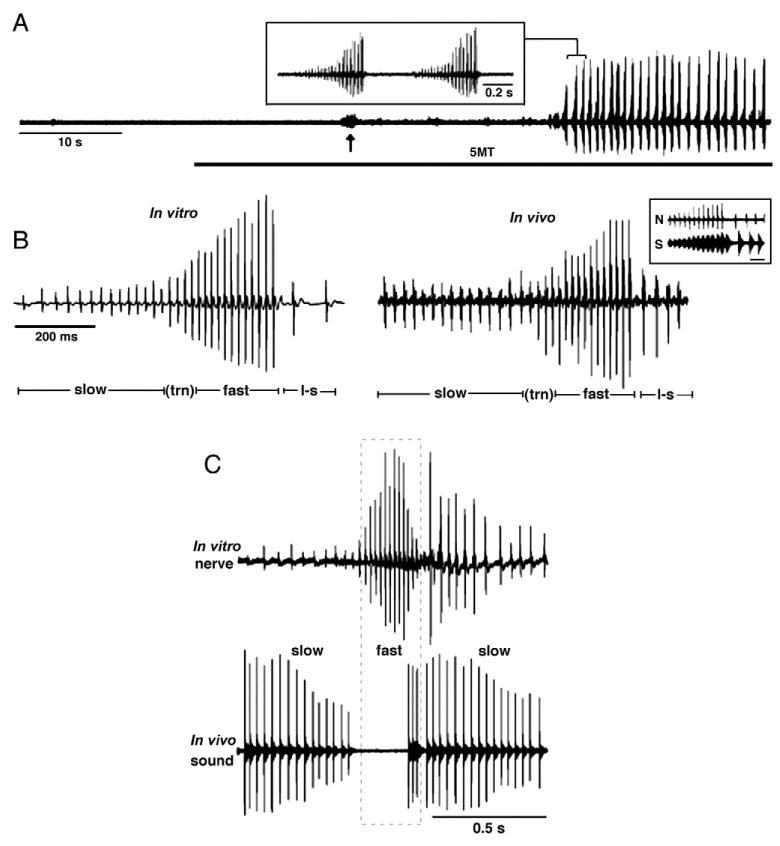
Fictive calling in X. laevis (adapted from Rhodes et al., 2007, copyright 2007 by the Society for Neuroscience). Laryngeal nerve recordings from males show that the temporal pattern of fictive advertisment-like calls recorded in an in vitro, isolated brain preparation closely compare to the nerve records obtained in vivo. (A) Nerve records from isolated brain preparation show induction of a fictive advertisement call 35 s after bath application of serotonin (5HT, gray bar). 5HT also evokes separate bursts of respiratory-related activity (arrow). The inset is an expansion of the region bracketed in ‘A’ showing that each large-amplitude burst is a single bout of a fictive advertisement call. (B) Shown here are in vitro (left) and in vivo (right) nerve recordings of male advertisement call to illustrate slow, fast, and loud–slow (l–s) trills, and the pulses that occur during the transition (trn) from slow to fast trills. The inset shows simultaneous nerve recordings (N) and a portion of fast and loud–slow trill in a freely behaving male (S). Each fictive sound pulse in the nerve record is followed by the production of a click sound several milliseconds later. Bar scale for the inset represents 50 ms. (C) Testosterone-treated females produce male-like vocal patterns in vivo and in vitro. Shown here are recordings of fictive (top) and natural (bottom) calls from a female 8 weeks after testosterone treatment; slow and fast trill segments are indicated. The fictive call of this female more closely resembles the advertisement call of a male than the natural call previously recorded from this same testosterone-treated female (the natural call has an abnormal pause between the slow and fast trill portions of the call).
Androgen target cells in vocal motor systems
Several investigations of vertebrates since the 1970s have identified CNS sites with an abundance of steroid hormone receptors, including those for androgens. While such studies include non-vocal and vocal teleosts (Morrell et al., 1975; see Bass et al., 1986 and Fine et al., 1996 for later reports and reviews), it is only with the recent mapping of a vocal motor network that the location of androgen receptors, and steroid receptors in general (Forlano et al., 2005a,b), can be directly linked to vocal nuclei (Fig. 1A). Sites of androgen receptors in vocal nuclei have long been well-documented for amphibians (Fig. 1B; Kelley, 1986; Perez et al., 1996). Although this review does not focus on birds, they are among the most extensively studied group of vocal vertebrates for the mapping of steroid receptor sites and so we show the avian vocal nuclei that contain androgen receptors to provide a broader comparative context (Fig. 1C; Gahr, 2001; Ball et al., 2002; Schlinger and Brenowitz, 2002; also see Belle et al., 2003 and Kim et al., 2004). To our knowledge, studies of mammals have not explicitly identified androgen receptor sites within vocal nuclei (but see Simerly et al., 1990 for androgen receptors and Jürgens, 2002 for vocal nuclei).
Perhaps most germane to the current discussion is that teleost fish, amphibians and birds show a common pattern of androgen receptors in brainstem vocal nuclei (asterisks, Fig. 1). A recent report for midshipman fish (Forlano et al., 2005b) identifies abundant androgen receptor mRNA in motor nuclei that innervate the vocal swim bladder muscles (also have abundant androgen receptors), the prepacemaker (ventral medullary) nucleus and the midbrain periaqueductal gray (part of mVAC in Fig. 1A). Labeling in the prepacemaker nucleus is consistent with a previous autoradiographic study showing androgen-binding sites in a comparable region in toadfish (Fine et al., 1996; A. Bass, unpublished observations). Among frogs (Fig. 1B), androgen target vocal nuclei include the motor nucleus IX–X that innervates laryngeal muscles (also have abundant androgen receptors), the adjacent reticular formation, and DTAM (Kelley, 1986; Perez et al., 1996). For songbirds and hummingbirds (Fig. 1C), androgen receptors are abundant in the tracheosyringeal division of the hypoglossal motor nucleus (XIIts) that innervates syringeal muscles (also have abundant androgen receptors), the midbrain's nucleus intercollicularis (not shown in Fig. 1C) and the hindbrain's nucleus retroambigualis, both of which innervate XIIts (see Wild, 2004 for circuitry; see Gahr, 2001; Ball et al., 2002 and Schlinger and Brenowitz, 2002 for androgen receptors).
Androgen receptor mRNA in the midshipman's vocal motor nucleus is concentrated over a peripheral rim of glial cells that express aromatase, the enzyme that converts testosterone to estrogen (Forlano et al., 2001; see Forlano and Bass, 2005a,b for androgen-dependent regulation of sexually dimorphic aromatase expression). By contrast, estrogen receptor mRNA is concentrated over the somata of motoneurons (Forlano et al., 2005a), consistent with estrogen-dependent modulation of the vocal CPG (Remage-Healey and Bass, 2004, 2007). The anatomical distribution of androgen receptors is broadly similar among the three adult midshipman morphs, and this is also true of estrogen receptors (Forlano et al., 2005a,b). While the vocal motoneurons of songbirds and Xenopus have androgen receptors, they are not reported for the vocal motoneurons of budgerigars (parrots) and various species of nonpasserines (Gahr, 2000). For songbirds and frogs, it is not yet known whether androgens influence either the morphology or physiology of vocal motoneurons by acting directly on those neurons independently of androgen effects on the vocal muscles they innervate (Tramontin and Brenowitz, 2000; Kelley, 2002; but see Morris et al. 2004 for discussion of spinal motor systems in rodents). As discussed below, androgen-dependent modulation of vocal CPG physiology in teleosts may begin to explain, in part, the functional significance of abundant androgen receptor expression in vocal control nuclei.
Androgen-dependent plasticity of vocal CPG activity
Androgens influence behavioral and neural traits over widely varying time frames. We first comment on long-term (weeks–months) androgen effects on vocal behavior, morphology and neurophysiology in teleost fish and amphibians, the two groups that are the focus of our commentary (see Tramontin and Brenowitz, 2000; Schlinger and Brenowitz, 2002; Ball et al. 2002 for studies of songbirds). We then concentrate on recent studies revealing rapid androgen effects on behavioral and neurophysiological mechanisms in teleosts.
Long-term androgen actions
While numerous studies of teleosts associate increased calling behavior among territorial males with elevated levels of circulating androgens during the breeding season (see below), investigations of long-term androgen effects on natural vocal behavior are apparently rare. Winn and Stout (1960) comment in their field study of satinfin shiners (Notropis analostanus) that 5–10 minutes after receiving a testosterone injection (details not provided), males were more vocal than either non-injected or sesame oil controls. While not a focus of their study, Lee and Bass (2005) note that after 39 day implants of 11-ketotestosterone, a teleost-specific androgen (see below), type II sneaker males did not produce the advertisement hums typical of territorial type I males (Fig. 2A). However, androgen-treated type II males may yet generate the long duration trains of agonistic grunts that also characterize type I males (Fig. 2A).
Pilot studies in midshipman fish show long-term androgen effects on the discharge frequency of fictive calls that establishes the fundamental frequency/pulse repetition rate of natural calls (see earlier section). Fictive call discharge frequency among juvenile males (∼95–100 Hz) is similar to that of females and type II, sneaker males (Bass and Baker, 1990; A. Bass, unpublished observations). After 8–9 weeks of testosterone propionate implants (methods after Brantley et al., 1993a), the discharge frequency increases by 13% in androgen-treated juveniles (n=4) compared to size-matched, untreated controls (n=7) (Bass, 1995; A. Bass, unpublished observations). The latter increase compares closely with inter- and intrasexual differences in discharge frequency which is 15–20% higher in type I males compared to type II males and females (Bass and Baker, 1990). Comparable testosterone treatments of juvenile males also induce increases in the size of vocal muscles (Brantley et al., 1993b; Lee and Bass, 2005; see Connaughton and Taylor, 1995 for other teleosts) along with vocal motoneuron soma size and aromatase expression levels in the motor nucleus (Bass and Forlano, 2008; the vocal muscle and motor and premotor neurons are dimorphic; Brantley et al., 1993b; Bass et al., 1996).
The long-term influences of androgens on vocal systems in amphibians have been most completely assessed in X. laevis (Kelley, 2002; Moore et al., 2005). This includes the demonstration of androgen-dependent masculinization of calling behaviors in females and juveniles, and the sexually dimorphic larynx, laryngeal synapses and motoneurons (Kelley, 2002; Potter et al., 2005). For example, the most recent study of Xenopus (Potter et al., 2005) shows that 8 week implants of testosterone can induce adult females to produce calls with temporal properties like those of males. Potter et al. (2005) also examined the temporal onset of the masculinization of laryngeal muscle contractile properties (also see Tobias and Kelley, 1987) and present new evidence for androgen-induced increases in the size of laryngeal motoneuron somata that compares closely to the size of male motoneurons. Both the laryngeal muscle and motoneurons showed male-like properties prior to the onset of male-like calling behaviors.
Schmidt (1980, 1983) identified the masculinizing effects of androgen treatments (8–9 day injections of testosterone propionate and/or dihydrotestosterone) on fictive mate calling in the isolated brains of juvenile and female Northern leopard frogs. The recent study of fictive calling in the isolated brain preparation from Xenopus (see earlier section) revealed that serotonin could rapidly evoke both male, advertisement-like and female, release-like fictive calls from the brains of adult females treated with testosterone for either 8 weeks or 5 months (Fig. 4B; Rhodes et al., 2007). In comparison to untreated females, the brains of testosterone-treated females, like those of males, showed a briefer delay between serotonin application and the onset of fictive calling. Besides providing a wonderful new experimental tool for inducing fictive calls in an isolated brain preparation, these new results for Xenopus show that the vocal CPG's sensitivity to rapid modulation by serotonin is also subject to the long-term influences of androgens.
Rapid, short-term androgen actions
The most recent reports of androgen actions on vocal mechanisms in teleosts emphasize rapid (minutes–hours) effects on both natural vocal behavior and the neurophysiological output of the CPG network (see Remage-Healey and Bass, 2006a for a companion review that considered, more generally, rapid steroid regulation of vertebrate reproductive and courtship behaviors).
11-Ketotestosterone (11kT, structure shown in Fig. 5) is the major circulating androgen in male teleost fish (Oliveira et al., 2005), including type I male midshipman and several toadfish species (Brantley et al., 1993a; Knapp et al., 1999; Modesto and Canario, 2003; Fine et al., 2004; Sisneros et al., 2004). Testosterone is the predominant circulating androgen in female toadfish, and female and type II male midshipman fish, while 11kT is low or non-detectable (Brantley et al., 1993a; Knapp et al., 1999; Sisneros et al., 2004; Fine et al., 2004). The divergent levels of androgens between type I male midshipman vs. type II males and females is consistent with observations in other teleosts with alternative male reproductive tactics (Brantley et al., 1993a; Oliveira et al., 2005; see Knapp and Neff, 2007 for recent study in a sunfish).
Fig. 5.
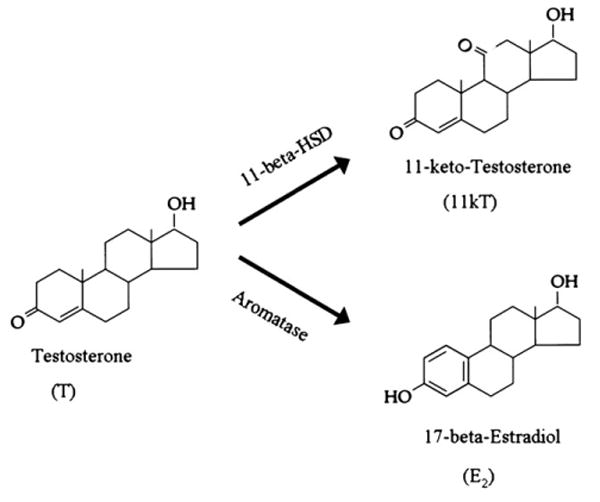
Chemical structure of testosterone (T), 11-ketotestosterone (11kT), and 17-beta-estradiol (E2) in teleost fishes, showing the enzymatic synthetic pathways that oxidize testosterone into either 11kT (11-beta-hydroxysteroid-dehydrogenase) or E2 (aromatase). Adapted in part from Bentley (1998) with the permission of Cambridge University Press.
11-Ketotestosterone is a potent non-aromatizable androgen derived from testosterone (Fig. 5, like dihydrotestosterone in tetrapods) that can masculinize secondary sex characters (Oliveira et al., 2005; also see Lee and Bass, 2005 for most recent study of midshipman fish). Both male toadfish and type I male midshipman fish exhibit elevated plasma 11kT levels during vocal advertisement calling compared to non-calling contexts (Knapp et al., 2001; Remage-Healey and Bass, 2005). When male toadfish are exposed to simulated territorial challenges (via acoustic playbacks mimicking the calls of conspecific males), plasma 11kT levels rise in concert with fast increases in vocal signaling (within 5–20 min; Remage-Healey and Bass, 2005), suggesting that 11kT may regulate advertisement calling (and the vocal CPG) in a rapid manner.
Taking advantage of the fictive call preparation (Fig. 2D), we have now shown that the vocal CPG of midshipman and toadfish is rapidly regulated by androgens in a steroid-and sex-specific manner. Male toadfish and type I male midshipman are uniquely responsive to 11kT. Following injection into the dorsal trunk muscle, 11kT causes rapid (within 5 min) and sustained (up to 120 min) increases in the duration of fictive vocalizations in both species (Remage-Healey and Bass, 2004; 2006b). There are no changes in the discharge frequency of the fictive call (see Fig. 2D). The rapid responses to 11kT are restricted to advertising males only; the fictive calls of females of both species and type II male midshipman do not respond to 11kT (Remage-Healey and Bass, 2006b; 2007). These observations are consistent with the behavioral field studies discussed above. First, they reflect that circulating 11kT levels are only evident in type I male midshipman and male toadfish. Second, 11kT levels fluctuate during changing social and vocalization contexts in type I male midshipman and male toadfish. Third, the fast time-course of androgen-sensitive fictive calling is consistent with the similarly rapid and concurrent changes in calling and circulating 11kT levels observed in studies that simulate territorial challenges in toadfish. Lastly, the time-course of 11kT-dependent shifts in fictive calling are consistent with the rapid effects of experimentally-induced elevations in circulating 11kT levels (using food containing 11kT crystals) on elevations in the calling behavior of advertising male toadfish (Remage-Healey and Bass, 2006b).
Type II male and female midshipman are rapidly responsive to testosterone, rather than 11kT. Following injection into the trunk muscle, testosterone causes rapid (within 5 min) and sustained (up to 45 min) increases in the duration of fictive vocalizations (Remage-Healey and Bass, 2007). These results are consistent with testosterone being the predominant circulating androgen in type II male and female midshipman (see above). Neither type I male midshipman nor male toadfish are responsive to testosterone, consistent with the low circulating levels of testosterone in these groups. Interestingly, female toadfish are likewise unresponsive to testosterone (Remage-Healey and Bass, 2006b). To date, we have no information on circulating levels of testosterone in our study population of female O. beta toadfish, but based on the lack of rapid actions on vocal CPG activity, we predict low circulating levels of testosterone in breeding females. A summary of the information presented above can be found in Table 1.
Table 1.
A summary of the rapid effects of the steroids 11-ketotestosterone (11kT), testosterone (T) and 17-beta-estradiol (E2) on fictive call duration in the plainfin midshipman's three reproductive morphs and in Gulf toadfish
| Midshipman | Toadfish | ||||
|---|---|---|---|---|---|
| Type I male | Type II male | Female | Male | Female | |
| Predominant circulating androgen | 11kT | T | T | 11kT | ? |
| Rapid 11kT Effect | ⬆ | ⬄ | ⬄ | ⬆ | ⬄ |
| Rapid T Effect | ⬄ | ⬆ | ⬆ | ⬄ | ⬄ |
| Rapid E2 Effect | ⬆ | ⬆ | ⬆ | ⬄ | ⬄ |
| Androgen action blocked by CA? | Yes | Yes | No | ? | ? |
| Androgen action blocked by FAD? | No | No | Yes | ? | ? |
Also shown for midshipman are the effects of cyproterone acetate (CA), an androgen receptor antagonist, and fadrozole (FAD), an aromatase inhibitor. See Remage-Healey and Bass (2004, 2006b, 2007) for further discussion and relevant references.
The above work in toadfish and midshipman together suggests that androgens interact acutely with the vocal CPG. To explicitly test this hypothesis in both species, the hindbrain-spinal region that contains the vocal CPG (see Figs. 1, 2) was isolated in vivo from rostral and caudal input via surgical transections (Remage-Healey and Bass, 2004, 2006b). In this preparation, fictive vocalizations were elicited from the CPG via brief electrical microstimulation in the region of the prepacemaker nucleus (see Fig. 1A) and 11kT was injected into the trunk muscle as in other studies. The rapid effects (5–45 min) of 11kT on fictive call duration were observed in the isolated CPG of both type I male midshipman and male toadfish (Fig. 6). However, the sustained effects of 11kT that were observed in the intact preparation (45 to 120 min) were not observed, suggesting that descending input from the midbrain and/or forebrain (candidate loci include androgen receptor-expressing nuclei such as the midbrain PAG and forebrain preoptic area; Fig. 1A) is necessary for the sustained modulation of CPG activity (Remage-Healey and Bass, 2004, 2006b). These results strongly indicate that androgens modulate the activity of brainstem CPGs via direct (or locally indirect) mechanisms.
Fig. 6.
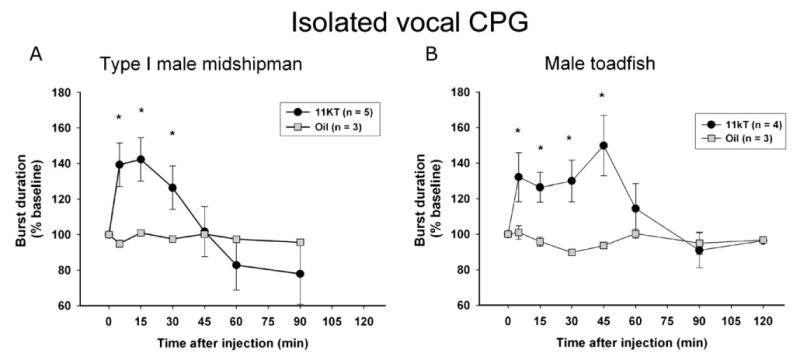
Rapid actions of androgens on the vocal central pattern generator (CPG; adapted from Remage-Healey and Bass, 2004 — copyright 2004 by the Society for Neuroscience; and Remage-Healey and Bass, 2006b). The vocal CPG of type I male midshipman (A) and male toadfish (B) was isolated via surgical transection from both rostral and caudal inputs. Once stable, CPG activity was elicited via brief electrical microstimulation of the prepacemaker region (see Fig. 1A) and either 11kT or oil vehicle were injected into dorsal trunk musculature. 11kT treatment rapidly induced an increase in fictive call duration in males of both species. Fictive call (burst) duration was normalized to 100% of baseline output at multiple time points following injection (0 min). Number of animals (n) is indicated for each set of experiments (*p < 0.05 for repeated-measures ANOVA, followed by Tukey's multiple comparison tests for differences among sampling times).
Other studies of fictive calling show that estrogen can also induce rapid increases in fictive grunt duration in all three midshipman reproductive morphs, although the magnitude of the estrogen effect in all cases is reduced compared to that for 11kT in type I males and testosterone in type II males (Table 1; Remage-Healey and Bass, 2004, 2007). Additional experiments using an androgen receptor antagonist (cyproterone acetate, CA) and the aromatase inhibitor fadrozole that rapidly blocks the conversion of testosterone to estrogen in midshipman fish (Schlinger et al., 1999) reveal that the rapid testosterone effect in type II males and females is dependent, respectively, on androgen- and estrogen-dependent mechanisms (CA also blocks the 11kT effect in type I males, see Table 1). Thus, CA and fadrozole completely block androgen modulation of fictive calls in, respectively, male vs. female midshipman. Thus, while estrogen can modulate fictive call duration in all three reproductive morphs (see Table 1), aromatization of testosterone to estrogen only accounts for testosterone's actions in females.
The divergent sensitivity to 11kT (only in type I male midshipman and male toadfish) vs. T (only in type II male and female midshipman) suggests separate binding sites for these two androgens, but that these sites are similarly sensitive to antagonism by CA. Recent discoveries of an androgen receptor specifically activated by 11kT (Olsson et al., 2005) and an 11kT-responsive membrane androgen receptor (Thomas et al., 2006) lend support to the hypothesis that multiple types of androgen-binding sites occur in teleost brain. Our findings do not rule out the possibility, however, that a common binding site (e.g., membrane androgen receptor) is responsible for both the 11kT and T effects, and that the androgen sensitivity of this receptor is uniquely modified in, for example, type I vs. type II male midshipman. Steroid receptor acetylation can alter ligand sensitivity (e.g. Fu et al., 2004), so that a common androgen receptor could be similarly modified to confer differential androgen sensitivity.
The effects of androgens (11kT and testosterone) on fictive grunt duration in midshipman fish are consistent with the “compensation hypothesis” of De Vries and colleagues proposing that sex differences in brain structure may encode either sexually differentiated or undifferentiated behavior (De Vries, 2004). As De Vries (2004) comments, “the neurochemical and hormonal underpinnings of social behavior may differ even in cases where these behaviors are remarkably similar between the sexes.” Thus, the effect of androgens on fictive grunts is similarly “undifferentiated” in all three midshipman reproductive morphs; androgens rapidly increase fictive call duration. However, the underlying receptor mechanism diverges; thus, androgens act through androgen receptor-like mechanisms in males (type I and II) and via aromatization in females.
The effects of androgens on fictive calling in midshipman fish compare with neuropeptide actions. The neuropeptides arginine vasotocin (AVT, the non-mammalian homologue of mammalian arginine vasotocin) and isotocin (the non-mammalian homologue of mammalian oxytocin) inhibit fictive calling (call duration and/or rate) within minutes in, respectively, type I males vs. type II males and females, whereas the appropriate antagonists facilitate calling (Goodson and Bass, 2000). The results suggest decreases in neuropeptide release within the vocal motor system (see Goodson et al., 2003 for anatomical distribution) when androgens are elevated.
Concluding comments
Studies of androgen modulation of vocal patterning remain to investigate (also see Bass, in press; Remage-Healey and Bass, 2007; Rhodes et al., 2007) how androgen-induced plasticity is dependent on changes in synaptic and network properties (e.g., see White et al., 1999), as well as ligand (steroid)-dependent and independent (via second messenger pathways) events (e.g., see Blaustein, 2004; Ronnekleiv and Kelly, 2005). While multiple steroids (Table 1; also see Remage-Healey and Bass, 2004, 2006b, 2007 for rapid cortisol modulation) have rapid, but distinct, effects on fictive calling in batrachoidids, it remains to be shown if different steroids interact along a rapid time-course with each other. Steroids may also have fast interactions with other neurochemicals such as serotonin and neuropeptides that have already been shown to quickly change the output of the vocal CPGs in, respectively, anurans and batrachoidids (see earlier sections; also see Knapp, 2004; Moore et al., 2005). Lastly, we need to establish if steroids have long-term, organizational-like effects on their own rapid actions, comparable to testosterone's influence on serotonin modulation of the vocal CPG in amphibians (see last section).
Rapid, androgen-dependent regulation of signal duration may be widespread among the multitude of vocal fishes, especially in light of the observation that androgens increase fictive grunt duration in both midshipman and toadfish. Given the widespread occurrence of grunt-like signals among teleosts (Fish and Mowbray, 1970), the frequent association of grunts with agonistic encounters (Ladich et al., 2006), and the acute sensitivity of teleosts to signal duration (Bass and McKibben, 2003), androgen modulation of grunt duration may represent a species-wide, sex-independent mechanism to rapidly regulate vocal/ social signaling within the narrow time frame of aggressive encounters.
As we comment extensively elsewhere (Remage-Healey and Bass, 2007), the studies in midshipman fish may prove especially relevant to our understanding of the relationship between divergent endocrine profiles within a single sex and steroid actions at the level of neuronal networks for social behavior. This perspective may have important implications for the atypical neuroendocrine phenotypes associated with intrasexual divergence in social behavior among tetrapods (Rhen and Crews 2002; Roselli et al., 2004), including humans and other primates (Gladue et al., 1984; Maggioncalda et al., 2002; Morris et al., 2004). Divergent androgen mechanisms on CPGs may be particularly important, since the rapid actions of 11kT and testosterone appear to be highly steroid-specific in midshipman (Remage-Healey and Bass, 2007). With the foundation of these studies, there now exists the opportunity to examine how other steroid-sensitive, rhythmically-active circuits, ranging from those controlling GnRH release (Sisk and Foster, 2004) to copulatory behavior (e.g., see Cohen et al., 1985; Murphy and Hoffman, 2001; Morris et al., 2004) and the electric organ discharges of weakly electric fish (Bass and Zakon, 2005), are acutely regulated by androgenic steroid hormones.
Acknowledgments
Research support during preparation of this review from NSF IOB-0516748 (AHB) and NIH NRSA F32NS058009-01 (LRH). Given the especially wide scope of this essay, the reference list is by no means exhaustive and reviews were cited wherever possible. The authors thank Cynthia Jordan and Lydia DonCarlos for their generous invitation to contribute to this special issue; E. Brenowitz for the comments on the text; H. Rhodes for Fig. 4; M. Marchaterre and K. Rohmann for the assistance with the figures; L. Miller and T. Natoli for their help with the references.
References
- Arnold AP, Nottebohm F, Pfaff DW. Hormone concentrating cells in vocal control and other areas of the brain of the zebra finch (Poephila guttata) J Comp Neurol. 1976;165:487–512. doi: 10.1002/cne.901650406. [DOI] [PubMed] [Google Scholar]
- Ashmore RC, Wild JM, Schmidt MF. Brainstem and forebrain contributions to the generation of learned motor behaviors for song. J Comp Neurol. 2005;25:8543–8554. doi: 10.1523/JNEUROSCI.1668-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front Neuroendocrinol. 2002;23:137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- Bass AH. The evolution of vertebrate motor systems for acoustic and electric communication: peripheral and central elements. Brain Behav Evol. 1989;33:237–247. doi: 10.1159/000115931. [DOI] [PubMed] [Google Scholar]
- Bass AH. Dimorphic male brains and alternative reproductive tactics in a vocalizing fish. Trends Neurosci. 1992;15:139–145. doi: 10.1016/0166-2236(92)90356-d. [DOI] [PubMed] [Google Scholar]
- Bass AH. Alternative life history strategies and dimorphic males in an acoustic communication system. Fifth Int. Symp. Reprod. Physiol. Fish FishSymp; 1995. pp. 258–260. [Google Scholar]
- Bass AH. Shaping brain sexuality. Am Sci. 1996;84:352–363. [Google Scholar]
- Bass AH. Steroid-dependent plasticity of vocal motor systems: novel insights from teleost fish. Brain Res Rev. doi: 10.1016/j.brainresrev.2007.04.006. in press. Electronic publication ahead of print. [DOI] [PubMed] [Google Scholar]
- Bass AH, Marchaterre MA. Sound-generating (vocal) motor system in a teleost fish (Porichthys notatus): sexual polymorphism in the ultrastructure of myofibrils. J Comp Neurol. 1989;286:141–153. doi: 10.1002/cne.902860202. [DOI] [PubMed] [Google Scholar]
- Bass AH, Baker R. Sexual dimorphisms in the vocal control system of a teleost fish: morphology of physiologically identified cells. J Neurobiol. 1990;21:1155–1168. doi: 10.1002/neu.480210802. [DOI] [PubMed] [Google Scholar]
- Bass AH, Baker R. Adaptive modification of homologous vocal control traits in teleost fishes. Brain Behav Evol. 1991;38:240–254. doi: 10.1159/000114391. [DOI] [PubMed] [Google Scholar]
- Bass AH, Baker R. Phenotypic speciation of hindbrain rhombomeres and the origins of rhythmic circuits in vertebrates. Brain Behav Evol. 1997;50:3–16. doi: 10.1159/000113351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog Neurobiol. 2003;69:1–26. doi: 10.1016/s0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Bass AH, Zakon HH. Vocal and electric fish: at the crossroads of neuroethology and behavioral neuroendocrinology. Horm Behav. 2005;48:360–372. doi: 10.1016/j.yhbeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Bass AH, Forlano PM. Neuroendocrine mechanisms of alternative reproductive tactics: the chemical language of social plasticity. In: Oliveira RF, Taborsky M, Brockmann J, editors. Alternative Tactics — An Integrative Approach. Cambridge Univ. Press; 2008. pp. 109–131. [Google Scholar]
- Bass AH, Segil N, Kelley DB. A steroid-sensitive electromotor pathway in mormyrid fish: steroid autoradiography and receptor biochemistry. J Comp Physiol. 1986;159:535–544. doi: 10.1007/BF00604173. [DOI] [PubMed] [Google Scholar]
- Bass AH, Marchaterre MA, Baker R. Vocal-acoustic pathways in a teleost fish. J Neurosci. 1994;14:4025–4039. doi: 10.1523/JNEUROSCI.14-07-04025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH, Horvath BJ, Brothers E. Nonsequential developmental trajectories lead to simorphic vocal circuitry for males with alternative reproductive tactics. J Neurobiol. 1996;30:493–504. doi: 10.1002/(SICI)1097-4695(199608)30:4<493::AID-NEU5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Bass AH, Bodnar DA, Marchaterre MA. Complementary explanations for existing phenotypes in an acoustic communication system. In: Hauser M, Konishi M, editors. Neural Mechanisms of Communication. Chapter 17 MIT Press; 1999. pp. 493–514. [Google Scholar]
- Bass AH, Bodnar DA, Marchaterre MA. Midbrain acoustic circuitry in a vocalizing fish. J Comp Neurol. 2000;419:505–531. doi: 10.1002/(sici)1096-9861(20000417)419:4<505::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bass AH, Gilland E, Baker R. Program No 1001.5 2005. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2005a. Early development of teleost vocal circuitry. Online. [Google Scholar]
- Bass AH, Rose GJ, Pritz MB. Auditory midbrain of fish, amphibians and reptiles: model systems for understanding auditory function. In: Winer JW, Schreiner CE, editors. The Inferior Colliculus. Springer-Verlag; New York: 2005b. pp. 459–492. [Google Scholar]
- Belle MD, Tsutsui K, Lea RW. Sex steroid communication in the ring dove brain during courtship. Can J Physiol Pharmacol. 2003;81:359–370. doi: 10.1139/y03-036. [DOI] [PubMed] [Google Scholar]
- Bentley PJ. Comparative vertebrate endocrinology. Cambridge Univ. Press; Cambridge: 1998. [Google Scholar]
- Blaustein JD. Neuronal steroid hormone receptors: they're not just for hormones anymore. Endocrinol. 2004;145:1075–1081. doi: 10.1210/en.2003-1485. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Animal communication. Sinauer Assoc., Inc.; Sunerland, Mass: 1998. [Google Scholar]
- Brantley RK, Bass AH. Alternative male spawning tactics and acoustic signaling in the plainfin midshipman fish, Porichthys notatus. Ethology. 1994;96:213–232. [Google Scholar]
- Brantley RK, Wingfield J, Bass AH. Hormonal bases for male teleost dimorphisms: sex steroid levels in Porichthys notatus, a fish with alternative reproductive tactics. Horm Behav. 1993a;27:332–347. doi: 10.1006/hbeh.1993.1025. [DOI] [PubMed] [Google Scholar]
- Brantley RK, Marchaterre MA, Bass AH. Androgen effects on vocal muscle structure in a teleost fish with inter and intrasexual dimorphisms. J Morphol. 1993b;216:305–318. doi: 10.1002/jmor.1052160306. [DOI] [PubMed] [Google Scholar]
- Capranica RR. The untuning of the tuning curve: is it time? Neurosciences. 1992;4:401–408. [Google Scholar]
- Cohen MJ, Winn HE. Electrophysiological observations on hearing and sound production in the fish, Porichthys notatus. J Exp Zool. 1967;165:355–369. doi: 10.1002/jez.1401650305. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Schwartz-Giblin S, Pfaff DW. The pudendal nerve-evoked response in axial muscle. Exp Brain Res. 1985;61:175–185. doi: 10.1007/BF00235633. [DOI] [PubMed] [Google Scholar]
- Connaughton MA, Taylor MH. Effects of exogenous testosterone on vocal muscle mass in the weakfish, Cynoscion regalis. Gen Comp Endocrinol. 1995;100:238–245. doi: 10.1006/gcen.1995.1153. [DOI] [PubMed] [Google Scholar]
- De Vries G. Sex differences in adult and developing brains; compensation, compensation, compensation. Endocrinol. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- Demski LS, Gerald JW. Sound production evoked by electrical stimulation of the brain in toadfish (Opsanus beta) Anim Behav. 1972;20:507–513. doi: 10.1016/s0003-3472(72)80015-0. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nature. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine ML, Perini MA. Sound production evoked by electrical stimulation of the forebrain in the oyster toadfish. J Comp Phys A. 1994;174:173–185. doi: 10.1007/BF00193784. [DOI] [PubMed] [Google Scholar]
- Fine ML, Johnson MS, Matt DW. Seasonal variation in androgen levels in the oyster toadfish. Copeia. 2004;2:235–244. [Google Scholar]
- Fine ML, Chen FA, Keefer DA. Autoradiographic localization of dihydrotestosterone and testosterone concentrating neurons in the brain of the oyster toadfish. Brain Res. 1996;709:65–80. doi: 10.1016/0006-8993(95)01275-3. [DOI] [PubMed] [Google Scholar]
- Fish MP, Mowbray WH. Sounds of Western North Atlantic fishes. Johns Hopkins; Baltimore and London: 1970. [Google Scholar]
- Forlano PM, Bass AH. Seasonal plasticity of brain aromatase mRNA expression in glia: divergence across sex and vocal phenotypes. J Neurobiol. 2005a;65:37–49. doi: 10.1002/neu.20179. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Bass AH. Steroid regulation of brain aromatase expression in glia: female preoptic and vocal motor nuclei. J Neurobiol. 2005b;65:50–58. doi: 10.1002/neu.20178. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Myers DA, Bass AH. Neuroanatomical basis for high aromatase levels in teleost fish: aromatase enzyme and mRNA expression identify glia as source. J Neurosci. 2001;21:8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J Comp Neurol. 2005a;483:91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Marchaterre MA, Deitcher DL, Bass AH. Program No 1001.6. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Soc. Neurosci.; 2005b. Distribution of androgen receptor mRNA in vocal and non-vocal circuitry of a teleost fish. Online. [Google Scholar]
- Frost DR. Amer. Mus. Nat. Hist.; New York: 2006. Amphibian species of the world. http://research.amnh.org/herpetology/amphibia/index.php. [Google Scholar]
- Fu MF, Wang CG, Zhang XP, Pestell RG. Acetylation of nuclear receptors in cellular growth and apoptosis. Biochem Pharmacol. 2004;68:1199–1208. doi: 10.1016/j.bcp.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Gahr M. Neural song control system of hummingbirds: comparison to swifts, vocal learning (songbirds) and nonlearning (suboscines) passerines, and vocal learning (budgerigars) and nonlearning (dove, owl, gull, quail, chicken) nonpasserines. J Comp Neurol. 2000;426:182–196. [PubMed] [Google Scholar]
- Gahr M. Distribution of sex steroid hormone receptors in the avian brain: functional implications for neural sex differences and sexual behaviors. Microsc Res Tech. 2001;55:1–11. doi: 10.1002/jemt.1151. [DOI] [PubMed] [Google Scholar]
- Gans C. Biomechanics. Lippincott, Inc.; Philadelphia: 1974. [Google Scholar]
- Getting PA. Emerging principles governing the operation of neural networks. Ann Rev Neurosci. 1989;12:185–204. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- Gladue BA, Green R, Hellman RE. Neuroendocrine response to estrogen and sexual orientation. Science. 1984;225:1496–1499. doi: 10.1126/science.6089349. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Forebrain peptide modulation of sexually polymorphic vocal motor circuitry. Nature. 2000;403:769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. J Comp Neurol. 2002;448:298–321. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Bass AH. Isotocin distributions in vocal fish: relationship to vasotocin and vocal-acoustic circuitry. J Comp Neurol. 2003;462:1–14. doi: 10.1002/cne.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nature. 2003;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Jürgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Jürgens U, Hage SR. On the role of the reticular formation in vocal pattern generation. Behav Brain Res. 2006;182:308–314. doi: 10.1016/j.bbr.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Kelley DB. Neuroeffectors for vocalization in Xenopus laevis: hormonal regulation of sexual dimorphism. J Neurobiol. 1986;17:231–248. doi: 10.1002/neu.480170307. [DOI] [PubMed] [Google Scholar]
- Kelley DB. Hormonal regulation of motor output in amphibians: Xenopus laevis vocalizations as a model system. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain, and Behavior. Vol. 2. Academic Press; New York: 2002. pp. 445–468. [Google Scholar]
- Kelley DB, Brenowitz E. Hormonal influences on courtship behaviors. In: Becker J, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral endocrinology. MIT Press; Cambridge, MA: 2002. pp. 289–329. [Google Scholar]
- Kim YH, Perlman WR, Arnold AP. Expression of androgen receptor mRNA in zebra finch song system: developmental regulation by estrogen. J Comp Neurol. 2004;469:535–547. doi: 10.1002/cne.11033. [DOI] [PubMed] [Google Scholar]
- Kittelberger JM, Land BR, Bass AH. The midbrain periaqueductal gray and vocal patterning in a teleost fish. J Neurophysiol. 2006;96:71–85. doi: 10.1152/jn.00067.2006. [DOI] [PubMed] [Google Scholar]
- Knapp R. Endocrine mediation of vertebrate male alternative reproductive tactics: the next generation of studies. Integr Comp Biol. 2004;43:658–668. doi: 10.1093/icb/43.5.658. [DOI] [PubMed] [Google Scholar]
- Knapp R, Neff BD. Steroid hormones in bluegill, a species with male alternative reproductive tactics including female mimicry. Biol Lett. 2007 doi: 10.1098/rsbl.2007.0379. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp R, Wingfield JC, Bass AH. Steroid hormones and paternal care in the midshipman fish (Porichthys notatus) Horm Behav. 1999;35:81–89. doi: 10.1006/hbeh.1998.1499. [DOI] [PubMed] [Google Scholar]
- Knapp R, Marchaterre MM, Bass AH. Relationship between courtship behavior and steroid hormone levels in parental male plainfin midshipman fish. Horm Behav. 2001;39:335. [Google Scholar]
- Ladich F, Bass AH. Vocal/vocal motor pathways in piranhas (Characidae) with a comparison to other teleosts. Brain Behav Evol. 2005;66:167–176. doi: 10.1159/000087157. [DOI] [PubMed] [Google Scholar]
- Ladich F, Collin SP, Moller P, Kapoor BG, editors. Communication in fishes. Vol. 1. Science Pubs.; Enfield, NH: 2006. [Google Scholar]
- Lee JSF, Bass AH. Differential effects of 11-ketotestosterone on dimorphic traits in a teleost with alternative male reproductive morphs. Horm Behav. 2005;47:523–531. doi: 10.1016/j.yhbeh.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Lee JSF, Bass AH. Dimorphic male midshipman fish: reduced sexual selection or sexual selection for reduced characters? Behav Ecol. 2006;17:670–675. [Google Scholar]
- Leonardo A, Fee MS. Ensemble coding of vocal control in birdsong. J Neurosci. 2005;25:652–661. doi: 10.1523/JNEUROSCI.3036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggioncalda AN, Czekala NM, Sapolsky RM. Male orangutan subadulthood: a new twist on the relationship between chronic stress and developmental arrest. Am J Phys Anthropol. 2002;118:25–32. doi: 10.1002/ajpa.10074. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Cur Biol. 2001;11:R986–R996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;3:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Perkel DJ, Brenowitz EA. Seasonal changes in intrinsic electrophysiological activity of song control neurons in wild song sparrows. J Comp Physiol A. 2007;193:677–683. doi: 10.1007/s00359-007-0222-1. [DOI] [PubMed] [Google Scholar]
- Modesto T, Canario AV. Morphometric changes and sex steroid levels during the annual reproductive cycle of the lusitanian toadfish, Halobatrachus didactylus. Gen Comp Endocrinol. 2003;13:220–231. doi: 10.1016/s0016-6480(03)00027-3. [DOI] [PubMed] [Google Scholar]
- Moore FL, Boyd SK, Kelley DB. Hormonal regulation of behaviors in amphibians. Horm Behav. 2005;48:373–383. doi: 10.1016/j.yhbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Morrell JI, Kelley DB, Pfaff DW. Sex steroid binding in the brains of vertebrates. In: Knigge KM, Scott DE, Kobayashi H, Miura-shi S, editors. Brain-Endocrine Interaction II. The Ventricular System. S. Karger AG; Basel: 1975. pp. 230–256. [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nature Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Murphy AZ, Hoffman GE. Distribution of gonadal steroid receptor-containing neurons in the preoptic-periaqueductal gray-brainstem pathway: a potential circuit for the initiation of male sexual behavior. J Comp Neurol. 2001;438:191–212. doi: 10.1002/cne.1309. [DOI] [PubMed] [Google Scholar]
- Nelson JS. Fishes of the World. Wiley; New York: 1994. [Google Scholar]
- Northcutt RG. The forebrain of gnathostomes: in search of a morphotype. Brain Behav Evol. 1995;46:275–318. doi: 10.1159/000113279. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary (Serinus canarius) J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Westneat M, Hoese W. Birdsong: motor function and the evolution of communication. Sem Neurosci. 1992;4:385–390. [Google Scholar]
- Oliveira RF, Ros AFH, Goncalves DM. Intra-sexual variation in male reproduction in teleost fish: a comparative approach. Horm Behav. 2005;48:430–439. doi: 10.1016/j.yhbeh.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Olsson PE, Berg AH, von Hofsten J, Grahn B, Hellqvist A, Larsson A, Karlsson J, Modig C, Borg B, Thomas P. Molecular cloning and characterization of a nuclear androgen receptor activated by 11-ketotestosterone. Reprod Biol Endocrinol. 2005;3:37–54. doi: 10.1186/1477-7827-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuki A, Somiya H. Innervation of vocal muscles in teleosts: occipital vs. spinal nerves. Brain Behav Evol. 2007;69:132–141. doi: 10.1159/000095202. [DOI] [PubMed] [Google Scholar]
- Pappas GD, Bennett MVL. Specialized junctions involved in electrical transmission between neurons. Ann NY Acad Sci. 1966;137:495–508. doi: 10.1111/j.1749-6632.1966.tb50177.x. [DOI] [PubMed] [Google Scholar]
- Park KH, Meitzen J, Moore IT, Brenowitz EA, Perkel DJ. Seasonal-like plasticity of spontaneous firing rate in a songbird pre-motor nucleus. J Neurobiol. 2005;64:181–191. doi: 10.1002/neu.20145. [DOI] [PubMed] [Google Scholar]
- Perez J, Cohen MA, Kelley DB. Androgen receptor mRNA expression in Xenopus laevis CNS: sexual dimorphism and regulation in laryngeal motor nucleus. J Neurobiol. 1996;30:556–568. doi: 10.1002/(SICI)1097-4695(199608)30:4<556::AID-NEU10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Potter KA, Bose T, Yamaguchi A. Androgen-induced vocal transformation in adult female African clawed frogs. J Neurophysiol. 2005;94:415–428. doi: 10.1152/jn.01279.2004. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Mello CV, Jarvis ED. Songbirds and the revised avian brain nomenclature. Ann NY Acad Sci. 2004;1016:77–108. doi: 10.1196/annals.1298.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Simultaneous, rapid, elevations in steroid hormones and vocal signaling during playback challenge: a field experiment in Gulf toadfish. Horm Behav. 2005;47:297–305. doi: 10.1016/j.yhbeh.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. A rapid, neuromodulatory role for steroid hormones in the control of reproductive behavior. Brain Res. 2006a;1126:27–35. doi: 10.1016/j.brainres.2006.06.049. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. From social behavior to neurons: steroid hormones rapidly modulate advertisement calling via a vocal pattern generator. Horm Behav. 2006b;50:432–441. doi: 10.1016/j.yhbeh.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci. 2007;27(5):1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T, Crews D. Variation in reproductive behaviour within a sex: neural systems and endocrine activation. J Neuroendocrinol. 2002;14:517–531. doi: 10.1046/j.1365-2826.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- Rhodes HJ, Yu HJ, Yamaguchi A. Xenopus vocalizations are controlled by a sexually differentiated hindbrain central pattern generator. J Neurosci. 2007;27:1485–1497. doi: 10.1523/JNEUROSCI.4720-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ. Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol. 2005;26:65–84. doi: 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Roselli CR, Larkin K, Schrunk JM, Stormshak F. Sexual partner preference, hypothalamic morphology and aromatase in rams. Physiol Behav. 2004;83:233–245. doi: 10.1016/j.physbeh.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Brenowitz E. Neural and hormonal control of birdsong. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain, and Behavior. Vol. 2. Academic Press; New York: 2002. pp. 799–839. [Google Scholar]
- Schlinger BA, Greco C, Bass AH. Aromatase activity in hindbrain vocal control region: divergence between “singing” and “sneaking” males. Proc Roy Soc, Series B (London) 1999;266:131–136. [Google Scholar]
- Schmidt RS. Neural correlates of frog calling. Isolated brainstem. J Comp Physiol. 1976;108:99–113. [Google Scholar]
- Schmidt RS. Development of anuran calling circuits: effect of testosterone propionate injections. Gen Comp Endocrinol. 1980;41:80–83. doi: 10.1016/0016-6480(80)90035-0. [DOI] [PubMed] [Google Scholar]
- Schmidt RS. Neural correlates of frog calling. Masculinization by androgens. Horm Behav. 1983;17:94–102. doi: 10.1016/0018-506x(83)90019-3. [DOI] [PubMed] [Google Scholar]
- Schmidt RS. Neural correlates of frog calling: production by two semi-independent generators. Behav Brain Res. 1992;50:17–30. doi: 10.1016/s0166-4328(05)80284-0. [DOI] [PubMed] [Google Scholar]
- Simerly R, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–85. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolesscence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisneros J, Forlano P, Knapp R, Bass AH. Seasonal variation of steroid hormone levels in an intertidal-nesting fish, the vocal plainfin midshipman. Gen Comp Endocrinol. 2004;136:101–116. doi: 10.1016/j.ygcen.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Skoglund CR. Functional analysis of swim-bladder muscles engaged in sound production of the toadfish. J Biophys Biochem Cytol. 1961;10:187–200. doi: 10.1083/jcb.10.4.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis MM, Perkel DJ. Rhythmic activity in a forebrain vocal control nucleus in vitro. J Neurosci. 2005;25:2811–2822. doi: 10.1523/JNEUROSCI.5285-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturdy CB, Wild JM, Mooney R. Respiratory and telencephalic modulation of vocal motor neurons in the Zebra Finch. J Neurosci. 2003;23:1072–1086. doi: 10.1523/JNEUROSCI.23-03-01072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavolga WN. Sound production and detection. In: Hoar WS, Randall DJ, editors. Fish Physiology. Vol. 5. Academic, Elsevier; New York: 1971. pp. 135–205. [Google Scholar]
- Thomas P, Dressing G, Pang Y, Berg H, Tubbs C, Benninghoff A, Doughty K. Progestin, estrogen and androgen G-protein coupled receptors in fish gonads. Steroids. 2006;71:310–316. doi: 10.1016/j.steroids.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Tobias ML, Kelley DB. Vocalizations by a sexually dimorphic isolated larynx: peripheral constraints on behavioral expression. J Neurosci. 1987;7:3191–3197. doi: 10.1523/JNEUROSCI.07-10-03191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Vasilakos K, Wilson RJA, Kimura N, Remmers JE. Ancient gill and lung oscillators may generate the respiratory rhythm of frogs and rats. J Neurobiol. 2004;62:369–385. doi: 10.1002/neu.20102. [DOI] [PubMed] [Google Scholar]
- Walsh PW, Mommsen TP, Bass AH. Biochemical and molecular aspects of singing in batrachoidid fishes. In: Hochachka PW, Mommsen TP, editors. Biochemistry and Molecular Biology of Fishes. Metabolic Biochemistry. Vol. 4. 1995. pp. 279–289. [Google Scholar]
- Weiser M, Bennett MVL, Baker R. Toadfish vocal motor system. III. Localization of the command nucleus. Biol Bull. 1986;171:498. [Google Scholar]
- Wetzel DM, Haerter UL, Kelley DB. A proposed neural pathway for vocalization in South African clawed frogs, Xenopus laevis. J Comp Physiol A. 1985;157:749–761. doi: 10.1007/BF01350072. [DOI] [PubMed] [Google Scholar]
- White SA, Livingston FS, Mooney R. Androgens modulate NMDA receptor-mediated EPSCs in the Zebra Finch song system. J Neurophysiol. 1999;82:221–2234. doi: 10.1152/jn.1999.82.5.2221. [DOI] [PubMed] [Google Scholar]
- Wild M. Functional neuroanatomy of the sensorimotor control of singing. Ann NY Acad Sci. 2004;1016:438–462. doi: 10.1196/annals.1298.016. [DOI] [PubMed] [Google Scholar]
- Winn HE, Stout JF. Sound production by the satinfin shiner, Notropis analostanus, and related fishes. Science. 1960;132:222–223. doi: 10.1126/science.132.3421.222. [DOI] [PubMed] [Google Scholar]
- Yager DD. A unique sound production mechanism in the pipid anuran Xenopus borealis. Zool J Linn Soc. 1992;104:351–375. [Google Scholar]
- Yamaguchi A, Kaczmarek LK, Kelley DB. Functional specialization of male and female motoneurons. J Neurosci. 2003;23:11568–11576. doi: 10.1523/JNEUROSCI.23-37-11568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond RE, Nottebohm F, Pfaff DW. Androgen-concentrating cells in the midbrain of a songbird. Science. 1973;179:1005–1007. doi: 10.1126/science.179.4077.1005. [DOI] [PubMed] [Google Scholar]
- Zornik E, Kelley DB. Breathing and calling: neuronal networks in the Xenopus laevis hindbrain. J Comp Neurol. 2007;501:303–315. doi: 10.1002/cne.21145. [DOI] [PubMed] [Google Scholar]


