Abstract
Bcl-2 and other closely related members of the Bcl-2 family of proteins inhibit the death of neurons and many other cells in response to a wide variety of pathogenic stimuli. Bcl-2 inhibition of apoptosis is mediated by its binding to pro-apoptotic proteins, e.g., Bax and tBid, inhibition of their oligomerization, and thus inhibition of mitochondrial outer membrane pore formation, through which other pro-apoptotic proteins, e.g., cytochrome c, are released to the cytosol. Bcl-2 also exhibits an indirect antioxidant activity caused by a sub-toxic elevation of mitochondrial production of reactive oxygen species and a compensatory increase in expression of antioxidant gene products. While classic approaches to cytoprotection based on Bcl-2 family gene delivery have significant limitations, cellular protein transduction represents a new and exciting approach utilizing peptides and proteins as drugs with intracellular targets. The mechanism by which proteins with transduction domains are taken up by cells and delivered to their targets is controversial but usually involves endocytosis. The effectiveness of transduced proteins may therefore be limited by their release from endosomes into the cytosol.
Keywords: Apoptosis, Bcl-2, mitochondria, protein transduction, endocytosis
Bcl-2 AND NEUROPROTECTION
Bcl-2 is a 26 kDa protein that protects many different types of cells from death caused by a wide variety of insults. Although generally described as an anti-apoptotic protein, we and other investigators find it to also be effective against rapid, necrotic cell death, including that associated with in vitro ischemia (chemical hypoxia plus glucose deprivation) (Myers et al., 1995; Kane et al., 1995). Bcl-2 is a membrane protein located at mitochondria, the endoplasmic reticulum and the nuclear envelope. Bcl-2 is expressed at low basal levels in neurons in the adult brain (Merry and Korsmeyer, 1997), but is induced in many neurons following ischemia (Chen et al., 1995), and is elevated in aged animals (Kaufmann et al., 2001). Upregulation of Bcl-2 following brief, sublethal cerebral ischemia increases the resistance of neurons to a subsequent longer period of ischemia (Shimizu et al., 2001). In addition, Bcl-2 overexpression in neurons ameliorates cerebral ischemic injury (Martinou et al., 1994). Bcl-2 gene deletion (Hata et al., 1999) or antisense treatment increases brain damage following ischemia/reperfusion (Chen et al., 2000). Studies demonstrating that delivery of the bcl-2 gene to the brain via Adenoviral or Herpes virus vectors, or by liposome-mediated transfer, reduces the severity of ischemic injury suggest that Bcl-2 could be used therapeutically (Shimazaki et al., 2000; Linnik et al., 1995).
Mitochondrial Mechanisms of Action of Anti-Apoptotic Bcl-2 Family Proteins
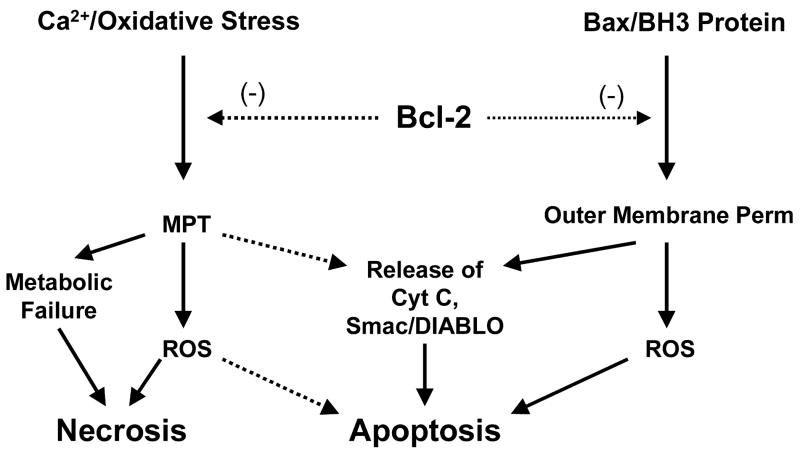
Murphy et al. (1996a, 1996b) were the first to report a direct effect of Bcl-2 on mitochondrial function, namely that overexpression increases mitochondrial Ca2+ buffering capacity and protects against Ca2+-induced mitochondrial respiratory dysfunction (Murphy et al., 1996a). These observations were extended to demonstrate protection by Bcl-2 against cell death caused by elevated intracellular Ca2+ (Murphy and Fiskum, 1999). Kowaltowski and Fiskum then demonstrated inhibition by Bcl-2 of the mitochondrial inner membrane permeability transition (MPT) induced by Ca2+ together with oxidative stress elicited by the addition of hydroperoxides (Kowaltowski et al., 2000). The MPT results in uncoupling of oxidative phosphorylation, mitochondrial swelling, and release of cytochrome c through the disrupted outer membrane. Bcl-2 inhibits the MPT through its influence over NAD(P)H and glutathione redox state as it is not effective when the MPT is activated by direct chemical oxidation of protein thiols (Fig. 1) (Kowaltowski et al., 2000). This effect may be specific for neural cells, as it was not observed in liver mitochondria from Bcl-2 transgenic mice (Yang et al., 2000). The redox mode of MPT inhibition by Bcl-2 is consistent with the findings that Bcl-2 overexpressing neural cells display a relatively reduced redox state and a resistance to mitochondrial injury and cell death induced by oxidative/metabolic stress (Myers et al., 1995; Murphy et al., 1996b; Ellerby et al., 1996). When such stress is excessive, Bcl-2 protects against acute necrotic cell death, while when the duration of stress is limited, Bcl-2 protects against delayed, apoptotic cell death (Myers et al., 1995). While for many years the MPT was widely touted as a pro-apoptotic event (Zamzami and Kroemer, 2001), recent results obtained with tissues from knockout animals that do not express the MPT-associated protein cyclophilin D indicate that MPT is important for necrotic but not for many forms of apoptotic cell death (Nakagawa et al., 2005; Basso et al., 2005).
Fig. 1.
Mitochondrial mechanisms of cytoprotection by Bcl-2. Expression of Bcl-2 causes upregulation of antioxidant gene expression resulting in protection from oxidative stress. One consequence of this form of protection is inhibition of the mitochondrial permeability transition (MPT), thus protection cells against MPT-induced metabolic failure, generation of reactive O2 species (ROS), and rupture of the outer membrane resulting in loss of cytochrome c, Smac/DIABLO, and other intermembrane pro-apoptotic proteins. In addition, by binding to Bax and BH3-only proteins, e.g., tBid, Bcl-2 inhibits mitochondrial outer membrane pore formation, thereby inhibiting release of apoptotic proteins and mitochondrial ROS formation.
In contrast to the mechanism by which Bcl-2 protects against Ca2+-induced mitochondrial cytochrome c release, we and other investigators found that inhibition by Bcl-2 of cytochrome c release induced by Bax in the presence of BH3 death domain only proteins, e.g., tBid, is independent of MPT inhibition (Polster et al., 2001; Jurgensmeier et al., 1998). Bax-mediated cytochrome c release occurs by a selective increase in the permeability of the mitochondrial outer membrane with no necessity for an increase in inner membrane permeability (Polster et al., 2001). Bax oligomerization and insertion in the outer membrane results in the formation of pores sufficiently large to allow the escape of cytochrome c, SMAC-Diablo, and other pro-apoptotic proteins from the mitochondrial intermembrane space into the cytosol. The Bax mechanism of release is insensitive to changes in mitochondrial redox state (Fiskum et al., 2000), whereas the MPT mechanism is highly sensitive to activation by an oxidized shift in redox state.
Considerable evidence indicates that Bcl-2 inhibits Bax-mediated cytochrome c release by heterodimerizing with Bax via the BH3 “death domain” common to all Bcl-2 family proteins and by sequestering BH3 only proteins (Shangary and Johnson, 2002). Different BH3 domain only proteins have different affinities for Bcl-2 and Bcl-XL (Chen et al., 2005) and some, e.g., BimEL, may stimulate Bax-induced cytochrome c release independent of their ability to interact with Bcl-2 or Bcl-XL (Yamaguchi and Wang, 2002). According to a recent model, activator BH3-only proteins, e.g., tBid, can directly bind and induce Bax oligomerization and mitochondrial outer membrane permeabilization, while sensitisers (e.g., Bad) bind to Bcl-2 and disrupt its heterodimerization with Bax (Fig. 1) (Letai et al., 2002).
THERAPEUTIC TARGETING OF Bcl-2 FAMILY PROTEINS
Extensive experimental evidence supports the concept of targeting Bcl-2 family proteins to modulate cell death in diverse pathologic conditions such as cancer or acute and chronic neurodegeneration. Despite substantial progress in understanding the molecular mechanisms of action of Bcl-2 family proteins, translation of this knowledge into effective therapies is limited. While useful in experimental settings, the therapeutic use of gene-based strategies is currently limited. Poor delivery, unequal rates of expression, toxicity and safety are several problems associated with this approach (Ferber, 2001).
Several other approaches are pursued, mostly for targeting anti-apoptotic Bcl-2/Bcl-XL proteins, and hold promise for generating efficient therapeutic tools for many forms of cancer (reviewed in Reed and Pellecchia, 2005). An important recent development is the discovery of several classes of small-molecular inhibitors of Bcl-2 and Bcl-XL through structure-based computer database screening and high-throughput screening of small-molecule libraries (reviewed in O’Neill et al., 2004; Reed and Pellecchia, 2005).
Therapeutic delivery of “information-rich” macromolecular compounds is another promising approach explored for delivery of full-length Bcl-2 family proteins and delivery of related peptides. Delivery of biologically active peptides or proteins has until recently been limited by their poor bioavailability imposed by the plasma membrane barrier. Several strategies, including linkage to non-toxic fragments of cell-penetrating toxins and receptor ligands such as transferrin, and incorporation into liposomes or other lipid-based delivery systems, have been explored to overcome this barrier (Dalkara et al., 2004; Stenmark et al., 1991). Delivery of the anti-apoptotic Bcl-XL (Liu et al., 1999), or pro-apoptotic proteins such as Bad have been reported using these proteins as fusion proteins with diphtheria toxin (DT) fragments (Ichinose et al., 2002).
Protein transduction is a strategy developed during the last decade that allows the delivery of various membrane-impermeable cargoes in a receptor-independent manner in virtually any cell type, including primary cell cultures, and in vivo in various tissues including the brain (reviewed in Wadia and Dowdy, 2003; Joliot and Prochiantz, 2004). This new technology has a tremendous potential for overcoming the barrier imposed by the plasma membrane to deliver therapeutic macromolecules.
Protein Transduction
The concept of protein transduction originated from observations that certain proteins, e.g., the HIV-1 TAT (Frankel and Pabo, 1988; Green and Loewenstein, 1988) and the homeodomain protein Antennapedia (Antp) (Derossi et al., 1994) can enter cells in a receptor independent-manner. Based on work with homeodomain proteins, the concept of “messenger” proteins was proposed, according to which these proteins would regulate neighboring cells in a paracrine mode (Joliot and Prochiantz, 2004). The ability of Antp and TAT to enter cells was mapped to short domains, named protein transduction domains (PTD). PTDs, also known as cell-penetrating peptides (CPPs), are comprised of short basic peptides of various origins that can cross biological membranes. When fused to other molecules, some of these PTDs promote the delivery of attached cargoes (protein and non-protein) into cells. The highly cationic HIV-1 TAT-PTD, the third helix of Antennapedia homeodomain protein and polyarginine are the best characterized PTDs.
The TAT(47–57) PTD is a 10 aminoacids (YGRKKRRQRR) peptide derived from the human immunodefficiency virus (HIV)-1 transactivator of transcription (TAT) protein. In 1988, two groups reported independently that the HIV-1 TAT protein can translocate inside cells (Frankel and Pabo, 1988; Green and Loewenstein, 1988). Fawell et al. showed that heterologous proteins can be delivered into cells when chemically cross-linked with a 36 aminoacids peptide from HIV-1 TAT(37–72) and demonstrated the applicability of this approach to deliver proteins in vivo in mice (Fawell et al., 1994). Vives et al., determined that the basic domain (residues 49–60) in the HIV-1 TAT retains the transduction potential (Vives et al., 1997). Using the TAT(47–57) PTD, Dowdy’s group developed a convenient method for transduction of proteins based on a in frame fusion strategy that facilitated the delivery of a large variety of proteins. Delivery of large protein cargoes (up to 120 kDa) occurs both in cells, and in vivo in mice, and most tissues including the brain are transduced without toxic effects (Schwarze et al., 1999).
The third helix of the Drosophila melanogaster homeodomain protein Antennapedia also translocates inside cells in a receptor and energy-independent manner (Derossi et al., 1994). The 16 aminoacids transduction domain of Antennapedia (aminoacids 43–58; pAntp), also known as Penetratin, was used to deliver various cargoes such as peptides, proteins and anti-sense oligonucleotides (Dietz and Bahr, 2004; Joliot and Prochiantz, 2004). The Herpes simplex virus tegument protein VP22 is another cell translocating protein. Unlike the TAT-PTD and Antennapedia, VP22 mediates intercellular transport upon being secreted from cells (Elliott and O’Hare, 1997).
Numerous other PTDs have been reported and are derived from viral and cellular proteins or antibacterial peptides; synthetic CPPs have also been designed (Dietz and Bahr, 2004; Futaki, 2002). On the basis of observation that PTDs such as TAT-PTD and pAntp are highly enriched in basic aminoacids, especially arginine, arginine polymers of various lengths were tested and the R8 and R9 oligomers were found to transduce cells even more efficiently than the original TAT-PTD peptide (Wender et al., 2000; Futaki, 2002). To facilitate protein delivery without the need of covalent linkage to the cargo, Morris et al. designed the 21-mer Pep-1 peptide that can bind proteins and promote cellular transduction (Morris et al., 2001). One study indicated that Pep-1 is also efficient when used as a fusion protein and transduces superoxide dismutase into the brains of mice (Sik et al., 2004).
Transduction of both PTDs and attached cargoes was initially reported to occur rapidly (minutes) in a receptor and temperature-independent manner. Other studies examining the transduction of protein cargoes reported a much slower rate of uptake (hours) (Fittipaldi et al., 2003). PTD-mediated delivery is dose-dependent and cellular uptake occurs uniformly in close to 100% of the cells exposed. While transduction occurs into almost all cell types, internalization is not observed in some cell types (MDCK, CaCo-2) (Violini et al., 2002; Kramer and Wunderli-Allenspach, 2003). PTDs are usually used at μM concentrations (1–10 μM) and most applications with fused proteins use nM concentrations (~100 nM). Transduction of a biologically active modified Bcl-XL can occur even at pM concentrations (Asoh et al., 2002). Most of the PTDs lack toxic effects at these doses. Toxicity occurs only at very high doses of TAT basic domain and pAntp (Jia et al., 2001; Bolton et al., 2000) and might be a concern for CPPs derived from pore-forming antibacterial peptides (Takeshima et al., 2003).
The list of cargoes delivered and the number of potential applications is continually increasing, and was recently reviewed in detail by Dietz and Bahr (2004). Beside proteins, other notable examples of cargoes include plasmid DNA complexed with TAT monomers or TAT oligomers and antisense oligonucleotides. Other small non-protein cargoes such as drugs, doxorubicin, cyclosporin A or large cargoes such as liposomes, phage particles and even nanoparticles were also delivered. In addition, the TAT-PTD can facilitate viral-mediated gene expression (Dietz and Bahr, 2004).
MODULATION OF MITOCHONDRIA-DEPENDENT CELL DEATH THROUGH PROTEIN TRANSDUCTION
Delivery of Pro-Apoptotic and Anti-Apoptotic Peptides
The ability of PTDs to facilitate intracellular delivery has been utilized to modulate apoptotic cell death by transduction of small PTD-linked peptides. The BH3 domain, encompassing an amphipathic α-helix, is shared by all Bcl-2 family proteins and is required for the dimerization and death-inducing ability of pro-apoptotic Bcl-2 proteins, especially the BH3-only proteins. The BH3 domain of pro-apoptotic proteins binds to the hydrophobic groove created by the BH3, BH2 and BH1 domains of anti-apoptotic Bcl-2-like proteins (reviewed in Petros et al., 2004). Synthetic BH3 peptides (~16-mer) can mimic the functionality of BH3-only proteins, induce Bax/Bak oligomerization, and antagonize anti-apoptotic Bcl-2 and Bcl-XL proteins by disrupting their complexes with pro-apoptotic Bax and Bak. The functionality of several BH3 peptides was demonstrated in cells and in isolated mitochondria, where they are able to trigger the release of apoptogenic factors (Cyt C, EndoG, Smac/DIABLO and AIF) from the mitochondrial intermembrane space (Polster et al., 2001; Letai et al., 2002).
Unlike the BH3 peptides alone, BH3 domain peptides fused to TAT-PTD, pAntp or polyarginine are rapidly internalized into cells through protein transduction, and subsequently induce apoptosis (Letai et al., 2002; Holinger et al., 1999). While useful experimental tools, cell-penetrating BH3 peptides derived from pro-apoptotic Bcl-2 proteins are also explored as a potential treatment for various forms of cancer. Using a hydrocarbon stapling strategy, Korsmeyer’s group generated BH3 peptides with improved stability and affinity that are effective at inhibiting the growth of leukemia xenografts in vivo (Walensky et al., 2004). The stapled peptides are internalized through macropinocytosis, a mechanism shown recently to mediate internalization of the TAT-PTD (Wadia et al., 2004) and polyarginine (Nakase et al., 2004).
PTD-mediated delivery of small peptides is also used to inhibit apoptotic cell death. The BH4 domain, shared only by anti-apoptotic Bcl-2 proteins, is required for interaction with several non-Bcl-2 family proteins and for anti-apoptotic activity. Shimizu et al. reported that a synthetic peptide corresponding to the BH4 domain of Bcl-XL can enter cells and inhibit etoposide-induced apoptotic death when fused to TAT-PTD (Shimizu et al., 2000). TAT-BH4 is also effective in vivo and inhibits X-ray-induced apoptosis, Fas-induced fulminant hepatitis in mice, and ischemia-reperfusion injury in isolated rat heart (Sugioka et al., 2003; Chen et al., 2002). The BH4 domain of the anti-apoptotic Bcl-2 is also effective at protecting coronary endothelial cells against oxidative stress-induced death (Cantara et al., 2004).
Activation of the multidomain Bax/Bak proteins is required for activation of the intrinsic, mitochondria-dependent cell death pathway. Cells lacking both these proteins display long-term protection against multiple apoptotic stimuli (Wei et al., 2001). While protein–protein interaction and structural studies of Bcl-2 proteins provide a strong support for use of BH3 peptides as death inducers, no such candidate protein domains emerged, until recently, for inhibition of Bax/Bak activation. Recent studies revealed that Bax translocation from cytosol to mitochondria is suppressed by the DNA repair protein Ku70 (Sawada et al., 2003) and a similar role was demonstrated for the short peptide humanin (Guo et al., 2003). A pentapeptide derived from the Bax-binding sequence of Ku70, termed BIP (Bax inhibitory peptide), was tested for potential suppression of Bax activity. The BIP peptide is cell-permeable and protects against cell death induced by cytotoxic drugs (Sawada et al., 2003).
Humanin, a 24 aminoacids endogenous peptide, initially discovered as an inhibitor of amyloid-β induced neuronal death (Hashimoto et al., 2001), binds and stabilizes Bax in the cytosol in an inactive conformation (Guo et al., 2003). Reed’s group also demonstrated that in addition to Bax, humanin binds to and inhibits the pro-apoptotic activity of the BH3-only proteins Bid (Zhai et al., 2005) and Bim-EL (Luciano et al., 2005). The ability of humanin to target and antagonize the activity of multiple pro-apoptotic proteins makes it an attractive candidate for cytoprotection. These studies also demonstrated that polyarginine-mediated transduction of the humanin peptide into cells is effective at inhibiting Bid and BimEL-induced death (Zhai et al., 2005; Luciano et al., 2005).
Delivery of Bcl-2 Family Proteins
The anti-death Bcl-2 family members Bcl-2 and Bcl-XL inhibit apoptosis and are also effective at protecting against necrotic forms of cell death (Myers et al., 1995; Kane et al., 1995). Korsmeyer’s group proposed over a decade ago the rheostat model, according to which the anti-apoptotic Bcl-2/Bcl-XL bind and neutralize pro-apoptotic Bcl-2 family proteins and their relative balance determines cell death or survival (Korsmeyer et al., 1993). In addition to antagonizing the pro-apoptotic activity of multidomain Bax/Bak and to sequester BH3-only proteins (Bid, Bim, Bad and others), early studies indicated that the cytoprotective activity of Bcl-2 and more recently that of Bcl-XL and Mcl-1, is at least in part due to additional mechanisms including their ability to increase protection against oxidative stress (Hockenbery et al., 1993; Kowaltowski et al., 2000, 2004). The multitasking nature of Bcl-2 cytoprotective activity cannot be mimicked by small drugs or peptide domains. Therefore delivery of “information-rich” macromolecules, i.e., full-length proteins, should be the most effective approach at cytoprotection.
Several groups have employed protein transduction to deliver anti-apoptotic Bcl-2 family proteins into cultured cells and in vivo into the brain in models of neural cell death. A TAT-PTD fused Bcl-XL was efficiently transduced and protected retinal ganglion cells following optic nerve transsection (Dietz et al., 2002). This group also demonstrated that pretreatment with TAT-Bcl-XL by intravenous injection reduces ischemia/reperfusion brain injury in mice (Kilic et al., 2002). Using a TAT-fused Bcl-XL construct, Cao et al. also showed that transduction and neuroprotection can be achieved both in vitro and in vivo. In this study, a significant protection against brain ischemia/reperfusion was observed even when the protein was injected intraperitoneally after the ischemic period (Cao et al., 2002). Another study used TAT-mediated transduction of a mutated Bcl-XL (FNK) with increased anti-apoptotic activity and showed greater protection in cultured cells and in vivo against ischemia/reperfusion than that obtained with PTD-Bcl-XL (Asoh et al., 2002). The same approach was also used to deliver Bcl-XL to pancreatic islets (Embury et al., 2001) and FNK to chondrocytes in cartilage slice culture (Ozaki et al., 2004).
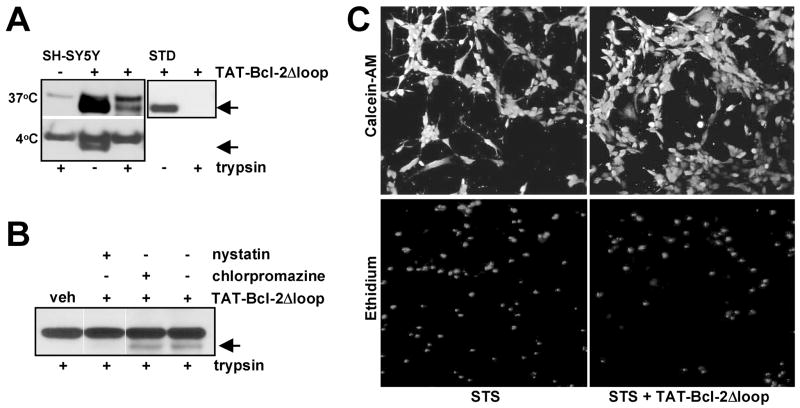
While these studies indicate that protein transduction of Bcl-2 family proteins or peptides could become an effective therapeutic tool, a detailed understanding of the mechanisms of intracellular delivery of functional proteins is needed, as several practical limitations have emerged. We recently explored the transduction of a modified Bcl-2 protein in neural cells, and investigated the mechanisms involved in intracellular delivery. For this purpose, a TAT-fusion protein was generated with a loop deleted Bcl-2 protein. Transducible TAT-Bcl-2Δloop protein confers significant protection in several neuronal cell lines against staurosporine or trophic factor withdrawal-induced death (Fig. 3 and unpublished results). Similar to recent findings from other laboratories, our results indicate that transduction of Bcl-2 in neuronal cell lines is mediated through an endocytotic pathway rather than through transduction mechanism.
Fig. 3.
Transduction of TAT-Bcl-2Δloop protein. (A) Transduction of TAT-Bcl-2Δloop was examined in SH-SY5Y neuroblastoma cells incubated with the protein (200 nM) for 1 h at 37° C or 4° C. TAT-Bcl-2Δloop uptake was examined by immunoblotting with an anti-Bcl-2 antibody and was detected as an additional band with a lower molecular weight than endogenous Bcl-2 (upper band). Trypsin treatment was performed at the end of incubation to eliminate the cell-surface bound protein and indicated that TAT-Bcl-2Δloop is internalized at 37° C but not at 4° C. A control protein (STD) was completely digested in the same conditions in the absence of cells. (B) The cells were pretreated with an inhibitor of clathrin-dependent endocytosis (chlorpromazine, 10 μM) or with the lipid raft disrupting agent nystatin (50 μg/ml) and the internalization of TAT-Bcl-2Δloop (100 nM; 1 h, 37° C) examined as in (A). Disruption of lipid rafts but not of clathrin-dependent endocytosis inhibited internalization of TAT-Bcl-2Δloop. (C) SH-SY5Y cells pretreated with TAT-Bcl-2Δloop (100 nM) were exposed to staurosporine (STS; 100 nM) for 18 h then cell survival examined by the live/dead assay by staining the cells with Calcein-AM (viable cells; upper panel) and ethidium homodimer (dead cells; lower panel). Pretreatment with TAT-Bcl-2Δloop resulted in significant protection (48.15%) against STS-induced death (n = 4, p < 0.05).
MECHANISM OF PTD-MEDIATED PROTEIN DELIVERY
Direct Membrane Translocation of PTDs
The mechanism of PTD-mediated transduction is not entirely understood. The full-length HIV-1 TAT enters cells through adsorptive endocytosis in a receptor-independent manner (Mann and Frankel, 1991). In contrast, internalization of TAT-PTD and pAntp involves a distinct process named “transduction”. Early reports indicated that transduction is effective even at 4° C, in the absence of endocytosis, and is energy and receptor-independent (Vives et al., 1997; Derossi et al., 1994). While it is known that polybasic peptides, e.g., lysine polymers, stimulate the uptake of cargoes into cells through adsorptive endocytosis (Ryser et al., 1978), the energy and receptor-independent characteristics of PTD uptake, appeared to distinguish transduction from most classic forms of internalization.
The numerous biochemical and biophysical studies aimed at elucidating the process of PTD internalization suggest that transduction is a multistep process initiated by interaction of CPPs with the cell surface (either with membrane lipids or negatively charged cell surface constituents). Binding is followed by internalization through either an undefined mechanism involving a direct membrane translocation step or by the better characterized process of endocytosis (Fig. 2). Although exhibiting diverse structures, most CPP share a high content of basic amino acids. The polycationic character of CPP is one of the most important structural requirements, as indicated by the correlation between the number of basic residues and efficiency of transduction (Wender et al., 2000). While positive charge is important, arginine appears to play a unique role. The guanidium group of arginine, rather than simply a positive charge, is required for efficient internalization, and replacement by an ammonium group reduces translocation (Wender et al., 2000). The uptake of oligoarginine is consistently more efficient than that of lysine or histidine oligomers (Mitchell et al., 2000). The presence of arginine may facilitate a transduction mechanism, while lysine-rich CPPs promote endocytosis (Zaro and Shen, 2003). Hydrophobicity and amphipathicity also play a role in transduction of some CPPs. Unlike the TAT-PTD and polyarginine, pAntp contains several hydrophobic residues. The central hydrophobic core composed of the W6, F7 aminoacids appears critical for transduction (Dom et al., 2003).
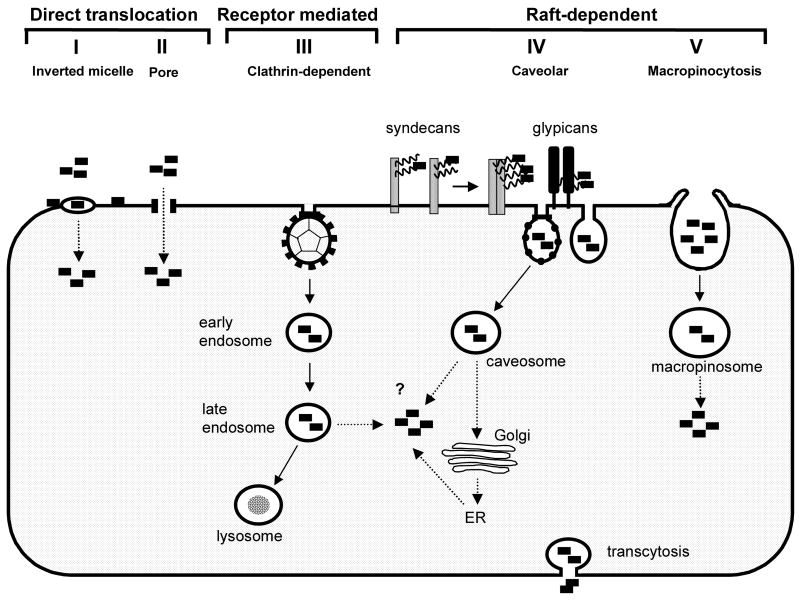
Fig. 2.
Mechanism of protein transduction. Direct membrane translocation. Cationic PTDs bind to negatively charged membrane lipids and translocate across plasma membrane into cytosol. Direct translocation occurs through either (I) transient formation of inverted micelles or (II) formation of a pore-like structure allowing direct cytosolic delivery. Endocytotic uptake. Binding to the negatively charged cell-surface HSPG (syndecans or glypicans) is followed by endocytotic uptake of PTDs through (III) a clathrin-dependent, receptor-mediated pathway or a lipid raft-dependent pathway. Lipid raft-dependent endocytosis can occur either through (IV) a caveolar/caveolar-like pathway or (V) through raft-dependent macropinocytosis. Cytosolic delivery of PTDs (dotted line) might involve a direct translocation process where in this case the mechanisms (I) and (II) might take place at the endosomal membrane. PTD-induced endosome disruption or constitutive endosomal leakage could also mediate cytosolic delivery. Another possible pathway involves retrograde transport to Golgi and ER with subsequent cytosolic release.
Several models have been proposed to explain the apparent ability of PTDs to translocate across cellular membranes (Fig. 2). Transient formation of inverted micelles initiated by binding of the basic PTDs to negatively charged membrane lipids is suggested for pAntp, TAT-PTD and Pep-1 (Derossi et al., 1994; Vives et al., 1997; Henriques and Castanho, 2004). Another mechanism involves formation of a pore allowing passage of small peptides through membranes, as proposed for Pep-1 and for CPPs derived from pore-forming antimicrobial peptides (Deshayes et al., 2004; Takeshima et al., 2003). However, PTDs such as TAT-PTD and polyarginine are highly charged and do not contain hydrophobic residues required for formation of inverted micelles. It is also difficult to explain through these models the internalization of very large cargoes as shown for the TAT-PTD. While TAT-PTD can interact with negatively charged lipids, binding to negatively charged heparan sulfate proteoglycans (HSPG) at the cell surface appears more likely (Ziegler et al., 2003). Partial insertion of pAntp into lipid membranes is also documented (Joliot and Prochiantz, 2004). Despite evidence for binding to membrane lipids, actual translocation across model lipid membranes or intact plasma membrane of cells was not observed for TAT-PTD (Kramer and Wunderli-Allenspach, 2003). For pAntp and for Pep-1, a few studies support a direct translocation model (Dom et al., 2003), while others indicate that actual internalization occurs through endocytosis (Drin et al., 2003).
PTD-Mediated Endocytosis
Studies performed in living cells without fixation indicate that contrary to initial reports, PTDs (TAT-PTD, polyarginine and pAntp) and PTD-fused proteins are unable to enter cells at 4° C (Richard et al., 2003; Lundberg et al., 2003; Drin et al., 2003). Strong cell-surface association of polycationic PTDs and artifactual redistribution following fixation result in apparent uptake at 4° C and cytosolic or nuclear staining. Moreover, PTDs and fused proteins have been detected in vesicular structures inside the cells, suggesting endocytotic uptake (Richard et al., 2003; Lundberg et al., 2003; Drin et al., 2003).
The clathrin-dependent, receptor-mediated endocytotic pathway is the best-characterized form of internalization of membranes and proteins. Clathrin-independent endocytotic pathways include caveolar-endocytosis and macropinocytosis. Unlike clathrin-dependent endocytosis, most of these internalization pathways are sensitive to disruption of the plasma membrane lipid rafts (Nichols and Lippincott-Schwartz, 2001). The lipid-raft dependent caveolar pathway is involved in internalization of the full length HIV-1 TAT and of a TAT-PTD fused GFP protein in HeLa cells (Fittipaldi et al., 2003). Caveolae, first identified in endothelial cells (Palade, 1953), are found in many other cell types, although they have not been detected in lymphocytes and neurons (Razani et al., 2002), suggesting that endocytosis of TAT-fusion proteins might occur in a cell-specific manner. For instance, in lymphoid cells, an alternate, lipid raft-dependent, macropinocytotic pathway is involved in the internalization of both TAT-PTD and TAT-fusion proteins (Wadia et al., 2004; Kaplan et al., 2005). In neurons, internalization of full-length HIV-1 TAT occurs through a LRP receptor-dependent pathway, but the mechanism of TAT-PTD mediated uptake has not been examined. Since binding of full length TAT to LRP occurs through a different domain of TAT (34–47) than its PTD (47–57) (Liu et al., 2000), it is likely that such a receptor-mediated pathway is not involved in internalization of TAT-PTD or of TAT-fusion proteins.
We recently examined the mechanisms involved in TAT-mediated internalization of Bcl-2 using neural cells. Using trypsinization to eliminate the cell-surface bound protein, we found that internalization of TAT-Bcl-2Δloop protein (35% of total cellular) occurs at 37° C but not at 4° C (Fig. 3A). Since all forms of endocytosis are inhibited at 4° C, this finding suggests that an endocytotic process rather than a temperature-independent transduction mechanism is involved. Consistent with this hypothesis, fluorescence microscopy of live cells transduced with both an FITC-labeled TAT-Bcl-2Δloop protein or a TAT-YFP protein reveal a vesicular distribution (Soane and Fiskum, unpublished).
The lipid raft-disrupting agent nystatin, that sequesters plasma membrane cholesterol, was used to test for possible involvement of a lipid raft-dependent endocytotic pathway in internalization of TAT-Bcl-2Δloop. A marked inhibition of internalization was observed in SH-SY5Y neuroblastoma cells (Fig. 3B). In addition, inhibition of clathrin-mediated endocytosis with the specific inhibitor chlorpromazine does not affect the internalization of TAT-Bcl-2Δloop (Fig. 3B). Fluorescence microscopy of transduced TAT-YFP also indicates that the protein does not colocalize with transferrin, a marker of clathrin-mediated endocytosis. Moreover, significant colocalization was observed with 70 kDa Dextran, a marker of fluid phase endocytosis (Soane and Fiskum, unpublished). Thus, like in other cells, TAT-mediated protein internalization of Bcl-2 in neural cells also occurs through a clathrin-independent and raft-dependent pathway.
While the direct membrane translocation mechanism might still be involved in internalization of PTDs and small cargoes, the delivery of protein cargoes is most likely mediated through endocytosis. Internalization of TAT-fusion proteins is apparently mediated in most cell types through endocytotic pathways originating at the plasma membrane lipid rafts, and can follow a caveolar or non-caveolar route, e.g., macropinocytosis or other less well characterized raft-dependent pathways (Fig. 2). Raft dependence of internalization was also noted in some cell types for other cargoes transduced by TAT-PTD, such as plasmid DNA and phage particles (Ignatovich et al., 2003; Eguchi et al., 2001). In addition, similar to the TAT-PTD, polyarginine was also shown recently to be internalized through macropinocytosis in HeLa cells (Nakase et al., 2004).
Contrary to this model, however, are observations of partial colocalization with markers of clathrin-dependent endocytosis as reported for PTDs (Richard et al., 2003; Potocky et al., 2003) and for fusion proteins (Sengoku et al., 2004). In this later study, however, the proteins appeared inactive and sequestered in endosomes. Some studies also report a lack of inhibition of TAT-PTD or pAntp uptake by cholesterol-sequestering agents and suggest that internalization of PTDs is not limited to raft-dependent pathways (Drin et al., 2003; Richard et al., 2005). Consistent with this interpretation, TAT-PTD can enter at least HeLa cells through a clathrin-mediated pathway (Richard et al., 2005). However, in lymphoid cells, macropinocytosis is involved in internalization of both TAT-PTD and TAT-Cre fusion protein (Wadia et al., 2004; Kaplan et al., 2005). On the other hand, using the same cell type (HeLa cells) different endocytotic mechanisms are involved in internalization of TAT-PTD (clathrin-mediated) (Richard et al., 2005) and polyarginine (macropinocytosis) (Nakase et al., 2004).
The characteristics of PTD-mediated internalization are reminiscent of the cholera toxin can be internalized by both clathrin-dependent and independent pathways. However, cholera toxin is active only when it is endocytosed through non-clathrin and raft-dependent endocytosis, leading to Golgi localization and subsequent cytosolic delivery (Nichols and Lippincott-Schwartz, 2001). Sequence similarities between the TAT-PTD and several bacterial toxins that use the retrograde transport system to reach the ER and Golgi were noted recently (Fischer et al., 2004). Most of these arginine-rich motifs belong to toxins or proteins known to utilize a non-clathrin-mediated and cholesterol-sensitive endocytotic pathway for internalization. Currently, no explanation is available for these differences in the endocytotic pathways mediating transduction, other than the possible influence of the attached cargo and differences in the structure of the PTDs used. One possibility is that the internalization pathway is modulated by specific interaction of PTDs with cell-surface proteoglycans.
Role of Heparan Sulfate Proteoglycans
Heparan sulfate proteoglycans (HSPG) can mediate endocytotic uptake of various endogenous ligands, including basic fibroblast growth factor (FGF), lipoproteins, or pathogens, e.g., bacteria (Belting et al., 2003). Reports indicating that heparin, a structural analogue of heparan sulfate, inhibits internalization of HIV-1 TAT and that the TAT-PTD can bind heparin suggest a possible role of HSPG in PTD-mediated internalization (Mann and Frankel, 1991; Hakansson et al., 2001). Using CHO cells defective in heparan sulfate (HS) synthesis, Tyagi et al. clearly demonstrated that internalization of TAT and TAT-PTD fused proteins is dependent on binding to HSPG. Heparan sulfate but not chondroitin sulfate (CS) was required for internalization (Tyagi et al., 2001). Other studies have shown that binding to HSPG is also required for internalization of the TAT-PTD and polyarginine (Suzuki et al., 2002; Richard et al., 2005).
HS is present on cells mainly on two classes of membrane-anchored proteoglycans (PG), i.e., syndecans and glypicans. Expression of PG is affected by multiple factors and is developmentally regulated (Bandtlow and Zimmermann, 2000). Most cells contain PG of both types and their ubiquitous distribution might explain the ability of PTDs to transduce virtually any cell type. Glypicans are enriched in lipid rafts and are therefore logical candidates for raft-dependent internalization of TAT-delivered Bcl-2 or other proteins and PTDs. However, ligand binding to the HS chains of syndecans can also induce their clustering and raft-dependent endocytosis (Fuki et al., 2000). The TAT-PTD was shown to induce aggregates on the cell surface in an HS-dependent manner (Ziegler et al., 2005). The presence and binding of HSPG is not necessarily equivalent with internalization, as indicated by studies in MDCK and CaCo-2 cells in which no intracellular/transcellular TAT-PTD transport could be detected, despite the presence of HSPGs (Violini et al., 2002).
Cytosolic Delivery of Transduced Proteins
Despite recent evidence for involvement of endocytosis in protein transduction, demonstration of specific biologic effects of transduced proteins indicates that at least a fraction of the PTD-fused proteins are released from endosomes. Among Bcl-2 family proteins, both Bcl-2 (Fig. 3C and unpublished results) and Bcl-XL are transduced in a functional form (Cao et al., 2002; Kilic et al., 2002; Asoh et al., 2002).
For the full length HIV-1 TAT protein, endocytotic internalization leads to endosomal localization and subsequent cytosolic release through a mechanism requiring acidification and relying on Hsp90 (Vendeville et al., 2004). The mechanisms of endosomal release of PTDs and attached cargoes are, however, unclear. Some of the potential endosome release mechanisms involve a direct translocation or transport of PTDs across endosomal membranes, or formation of inverted micelle or pore-like structures in endosomes rather than in the plasma membrane (Fig. 2). Endosomal escape might also involve PTD-induced endosome disruption, or constitutive release from a fraction of leaky endosomes, e.g., macropinosomes (Wadia et al., 2004). Similar to cholera toxin, the retrograde transport to Golgi/ER and subsequent cytosolic exit through retro-translocation could also be involved. An acidification-dependent release from endosomes was suggested for TAT-PTD by the observation that neutralization of endosomal pH inhibits its cytosolic delivery (Potocky et al., 2003). Studies using brefeldin A, that disrupts Golgi trafficking, provide support for the possibility that the transduced proteins are released following retrograde transport to Golgi (Fischer et al., 2004; Fittipaldi et al., 2003). Another possible explanation is that the endosomal release is facilitated by some of the protein cargoes and not by the PTDs. Bcl-2 family proteins are a class of cargoes that could induce their own endosomal escape, since they can form ion channels or pores in membranes. Channel formation by Bcl-2 proteins is augmented by low pH (Schendel et al., 1998) and might therefore be activated in endosomes after acidification. This suggests that endosomal escape of TAT-delivered Bcl-2 or Bcl-XL can occur in a manner similar to that of the pH-dependent pore-forming toxin Diphtheria toxin (DT), with which Bcl-2 proteins share structural homology. Yet another possibility is that Bcl-2 proteins delivered through transduction reach the ER through the retrograde transport mechanism and exert their effect at the ER without being released into the cytosol and localize to mitochondria. This possibility was also suggested by the studies on the DTR fused Bcl-XL (Liu et al., 1999).
Recognition of the involvement of endocytosis in PTD-mediated transduction indicates that efficient delivery will only be achieved if this barrier is eliminated. The efficacy of PTDs to promote endosomal escape, at least for protein cargoes, appears quite limited in some cases (Sengoku et al., 2004). Several studies demonstrate that enhancing endosomal escape results in increased functional activity of transduced proteins. Chloroquine, known to enhance transactivation by full-length TAT and sucrose, was used to disrupt endosomes, resulting in an increase in the activity of a TAT-Cre protein (Caron et al., 2004; Wadia et al., 2004). Photo-acceleration of PTD release (TAT-PTD, polyarginine and pAntp) from endosomes following exposure to fluorescent light (480 nm) was also reported. The same method was efficient at increasing the cytosolic release of a polyarginine-transduced p53 protein (Matsushita et al., 2004). Similarly, laser illumination was also reported to increase redistribution of CPPs from endosomes to cytosol (Maiolo et al., 2004).
pH-sensitive toxins or fusogenic peptides can enhance endosomal escape through pH-induced endosomolysis and are used to increase the efficiency of non-viral DNA delivery methods (Cho et al., 2003). The utility of such a strategy at improving PTD-mediated delivery and endosomal escape was demonstrated by Wadia et al. by using a TAT-HA2 pH-sensitive fusogenic peptide that was co-transduced with a TAT-Cre protein, resulting in increased TAT-Cre activity (Wadia et al., 2004). This approach was also utilized for generation of a p53 fusion protein with both the TAT and HA2 peptides, and resulted in improved activity of transduced p53 (Michiue et al., 2005). The mechanism of endosomal release of transduced proteins remains one of the critical issues for PTD-mediated transduction and further improvement of cytosolic delivery might greatly enhance the therapeutic potential of this approach.
Acknowledgments
The authors and some of their work described in this article were supported by grants from the NIH (NS34152 and NS45038) and by the U.S. Department of Defense (DAMD 17-99-1-9483).
Abbreviations
- Antp
Antennapedia
- Bcl
B-cell lymphoma
- BH domain
Bcl-2 homology domain
- CPP
cell penetrating peptide
- ER
endoplasmic reticulum
- HIV
human immunodefficiency virus
- HS
heparan sulfate
- HSPG
heparan sulfate proteoglycan
- PTD
protein transduction domain
- STS
staurosporine
- TAT
transactivator of transcription
References
- Asoh S, Ohsawa I, Mori T, Katsura K, Hiraide T, Katayama Y, Kimura M, Ozaki D, Yamagata K, Ohta S. Proc Natl Acad Sci USA. 2002;99:17107–17112. doi: 10.1073/pnas.262460299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow CE, Zimmermann DR. Physiol Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. J Biol Chem. 2005 doi: 10.1074/jbc.C500089200. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Belting M, Mani K, Jonsson M, Cheng F, Sandgren S, Jonsson S, Ding K, Delcros JG, Fransson LA. J Biol Chem. 2003;278:47181–47189. doi: 10.1074/jbc.M308325200. [DOI] [PubMed] [Google Scholar]
- Bolton SJ, Jones DN, Darker JG, Eggleston DS, Hunter AJ, Walsh FS. Eur J Neurosci. 2000;12:2847–2855. doi: 10.1046/j.1460-9568.2000.00171.x. [DOI] [PubMed] [Google Scholar]
- Cantara S, Donnini S, Giachetti A, Thorpe PE, Ziche M. J Vasc Res. 2004;41:202–207. doi: 10.1159/000077408. [DOI] [PubMed] [Google Scholar]
- Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, Lu A, Ran R, Graham SH, Chen J. J Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron NJ, Quenneville SP, Tremblay JP. Biochem Biophys Res Commun. 2004;319:12–20. doi: 10.1016/j.bbrc.2004.04.180. [DOI] [PubMed] [Google Scholar]
- Chen J, Graham SH, Chan PH, Lan J, Zhou RL, Simon RP. Neuroreport. 1995;6:394–398. doi: 10.1097/00001756-199501000-00040. [DOI] [PubMed] [Google Scholar]
- Chen J, Simon RP, Nagayama T, Zhu R, Loeffert JE, Watkins SC, Graham SH. J Cereb Blood Flow Metab. 2000;20:1033–1039. doi: 10.1097/00004647-200007000-00002. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Chen M, Won DJ, Krajewski S, Gottlieb RA. J Biol Chem. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- Cho YW, Kim JD, Park K. J Pharm Pharmacol. 2003;55:721–734. doi: 10.1211/002235703765951311. [DOI] [PubMed] [Google Scholar]
- Dalkara D, Zuber G, Behr JP. Mol Ther. 2004;9:964–969. doi: 10.1016/j.ymthe.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Derossi D, Joliot AH, Chassaing G, Prochiantz A. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- Deshayes S, Heitz A, Morris MC, Charnet P, Divita G, Heitz F. Biochemistry. 2004;43:1449–1457. doi: 10.1021/bi035682s. [DOI] [PubMed] [Google Scholar]
- Dietz GP, Bahr M. Mol Cell Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Dietz GP, Kilic E, Bahr M. Mol Cell Neurosci. 2002;21:29–37. doi: 10.1006/mcne.2002.1165. [DOI] [PubMed] [Google Scholar]
- Dom G, Shaw-Jackson C, Matis C, Bouffioux O, Picard JJ, Prochiantz A, Mingeot-Leclercq MP, Brasseur R, Rezsohazy R. Nucleic Acids Res. 2003;31:556–561. doi: 10.1093/nar/gkg160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. J Biol Chem. 2003;278:31192–31201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- Eguchi A, Akuta T, Okuyama H, Senda T, Yokoi H, Inokuchi H, Fujita S, Hayakawa T, Takeda K, Hasegawa M, Nakanishi M. J Biol Chem. 2001;276:26204–26210. doi: 10.1074/jbc.M010625200. [DOI] [PubMed] [Google Scholar]
- Ellerby LM, Ellerby HM, Park SM, Holleran AL, Murphy AN, Fiskum G, Kane DJ, Testa MP, Kayalar C, Bredesen DE. J Neurochem. 1996;67:1259–1267. doi: 10.1046/j.1471-4159.1996.67031259.x. [DOI] [PubMed] [Google Scholar]
- Elliott G, O’Hare P. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- Embury J, Klein D, Pileggi A, Ribeiro M, Jayaraman S, Molano RD, Fraker C, Kenyon N, Ricordi C, Inverardi L, Pastori RL. Diabetes. 2001;50:1706–1713. doi: 10.2337/diabetes.50.8.1706. [DOI] [PubMed] [Google Scholar]
- Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, Barsoum J. Proc Natl Acad Sci USA. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber D. Science. 2001;294:1638–1642. doi: 10.1126/science.294.5547.1638. [DOI] [PubMed] [Google Scholar]
- Fischer R, Kohler K, Fotin-Mleczek M, Brock R. J Biol Chem. 2004;279:12625–12635. doi: 10.1074/jbc.M311461200. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Polster BM, Kowaltowski AJ. In: Pharmacology of Cerebral Ischemia 2000. Krieglstein J, Klumpp S, editors. Medpharm Scientific Publishers; Stuttgart: 2000. [Google Scholar]
- Fittipaldi A, Ferrari A, Zoppe M, Arcangeli C, Pellegrini V, Beltram F, Giacca M. J Biol Chem. 2003;278:34141–34149. doi: 10.1074/jbc.M303045200. [DOI] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Fuki IV, Meyer ME, Williams KJ. Biochem J. 2000;351:607–612. [PMC free article] [PubMed] [Google Scholar]
- Futaki S. Int J Pharm. 2002;245:1–7. doi: 10.1016/s0378-5173(02)00337-x. [DOI] [PubMed] [Google Scholar]
- Green M, Loewenstein PM. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- Hakansson S, Jacobs A, Caffrey M. Protein Sci. 2001;10:2138–2139. doi: 10.1110/ps.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Ito Y, Niikura T, Shao Z, Hata M, Oyama F, Nishimoto I. Biochem Biophys Res Commun. 2001;283:460–468. doi: 10.1006/bbrc.2001.4765. [DOI] [PubMed] [Google Scholar]
- Hata R, Gillardon F, Michaelidis TM, Hossmann KA. Metab Brain Dis. 1999;14:117–124. doi: 10.1023/a:1020709814456. [DOI] [PubMed] [Google Scholar]
- Henriques ST, Castanho MA. Biochemistry. 2004;43:9716–9724. doi: 10.1021/bi036325k. [DOI] [PubMed] [Google Scholar]
- Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Holinger EP, Chittenden T, Lutz RJ. J Biol Chem. 1999;274:13298–13304. doi: 10.1074/jbc.274.19.13298. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Liu XH, Hagihara N, Youle RJ. Cancer Res. 2002;62:1433–1438. [PubMed] [Google Scholar]
- Ignatovich IA, Dizhe EB, Pavlotskaya AV, Akifiev BN, Burov SV, Orlov SV, Perevozchikov AP. J Biol Chem. 2003;278:42625–42636. doi: 10.1074/jbc.M301431200. [DOI] [PubMed] [Google Scholar]
- Jia H, Lohr M, Jezequel S, Davis D, Shaikh S, Selwood D, Zachary I. Biochem Biophys Res Commun. 2001;283:469–479. doi: 10.1006/bbrc.2001.4790. [DOI] [PubMed] [Google Scholar]
- Joliot A, Prochiantz A. Nat Cell Biol. 2004;6:189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane DJ, Ord T, Anton R, Bredesen DE. J Neurosci Res. 1995;40:269–275. doi: 10.1002/jnr.490400216. [DOI] [PubMed] [Google Scholar]
- Kaplan IM, Wadia JS, Dowdy SF. J Control Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Kaufmann JA, Bickford PC, Taglialatela G. J Neurochem. 2001;76:1099–1108. doi: 10.1046/j.1471-4159.2001.00118.x. [DOI] [PubMed] [Google Scholar]
- Kilic E, Dietz GP, Hermann DM, Bahr M. Ann Neurol. 2002;52:617–622. doi: 10.1002/ana.10356. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ, Shutter JR, Veis DJ, Merry DE, Oltvai ZN. Semin Cancer Biol. 1993;4:327–332. [PubMed] [Google Scholar]
- Kowaltowski AJ, Fenton RG, Fiskum G. Free Radic Biol Med. 2004;37:1845–1853. doi: 10.1016/j.freeradbiomed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Vercesi AE, Fiskum G. Cell Death Differ. 2000;7:903–910. doi: 10.1038/sj.cdd.4400722. [DOI] [PubMed] [Google Scholar]
- Kramer SD, Wunderli-Allenspach H. Biochim Biophys Acta. 2003;1609:161–169. doi: 10.1016/s0005-2736(02)00683-1. [DOI] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Linnik MD, Zahos P, Geschwind MD, Federoff HJ. Stroke. 1995;26:1670–1674. doi: 10.1161/01.str.26.9.1670. [DOI] [PubMed] [Google Scholar]
- Liu XH, Castelli JC, Youle RJ. Proc Natl Acad Sci USA. 1999;96:9563–9567. doi: 10.1073/pnas.96.17.9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Luciano F, Zhai D, Zhu X, Bailly-Maitre B, Ricci JE, Satterthwait A, Reed JC. J Biol Chem. 2005;280:15825–15835. doi: 10.1074/jbc.M413062200. [DOI] [PubMed] [Google Scholar]
- Lundberg M, Wikstrom S, Johansson M. Mol Ther. 2003;8:143–150. doi: 10.1016/s1525-0016(03)00135-7. [DOI] [PubMed] [Google Scholar]
- Maiolo JR, Ottinger EA, Ferrer M. J Am Chem Soc. 2004;126:15376–15377. doi: 10.1021/ja044867z. [DOI] [PubMed] [Google Scholar]
- Mann DA, Frankel AD. EMBO J. 1991;10:1733–1739. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Noguchi H, Lu YF, Tomizawa K, Michiue H, Li ST, Hirose K, Bonner-Weir S, Matsui H. FEBS Lett. 2004;572:221–226. doi: 10.1016/j.febslet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Merry DE, Korsmeyer SJ. Annu Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- Michiue H, Tomizawa K, Wei FY, Matsushita M, Lu YF, Ichikawa T, Tamiya T, Date I, Matsui H. J Biol Chem. 2005;280:8285–8289. doi: 10.1074/jbc.M412430200. [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, Kim DT, Steinman L, Fathman CG, Rothbard JB. J Pept Res. 2000;56:318–325. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- Morris MC, Depollier J, Mery J, Heitz F, Divita G. Nat Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G. Proc Natl Acad Sci USA. 1996a;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AN, Fiskum G. Biochem Soc Symp. 1999;66:33–41. doi: 10.1042/bss0660033. [DOI] [PubMed] [Google Scholar]
- Murphy AN, Myers KM, Fiskum G. In: Pharmacology of Cerebral Ischemia. Krieglstein J, editor. Wissenschaftliche Verlugsgesellschaft; Stuttgart, Germany: 1996b. pp. 163–172. [Google Scholar]
- Myers KM, Fiskum G, Liu Y, Simmens SJ, Bredesen DE, Murphy AN. J Neurochem. 1995;65:2432–2440. doi: 10.1046/j.1471-4159.1995.65062432.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, Takehashi M, Tanaka S, Ueda K, Simpson JC, Jones AT, Sugiura Y, Futaki S. Mol Ther. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Lippincott-Schwartz J. Trends Cell Biol. 2001;11:406–412. doi: 10.1016/s0962-8924(01)02107-9. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Manion M, Schwartz P, Hockenbery DM. Biochim Biophys Acta. 2004;1705:43–51. doi: 10.1016/j.bbcan.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Ozaki D, Sudo K, Asoh S, Yamagata K, Ito H, Ohta S. Biochem Biophys Res Commun. 2004;313:522–527. doi: 10.1016/j.bbrc.2003.11.144. [DOI] [PubMed] [Google Scholar]
- Palade GE. J Appl Phys. 1953;24:1424–1436. [Google Scholar]
- Petros AM, Olejniczak ET, Fesik SW. Biochim Biophys Acta. 2004;1644:83–94. doi: 10.1016/j.bbamcr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Polster BM, Kinnally KW, Fiskum G. J Biol Chem. 2001;276:37887–37894. doi: 10.1074/jbc.M104552200. [DOI] [PubMed] [Google Scholar]
- Potocky TB, Menon AK, Gellman SH. J Biol Chem. 2003;278:50188–50194. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- Razani B, Woodman SE, Lisanti MP. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- Reed JC, Pellecchia M. Blood. 2005:2004–2007. doi: 10.1182/blood-2004-07-2761. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik LV. J Biol Chem. 2005;280:15300–15306. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- Ryser HJ, Shen WC, Merk FB. Life Sci. 1978;22:1253–1260. doi: 10.1016/0024-3205(78)90094-2. [DOI] [PubMed] [Google Scholar]
- Sawada M, Hayes P, Matsuyama S. Nat Cell Biol. 2003;5:352–357. doi: 10.1038/ncb955. [DOI] [PubMed] [Google Scholar]
- Schendel SL, Montal M, Reed JC. Cell Death Differ. 1998;5:372–380. doi: 10.1038/sj.cdd.4400365. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Sengoku T, Bondada V, Hassane D, Dubal S, Geddes JW. Exp Neurol. 2004;188:161–170. doi: 10.1016/j.expneurol.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Shangary S, Johnson DE. Biochemistry. 2002;41:9485–9495. doi: 10.1021/bi025605h. [DOI] [PubMed] [Google Scholar]
- Shimazaki K, Urabe M, Monahan J, Ozawa K, Kawai N. Gene Ther. 2000;7:1244–1249. doi: 10.1038/sj.gt.3301211. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Konishi A, Kodama T, Tsujimoto Y. Proc Natl Acad Sci USA. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Nagayama T, Jin KL, Zhu L, Loeffert JE, Watkins SC, Graham SH, Simon RP. J Cereb Blood Flow Metab. 2001;21:233–243. doi: 10.1097/00004647-200103000-00007. [DOI] [PubMed] [Google Scholar]
- Sik EW, Won KD, Koo HI, Yoo KY, Kang TC, Ho JS, Soon CH, Hyun CS, Hoon KY, Young KS, Yil KH, Hoon KJ, Kwon OS, Cho SW, Soo LK, Park J, Ho WM, Young CS. Free Radic Biol Med. 2004;37:1656–1669. doi: 10.1016/j.freeradbiomed.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Moskaug JO, Madshus IH, Sandvig K, Olsnes S. J Cell Biol. 1991;113:1025–1032. doi: 10.1083/jcb.113.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka R, Shimizu S, Funatsu T, Tamagawa H, Sawa Y, Kawakami T, Tsujimoto Y. Oncogene. 2003;22:8432–8440. doi: 10.1038/sj.onc.1207180. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Futaki S, Niwa M, Tanaka S, Ueda K, Sugiura Y. J Biol Chem. 2002;277:2437–2443. doi: 10.1074/jbc.M110017200. [DOI] [PubMed] [Google Scholar]
- Takeshima K, Chikushi A, Lee KK, Yonehara S, Matsuzaki K. J Biol Chem. 2003;278:1310–1315. doi: 10.1074/jbc.M208762200. [DOI] [PubMed] [Google Scholar]
- Tyagi M, Rusnati M, Presta M, Giacca M. J Biol Chem. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- Vendeville A, Rayne F, Bonhoure A, Bettache N, Montcourrier P, Beaumelle B. Mol Biol Cell. 2004;15:2347–2360. doi: 10.1091/mbc.E03-12-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violini S, Sharma V, Prior JL, Dyszlewski M, Piwnica-Worms D. Biochemistry. 2002;41:12652–12661. doi: 10.1021/bi026097e. [DOI] [PubMed] [Google Scholar]
- Vives E, Brodin P, Lebleu B. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Dowdy SF. Curr Protein Pept Sci. 2003;4:97–104. doi: 10.2174/1389203033487289. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Stan RV, Dowdy SF. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. Proc Natl Acad Sci USA. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Wang HG. J Biol Chem. 2002;277:41604–41612. doi: 10.1074/jbc.M207516200. [DOI] [PubMed] [Google Scholar]
- Yang JC, Kahn A, Cortopassi G. Toxicology. 2000;151:65–72. doi: 10.1016/s0300-483x(00)00298-5. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Kroemer G. Nat Rev Mol Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- Zaro JL, Shen WC. Biochem Biophys Res Commun. 2003;307:241–247. doi: 10.1016/s0006-291x(03)01167-7. [DOI] [PubMed] [Google Scholar]
- Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC, Reed JC. J Biol Chem. 2005;280:15815–15824. doi: 10.1074/jbc.M411902200. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Blatter XL, Seelig A, Seelig J. Biochemistry. 2003;42:9185–9194. doi: 10.1021/bi0346805. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Nervi P, Durrenberger M, Seelig J. Biochemistry. 2005;44:138–148. doi: 10.1021/bi0491604. [DOI] [PubMed] [Google Scholar]