Abstract
Cytosolic sulphotransferase SULT1A1 plays a dual role in the activation of some carcinogens and inactivation of others. A functional polymorphism leading to Arg213His substitution (SULT1A1*2) affects its catalytic activity and thermostability. To study the association of SULT1A1*2 polymorphism with tobacco-related cancers (TRCs), a case–control study comprising 132 patients with multiple primary neoplasm (MPN) involving TRC and 198 cancer-free controls was carried out. One hundred and thirteen MPN patients had at least one cancer in upper aerodigestive tract including lung (UADT-MPN). SULT1A1*2 showed significant risk association with UADT-MPN (odds ratio (OR)=5.50, 95% confidence interval (CI): 1.09, 27.7). Meta-analysis was conducted combining the data with 34 published studies that included 11 962 cancer cases and 14 673 controls in diverse cancers. The SULT1A1*2 revealed contrasting risk association for UADT cancers (OR=1.62, 95% CI: 1.12, 2.34) and genitourinary cancers (OR=0.73, 95% CI: 0.58, 0.92). Furthermore, although SULT1A1*2 conferred significant increased risk of breast cancer to Asian women (OR=1.91, 95% CI: 1.08, 3.40), it did not confer increased risk to Caucasian women (OR=0.92, 95% CI: 0.71, 1.18). Thus risk for different cancers in distinct ethnic groups could be modulated by interaction between genetic variants and different endogenous and exogenous carcinogens.
Keywords: multiple primary neoplasm, SULT1A1, meta-analysis, single nucleotide polymorphism, tobacco related cancer, upper aerodigestive tract cancer
Tobacco-related cancer (TRC) accounts for almost half the global burden of cancer and arises from a complex gene–environment interaction. Traditionally, only lung, oesophageal, and head and neck cancers were described as TRCs. However, based on several studies, the International Association of Research in Cancer (IARC) has broadened the definition of TRC to include carcinoma of the cervix, bladder, stomach, kidney, pancreas, liver and myeloid leukaemia (Doll, 1999; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2004; Hung et al, 2005). Although prolonged exposure to tobacco with or without other carcinogens plays a central role in the genesis of these cancers, various host genetic factors could significantly modulate the risk of developing TRC.
Several genetic alterations in the genes coding for xenobiotic-metabolising enzymes (XMEs), DNA repair, cell cycle regulation and apoptotic pathway confer a low-penetrance genetic susceptibility to tobacco carcinogens (Kotnis et al, 2005). There is a large body of evidence, including meta-analyses to support the association of various isoforms of glutathione-S transferases (GSTs), cytochrome P450 and N-acetyl transferase with tobacco carcinogenesis. Although sulphotransferase (SULT) enzymes could play an equally important role in detoxifying tobacco carcinogens, there are very few, mostly inconclusive, studies examining the association of genetic alteration in the genes coding for this super family of multifunctional enzymes with TRC (Seth et al, 2000; Hung et al, 2004; Sellers et al, 2005; Dandara et al, 2006).
Sulphotransferase enzymes catalyse sulphation by transferring sulphonate (sulphuryl) group from cofactor 3′-phosphoadenosine 5′-phosphosulphate to a nucleophilic acceptor substrate to form either a sulphate ester or a sulphamate. These sulphate conjugates are more polar and less reactive than the parent compound and facilitate their excretion (Glatt et al, 2001). However, some sulpho conjugates are strong electrophiles and may covalently bind to DNA and proteins (Glatt et al, 2001).
SULT1A1 gene is one of the most important and well-studied members of the SULT family and is abundant in a wide variety of tissues. SULT1A1 plays a major role in biotransformation of numerous substrates including several carcinogens, neurotransmitters, steroid hormones and drugs (Raftogianis et al, 1999; Hildebrandt et al, 2007). Although sulphation is an important property of SULT1A1 in the inactivation of carcinogens, it also plays an important role in toxification of dietary and environmental mutagens (Glatt et al, 2001; Al-Buheissi et al, 2006). Of the various polymorphisms in SULT1A1, the Arg → His polymorphism at position 213, in exon 7 (SULT1A1*2), has a twofold lower catalytic activity and thermo stability than its high-activity Arg213 counterpart as demonstrated in platelet cytosol (Raftogianis et al, 1997; Nowell et al, 2000; Nagar et al, 2006). Although several reports show risk association of the SULT1A1 variant with different cancers (Sun et al, 2005; Dandara et al, 2006; Pachouri et al, 2006; Fan et al, 2007; Lilla et al, 2007; Bardakci et al, 2008), others show either no effect (Sellers et al, 2005) or a protective effect (Cheng et al, 2005).
In this case–control study, we have examined the association of SULT1A1 Arg213His polymorphism with tobacco carcinogenesis using a unique group of individuals with multiple tobacco-related primary cancers and have used a meta-analysis approach to confirm our findings. Considering that His213 variant of SULT1A1 has a lower activity than Arg213 variant (Raftogianis et al, 1997; Nagar et al, 2006), we hypothesise that if tobacco carcinogenesis is significantly modulated by the Arg213His polymorphism, it would demonstrate a significant association with TRC and this association may be stronger in individuals with multiple primary TRC.
Materials and methods
Study subjects
A registry of patients with multiple primary cancers or familial cancers was established at the Tata Memorial Hospital, Mumbai, in 1996 by one of the authors (RS). From this registry, 132 consecutive multiple primary neoplasm (MPN) patients where one or both the primaries were tobacco related, and their genomic DNA and consent were available, were taken up for this study. Histological or cytological confirmation of each primary cancer was available and each of the cancers was classified as TRC or non-TRC as per the IARC criteria (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2004). There was no restriction for age at diagnosis, gender or carcinogen exposure. For defining two cancers as distinct multiple primaries, modified Hong's criteria (Hong et al, 1990) was used, which states that – (a) there is >2 cm of normal intervening mucosa between two primaries in head and neck region; (b) lung as second primary if present, should be of different histology, or be solitary and with characteristic radiology of lung cancer; and (c) there is no evidence of haematogenous spread. Bilateral cancers in paired organs such as breast, ovaries or kidneys were not classified as MPN.
Majority of the MPN cases in the registry hailed from the western and northern parts of India. The cancer-free controls (n=198) were volunteers who consented to donate blood or buccal washes for the study. The controls were also from the same region and were free of any cancer or pre-cancerous condition. They were either visiting our hospital in the Preventive Oncology Department for cancer screening (n=124) or visiting government dental college for various non-malignant, dental ailments (n=68). A few were healthy, ethnically matched workers from Mumbai (n=6). Detailed questionnaire including ethnicity and lifetime history of tobacco and alcohol use was obtained from all cases and controls. A majority of them were tobacco users. Family history of cancer was obtained for all MPN cases and from majority of the cancer-free controls. After obtaining informed consent, 3–6 ml of peripheral blood was collected from each subject. Exfoliated buccal cells (mouthwash samples) were collected in sterile phosphate-buffered saline from control individuals who were reluctant to give blood (n=68). The study was approved by our Hospital Ethics Committee.
DNA extraction and genotyping
Genomic DNA was extracted from peripheral blood/mouthwash samples using phenol chloroform method standardised in our laboratory (Koppikar and Mulherkar, 2006). PCR for SULT1A1 genotyping followed by RFLP using HaeII restriction enzyme was carried out as described by Wang et al (2002). The authenticity of the PCR products was confirmed by sequencing at least five PCR products at random on an automated DNA sequencer (ABI Prism 3100 Avant) using the Big Dye terminator kit (ABI Prism, Foster City, CA, USA) as per the manufacturer's instructions.
Identification and analysis of studies for meta-analysis
PUBMED searches were conducted to identify studies on SULT using the search words ‘SULT1A1, SULT AND polymorphism’ and ‘SULT AND cancer’. The inclusion criteria were case–control studies examining associations of SULT1A1 Arg213His polymorphism either alone or in combination with other genes, published until July 2007. For every study, publication date, country of origin, demographics, genotyping methodology, ethnicity, source and genotype frequency of study subjects were reviewed. In case of missing information, the authors were contacted and requested to provide the data. One study was excluded as all the required information could not be obtained (Peng et al, 2003). Genotyping studies on only cancer cases or exclusively on healthy subjects were excluded as comparison of cancer patients with matched controls was a prerequisite for studying association of a particular genotype with cancer risk (Nowell et al, 2002b, 2005; Magagnotti et al, 2003; Sparks et al, 2004; Shatalova et al, 2005; Grabinski et al, 2006).
Statistical analysis
The risk (odds ratio, OR) was estimated by comparison of the variant 213His genotype vs the wild-type 213Arg allele using dominant model ((Arg/His+His/His) vs Arg/Arg), recessive model (His/His vs (Arg/His+Arg/Arg)) as well as the extreme model (His/His vs Arg/Arg). The risk was adjusted for age and habit using unconditional logistic regression analysis using SPSS v14.0. Hardy–Weinberg equilibrium in the controls was evaluated for each study using χ2 test. For each genetic contrast, the between-study heterogeneity was estimated across all eligible comparisons using Q statistics. Funnel plots and Egger’s test were used to assess potential publication bias, which results from non-publication of small studies with negative results (Egger et al, 1997). This test detects funnel plot asymmetry by determining whether the intercept deviates significantly from zero in a regression of the standardised effect estimates against their precision. Influence analysis was also carried to assess whether summary OR was driven by any one study in the recessive model of meta-analysis (Sterne et al, 2002). Stratification by ethnicity (Asian, Caucasian and Others), total study size of cases and controls (up to 500 or more), Hardy–Weinberg equilibrium (yes/no), primary site (upper aerodigestive tract (UADT) and lung, breast, colorectal, genitourinary and other sites), source of control (population/hospital) and carcinogen exposure studied (yes/no) were pre-specified as characteristics for the assessment of heterogeneity. Meta-analysis was carried out using Review Manager Version 4.2 (Cochrane Collaboration) and STATA software for meta-regression analysis. P-values were two sided.
Results
In this case–control study, we have examined the association of SULT1A1 Arg213His (SULT1A1*2) polymorphism in 132 patients with tobacco-related multiple primary cancers and 198 cancer-free controls. For selection of MPN patients, stringent modified Hong's criteria were used to minimise the possibility of misclassifying metastasis, recurrences, skip lesions and radiation-induced cancers, as second primaries. Radiation therapy was used in the management of first primary in 60% of patients but none of the second primaries were classified as radiation-associated sarcomas (Huber et al, 2007) or meningiomas (King et al, 2007).
Using the IARC definition of TRC (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2004), these 132 MPN patients with at least one TRC primary were further subclassified as those with at least one primary in the UADT (UADT-MPN, n=113, Table 1) and those having none of the primaries in UADT (n=19). Majority of the patients (n=74) had both the primaries within the UADT region. The characteristics of these 113 patients with at least one primary in UADT and the healthy controls are shown in Table 1. Of the 113 patients with UADT-MPN, 96 (85%) reported tobacco use and majority (68%) had tobacco-chewing habit (Supplementary Table s1). The most common form of tobacco was chewing of tobacco quid with lime or with betel leaf or application of roasted tobacco (masheri) over gums. Quanta and duration of tobacco chewing were not available for all the participants and hence were not included in the analysis.
Table 1. Demographics of study subjects.
| Category | UADT TRCa (n=113) (%) | Cancer-free controlsb (n=198) (%) |
|---|---|---|
| Gender | ||
| Males | 74 (65) | 129 (65) |
| Females | 39 (35) | 69 (35) |
| Age c | ||
| Median | 50 | 46 |
| Range | (26–75) | (20–84) |
| Type of MPN | ||
| Synchronousd | 32 (28) | — |
| Metachronous | 79 (70) | — |
| Oral – 90 | — | |
| Oesophagus – 28 | — | |
| Larynx/hypopharynx – 24 | — | |
| Primary cancer sites (226 cancers in 113 cases) | Oropharynx – 24 | — |
| Lung – 15 | — | |
| Cervix – 15 | — | |
| Others – 30 | — | |
| Tobacco habit | ||
| No habit | 14 (12) | 14 (7) |
| Only T | 70 (62) | 156 (79) |
| T+A | 26 (23) | 27 (14) |
| No information | 3 (3) | 1 (<1) |
| SULT1A1 genotypes | ||
| Arg/Arg | 60 (53) | 135 (68) |
| Arg/His | 47 (42) | 61 (31) |
| His/His | 6 (5) | 2 (1) |
A=alcohol; MPN=multiple primary neoplasm; T=tobacco; TRC=tobacco-related cancer; UADT=upper aerodigestive tract.
Tobacco-related cancers were as defined by IARC (2004) and included UADT (including nasopharynx), cervix, bladder, stomach, kidney, liver, pancreas and myeloid leukaemia.
At least one primary in the UADT.
Controls were enrolled mainly from the Preventive Oncology Department, Tata Memorial Hospital and Government Dental College, Mumbai.
Age (years) at the diagnosis of the index cancer of the patients or age at accrual for the controls.
Synchronous – cancers occurring within 6 months of diagnosis of first primary site.
The genotype distribution of SULT1A1*2 as His/His (homozygous variant), Arg/His (heterozygous) and Arg/Arg (homozygous wild type) was compared in cases and controls using dominant, recessive and extreme models. These models were based on the biological plausibility that His213 variant allele is risk conferring compared with Arg213 allele. Thus His/His213 genotype was considered risk conferring whereas Arg/Arg213 would confer protection. However, activity of the SULT1A1 allozymes in platelets from heterozygous (Arg/His) individuals has been reported to be only slightly lower than that from the Arg/Arg individuals but much higher than that from His/His individuals (Raftogianis et al, 1997; Nowell et al, 2000). Hence recessive model (Arg/Arg+Arg/His vs His/His) was considered in the study.
The risk association of SULT1A1*2 was evaluated in 113 MPN patients with at least one UADT TRC. The remaining 19 patients with both TRCs outside UADT were analysed as a separate group as well as a combined TRC group (n=132) (Table 2). The results of the TRC outside UADT group (n=19) and the combined group (n=132) are not included in the meta-analysis due to small sample size (n=19) with very diverse TRCs (cervix, bladder and stomach and so on). After adjusting for age and tobacco use, a significant risk association of SULT1A1*2 with UADT TRC was seen in dominant, recessive as well as extreme models. In all three models, there was a significant increased risk associated with His213 genotype (Table 2).
Table 2. Analysis of risk association in tobacco-related MPN patients using genetic models.
| Cancer-free controls (n=198) | TRC outside UADT (n=19) | At least one in UADT (n=113) | All TRCs (n=132) | ||
|---|---|---|---|---|---|
| Category | Genotype | N | n (ORa (95% CI)) | n (ORa (95% CI)) | n (ORa (95% CI)) |
| Dominant | Arg/Arg | 135 | 6 (ref) | 60 (ref) | 66 (ref) |
| His/His,His/Arg | 2, 61 | 0, 13 (7.91 (2.06, 30.39)) | 6, 47 (1.94 (1.20, 3.14)) | 6, 60 (2.12 (1.3, 3.44)) | |
| Recessive | His/Arg,Arg/Arg | 61, 135 | 13, 6 (ref) | 47, 60 (ref) | 60, 66 (ref) |
| His/His | 2 | 0 | 6 (6.07 (1.20, 30.66)) | 6 (7.43 (1.42, 38.820) | |
| Extreme | Arg/Arg | 135 | 6 (ref) | 60 (ref) | 66 (ref) |
| His/His | 2 | 0 | 6 (7.84 (1.53, 40.15)) | 6 (8.92 (1.71, 46.66)) |
MPN=multiple primary neoplasm; TRC=tobacco-related cancer; UADT=upper aerodigestive tract.
Odds ratio (OR) adjusted for age and tobacco habit; 95% CI – 95% confidence interval.
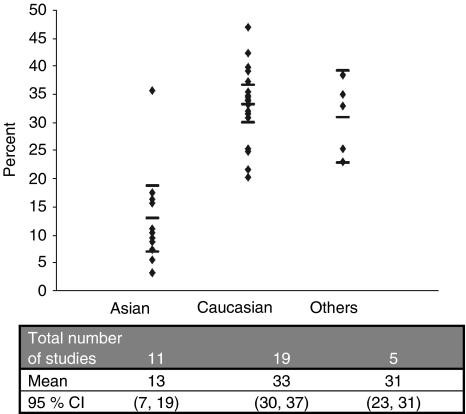
To compare these observations with the published studies, a meta-analysis of studies evaluating association of SULT1A1 Arg213His with different cancers was performed. From the Medline search using the search terms described earlier, we identified 34 case–control studies for SULT1A1 Arg213His polymorphism (Seth et al, 2000; Steiner et al, 2000; Bamber et al, 2001; Zheng et al, 2001, 2003; Ozawa et al, 2002; Wang et al, 2002; Nowell et al, 2002a, 2004; Tang et al, 2003; Wu et al, 2003; Chacko et al, 2004; Hung et al, 2004; Langsenlehner et al, 2004; Liang et al, 2004; Tiemersma et al, 2004; Tsukino et al, 2004; Boccia et al, 2005, 2006; Cheng et al, 2005; Choi et al, 2005; Han et al, 2005; Jerevall et al, 2005; Le Marchand et al, 2005; Lilla et al, 2005; Moreno et al, 2005; Pereira et al, 2005; Sellers et al, 2005; Sun et al, 2005; Yang et al, 2005; Dandara et al, 2006; Kellen et al, 2006; Mikhailova et al, 2006; Pachouri et al, 2006). Including the 113 patients with UADT-MPN from this study, there were 11 962 cancer cases and 14 673 cancer-free controls. To specifically examine the risk association of His213 with different types of cancers including UADT TRC, which is biologically more plausible, the studies included in meta-analysis were categorised according to the site of primary cancer as UADT TRC, genitourinary, breast, colorectal and other cancer sites. All the studies were further analysed with respect to genotypes, source of controls, ethnicity and carcinogen exposure. The study characteristics (Supplementary Table s2) showed that 13 studies had accrued controls from general population whereas 20 studies had hospital-based controls and one had mixed source of controls. In 23 studies, the distribution of genotypes in controls was consistent with Hardy–Weinberg equilibrium (Supplementary Table s3). It was noteworthy that the His213 allele occurred at a significantly lower frequency amongst Asians (13%; 95% confidence interval (CI): 7–19) as compared with Caucasians (33%; 95% CI: 30–37) and other ethnic groups (31%; 95% CI: 23–31) (Figure 1). However, it is possible that the variation is even larger between different Asian populations.
Figure 1.
Allele frequencies from the meta-analysis studies in control groups of different ethnicities.
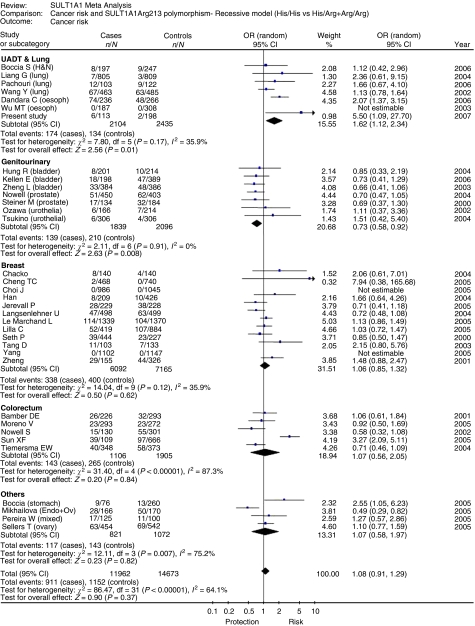
All the studies were analysed using the three models, namely recessive (Figure 2), which was biologically more plausible (Raftogianis et al, 1997), as well as dominant and extreme models (Supplementary Figure s1, s2 and Supplementary Table s3). All the three models showed a high degree of statistical heterogeneity among the 35 studies, including this study. Meta-regression analysis was performed to investigate the source for statistical heterogeneity (Supplementary Table s3). No obvious source of heterogeneity was identified except for ethnicity in the dominant model.
Figure 2.
Meta-analysis recessive model.
Symmetrical Funnel plot suggested the absence of publication bias for all the three models (Egger's test P-value >0.05; Supplementary Figure s3). Influence analysis was carried out to study the effect of individual studies in the meta-analysis on the overall outcome (Supplementary Figure s4). None of the studies affected the outcome of the meta-analysis significantly. When different ethnic groups were analysed separately irrespective of cancer site, the Asians showed a significant increased risk (OR=1.84, 95% CI: 1.20, 2.83) as compared with Caucasians (OR=1.03, 95% CI: 0.82, 1.29) (Supplementary Table s3) in the recessive model.
The effect of ethnicity for specific cancer sites could be examined separately only for breast cancer where ethnicity was reported in sufficient number of studies (n=11) (Supplementary Figure s5). Effect of ethnicity could not be evaluated in other cancer sites due to the small number of studies. Although SULT1A1*2 conferred significant increased risk of breast cancer to Asian women (OR=1.91, 95% CI: 1.08, 3.40), it did not confer increased risk to Caucasian women (OR=0.92, 95% CI: 0.71, 1.18).
Stratified meta-analysis according to cancer site, irrespective of ethnicity or any other factor, showed a 1.46- to 1.62-fold risk for UADT cancer in all the three models, whereas the cancers in the genitourinary site showed a significant protection with an OR of 0.67–0.81 in the three models (Figure 2, Supplementary Figure s1 and s2). Other tumour sites, however, did not show any significant association.
Discussion
Despite decades of public health programmes, TRCs remain the leading cause of cancer morbidity and mortality worldwide. Epidemiological studies over the past 50 years have clearly established how tobacco contributes to cancer risk not only in the directly exposed and anatomically related regions of the upper aerodigestive tract and lung but also in distant organs such as cervix, bladder, kidney, pancreas and so on (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2004). Tobacco is implicated as the single most important environmental factor for several TRCs (lung, head and neck, oesophagus, bladder, kidney and pancreas). It also confers significant risk for cancers even where viral or bacterial oncogenesis plays a predominant role (e.g., cervix, stomach, liver and nasopharynx). Weak genetic susceptibility in tobacco-exposed population is conferred by a large number of low-penetrance genes. However, there is paucity of systematic studies of all the important genes that may predispose to tobacco carcinogenesis.
The focus of research to elucidate genetic susceptibility to tobacco carcinogenesis has been on phase I and phase II detoxifying enzymes and to a lesser extent on the genes that regulate DNA repair, apoptosis and other relevant pathways. In contrast to the GST super family of enzymes that have been studied extensively for tobacco carcinogenesis (Nakajima et al, 1995; Cheng et al, 1999; Buch et al, 2002; Jhavar et al, 2004, 2005), other phase II metabolising enzymes such as the SULT have been less extensively studied (Hung et al, 2004; Dandara et al, 2006; Pachouri et al, 2006). There also has not been any collation of published data or a meta-analysis of case–control studies evaluating SULT enzyme in cancers.
Yasuda et al (2007) have reported that of the 11 known human cytosolic sulphotransferases, SULT1A1 is one of the four major SULT enzymes responsible for sulphation of tobacco carcinogens. The role of SULT1A1 in the biotransformation of tobacco carcinogens and its association with lung cancer has been previously reported (Liang et al, 2004). There are reports of SULT1A1*2 association with increased risk for oesophageal cancers (Dandara et al, 2006) as well as gastric cancer (Boccia et al, 2005) in individuals who consume alcohol and smoke tobacco.
To elucidate the effect of Arg213His polymorphism of SULT1A1 in tobacco users, we have studied a group of patients with multiple primary cancers, where at least one of the primary cancers was a TRC. We have postulated that as opposed to patients with a single primary TRC, those who develop multiple primary cancers are likely to show more pronounced gene–environment interactions (Kotnis et al, 2005). Hence, this may be a better clinical model to detect significant association of low-penetrance genes, even in smaller number of patients. In this case–control study, we show a strong association of the Arg213His polymorphism of SULT1A1 with the development of tobacco-related UADT cancers. These findings are further supported by the results of the meta-analysis examining the association of this polymorphism with cancer risk.
The meta-analysis also brings out the markedly lower mean frequency of SULT1A1 His213 in the Asian population as compared with the Caucasian population. However, it is possible that the variation is even larger if different Asian populations are taken in the study separately. We (Jhavar et al, 2004, 2005) and others from India (Buch et al, 2002; Anantharaman et al, 2007) have reported a markedly lower frequency of GSTT1 null genotype in the Indian population as compared with that in the Japanese, Chinese and Korean population (Raimondi et al, 2006). Although marked geoethnic variation in the incidence of different cancers is attributed largely to the differences in carcinogenic exposure and diet, marked differences in the population frequency of the risk-conferring genotype of some XMEs could also influence cancer risk.
The results of this meta-analysis are intriguing as they demonstrate opposite effects of SULT1A1 polymorphism on two distinct anatomical sites of TRCs. Thus in the meta-analysis, in contrast to the seven studies where UADT and lung cancers showed an increased risk association with SULT1A1*2, seven studies on genitourinary cancers showed a protective effect. This could perhaps be explained by the dual role of SULT1A1 in the bioactivation as well as detoxification of carcinogens (Glatt, 2000). Thus, detoxification of exogenous and endogenous carcinogens confers a protective effect for cancer (Glatt et al, 2001), whereas bioactivation of promutagens could increase the risk of certain cancers (Zheng et al, 2003; Tiemersma et al, 2004). The risk association of SULT1A1*2 with cancers of the UADT and lung is expected from its known role in tobacco detoxification (Al-Buheissi et al, 2006). Nowell et al (2004) has reported that SULT1A1 could contribute to prostate cancer risk, and the magnitude of the association may depend on ethnicity and meat consumption. It has been reported that the carcinogens are transferred to the kidney and ureter (Meinl et al, 2006) although their levels are substantially lower in the kidney than in the liver. However, it is difficult to explain the protective role of SULT1A1*2 His213 variants with cancer in the genitourinary cancers. Detailed biochemical studies in different human tissues, especially in the genitourinary vs UADT region, might explain the opposing tissue-specific effects of SULT1A1.
Another important aspect that has emerged from the meta-analysis is the difference in the risk of breast cancer conferred by SULT1A1*2 variant to Asian women compared with Caucasian women. A similar phenomenon has been reported for GSTM1 polymorphism. Carlsten et al (2008) have reported that although GSTM1 null status conferred a significantly increased risk of lung cancer to East Asians it did not confer increased risk to Caucasians. Thus, in distinct ethnic groups, risk for different cancers could be modulated by interaction between genetic variants and different endogenous and exogenous carcinogens.
There are several limitations in the present meta-analysis as is often the case. Contribution of possible sources of heterogeneity such as site of cancer, ethnicity, Hardy–Weinberg equilibrium, source of controls, sample size/power and carcinogen exposure were considered. However, meta-regression analysis demonstrated a significant heterogeneity due to ethnicity alone. This was also reflected in the allele frequency where Asians and Caucasians showed a striking difference. Hence, the actual source of heterogeneity could not be investigated due to the complexities of the confounding variables. In addition, meta-analysis in general looks at the crude OR instead of adjusted OR as the adjustment and matching factors differ across the studies. The residual confounders might have influenced our analysis.
This study encourages detailed biochemical investigation on the-tissue specific influence of SULT1A1 Arg213His enzyme in metabolism of tobacco carcinogens. This is the first meta-analysis that provides significant and contrasting association of SULT1A1 Arg213His polymorphism on cancer risk in distinct sites of TRCs namely UADT and genitourinary and an increased risk for breast cancer in Asians.
Acknowledgments
The work was supported in part by a grant from Department of Biotechnology (no. BT/PR5588/Med/12/231/2004 dated 25.3.2005), Government of India and Cancer Genetics Unit Research Fund (Tata Memorial Centre-1668). AK was supported by a Senior Research Fellowship of Council of Scientific and Industrial Research (India).
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Al-Buheissi SZ, Patel HR, Meinl W, Hewer A, Bryan RL, Glatt H, Miller RA, Phillips DH (2006) N-Acetyltransferase and sulfotransferase activity in human prostate: potential for carcinogen activation. Pharmacogenet Genomics 16: 391–399 [DOI] [PubMed] [Google Scholar]
- Anantharaman D, Chaubal PM, Kannan S, Bhisey RA, Mahimkar MB (2007) Susceptibility to oral cancer by genetic polymorphisms at CYP1A1, GSTM1 and GSTT1 loci among Indians: tobacco exposure as a risk modulator. Carcinogenesis 28: 1455–1462 [DOI] [PubMed] [Google Scholar]
- Bamber DE, Fryer AA, Strange RC, Elder JB, Deakin M, Rajagopal R, Fawole A, Gilissen RA, Campbell FC, Coughtrie MW (2001) Phenol sulphotransferase SULT1A1*1 genotype is associated with reduced risk of colorectal cancer. Pharmacogenetics 11: 679–685 [DOI] [PubMed] [Google Scholar]
- Bardakci F, Arslan S, Bardakci S, Binatli AO, Budak M (2008) Sulfotransferase 1A1 (SULT1A1) polymorphism and susceptibility to primary brain tumors. J Cancer Res Clin Oncol 134: 109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia S, Cadoni G, La Torre G, Arzani D, Volante M, Cattel C, Gianfagna F, Paludetti G, Almadori G, Ricciardi G (2006) A case–control study investigating the role of sulfotransferase 1A1 polymorphism in head and neck cancer. J Cancer Res Clin Oncol 132: 466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia S, Persiani R, La Torre G, Rausei S, Arzani D, Gianfagna F, Romano-Spica V, D’Ugo D, Ricciardi G (2005) Sulfotransferase 1A1 polymorphism and gastric cancer risk: a pilot case–control study. Cancer Lett 229: 235–243 [DOI] [PubMed] [Google Scholar]
- Buch SC, Notani PN, Bhisey RA (2002) Polymorphism at GSTM1, GSTM3 and GSTT1 gene loci and susceptibility to oral cancer in an Indian population. Carcinogenesis 23: 803–807 [DOI] [PubMed] [Google Scholar]
- Carlsten C, Sagoo GS, Frodsham AJ, Burke W, Higgins JP (2008) Glutathione S-transferase M1 (GSTM1) polymorphisms and lung cancer: a literature-based systematic HuGE review and meta-analysis. Am J Epidemiol 167(7): 759–774 [DOI] [PubMed] [Google Scholar]
- Chacko P, Rajan B, Mathew BS, Joseph T, Pillai MR (2004) CYP17 and SULT1A1 gene polymorphisms in Indian breast cancer. Breast Cancer 11: 380–388 [DOI] [PubMed] [Google Scholar]
- Cheng L, Sturgis EM, Eicher SA, Char D, Spitz MR, Wei Q (1999) Glutathione-S-transferase polymorphisms and risk of squamous-cell carcinoma of the head and neck. Int J Cancer 84: 220–224 [DOI] [PubMed] [Google Scholar]
- Cheng TC, Chen ST, Huang CS, Fu YP, Yu JC, Cheng CW, Wu PE, Shen CY (2005) Breast cancer risk associated with genotype polymorphism of the catechol estrogen-metabolizing genes: a multigenic study on cancer susceptibility. Int J Cancer 113: 345–353 [DOI] [PubMed] [Google Scholar]
- Choi JY, Lee KM, Park SK, Noh DY, Ahn SH, Chung HW, Han W, Kim JS, Shin SG, Jang IJ, Yoo KY, Hirvonen A, Kang D (2005) Genetic polymorphisms of SULT1A1 and SULT1E1 and the risk and survival of breast cancer. Cancer Epidemiol Biomarkers Prev 14: 1090–1095 [DOI] [PubMed] [Google Scholar]
- Dandara C, Li DP, Walther G, Parker MI (2006) Gene–environment interaction: the role of SULT1A1 and CYP3A5 polymorphisms as risk modifiers for squamous cell carcinoma of the oesophagus. Carcinogenesis 27: 791–797 [DOI] [PubMed] [Google Scholar]
- Doll R (1999) The Pierre Denoix Memorial Lecture: nature and nurture in the control of cancer. Eur J Cancer 35: 16–23 [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Jin M, Chen K, Zhang Y, Zhang S, Liu B (2007) Case-only study of interactions between metabolic enzymes and smoking in colorectal cancer. BMC Cancer 7: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt H (2000) Sulfotransferases in the bioactivation of xenobiotics. Chem Biol Interact 129: 141–170 [DOI] [PubMed] [Google Scholar]
- Glatt H, Boeing H, Engelke CE, Ma L, Kuhlow A, Pabel U, Pomplun D, Teubner W, Meinl W (2001) Human cytosolic sulphotransferases: genetics, characteristics, toxicological aspects. Mutat Res 482: 27–40 [DOI] [PubMed] [Google Scholar]
- Grabinski JL, Smith LS, Chisholm GB, Drengler R, Rodriguez GI, Lang AS, Kalter SP, Garner AM, Fichtel LM, Hollsten J, Pollock BH, Kuhn JG (2006) Genotypic and allelic frequencies of SULT1A1 polymorphisms in women receiving adjuvant tamoxifen therapy. Breast Cancer Res Treat 95: 13–16 [DOI] [PubMed] [Google Scholar]
- Han DF, Zhou X, Hu MB, Xie W, Mao ZF, Chen DE, Liu F, Zheng F (2005) Polymorphisms of estrogen-metabolizing genes and breast cancer risk: a multigenic study. Chin Med J (Engl) 118: 1507–1516 [PubMed] [Google Scholar]
- Hildebrandt MA, Carrington DP, Thomae BA, Eckloff BW, Schaid DJ, Yee VC, Weinshilboum RM, Wieben ED (2007) Genetic diversity and function in the human cytosolic sulfotransferases. Pharmacogenomics J 7: 133–143 [DOI] [PubMed] [Google Scholar]
- Hong WK, Lippman SM, Itri LM, Karp DD, Lee JS, Byers RM, Schantz SP, Kramer AM, Lotan R, Peters LJ, Dimery IW, Brown BW, Goepfert H (1990) Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med 323: 795–801 [DOI] [PubMed] [Google Scholar]
- Huber GF, Matthews TW, Dort JC (2007) Radiation-induced soft tissue sarcomas of the head and neck. J Otolaryngol 36: 93–97 [DOI] [PubMed] [Google Scholar]
- Hung RJ, Boffetta P, Brennan P, Malaveille C, Hautefeuille A, Donato F, Gelatti U, Spaliviero M, Placidi D, Carta A, Scotto di Carlo A, Porru S (2004) GST, NAT, SULT1A1, CYP1B1 genetic polymorphisms, interactions with environmental exposures and bladder cancer risk in a high-risk population. Int J Cancer 110: 598–604 [DOI] [PubMed] [Google Scholar]
- Hung RJ, Hall J, Brennan P, Boffetta P (2005) Genetic polymorphisms in the base excision repair pathway and cancer risk: a HuGE review. Am J Epidemiol 162: 925–942 [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2004) Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum 83: 1–1438 [PMC free article] [PubMed] [Google Scholar]
- Jerevall PL, Ahmadi A, Bergman M, Stal O, Wingren S (2005) Sulfotransferase1A1 and risk of postmenopausal breast cancer. Anticancer Res 25: 2515–2517 [PubMed] [Google Scholar]
- Jhavar S, Sarin R, Mulherkar R, Benner A, Agarwal JP, Dinshaw K (2004) Glutathione S-transferase M1 or T1 null genotype as a risk factor for developing multiple primary neoplasms in the upper aero-digestive tract, in Indian males using tobacco. Oral Oncol 40: 84–91 [DOI] [PubMed] [Google Scholar]
- Jhavar SG, Sarin R, Chopra S, Kotnis A, Mulherkar R, A’Hern R, Agarwal JP, Shrivastava SK, Dinshaw KA (2005) Females with paired occurrence of cancers in the UADT and genital region have a higher frequency of either Glutathione S-transferase M1/T1 null genotype. J Carcinog 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellen E, Zeegers M, Paulussen A, Van Dongen M, Buntinx F (2006) Fruit consumption reduces the effect of smoking on bladder cancer risk. The Belgian case–control study on bladder cancer. Int J Cancer 118: 2572–2578 [DOI] [PubMed] [Google Scholar]
- King AD, Ahuja AT, Yeung DK, Wong JK, Lee YY, Lam WW, Ho SS, Yu SC, Leung SF (2007) Delayed complications of radiotherapy treatment for nasopharyngeal carcinoma: imaging findings. Clin Radiol 62: 195–203 [DOI] [PubMed] [Google Scholar]
- Koppikar P, Mulherkar R (2006) A simple method for extraction of high molecular weight genomic DNA from buccal cells in mouthwash. Ind J Biotech 5: 477–481 [Google Scholar]
- Kotnis A, Sarin R, Mulherkar R (2005) Genotype, phenotype and cancer: role of low penetrance genes and environment in tumour susceptibility. J Biosci 30: 93–102 [DOI] [PubMed] [Google Scholar]
- Langsenlehner U, Krippl P, Renner W, Yazdani-Biuki B, Eder T, Wolf G, Wascher TC, Paulweber B, Weitzer W, Samonigg H (2004) Genetic variants of the sulfotransferase 1A1 and breast cancer risk. Breast Cancer Res Treat 87: 19–22 [DOI] [PubMed] [Google Scholar]
- Le Marchand L, Donlon T, Kolonel LN, Henderson BE, Wilkens LR (2005) Estrogen metabolism-related genes and breast cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 14: 1998–2003 [DOI] [PubMed] [Google Scholar]
- Liang G, Miao X, Zhou Y, Tan W, Lin D (2004) A functional polymorphism in the SULT1A1 gene (G638A) is associated with risk of lung cancer in relation to tobacco smoking. Carcinogenesis 25: 773–778 [DOI] [PubMed] [Google Scholar]
- Lilla C, Risch A, Kropp S, Chang-Claude J (2005) SULT1A1 genotype, active and passive smoking, and breast cancer risk by age 50 years in a German case–control study. Breast Cancer Res 7: R229–R237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilla C, Risch A, Verla-Tebit E, Hoffmeister M, Brenner H, Chang-Claude J (2007) SULT1A1 genotype and susceptibility to colorectal cancer. Int J Cancer 120: 201–206 [DOI] [PubMed] [Google Scholar]
- Magagnotti C, Pastorelli R, Pozzi S, Andreoni B, Fanelli R, Airoldi L (2003) Genetic polymorphisms and modulation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-DNA adducts in human lymphocytes. Int J Cancer 107: 878–884 [DOI] [PubMed] [Google Scholar]
- Meinl W, Pabel U, Osterloh-Quiroz M, Hengstler JG, Glatt H (2006) Human sulphotransferases are involved in the activation of aristolochic acids and are expressed in renal target tissue. Int J Cancer 118: 1090–1097 [DOI] [PubMed] [Google Scholar]
- Mikhailova ON, Gulyaeva LF, Prudnikov AV, Gerasimov AV, Krasilnikov SE (2006) Estrogen-metabolizing gene polymorphisms in the assessment of female hormone-dependent cancer risk. Pharmacogenomics J 6: 189–193 [DOI] [PubMed] [Google Scholar]
- Moreno V, Glatt H, Guino E, Fisher E, Meinl W, Navarro M, Badosa JM, Boeing H (2005) Polymorphisms in sulfotransferases SULT1A1 and SULT1A2 are not related to colorectal cancer. Int J Cancer 113: 683–686 [DOI] [PubMed] [Google Scholar]
- Nagar S, Walther S, Blanchard RL (2006) Sulfotransferase (SULT) 1A1 polymorphic variants *1, *2, and *3 are associated with altered enzymatic activity, cellular phenotype, and protein degradation. Mol Pharmacol 69: 2084–2092 [DOI] [PubMed] [Google Scholar]
- Nakajima T, Elovaara E, Anttila S, Hirvonen A, Camus AM, Hayes JD, Ketterer B, Vainio H (1995) Expression and polymorphism of glutathione S-transferase in human lungs: risk factors in smoking-related lung cancer. Carcinogenesis 16: 707–711 [DOI] [PubMed] [Google Scholar]
- Nowell S, Ambrosone CB, Ozawa S, MacLeod SL, Mrackova G, Williams S, Plaxco J, Kadlubar FF, Lang NP (2000) Relationship of phenol sulfotransferase activity (SULT1A1) genotype to sulfotransferase phenotype in platelet cytosol. Pharmacogenetics 10: 789–797 [DOI] [PubMed] [Google Scholar]
- Nowell S, Coles B, Sinha R, MacLeod S, Luke Ratnasinghe D, Stotts C, Kadlubar FF, Ambrosone CB, Lang NP (2002a) Analysis of total meat intake and exposure to individual heterocyclic amines in a case–control study of colorectal cancer: contribution of metabolic variation to risk. Mutat Res 506–507: 175–185 [DOI] [PubMed] [Google Scholar]
- Nowell S, Ratnasinghe DL, Ambrosone CB, Williams S, Teague-Ross T, Trimble L, Runnels G, Carrol A, Green B, Stone A, Johnson D, Greene G, Kadlubar FF, Lang NP (2004) Association of SULT1A1 phenotype and genotype with prostate cancer risk in African-Americans and Caucasians. Cancer Epidemiol Biomarkers Prev 13: 270–276 [DOI] [PubMed] [Google Scholar]
- Nowell S, Sweeney C, Winters M, Stone A, Lang NP, Hutchins LF, Kadlubar FF, Ambrosone CB (2002b) Association between sulfotransferase 1A1 genotype and survival of breast cancer patients receiving tamoxifen therapy. J Natl Cancer Inst 94: 1635–1640 [DOI] [PubMed] [Google Scholar]
- Nowell SA, Ahn J, Rae JM, Scheys JO, Trovato A, Sweeney C, MacLeod SL, Kadlubar FF, Ambrosone CB (2005) Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat 91: 249–258 [DOI] [PubMed] [Google Scholar]
- Ozawa S, Katoh T, Inatomi H, Imai H, Kuroda Y, Ichiba M, Ohno Y (2002) Association of genotypes of carcinogen-activating enzymes, phenol sulfotransferase SULT1A1 (ST1A3) and arylamine N-acetyltransferase NAT2, with urothelial cancer in a Japanese population. Int J Cancer 102: 418–421 [DOI] [PubMed] [Google Scholar]
- Pachouri SS, Sobti RC, Kaur P, Singh J, Gupta SK (2006) Impact of polymorphism in sulfotransferase gene on the risk of lung cancer. Cancer Genet Cytogenet 171: 39–43 [DOI] [PubMed] [Google Scholar]
- Peng CT, Chen JC, Yeh KT, Wang YF, Hou MF, Lee TP, Shih MC, Chang JY, Chang JG (2003) The relationship among the polymorphisms of SULT1A1, 1A2 and different types of cancers in Taiwanese. Int J Mol Med 11: 85–89 [PubMed] [Google Scholar]
- Pereira WO, Paiva AS, Queiroz JW, Toma L, Dietrich CP, Nader HB, Jeronimo SM (2005) Genetic polymorphism in the sulfotransferase SULT1A1 gene in cancer. Cancer Genet Cytogenet 160: 55–60 [DOI] [PubMed] [Google Scholar]
- Raftogianis RB, Wood TC, Otterness DM, Van Loon JA, Weinshilboum RM (1997) Phenol sulfotransferase pharmacogenetics in humans: association of common SULT1A1 alleles with TS PST phenotype. Biochem Biophys Res Commun 239: 298–304 [DOI] [PubMed] [Google Scholar]
- Raftogianis RB, Wood TC, Weinshilboum RM (1999) Human phenol sulfotransferases SULT1A2 and SULT1A1: genetic polymorphisms, allozyme properties, and human liver genotype–phenotype correlations. Biochem Pharmacol 58: 605–616 [DOI] [PubMed] [Google Scholar]
- Raimondi S, Paracchini V, Autrup H, Barros-Dios JM, Benhamou S, Boffetta P, Cote ML, Dialyna IA, Dolzan V, Filiberti R, Garte S, Hirvonen A, Husgafvel-Pursiainen K, Imyanitov EN, Kalina I, Kang D, Kiyohara C, Kohno T, Kremers P, Lan Q, London S, Povey AC, Rannug A, Reszka E, Risch A, Romkes M, Schneider J, Seow A, Shields PG, Sobti RC, Sorensen M, Spinola M, Spitz MR, Strange RC, Stucker I, Sugimura H, To-Figueras J, Tokudome S, Yang P, Yuan JM, Warholm M, Taioli E (2006) Meta- and pooled analysis of GSTT1 and lung cancer: a HuGE-GSEC review. Am J Epidemiol 164: 1027–1042 [DOI] [PubMed] [Google Scholar]
- Sellers TA, Schildkraut JM, Pankratz VS, Vierkant RA, Fredericksen ZS, Olson JE, Cunningham J, Taylor W, Liebow M, McPherson C, Hartmann LC, Pal T, Adjei AA (2005) Estrogen bioactivation, genetic polymorphisms, and ovarian cancer. Cancer Epidemiol Biomarkers Prev 14: 2536–2543 [DOI] [PubMed] [Google Scholar]
- Seth P, Lunetta KL, Bell DW, Gray H, Nasser SM, Rhei E, Kaelin CM, Iglehart DJ, Marks JR, Garber JE, Haber DA, Polyak K (2000) Phenol sulfotransferases: hormonal regulation, polymorphism, and age of onset of breast cancer. Cancer Res 60: 6859–6863 [PubMed] [Google Scholar]
- Shatalova EG, Walther SE, Favorova OO, Rebbeck TR, Blanchard RL (2005) Genetic polymorphisms in human SULT1A1 and UGT1A1 genes associate with breast tumor characteristics: a case-series study. Breast Cancer Res 7: R909–R921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks R, Ulrich CM, Bigler J, Tworoger SS, Yasui Y, Rajan KB, Porter P, Stanczyk FZ, Ballard-Barbash R, Yuan X, Lin MG, McVarish L, Aiello EJ, McTiernan A (2004) UDP-glucuronosyltransferase and sulfotransferase polymorphisms, sex hormone concentrations, and tumor receptor status in breast cancer patients. Breast Cancer Res 6: R488–R498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M, Bastian M, Schulz WA, Pulte T, Franke KH, Rohring A, Wolff JM, Seiter H, Schuff-Werner P (2000) Phenol sulphotransferase SULT1A1 polymorphism in prostate cancer: lack of association. Arch Toxicol 74: 222–225 [DOI] [PubMed] [Google Scholar]
- Sterne JA, Juni P, Schulz KF, Altman DG, Bartlett C, Egger M (2002) Statistical methods for assessing the influence of study characteristics on treatment effects in ‘meta-epidemiological’ research. Stat Med 21: 1513–1524 [DOI] [PubMed] [Google Scholar]
- Sun XF, Ahmadi A, Arbman G, Wallin A, Asklid D, Zhang H (2005) Polymorphisms in sulfotransferase 1A1 and glutathione S-transferase P1 genes in relation to colorectal cancer risk and patients’ survival. World J Gastroenterol 11: 6875–6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Rundle A, Mooney L, Cho S, Schnabel F, Estabrook A, Kelly A, Levine R, Hibshoosh H, Perera F (2003) Sulfotransferase 1A1 (SULT1A1) polymorphism, PAH-DNA adduct levels in breast tissue and breast cancer risk in a case–control study. Breast Cancer Res Treat 78: 217–222 [DOI] [PubMed] [Google Scholar]
- Tiemersma EW, Bunschoten A, Kok FJ, Glatt H, de Boer SY, Kampman E (2004) Effect of SULT1A1 and NAT2 genetic polymorphism on the association between cigarette smoking and colorectal adenomas. Int J Cancer 108: 97–103 [DOI] [PubMed] [Google Scholar]
- Tsukino H, Kuroda Y, Nakao H, Imai H, Inatomi H, Osada Y, Katoh T (2004) Cytochrome P450 (CYP) 1A2, sulfotransferase (SULT) 1A1, and N-acetyltransferase (NAT) 2 polymorphisms and susceptibility to urothelial cancer. J Cancer Res Clin Oncol 130: 99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Spitz MR, Tsou AM, Zhang K, Makan N, Wu X (2002) Sulfotransferase (SULT) 1A1 polymorphism as a predisposition factor for lung cancer: a case–control analysis. Lung Cancer 35: 137–142 [DOI] [PubMed] [Google Scholar]
- Wu MT, Wang YT, Ho CK, Wu DC, Lee YC, Hsu HK, Kao EL, Lee JM (2003) SULT1A1 polymorphism and esophageal cancer in males. Int J Cancer 103: 101–104 [DOI] [PubMed] [Google Scholar]
- Yang G, Gao YT, Cai QY, Shu XO, Cheng JR, Zheng W (2005) Modifying effects of sulfotransferase 1A1 gene polymorphism on the association of breast cancer risk with body mass index or endogenous steroid hormones. Breast Cancer Res Treat 94: 63–70 [DOI] [PubMed] [Google Scholar]
- Yasuda S, Idell S, Fu J, Carter G, Snow R, Liu MC (2007) Cigarette smoke toxicants as substrates and inhibitors for human cytosolic SULTs. Toxicol Appl Pharmacol 221: 13–20 [DOI] [PubMed] [Google Scholar]
- Zheng L, Wang Y, Schabath MB, Grossman HB, Wu X (2003) Sulfotransferase 1A1 (SULT1A1) polymorphism and bladder cancer risk: a case–control study. Cancer Lett 202: 61–69 [DOI] [PubMed] [Google Scholar]
- Zheng W, Xie D, Cerhan JR, Sellers TA, Wen W, Folsom AR (2001) Sulfotransferase 1A1 polymorphism, endogenous estrogen exposure, well-done meat intake, and breast cancer risk. Cancer Epidemiol Biomarkers Prev 10: 89–94 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.