Abstract
L-DOPA-induced dyskinesia (LID) is one of the main limitations of long term L-DOPA use in Parkinson’s disease (PD) patients. We show that chronic L-DOPA treatment induces novel dyskinetic behaviors in aphakia mouse with selective nigrostriatal deficit mimicking PD. The stereotypical abnormal involuntary movements were induced by dopamine receptor agonists and attenuated by antidyskinetic agents. The development of LID was accompanied by preprodynorphin and preproenkephalin expression changes in the denervated dorsal striatum. Increased FosB-expression was also noted in the dorsal striatum. In addition, FosB expression was noted in the pedunculopontine nucleus and the zona incerta, structures previously not examined in the setting of LID. The aphakia mouse is a novel genetic model with behavioral and biochemical characteristics consistent with those of PD dyskinesia and provides a more consistent, convenient, and physiologic model than toxic lesion models to study the mechanism of LID and to test therapeutic approaches for LID.
Keywords: L-DOPA, Dyskinesia, Parkinson’s disease, FosB, Striatum, aphakia mouse
Introduction
L-DOPA-induced dyskinesia (LID) is one of the most significant limitations of chronic L-DOPA treatment in Parkinson’s disease (PD) patients, and extensive investigations have been undertaken to understand the mechanism of LID (Linazasoro, 2005) using both primate (Marin et al., 2006; Boyce et al., 1990a) and rodent models of PD (Cenci et al., 1998; Andersson et al., 1999; Lundblad et al., 2004; Winkler et al., 2002; Johnston et al., 2005; Kovoor et al., 2005; Picconi, et al. 2003). Primate models show behaviors that are closest to human LID (Marin et al., 2006), but the logistics of using primates limit the ability to use primates widely. Recent hemi-Parkinsonian rodent models created by unilateral toxic lesions of the nigrostriatal projection (Cenci, et al. 1998; Andersson et al., 1999; Lundblad et al., 2004; Lundblad et al., 2002) allow a more accessible model to study LID, but they have the limitation in generating consistent and selective lesions without high mortality (Barneoud et al., 2000; Chang et al., 1999; Truong et al., 2006). Pharmacological depletion of monoamines by reserpine has been proposed as a model to study LID, and has the advantage of a fast screening tool. However, this is an acute model whereas LID typically develops over time and the depletion of monoamine is more extensive than seen in PD (Johnston et al., 2005; Kovoor et al., 2005). An ideal model of LID should have: (1) selective loss of nigrostriatal lesions mimicking PD; (2) consistent and stable degree of lesion with minimal recovery, variability, and mortality; (3) high percentage of animals developing dyskinesia; (4) differentiation of acute and chronic L-DOPA effects on the behaviors.
The aphakia mice selectively lose dopamine neurons in the midbrain, especially in the substantia nigra (SN) as a consequence of a naturally occurring deletion of the promoter region and the noncoding exon 1 of the Pitx3 gene (Hwang et al., 2003; Nunes et al., 2003; van den Munckhof et al., 2003; Smidt et al., 2004). Consistent with the loss of DA neurons in the SN, there is significant DA denervation in the dorsal striatum (Hwang et al., 2003), which results in the motor deficits that are rescued by L-DOPA (Hwang et al., 2003; van den Munckhof et al., 2006). In addition, adaptation to the denervation and evidence for the supersensitivity of striatal dopamine receptors (DR) were noted (Hwang et al., 2005; van den Munckhof et al., 2006). Therefore, the aphakia mouse provides an animal model that has a consistent degree of lesion selectively of the nigrostriatal system without significant variability or mortality associated with toxic lesions (Hwang et al., 2003; van den Munckhof et al., 2003). Aphakia mice show evidence of striatal neuroadaptation to denervation of dopaminergic afferents both behaviorally and biochemically. Thus, we hypothesized that the aphakia mouse will develop dyskinetic behaviors with L-DOPA treatment. We present their behavioral and biochemical characteristics after chronic L-DOPA therapy. In addition, we report involvement of novel structures where changes in gene expression are associated with the presence of dyskinesia.
Materials and methods
Animals and drug treatment
The aphakia allele arose spontaneously in 1981 on the 129S1/Sv-p+ Tyr+ KitlSl-J/J strain at the Jackson Laboratory (Bar Harbor, ME) and was maintained in a C57BL background. Wild type C57BL/6 mice and mice homozygous for the retinal degeneration (rd1 or Pde6brd1) mutation in C57BL/6J background were used as control groups. All procedures of the experiments conducted were approved by IACUC of University of Chicago.
In experiment 1, six week old wild type (C57BL/6, Jackson laboratory) and aphakia mice were randomly divided into two groups in each genotype. One group received L-DOPA (Sigma, St. Louis, MO) twice daily for up to 7 weeks (n=10–11 of either genotype), while the second group received saline only for the same period of time (n=10 for both wild type and aphakia mice each). In the first week, the L-DOPA group received 10 mg/kg L-DOPA intraperitoneally (ip). Then from day 8, the dose of L-DOPA was increased to 25 mg/kg. Benserazide (12.5 mg/kg, Sigma, St. Lous, MO) was added to block peripheral conversion of L-DOPA in all experiments. The cylinder tests were performed 1 hr after L-DOPA challenge unless described specifically as we have seen the peak of behavioral effects at this point in unilateral PD mice with C57BL/6 background (Ding et al., 2003), and videotaped on day 1, day 4, day 7, day 8 and then once a week thereafter. Additional behavioral tests were performed as described below. The wild type mice only received chronic L-DOPA for 24 days because there was no noticeable behavior change observed during the repeated tests. Details about the behavioral test are described below.
During the last two week period of chronic treatment for aphakia mice, additional behavioral tests were performed using various reagents with or without L-DOPA. The reagents used alone for the behavioral test were amphetamine (2 mg/kg, Sigma), D2 dopamine receptor (DR) agonist quinpirole (0.5 mg/kg, RBI) and full D1 DR agonist SKF81297 (8 mg/kg, Sigma). Behavioral tests were performed 1 hr after quinpirole and 30 min after SKF81297 and amphetamine. The behavioral tests for buspirone, amantadine and ifenprodil (NR2B NMDA receptor antagonist, 10 mg/kg, Sigma) were performed during the last week of the chronic L-DOPA treatment. Buspirone and ifenprodil were given 30 min, while amantadine was injected 100 min before L-DOPA. For all pharmaceutical tests, the video was taken at baseline, immediately before and 1 hr after L-DOPA. On the day before sacrifice, the saline groups (n=10 for either genotype) were further divided into two groups: One received a single injection of L-DOPA (25 mg/kg), while the second group received only saline.
In experiment 2, blind mice with retinal degeneration (rd1 or Pde6brd1; The Jackson Laboratory, n=9) and aphakia mice (n = 10) at the age of 6 weeks old were treated with L-DOPA at the dose of 10 mg/kg for days 1–7, and then 25 mg/kg b.i.d. thereafter for an additional 4 weeks. Behavioral tests including both cylinder and balance beam (Hwang et al., 2005) were conducted on days 1, 4, 7, 14, 21, 28 and 35.
In experiment 3, six week old aphakia mice were divided into two groups. The first group (n=5) received chronic L-DOPA (25 mg/kg, b.i.d.), as in the first three weeks of previous experiments, while the second group (n=5) received the specific adenosine 2A receptor antagonist, KW-6002 (kindly provided by Jacques Petzer, North-West University, Potchefstroom, South Africa), at the dose of 3 mg/kg, once daily by i.p. for 3 weeks. Cylinder tests were performed on day 21 following chronic treatment with either L-DOPA or KW-6002. On day 22, the chronic L-DOPA group received acute KW-6002 (3 mg/kg) 20 min prior to L-DOPA, while L-DOPA (25 mg/kg) was given 20 min after KW-6002 for the chronic KW-6002 group. 1 hr after L-DOPA, cylinder tests were performed and videotaped for further behavioral analysis.
Behavioral tests and video analysis
Animals were placed in a clear plastic cylinder (10 cm in diameter and 12.5 cm in height) and videotaped for 3 min. Mirrors behind the cylinder were used to capture the behaviors when animals turned away from the camera angle. During the testing period, two procedures were employed occasionally to test distractability of the behaviors. Regular food pellets were introduced into the cylinder and the response of the animals was monitored. Two mice were placed in the same testing cylinder to note the effect of the social interaction on the behaviors. All of the behavioral tests were done between 10:00 am and 2:00 pm.
1. Cylinder test
Out of the 3 minute video recording of the cylinder test, only the middle two minutes were analyzed. The behaviors analyzed in the cylinder test included the frequency and duration of rearing and abnormal paw movements. Rearing movement was scored when mice reared on the two hind paws and raised to more than a half of their full body height. Abnormal paw movements consisted of three different categories, i.e. front paw dyskinesia, hind paw touch, and three paw dyskinesia. Front paw dyskinesia was noted when mice stood on both hind paws, with both front paws moving repeatedly up and down along the surface of the cylinder wall. Hind paw touch was noted when mice stood on their hind paws and repeatedly touched the surface of the cylinder wall with one hind paw. Three paw dyskinesia was noted when mice stood on their hind paws close to the wall of cylinder moving both front paws as noted above and repeatedly lifting the hind paws up and down in an alternating fashion while bearing their weight on the other hind paw. Under extreme circumstances, a few mice showed rapid up and down movements of front paws and both hind paws with their tail supporting their body weight. We labeled this as four paw dyskinesia, but did not include it in the final results of the behavioral tests because these behaviors were not frequent enough to analyze statistically. Mice that received amphetamine were videotaped at 60 min after injection. Instead of rearing and abnormal paw movements, these mice showed persistent oral stereotypy behavior. The total duration of the oral stereotypy was recorded.
2. Challenging beam test
The details of this procedure has been described previously (Fleming et al., 2004). Briefly, the beam (length, 1 m) started at a width of 3.5 cm and gradually narrowed to 0.5 cm in 1 cm increments. Animals were trained to traverse the length of the beam, starting at the widest section and ending at the narrowest section. Animals received 4 days of training before testing, and all training was performed without the mesh grid. On the day of the test, a mesh grid (1 cm square) of corresponding width was placed over the beam surface, leaving approximately a 1 cm space between the grid and the beam surface. Animals were videotaped while traversing the grid-surfaced beam for a total of five trials. Videotapes were viewed and scored for the time to traverse the beam over five trials by an investigator blind to the mouse genotype and drug treatment. Mean scores from the 5 trials were used for the analysis.
Tissue preparation
The mice were perfused through the ascending aorta with 4% paraformaldyde in 0.1 M phosphate buffer (pH 7.3) 24 hr after the last (acute) injection of L-DOPA or saline. The brains were post fixed overnight before being transferred into 30% sucrose. Serial coronal sections from the rostral striatum through the caudal extent of pedunculopontine nucleus (PPN) were made with a sliding microtome (Leica Jung Histoslide 2000) at 25 μm, collected into cryoprotectant and stored at −20 °C for future analysis. Some serial sections were mounted onto Superfrost/Plus microscope slides (Fisher Scientific, PA) and dried at room temperature before storage at −80 °C for in situ hybridization. Every eighth section through out the striatum and diencephalon and every other section of the brainstem were analyzed for immunohistochemistry as noted below.
Immunohistochemistry
Sections were first incubated in 0.5% Triton X-100 and 5% normal goat serum in 0.01 M PBS for 1 hr at RT before rabbit anti-FosB (1:500. Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or mouse anti-tyrosine hydroxylase (TH) antibody (1:500, Chemicon International, Temecula, CA) was applied for 72 hr at 4°C. After several washes in 0.01 M PBS, biotinylated anti-rabbit IgG (1:200, Vector Laboratories, Burlingame, CA) was applied for 2 hrs at RT followed by avidin–biotinylated peroxidase complex (Vectastain Elite ABC kit; Vector Laboratories) and diaminobenzidine (Sigma, Saint Louis, MS) as the developing agent. After immunohistochemistry, sections were mounted and coverslipped for analysis. The FosB antibody used in the current experiment recognizes both PAN FosB and all of its spliced forms of ΔFosB (Andersson et al., 1999). Only sections from WT and aphakia mice receiving chronic saline and chronic L-DOPA treatment were processed for FosB immunohistochemistry.
In situ hybridization
A 496-base pair fragment of the mouse preprodynorphin (Pdyn) cDNA (containing the sequence from 120 to 614 nucleotides, GenBank Acc. # U64968) and a 686-base pair fragment of the mouse preproenkephalin (PPE) cDNA (containing the sequence from 342 to 1025 nucleotides, GenBank Acc. # M13227) were obtained by PCR and cloned into the pCRs2.1-TOPOs plasmid (Invitrogen). The S35 labeled antisense RNA probes were prepared using in vitro transcription from linearized plasmids and purification kits (Promega Cat # P1450 and Promega Cat# Z3101, respectively). S35-UTP was purchased from Amersham (Catalog #: SJ130).
Sections mounted onto glass slides (SuperFrost, Fisher Scientific, PA) were first treated with 4% paraformaldehyde for 20 min after warming up to room temperature from −80°C. After 3 washes in PBS, 5 min each, sections were treated with proteinase K (5 μg/ml) for 20 min at RT. Then, the sections were treated with 0.1 M TCA and 0.01 M acetic anhydride for 10 min at RT after one wash in 0.5x SSC. Following incubation in 2x SSC for 5 min, sections were loaded with prehybridization solution (10 mM DTT, 0.3 M NaCl, 20 mM Tris-HCl, 5 mM EDTA, 1x Denhardt’s, 10% Dextran sulfate and 50% formamide) and incubated at 55°C for 3 hr. Next, hybridization solution composed of 0.2 mg/ml yeast tRNA and 6×105 cpm S35 labeled antisense preprodynorphin or preproenkephalin RNA probe was added onto sections and hybridization was performed over night at 55°C.
After hybridization, sections were rinsed with the following solutions in order: twice in 2x SSC for 10 min each, 20 ug/ml RNAse A for 40 min at RT, twice in 2x SSC containing for 10 min each, twice in 1x SSC for 10 min each, twice in 0.1x SSC for 10 min each at 55°C, twice in 0.5x SSC for 10 min each, 3 min in 50% ethanol, 3 min in 70% ethanol, 3 min in 70% ethanol and 3 min in 95% ethanol. All solutions described above contained 0.1% β-mercaptoethanol. After the sections were dried, they were exposed to Kodak BioMax MR film for 18 to 72 hr before development.
Image analysis
For the analysis of FosB expression in the striatum, one picture at the coordinate (Bregma 1.1 mm, see Franklin and Paxinos, 1997) was taken with a 10× objective from left and right striatum to cover the region with TH denervation in the dorsal striatum as shown in Fig 1. Then, the number of positive FosB expressing nuclei was counted separately in an area of 700×400 pixels from both left and right striatum by using the program MetaMorph (version 5.01). The number of FosB positive nuclei from both striatal sides was averaged and used as one data point for each mouse. For FosB expression in PPN, pictures covering the entire PPN were taken from serial coronal sections of each animal and the total number of positive FosB-immunoreactive nuclei from both left and right side were counted and added together as one sample.
Fig 1.

Selective dopaminergic denervation of the striatum (A–C) and the loss of dopaminergic neurons in the substantia nigra (D–I and D′–I′) in aphakia mice. The TH-immunoreactive fibers in the striatum are shown in three different planes representing rostral (A), middle (B) and caudal (C) striatum. TH-immunoreactive cells from the substantia nigra at six representative coronal planes show the loss of TH neurons, especially in the caudal substantia nigra (D–I) compared to the intact SN from the matched planes in the control RD mice (D′–I′). Scale bars are 500 μm for A–C and 100 μm for D–I and D′–I′.
Sections at the similar coordinate for the analysis of striatal FosB were also used for in situ analysis. The average optical intensity of areas located in the most dorsal striatum, i.e., the basal line of this area was the top one third of the lateral wall of the lateral ventricle and the top one quarter of the lateral border of the striatum, was obtained by using the MetaMorph image processing program. This area was chosen because: (1) Only this area showed the most significant TH positive fiber loss when compared to the other area of the striatum; (2) Only this area showed FosB expression in the chronic L-DOPA treated aphakia mice; and (3) Preliminary examination of the in situ signal showed that this area displayed substantial differences in the in situ signal for preprodynorphin.
Statistical analysis
SigmaStat (Version 2.03) was used for the statistical analysis. One way or two-way ANOVA followed by Tukey post hoc test was usually performed. p<0.05 was considered statistically significant.
Results
Selective denervation of dopaminergic afferents in the dorsal striatum of aphakia mice
In order to study the dyskinetic behaviors and the biochemical changes produced by L-DOPA treatment, it is essential to define the exact areas and the degree of dopamine denervation in the striatum of aphakia mice. The TH-immunoreactive fibers were almost completely absent in the dorsal striatum with a relative sparing in the ventral striatum (Fig. 1A–C) similar to the results of selective dopaminergic neuron loss in the substantia nigra as we previously reported (Hwang et al., 2005; van den Munckhof et al., 2006). The denervation of TH-immunoreactive fibers was noted throughout the whole dorsal striatum from rostral to caudal extent as shown in three representative coronal sections (Fig 1, A–C). The number of DA neurons in the substantia nigra of aphakia mice (Fig 1D–I) is significantly reduced compared to that of control RD mice (Fig 1D′–I′) as shown at several different coronal planes throughout the SN, which is consistent with our earlier findings (Hwang et al., 2005) and those of others (Nunes et al., 2003; Smidt et al., 2004).
L-DOPA improves akinesia in aphakia mice
We first determined doses of L-DOPA that could ameliorate the parkinsonian akinesia in aphakia mice so that these physiological doses could be used to study dyskinesia. We have previously described motor behavioral deficits in aphakia mice that were reversed with 25 mg/kg L-DOPA treatment (Hwang et al., 2005). We also used a lower dose of 10 mg/kg in this study and noted a similar effect of both doses on akinesia as noted below. Two different motor behavioral tests that were shown to be sensitive to dopamine-dependent motor functions in our previous study were employed. Rearing represents general activity level and may be more specific for dopaminergic status than horizontal activities (Zhuang et al., 2001). The balance beam test is a good indicator of fine motor coordination. The animals were first treated with 10 mg/kg for one week, and then the dose was increased to 25 mg/kg twice a day for 4 more weeks to increase the chance of developing dyskinesia (Fig. 2A). Low dose L-DOPA at 10 mg/kg increased rearing activity without further change with repeated testing for a week, while increasing the dose of L-DOPA to 25 mg/kg from the second week of treatment impaired rearing activity (Fig. 2A). This was due to the development of dyskinetic behaviors as noted below (Fig. 3). To determine whether this was due to the higher dose or increased response to repetitive treatment, naïve animals were exposed to 25 mg/kg L-DOPA (Fig. 2A). The response to the first dose of 25 mg/kg was similar to 10 mg/kg, indicating that this reduction of rearing was due to dyskinesia rather than the dose. In order to assess the akinesia improvement further, a fine motor activity was tested using a balance beam (Hwang et al., 2005). L-DOPA improved the balance beam performance of aphakia mice throughout the experimental period (Fig 2B). L-DOPA did not produce any locomotion changes in the rearing test in C57BL/6 mice (data not shown) nor in the balance beam test in control blind RD mice (Fig 2B).
Fig 2.
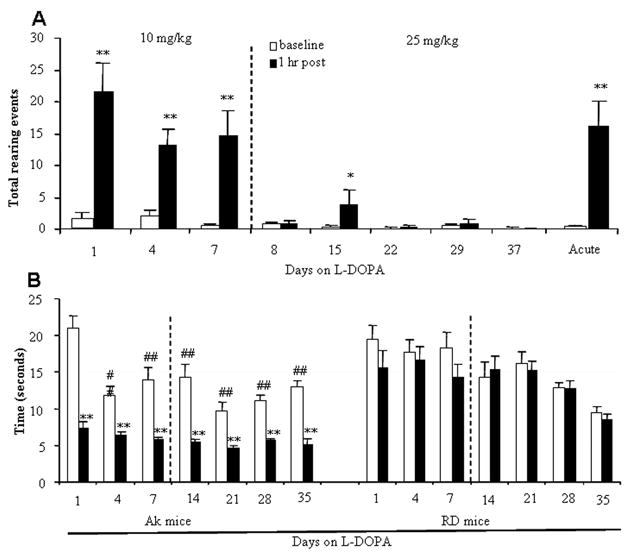
L-DOPA attenuated akinesia in aphakia mice measured by two different behavioral tests. A. Rearing activity before and one hour after acute L-DOPA. Mice were treated twice a day with 10 mg/kg of L-DOPA for one week and then with 25 mg/kg from day 8 to day 37 (n=11). A separate group of mice (n= 5) were treated with 25 mg/kg of L-DOPA for the first time to compare with the chronic treatment with 25 mg/kg. B. Time to cross the Balance Beam. The beam test was performed before and one hour post injection. Blind RD mice were used as a control. N=9–10 each group. *, p<0.05 and **, p<0.01 vs. baseline before L-DOPA. #, p<0.05 and ##, p<0.01 vs. the first day of L-DOPA treatment.
Fig 3.
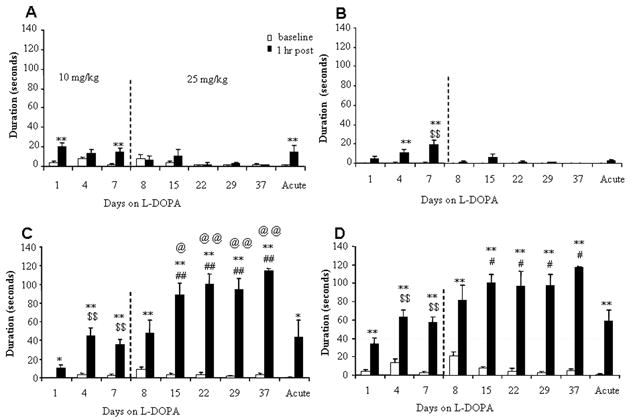
Duration of abnormal paw movements induced by L-DOPA in aphakia mice. The abnormal paw movements were classified into front paw dyskinesia (A), hind paw touch (B), and three paw dyskinesia (C), and total dyskinesia scores were computed as the total time animals spent in any of these movements (D). The animals were initially treated with 10 mg/kg of L-DOPA and the dose was increased to 25 mg/kg from day 8 as noted in Fig. 2. One half of the saline group received a single dose of 25 mg/kg L-DOPA before sacrifice to compare the effect of single dose of L-DOPA to that of the chronic L-DOPA. These periods are divided by a vertical line in the graph. n=5–11. *, p<0.05 and **, p<0.01 vs. baseline. $$, p<0.01 vs 1 hr post on day 1 of the first L-DOPA dose. #, p<0.05 and ##, p<0.01 vs. 1 hr post L-DOPA in the acute group given L-DOPA 25 mg/kg for the first time. @, p<0.05 and @@, p<0.01 vs. 1 hr post L-DOPA on day 8 of the first 25 mg/kg dose.
L-DOPA treatment induces abnormal involuntary movements in aphakia mice
We observed various types of L-DOPA-induced abnormal involuntary movements in aphakia mice as described in detail in the methods. One type of abnormal behavior was noted immediately after the first exposure to L-DOPA treatment. L-DOPA produced abnormal paw movements, such as front paw dyskinesia movement (Fig. 3A), which were rarely observed in aphakia mice without L-DOPA (Supplementary material, Table 2) and never seen in RD or in WT C57BL/6 control mice with L-DOPA (Supporting data, Table 1). Repeated L-DOPA treatment did not increase the duration of front paw dyskinesia (Fig. 3A). Hind paw touch on the wall was rarely present without L-DOPA or with the first dose of L-DOPA, but increased by 7 days of treatment (Fig. 3B). With repeated administration of L-DOPA, both front paw dyskinesia and hind paw touch were replaced by three paw dyskinesia, which consisted of simultaneous dyskinesia of both front paws and one hind paw on either side (see Supplementary material, video 1) (Fig. 3C). When treatment with the higher L-DOPA dose was continued, further increase in the duration of three paw dyskinesia was noted from day 8 to day 15, and the front paw dyskinesia and hind paw touch decreased in duration. This decrease was mainly due to replacement of these behaviors by three paw dyskinesia since three paw dyskinesia was present most of the time during recording, leaving little time for other movements. As seen in Fig. 3C, the three paw dyskinesia lasted 89–114 seconds out of the 120 seconds analyzed. Likewise, rearing activity decreased significantly as three paw dyskinesia increased to occupy most of the time (Fig. 2B). For wild type mice, neither acute nor long term (24 days) chronic L-DOPA treatment induced any noticeable abnormal paw movements (Supplementary material, Table 1). These behaviors, including front paw dyskinesia, hind paw touch, and three paw dyskinesia, were rarely observed in aphakia mice receiving chronic saline treatment (Supplementary material, Table 2). Occasionally, the dyskinetic mice showed four paw dyskinesia with their body weight supported only by the tail. This behavior was rare and only seen in a few dyskinetic aphakia mice, and therefore the data were not analyzed statistically.
We attempted to characterize the nature of three paw dyskinesia further by behavioral perturbation in the third week with 25 mg/kg L-DOPA. When food pellets were dropped into the cylinder during the test, the dyskinetic mice did not show any interruption from three paw dyskinesia. The dyskinetic mice often stood over the food pellet engaged in three paw dyskinesia completely ignoring the food. When two animals were put into the same cylinder, they did not show any interaction, but continued with three paw dyskinesia independently without interruption. The dyskinetic mice were also been placed into a larger cylinder or left in the home cage to determine whether the confined nature of the cylinder was responsible for the behaviors directed on the wall. The mice walked over to a wall, and started three paw dyskinesia as soon as they reached the wall.
Pharmacological characterization of dyskinesia
Dyskinesia was also characterized pharmacologically. Three paw dyskinesia was also induced by the full D1 DR agonist SKF81297 (8 mg/kg) and by the selective D2 DR agonist quinpirole (0.5 mg/kg) in aphakia mice that received chronic L-DOPA (Fig. 4A). Combined together, the results suggest that both D1 and D2 receptor activation mediates three paw dyskinesia in aphakia mice. We then tested compounds that have been shown to relieve L-DOPA-induced dyskinesia in patients and in animal models of dyskinesia. The glutamate NMDA antagonist, amantadine (60 mg/kg), and the 5HT-1A receptor agonist, buspirone (2 mg/kg), have both been shown to be effective in reducing L-DOPA-induced dyskinesia in PD patients (Del Dotto et al., 2001; Fox et al., 2002; Pahwa et al., 2006), PD monkeys (Blanchet et al., 1998) and 6-hydroxydopamine-induced hemi-parkinsonian rodents (Lundblad et al., 2002; Lundblad et al., 2005). A selective NR2B antagonist, ifenprodil has also shown to be effective in reducing dyskinesia (Nash et al., 2000; Steece-Collier et al., 2000; Gardoni et al., 2006). We found that all of the drugs tested including buspirone, amantadine and ifenprodil significantly attenuated L-DOPA induced three paw dyskinesia in the dyskinetic aphakia mice (Fig 4A). The baselines of three paw dyskinesia were similar before the administration of the anti-dyskinetic agents and L-DOPA (data not shown).
Fig 4.

Pharmacological responses of aphakia mice chronically treated with L-DOPA. A. Three paw dyskinesia in response to dopamine receptor agonists or L-DOPA with anti-dyskinetic drugs. B: The effect of acute amphetamine (2 mg/kg) on three paw dyskinesia and stereotypic behavior in aphakia mice receiving chronic L-DOPA. N=11 *, p<0.05 and **, p< 0.01 vs. baseline. $, p<0.05 and $$, p<0.01 vs. 1 hr post L-DOPA.
Stereotypic behaviors can be seen after dopaminergic stimulation. Psychostimulants such as amphetamine release dopamine from dopaminergic terminals and usually induce stereotypy behavior when used either acutely at high doses or repeatedly at both low and high doses. Amphetamine 2 mg/kg, which induces significant hyperactivity in normal mice (Rahman et al., 2003) did not induce any three paw dyskinesia in amphetamine-naïve aphakia mice with chronic L-DOPA treatment (Fig. 4B). Instead, amphetamine produced significant and continuous oral stereotypy behavior, which lasted about 90 seconds out of 120 seconds analyzed in aphakia mice (Fig 4B).
For experiment 2, a similar pattern of LID was observed in aphakia mice receiving chronic L-DOPA, but not in control RD mice (data not shown). In experiment 3, there was no induction of three paw dyskinesia in aphakia mice chronically treated with the selective adenosine 2A antagonist, KW-6002, for three weeks while aphakia mice treated in parallel with chronic L-DOPA showed significant performance of three paw dyskinesia (Fig 5). Acute KW-6002 did not attenuate three paw dyskinesia induced by L-DOPA in aphakia mice treated chronically with L-DOPA, while there was a lower, but significant induction of three paw dyskinesia when chronic KW-6002 treated aphakia mice received acute L-DOPA for the first time (Fig 5).
Fig 5.

Comparison of chronic L-DOPA and KW-6002 treatment on the induction of three paw dyskinesia. Aphakia mice (n=5) were treated with either chronic L-DOPA or KW-6002 (right hand panel) for 21 consecutive days and the cylinder tests were performed on day 21 with either 25 mg/kg L-DOPA or 3 mg/kg KW-6002 alone. The following day, all mice were pretreated with 3 mg/kg KW-6002 for 20 min before administration of 25 mg/kg L-DOPA. **, p<0.01 vs. baseline before L-DOPA.
Preprodynorphin and preproenkephalin expression is increased in the striatum of dyskinetic aphakia mice
It has been shown that the expression level of preprodynorphin/prodynorphin and preproenkephalin/proenkephalin is modulated in response to dopamine denervation as well as to the development of dyskinesia with L-DOPA therapy (Doucet et al., 1996; Cenci et al., 1998; Lundblad et al., 2004). Preprodynorphin (Fig 6A, B) and preproenkephalin (Fig 6C, D) mRNA levels in aphakia mice were not significantly different from WT mice that received chronic saline. Acute L-DOPA treatment had no significant impact on preprodynorphin (Fig. 6A, B) or preproenkephalin (Fig. 6C, D) expression in the striatum of either WT or aphakia mice. Chronic L-DOPA treatment increased the expression of preprodynorphin levels bilaterally in the striata of aphakia mice compared to the saline group and acute L-DOPA treatment group. Preproenkephalin levels were elevated in the chronic L-DOPA treated group compared to the saline group, but no further increase was observed compared to the acute L-DOPA group. L-DOPA had no effect on preprodynorphin/prodynorphin peptide gene expression in WT mice.
Fig 6.
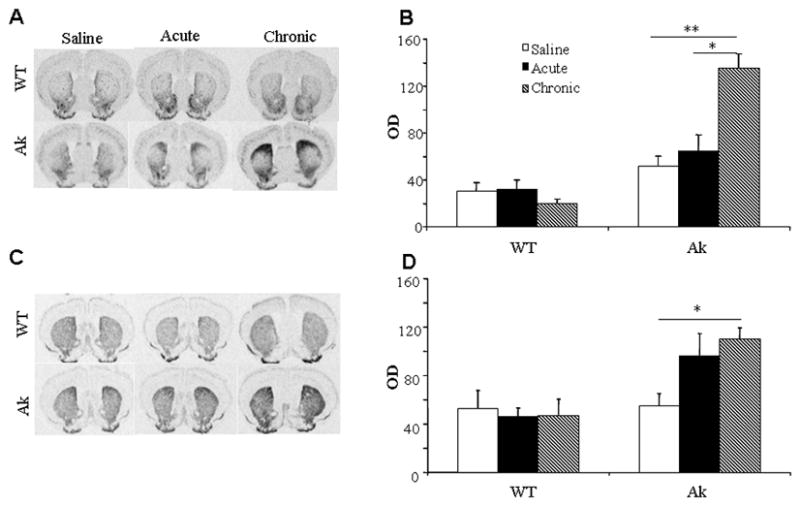
Prodynorphin (A and B) and proenkephalin (C and D) mRNA expression in the striatum after treatment with L-DOPA in wild type and aphakia mice. *, p<0.05 and **, p<0.01 as compared showing in B and D. N=3–4 for WT mice under each condition, while N=5 (saline), 3 (acute L-DOPA) and 11 (chronic L-DOPA) for aphakia mice.
FosB expression is induced in basal ganglia structures in dyskinetic aphakia mice
Chronic L-DOPA treatment increased the number of cells with FosB expression in the striatum bilaterally compared to saline treatment (Fig. 7, n=5–11, p<0.01). Negligible expression of FosB was noted in the aphakia mice who received chronic saline injection (Fig 7A and C, n=5) or in WT mice even with chronic L-DOPA treatment (Fig. 7D, n=10). FosB expression was localized to the dorsal striatum and throughout the whole rostral to caudal extent of striatum as shown in Fig. 7B.
Fig 7.

FosB expression in the striatum of aphakia and wild type mice. After chronic L-DOPA treatment, there was significant induction of FosB expression in the striatum of aphakia mice (B, C), but not in those that received chronic saline (A and C). Chronic treatment with L-DOPA did not induce any FosB expression in the striatum of wild type mice (D). **, p<0.01. Scale bar = 100 μm. ec: external capsule.
There was also a significant increase in FosB expression in PPN. As shown in Fig 8, unlike chronic saline treated aphakia mice (Fig 8A and D), chronic L-DOPA significantly enhanced FosB expression in PPN (Fig 8B–D). However, there was no FosB expression in the PPN of wild type mice treated with saline or chronic L-DOPA for 24 days (data not shown). In addition, significant FosB expression was also noted in the dorsal zona incerta (ZI) of aphakia mice treated with chronic L-DOPA, but not in chronic saline treated animals (Fig. 9).
Fig 8.
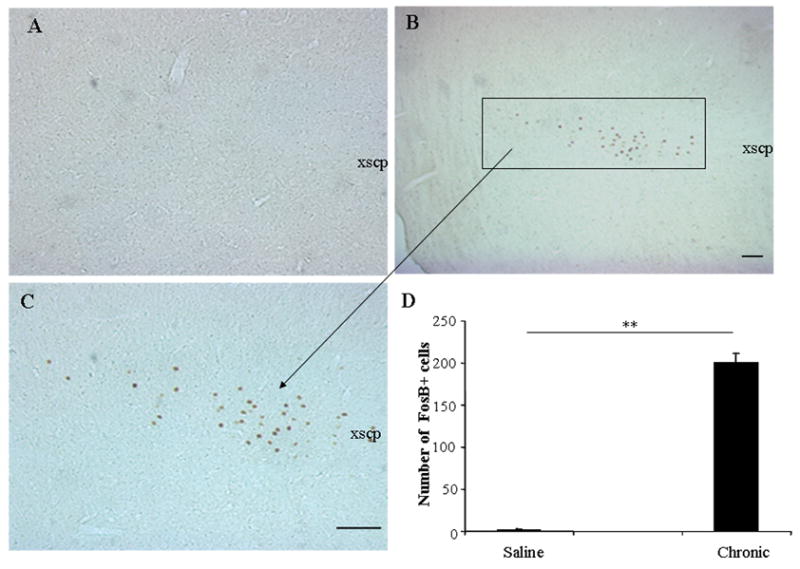
FosB expression was seen in the PPN of aphakia mice after L-DOPA treatment. In A–C, mice received saline or chronic L-DOPA, respectively. In B, C and E, chronic L-DOPA induced significant FosB expression in PPN. **, p<0.01. A and C were taken under 10x objective, while B was taken under 5x objective. Scale bar = 100 μm. xscp: decussation of the superior cerebellar peduncle.
Fig 9.

FosB expression was also seen in the Zona Incerta (ZI) after chronic L-DOPA (B–C) treatment, but not with chronic saline treatment (A). mt: mammillothalamic tract. Scale bar = 100 μm. mt: mammillothalamic tract.
Discussion
We describe for the first time, a model of PD dyskinesia based on a genetic mouse line with a selective loss of nigrostriatal projection that is stable and bilateral. Using this model, we demonstrate that abnormal involuntary movements develop with chronic repetitive exposure to L-DOPA. We show a spectrum of abnormal involuntary movements that are dependent on the duration and dose of L-DOPA treatment. These abnormal behaviors not only meet the behavioral criteria for LID, but also show pharmacological characteristics typical for LID. Dyskinetic movements are associated with biochemical markers of increased FosB, preprodynorphnin and preproenkephalin expression in the striatum. In addition, FosB expression changes were seen in the PPN and ZI of dyskinetic aphakia mice.
The core characteristics of dyskinetic movements in aphakia mice meet the criteria for L-DOPA-induced dyskinesia although the descriptive phenomenology is somewhat different from other models. The typical dyskinetic behaviors in PD patients include chorea and dystonia (Luquin et al., 1992b; Marconi et al., 1994). Similar phenomenology can be seen in L-DOPA treated MPTP lesioned primates (Clarke et al., 1989; Boyce et al., 1990b; Boyce et al., 1990a). Although the presence of dyskinetic phenomonology in rodents has been controversial, recent studies have noted various categories of movements, such as dystonia, limb, orolingual and neck dyskinesia in hemi-Parkinsonian rodents (Cenci et al., 1998; Lee et al., 2000). Rotational behavior induced by DA receptor agonists was initially considered to be similar to L-DOPA-induced dyskinesia as it shows enhancement with chronic L-DOPA treatment (Henry et al., 1998), but the significance and relevance of the rotational behaviors are not clear (Marin et al., 2006). The other type of LID in rodents is myoclonic jerk-like movements seen in mice treated with dopamine agonists after reserpine-induced dopamine depletion in RGS9-2 knock out animals (Kovoor et al., 2005) or increased vertical rearing in reserpine-treated mice (Johnston et al., 2005). The phenomenology of LID induced by L-DOPA in aphakia mice found in the current experiment is somewhat different from the unilateral 6-hydroxydopamine toxin models and more similar to the reserpine model (Johnston et al., 2005). The characteristic of these movements is analogous to LID in that these are excess stereotypical movements without apparent purpose. Voluntary movement of limb can suppress the LID temporarily in that limb, but could also increase LID elsewhere in the body, which is called an overflow dyskinesia. Similarly, the paw dyskinesia movements were interrupted when mice performed the balance beam task, but continued in the cylinder in the absence of focused voluntary tasks. When food pellets were introduced, the animals walked over the pellet, but did not exhibit any interest in the pellet and continued with their abnormal involuntary movements. The context in which these movements occur are also characteristic of LID. The movements were more likely to occur with a higher dose of L-DOPA, longer duration of treatment, and only in animals with nigrostriatal deficit. There is a hierarchy in the severity of movements. At the onset of L-DOPA treatment, two paw dyskinesia was present, then hind paw touch increased with repeated exposure to L-DOPA, and eventually with chronic treatment three paw dyskinesia predominated and other movements were not seen as much as a consequence.
All aphakia mice eventually developed LID similar to many other models of LID that show that all of their animals develop dyskinesia (Winkler et al., 2002; Carta et al., 2006). On the other hand, some models show only a proportion of rodents displaying dyskinesia with chronic L-DOPA treatment (Cenci et al., 1998; Picconi et al., 2003; Lundblad et al., 2004). There are several well known factors which might alter the proportion of animals developing LID: the dose of L-DOPA, target area and extent of lesion (Lundblad et al., 2004) and the delivery method of L-DOPA (Carta et al., 2006). The percentage of animals developing LID increases significantly with the dose escalation of L-DOPA in PD mice (Carta et al., 2006) and PD rats (Winkler et al., 2002). All PD rats with unilateral MFB lesion could develop LID with chronic L-DOPA (Winkler et al., 2002). All the hemi-Parkinsonian rats develop dyskinesia if L-DOPA is delivered directly into the striatum regardless of lesion degree and previous priming (Carta et al., 2006). Moreover, for the reserpine model of PD, both D1 and D2 agonists induce dyskinesia in all mice tested (Kovoor et al., 2005). The lack of non-dyskinetic animals in our model precludes the possibility to investigate potential individual susceptibility factor for dyskinesia development. However, aphakia mice allows us to focus on the changes that occurs with chronic L-DOPA exposure and compare them to those that occur with acute L-DOPA exposure.
The aphakia mouse provides a unique model of PD in several ways: (1) Dopamine depletion occurs selectively only in nigrostriatal system. (2) Unlike the hemi-Parkinsonian rodents, the denervation of striatal DA is bilateral. (3) The loss of nigrostriatal system occurs very early in development, which may facilitate compensatory mechanisms that may underlie the generation of dyskinesias. Similar to aphakia mice, early-onset PD patients are more likely to develop LID than late-onset PD. (4) Aphakia mouse represents a more consistent and less heterogeneous model of nigrostriatal dopamine depletion which would facilitate molecular and biochemical analysis of mechanisms underlying LID and provide a test model for therapeutic approaches.
LID in aphakia mice must be distinguished from stereotypic behaviors which are usually induced by dopamine agonists in normal animals including psychostimulants such as amphetamine. These behaviors are thought to occur from direct or indirect activation of dopamine receptors in the mesolimbic system, i.e. the ventral tegmental nucleus and nucleus accumbens (Andersson et al., 2001). Stereotypic behaviors include sniffing, grooming, rearing, and standing (Blanchard et al., 1998; Corda et al., 2005) and are different from the LID described here in L-DOPA treated aphakia mice. Amphetamine induced significant stereotypical behavior, but failed to induce dyskinesia movement in aphakia mice, further supporting the idea that the dyskinesia movement described here is distinct from psychostimulant-induced stereotypic behaviors. Furthermore, a drug that does not generate abnormal involuntary movements such as adenosine A2A antagonist, KW-6002 does not produce three paw dyskinesia when administered alone to aphakia mice.
The basic motor deficit of PD is akinesia, which is improved by dopaminergic agents, as demonstrated by several different behavioral models in rodents (Phillips et al., 1998; Chang et al., 1999; Johnson et al., 1999; Cenci et al., 2002; Woodlee et al., 2005). In aphakia mice, we used two measures of akinesia, rearing and balance beam traversal tests to demonstrate that the L-DOPA doses that were used to produce dyskinesia are in a physiologic range that improves performance of these motor tasks (Hwang et al., 2005; van den Munckhof et al., 2006). L-DOPA at the doses of 10–25 mg/kg significantly improved akinesia measured by the balance beam test at all time points tested up to 5 weeks, whereas initial dyskinetic movements were mildly manifested then became more prominent after chronic therapy. When aphakia mice were engaged in dyskinetic movements with front and hind paws, rearing was interrupted. On the other hand, the balance beam test (Fleming et al., 2004; Hwang et al., 2005) does not allow for manifestation of dyskinetic movements. As discussed earlier, this is characteristic of LID in that voluntary movement may suppress dyskinesia in the same limb whereas it may increase overflow dyskinesia in other body parts.
Pharmacologically, the abnormal movements were also produced by the selective D1 receptor agonist SKF81297 or the D2 agonist quinpirole in aphakia mice which is consistent with reports that both D1 and D2 DA receptors are involved in inducing dyskinesia in PD animals (Blanchet et al., 1995; Mehta et al., 2000; Kovoor et al., 2005; Monville et al., 2005), for review, see (Jenner, 1995)). The involvement of D2 DA receptor activation alone in dyskinesia in PD has been demonstrated in both primates (Clarke et al., 1989; Luquin et al., 1992a; Blanchet et al., 1995) and rodents (Kovoor et al., 2005; Monville et al., 2005). Treatment of aphakia mice with a D1 agonist appeared to be more potent in producing these movements a finding similar to that observed in other models (Monville et al., 2005). Drugs that have been shown to decrease dyskinesia in PD patients and animal models of PD have also decreased three paw dyskinesia movements. The selective adenosine A2A antagonist, KW-6002 (Bibbiani et al., 2003; Kase et al., 2003; Lundblad et al., 2005; Xiao et al., 2006), did not produce three paw dyskinesia in aphakia mice during the three week chronic treatment period nor did KW-6002 attenuate L-DOPA induced three paw dyskinesia once it was developed, consistent with a previous work in unilateral 6-OHDA lesion rats (Lundblad et al., 2003) and further supporting the distinctive characteristics of LID in aphakia mice.
Dyskinetic movement in aphakia mice is associated with changes in gene expression patterns in striatal neurons. Preprodynorphin and FosB expression increased after chronic treatment, but not after an acute dose of L-DOPA. Increased preproenkephalin expression was also noted with chronic L-DOPA treatment. Dynorphin is localized to D1 expressing striatal neurons of the direct pathway to SN, while enkephalin is in D2 expressing striatal neurons of the indirect pathway to the globus pallidus (Gerfen et al., 1990; Steiner and Gerfen, 1998; Nadjar et al., 2006). The expression of the precursor of dynorphin, prodynorphin, is under the tonic activation of D1 receptors, but the enkephalin precursor, proenkephalin, is under the tonic suppression of D2 receptors (Morris et al., 1988). Reports of striatal prodynorphin expression following unilateral acute 6-OHDA toxic lesion have been inconsistent, either no change (Morris et al., 1989) or a decrease in level (Gerfen et al., 1990; Gerfen et al., 1991; Cenci et al., 1998). The expression of prodynorphin has been noted to be unchanged (van den Munckhof et al., 2006) or decreased (Smits et al., 2005) in the striatum of aphakia mice as well. We found that there was no change in striatal dynorphin expression in aphakia mice at baseline or with acute L-DOPA treatment. Dynorphin expression, however, increased with chronic L-DOPA treatment similar to that seen previously in rats with unilateral 6-OHDA lesions where dynorphin expression was correlated with L-DOPA-induced dyskinesia (Cenci et al., 1998). Enkephalin expression has been shown to increase after complete lesion, but not with partial or bilateral 6-OHDA lesion of the dopaminergic nigrostriatal pathway (Salin et al., 1996). Striatal preproenkephalin expression in aphakia mice has been shown to either increase (van den Munckhof et al., 2006) or remain unchanged (Smits et al., 2005). We did not note any change in basal expression of preproenkephalin in the striatum of aphakia mice compared to the control mice, but chronic L-DOPA treatment increased preproenkephalin levels.
In hemi-Parkinsonian rodents, induction and level of striatal FosB expression is correlated with the degree of LID (Andersson et al., 1999; Cenci, 2002; Pavon et al., 2006). Inhibition of striatal FosB expression attenuates the severity of LID (Andersson et al., 1999). In the present study, FosB expression was also found to localize to the PPN and ZI of chronic L-DOPA treated aphakia mice. PPN is one of the main structures composing the midbrain motor area and the activity between PPN and basal ganglia are closely linked (for review, see (Pahapill and Lozano, 2000; Mena-Segovia et al., 2004). In hemi-Parkinsonian rats, the neuronal activity of PPN is significantly exaggerated (Carlson et al., 1999; Breit et al., 2001) and intra-PPN injection of a selective GABA-A antagonist ameliorates akinesia in PD monkeys (Nandi et al., 2002), suggesting that the PPN is involved in the pathophysiology of PD. However, the significance of FosB expression in PPN of dyskinetic animals remains to be addressed. Recently, ZI has been suggested as another target area for the treatment of PD (Benazzouz et al., 2004; Plaha et al., 2006).
In summary, the establishment of LID in aphakia mice provides an ideal model for mechanistic studies on the induction and maintenance of LID and for the therapeutic exploration for LID in PD. Long-lasting gene expression changes in PPN and ZI with LID raises interesting questions regarding their relevance to the pathogenesis of dyskinesia and warrant further studies as potential new target areas for surgical therapy.
Supplementary Material
Table 1 Duration (seconds) of abnormal paw movements in wild type mice treated with L-DOPA (n=10, mean ± SEM).
Table 2 Duration (seconds) of abnormal paw movements in aphakia mice treated with saline (n=5–10, mean ±SEM)
Video. An example of three paw dyskinesia induced by L-DOPA (25 mg/kg) in aphakia mice. Note that both front paw and one hind paw on either side were dyskinetic, moving up and down along the surface of the testing cylinder. This three paw dyskinesia lasted for the entire period of recording.
Acknowledgments
This work is supported by NIH R01 NS32080, R01 NS43286, R01 MH48866, T32 GM07839, and an International Grant from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, the Republic of Korea. We would like to thank Wilfredo Rosario, Kara A. Sands, and Hillary Schiff, for expert technical assistance and Dr. Hyun Chul Koh for his contribution to in situ hybridization experiments. Riboprobe plasmids were kindly provided by Drs. Barbara Cagniard and Dr. Xiaoxi Zhuang.
Abbreviation
- DR
dopamine receptor KW-6002 (E)-1,3-diethyl-8-(3,4-dimethoxy)-7-methylxanthine
- L-DOPA
L-3,4-dihydroxyphenylalanine
- LID
L-DOPA-induced dyskinesia
- PD
Parkinson’s disease
- PPN
pedunculopontine nucleus
- SN
substantia nigra
- ZI
zona incerta
- WT
wild type
- 3v
the third ventricle
- ec
external capsule i.p. intraperitoneal
- mt
mammillothalamic tract
- xscp
decussation of the superior cerebellar peduncle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol Dis. 1999;6:461–474. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- Andersson M, Konradi C, Cenci MA. cAMP response element-binding protein is required for dopamine-dependent gene expression in the intact but not the dopamine-denervated striatum. J Neurosci. 2001;21:9930–9943. doi: 10.1523/JNEUROSCI.21-24-09930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barneoud P, Descombris E, Aubin N, Abrous DN. Evaluation of simple and complex sensorimotor behaviours in rats with a partial lesion of the dopaminergic nigrostriatal system. Eur J Neurosci. 2000;12:322–336. doi: 10.1046/j.1460-9568.2000.00896.x. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Tai CH, Meissner W, Bioulac B, Bezard E, Gross C. High-frequency stimulation of both zona incerta and subthalamic nucleus induces a similar normalization of basal ganglia metabolic activity in experimental parkinsonism. Faseb J. 2004;18:528–530. doi: 10.1096/fj.03-0576fje. [DOI] [PubMed] [Google Scholar]
- Bibbiani F, Oh JD, Petzer JP, Castagnoli N, Jr, Chen JF, Schwarzschild MA, Chase TN. A2A antagonist prevents dopamine agonist-induced motor complications in animal models of Parkinson’s disease. Exp Neurol. 2003;184:285–294. doi: 10.1016/s0014-4886(03)00250-4. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hebert MA, Dulloog L, Kaawaloa N, Nishimura O, Blanchard DC. Acute cocaine effects on stereotype and defense: an ethoexperimental approach. Neurosci Biobehav Rev. 1998;23:179–188. doi: 10.1016/s0149-7634(98)00019-0. [DOI] [PubMed] [Google Scholar]
- Blanchet PJ, Konitsiotis S, Chase TN. Amantadine reduces levodopa-induced dyskinesias in parkinsonian monkeys. Mov Disord. 1998;13:798–802. doi: 10.1002/mds.870130507. [DOI] [PubMed] [Google Scholar]
- Blanchet PJ, Calon F, Martel JC, BŽdard PJ, Di Paolo T, Walters RR, Piercey MF. Continuous administration decreases and pulsatile administration increases behavioral sensitivity to a novel dopamine D2 agonist (U-91356A) in MPTP-exposed monkeys. JPharmacolExpTher. 1995;272:854–859. [PubMed] [Google Scholar]
- Boyce S, Rupniak NM, Steventon MJ, Iversen SD. Characterisation of dyskinesias induced by L-dopa in MPTP-treated squirrel monkeys. Psychopharmacology (Berl) 1990a;102:21–27. doi: 10.1007/BF02245739. [DOI] [PubMed] [Google Scholar]
- Boyce S, Clarke CE, Luquin R, Peggs D, Robertson RG, Mitchell IJ, Sambrook MA, Crossman AR. Induction of chorea and dystonia in parkinsonian primates. Mov Disord. 1990b;5:3–7. doi: 10.1002/mds.870050103. [DOI] [PubMed] [Google Scholar]
- Breit S, Bouali-Benazzouz R, Benabid AL, Benazzouz A. Unilateral lesion of the nigrostriatal pathway induces an increase of neuronal activity of the pedunculopontine nucleus, which is reversed by the lesion of the subthalamic nucleus in the rat. Eur J Neurosci. 2001;14:1833–1842. doi: 10.1046/j.0953-816x.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- Carlson JD, Pearlstein RD, Buchholz J, Iacono RP, Maeda G. Regional metabolic changes in the pedunculopontine nucleus of unilateral 6-hydroxydopamine Parkinson’s model rats. Brain Res. 1999;828:12–19. doi: 10.1016/s0006-8993(99)01268-8. [DOI] [PubMed] [Google Scholar]
- Carta M, Lindgren HS, Lundblad M, Stancampiano R, Fadda F, Cenci MA. Role of striatal L-DOPA in the production of dyskinesia in 6-hydroxydopamine lesioned rats. J Neurochem. 2006;96:1718–1727. doi: 10.1111/j.1471-4159.2006.03696.x. [DOI] [PubMed] [Google Scholar]
- Cenci MA. Transcription factors involved in the pathogenesis of L-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Amino Acids. 2002;23:105–109. doi: 10.1007/s00726-001-0116-4. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88:617–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- Clarke CE, Boyce S, Robertson RG, Sambrook MA, Crossman AR. Drug-induced dyskinesia in primates rendered hemiparkinsonian by intracarotid administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) J Neurol Sci. 1989;90:307–314. doi: 10.1016/0022-510x(89)90117-2. [DOI] [PubMed] [Google Scholar]
- Corda MG, Piras G, Lecca D, Fernandez-Teruel A, Driscoll P, Giorgi O. The psychogenetically selected Roman rat lines differ in the susceptibility to develop amphetamine sensitization. Behav Brain Res. 2005;157:147–156. doi: 10.1016/j.bbr.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Del Dotto P, Pavese N, Gambaccini G, Bernardini S, Metman LV, Chase TN, Bonuccelli U. Intravenous amantadine improves levadopa-induced dyskinesias: an acute double-blind placebo-controlled study. Mov Disord. 2001;16:515–520. doi: 10.1002/mds.1112. [DOI] [PubMed] [Google Scholar]
- Doucet JP, Nakabeppu Y, Bedard PJ, Hope BT, Nestler EJ, Jasmin BJ, Chen JS, Iadarola MJ, St-Jean M, Wigle N, Blanchet P, Grondin R, Robertson GS. Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of deltaFosB-like protein(s) in both the rodent and primate striatum. Eur J Neurosci. 1996;8:365–381. doi: 10.1111/j.1460-9568.1996.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24:9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SH, Henry B, Hill M, Crossman A, Brotchie J. Stimulation of cannabinoid receptors reduces levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate model of Parkinson’s disease. Mov Disord. 2002;17:1180–1187. doi: 10.1002/mds.10289. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Picconi B, Ghiglieri V, Polli F, Bagetta V, Bernardi G, Cattabeni F, Di Luca M, Calabresi P. A critical interaction between NR2B and MAGUK in L-DOPA induced dyskinesia. J Neurosci. 2006;26:2914–2922. doi: 10.1523/JNEUROSCI.5326-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, McGinty JF, Young WS., 3rd Dopamine differentially regulates dynorphin, substance P, and enkephalin expression in striatal neurons: in situ hybridization histochemical analysis. J Neurosci. 1991;11:1016–1031. doi: 10.1523/JNEUROSCI.11-04-01016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma J, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Henry B, Crossman AR, Brotchie JM. Characterization of enhanced behavioral responses to L-DOPA following repeated administration in the 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Exp Neurol. 1998;151:334–342. doi: 10.1006/exnr.1998.6819. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Ardayfio P, Kang UJ, Semina EV, Kim KS. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res. 2003;114:123–131. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Fleming SM, Ardayfio P, Moran-Gates T, Kim H, Tarazi FI, Chesselet MF, Kim KS. 3,4-dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: behavioral characterization of a novel genetic model of Parkinson’s disease. J Neurosci. 2005;25:2132–2137. doi: 10.1523/JNEUROSCI.3718-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P. The rationale for the use of dopamine agonists in Parkinson’s disease. Neurology. 1995;45:S6–12. doi: 10.1212/wnl.45.3_suppl_3.s6. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Schallert T, Becker JB. Akinesia and postural abnormality after unilateral dopamine depletion. Behav Brain Res. 1999;104:189–196. doi: 10.1016/s0166-4328(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Johnston TH, Lee J, Gomez-Ramirez J, Fox SH, Brotchie JM. A simple rodent assay for the in vivo identification of agents with potential to reduce levodopa-induced dyskinesia in Parkinson’s disease. Exp Neurol. 2005;191:243–250. doi: 10.1016/j.expneurol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Kase H, Aoyama S, Ichimura M, Ikeda K, Ishii A, Kanda T, Koga K, Koike N, Kurokawa M, Kuwana Y, Mori A, Nakamura J, Nonaka H, Ochi M, Saki M, Shimada J, Shindou T, Shiozaki S, Suzuki F, Takeda M, Yanagawa K, Richardson PJ, Jenner P, Bedard P, Borrelli E, Hauser RA, Chase TN. Progress in pursuit of therapeutic A2A antagonists: the adenosine A2A receptor selective antagonist KW6002: research and development toward a novel nondopaminergic therapy for Parkinson’s disease. Neurology. 2003;61:S97–100. doi: 10.1212/01.wnl.0000095219.22086.31. [DOI] [PubMed] [Google Scholar]
- Kovoor A, Seyffarth P, Ebert J, Barghshoon S, Chen CK, Schwarz S, Axelrod JD, Cheyette BN, Simon MI, Lester HA, Schwarz J. D2 dopamine receptors colocalize regulator of G-protein signaling 9–2 (RGS9-2) via the RGS9 DEP domain, and RGS9 knock-out mice develop dyskinesias associated with dopamine pathways. J Neurosci. 2005;25:2157–2165. doi: 10.1523/JNEUROSCI.2840-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Cenci MA, Schulzer M, Bjorklund A. Embryonic ventral mesencephalic grafts improve levodopa-induced dyskinesia in a rat model of Parkinson’s disease. Brain. 2000;123 (Pt 7):1365–1379. doi: 10.1093/brain/123.7.1365. [DOI] [PubMed] [Google Scholar]
- Linazasoro G. New ideas on the origin of L-dopa-induced dyskinesias: age, genes and neural plasticity. Trends Pharmacol Sci. 2005;26:391–397. doi: 10.1016/j.tips.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Vaudano E, Cenci MA. Cellular and behavioural effects of the adenosine A2a receptor antagonist KW-6002 in a rat model of l-DOPA-induced dyskinesia. J Neurochem. 2003;84:1398–1410. doi: 10.1046/j.1471-4159.2003.01632.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16:110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Usiello A, Carta M, Hakansson K, Fisone G, Cenci MA. Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Exp Neurol. 2005;194:66–75. doi: 10.1016/j.expneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Luquin MR, Laguna J, Obeso JA. Selective D2 receptor stimulation induces dyskinesia in parkinsonian monkeys. Ann Neurol. 1992a;31:551–554. doi: 10.1002/ana.410310514. [DOI] [PubMed] [Google Scholar]
- Luquin MR, Scipioni O, Vaamonde J, Gershanik O, Obeso JA. Levodopa-induced dyskinesias in Parkinson’s disease: clinical and pharmacological classification. Mov Disord. 1992b;7:117–124. doi: 10.1002/mds.870070204. [DOI] [PubMed] [Google Scholar]
- Marconi R, Lefebvre-Caparros D, Bonnet AM, Vidailhet M, Dubois B, Agid Y. Levodopa-induced dyskinesias in Parkinson’s disease phenomenology and pathophysiology. Mov Disord. 1994;9:2–12. doi: 10.1002/mds.870090103. [DOI] [PubMed] [Google Scholar]
- Marin C, Rodriguez-Oroz MC, Obeso JA. Motor complications in Parkinson’s disease and the clinical significance of rotational behavior in the rat: Have we wasted our time? Exp Neurol. 2006;197:269–274. doi: 10.1016/j.expneurol.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Mehta A, Thermos K, Chesselet MF. Increased behavioral response to dopaminergic stimulation of the subthalamic nucleus after nigrostriatal lesions. Synapse. 2000;37:298–307. doi: 10.1002/1098-2396(20000915)37:4<298::AID-SYN7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Monville C, Torres EM, Dunnett SB. Validation of the l-dopa-induced dyskinesia in the 6-OHDA model and evaluation of the effects of selective dopamine receptor agonists and antagonists. Brain Res Bull. 2005;68:16–23. doi: 10.1016/j.brainresbull.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Hollt V, Herz A. Dopaminergic regulation of striatal proenkephalin mRNA and prodynorphin mRNA: contrasting effects of D1 and D2 antagonists. Neuroscience. 1988;25:525–532. doi: 10.1016/0306-4522(88)90256-4. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Herz A, Hollt V. Localization of striatal opioid gene expression, and its modulation by the mesostriatal dopamine pathway: an in situ hybridization study. J Mol Neurosci. 1989;1:9–18. doi: 10.1007/BF02896851. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Brotchie JM, Guigoni C, Li Q, Zhou SB, Wang GJ, Ravenscroft P, Georges F, Crossman AR, Bezard E. Phenotype of striatofugal medium spiny neurons in parkinsonian and dyskinetic nonhuman primates: a call for a reappraisal of the functional organization of the basal ganglia. J Neurosci. 2006;26:8653–8661. doi: 10.1523/JNEUROSCI.2582-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi D, Aziz TZ, Giladi N, Winter J, Stein JF. Reversal of akinesia in experimental parkinsonism by GABA antagonist microinjections in the pedunculopontine nucleus. Brain. 2002;125:2418–2430. doi: 10.1093/brain/awf259. [DOI] [PubMed] [Google Scholar]
- Nash JE, Fox SH, Henry B, Hill MP, Peggs D, McGuire S, Maneuf Y, Hille C, Brotchie JM, Crossman AR. Antiparkinsonian actions of ifenprodil in the MPTP-lesioned marmoset model of Parkinson’s disease. Exp Neurol. 2000;165:136–142. doi: 10.1006/exnr.2000.7444. [DOI] [PubMed] [Google Scholar]
- Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci U S A. 2003;100:4245–4250. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain. 2000;123 (Pt 9):1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- Pahwa R, Factor SA, Lyons KE, Ondo WG, Gronseth G, Bronte-Stewart H, Hallett M, Miyasaki J, Stevens J, Weiner WJ. Practice Parameter: Treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006 doi: 10.1212/01.wnl.0000215250.82576.87. [DOI] [PubMed] [Google Scholar]
- Pavon N, Martin AB, Mendialdua A, Moratalla R. ERK Phosphorylation and FosB Expression Are Associated with L-DOPA-Induced Dyskinesia in Hemiparkinsonian Mice. Biol Psychiatry. 2005 doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Pavon N, Martin AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry. 2006;59:64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Latimer MP, Gupta S, Winn P, Brown VJ. Excitotoxic lesions of the subthalamic nucleus ameliorate asymmetry induced by striatal dopamine depletion in the rat. Behav Brain Res. 1998;90:73–77. doi: 10.1016/s0166-4328(97)00080-6. [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006;129:1732–1747. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- Rahman Z, Schwarz J, Gold SJ, Zachariou V, Wein MN, Choi KH, Kovoor A, Chen CK, DiLeone RJ, Schwarz SC, Selley DE, Sim-Selley LJ, Barrot M, Luedtke RR, Self D, Neve RL, Lester HA, Simon MI, Nestler EJ. RGS9 modulates dopamine signaling in the basal ganglia. Neuron. 2003;38:941–952. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- Salin P, Hajji MD, Kerkerian-le Goff L. Bilateral 6-hydroxydopamine-induced lesion of the nigrostriatal dopamine pathway reproduces the effects of unilateral lesion on substance P but not on enkephalin expression in rat basal ganglia. EurJNeurosci. 1996;8:1746–1757. doi: 10.1111/j.1460-9568.1996.tb01318.x. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Smits SM, Bouwmeester H, Hamers FP, Van Der Linden AJ, Hellemons AJ, Graw J, Burbach JP. Early developmental failure of substantia nigra dopamine neurons in mice lacking the homeodomain gene Pitx3. Development. 2004;131:1145–1155. doi: 10.1242/dev.01022. [DOI] [PubMed] [Google Scholar]
- Smits SM, Mathon DS, Burbach JP, Ramakers GM, Smidt MP. Molecular and cellular alterations in the Pitx3-deficient midbrain dopaminergic system. Mol Cell Neurosci. 2005;30:352–363. doi: 10.1016/j.mcn.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Steece-Collier K, Chambers LK, Jaw-Tsai SS, Menniti FS, Greenamyre JT. Antiparkinsonian actions of CP-101,606, an antagonist of NR2B subunit-containing N-methyl-d-aspartate receptors. Exp Neurol. 2000;163:239–243. doi: 10.1006/exnr.2000.7374. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Truong L, Allbutt H, Kassiou M, Henderson JM. Developing a preclinical model of Parkinson’s disease: a study of behaviour in rats with graded 6-OHDA lesions. Behav Brain Res. 2006;169:1–9. doi: 10.1016/j.bbr.2005.11.026. [DOI] [PubMed] [Google Scholar]
- van den Munckhof P, Gilbert F, Chamberland M, Levesque D, Drouin J. Striatal neuroadaptation and rescue of locomotor deficit by L-dopa in aphakia mice, a model of Parkinson’s disease. J Neurochem. 2006;96:160–170. doi: 10.1111/j.1471-4159.2005.03522.x. [DOI] [PubMed] [Google Scholar]
- van den Munckhof P, Luk KC, Ste-Marie L, Montgomery J, Blanchet PJ, Sadikot AF, Drouin J. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003;130:2535–2542. doi: 10.1242/dev.00464. [DOI] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Bjorklund A, Cenci MA. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson’s disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2002;10:165–186. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]
- Woodlee MT, Asseo-Garcia AM, Zhao X, Liu SJ, Jones TA, Schallert T. Testing forelimb placing “across the midline” reveals distinct, lesion-dependent patterns of recovery in rats. Exp Neurol. 2005;191:310–317. doi: 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Xiao D, Bastia E, Xu YH, Benn CL, Cha JH, Peterson TS, Chen JF, Schwarzschild MA. Forebrain adenosine A2A receptors contribute to L-3,4-dihydroxyphenylalanine-induced dyskinesia in hemiparkinsonian mice. J Neurosci. 2006;26:13548–13555. doi: 10.1523/JNEUROSCI.3554-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1 Duration (seconds) of abnormal paw movements in wild type mice treated with L-DOPA (n=10, mean ± SEM).
Table 2 Duration (seconds) of abnormal paw movements in aphakia mice treated with saline (n=5–10, mean ±SEM)
Video. An example of three paw dyskinesia induced by L-DOPA (25 mg/kg) in aphakia mice. Note that both front paw and one hind paw on either side were dyskinetic, moving up and down along the surface of the testing cylinder. This three paw dyskinesia lasted for the entire period of recording.


