Abstract
Platelets may play a role in human cerebral malaria (CM) pathogenesis, and have been shown to induce the clumping of Plasmodium falciparum-parasitised red blood cells (PRBC) in vitro. Both thrombocytopenia and platelet-induced PRBC clumping are associated with severe malaria and especially with CM. In this study, we have investigated the occurrence of the clumping phenomenon in CM patients by isolating and co-incubating their plasma and PRBC ex vivo.
Malawian children with CM all had a low platelet count, the degree of thrombocytopenia being directly proportional to the density of parasitaemia. Plasma from these patients subsequently induced weak PRBC clumping. When the assays were repeated having adjusted the plasma platelet concentrations to within the normal physiological range, massive clumping occurred.
These results suggest that thrombocytopenia may, through reduction of platelet mediated clumping of PRBC, provide a protective mechanism for the host during CM.
Keywords: paediatric cerebral malaria, Plasmodium falciparum, platelets, clumping, thrombocytopenia
INTRODUCTION
Severe malaria is a major cause of death in sub-Saharan African children. A pathological feature that is common to the diverse life-threatening syndromes that can result from Plasmodium falciparum infection, is the sequestration of large numbers of mature parasitised red blood cells (PRBC) within microvasculature of vital organs. Sequestration is generally attributed to cytoadherence of PRBC on microvascular endothelial cells [1]. However, post-mortem studies show that intravascular PRBC are not adjacent to the vascular endothelium [2], suggesting that some PRBC may be adherent to each other rather than to the vascular endothelium, and might have accumulated through a process of adhesion between PRBC.
Platelet accumulation can be found in the cerebral microvessels of patients who died from CM, but not in patients who died from severe malarial anaemia (SMA), suggesting a possible role of platelets in the pathogenesis of cerebral malaria (CM) [3]. Platelets can mediate the formation of PRBC clumps in vitro [4]. This phenomenon has been shown to be more common when using parasites from patients with severe malaria than with isolates from patients with mild malaria, both in Kenyan children [4] and in Thai adults [5]. The size of the clumps observed in vitro is sufficient, if the clumps are similarly sized in vivo, to be responsible for important rheological disturbances in infected patients.
Thrombocytopenia is a usual feature of P falciparum infection. The degree of thrombocytopenia was found to correlate with the risk of fatal outcome in one study [6], but not in another [7]. We postulated that the presence of abnormally low numbers of circulating platelets in malaria patients would restrict the formation of platelet-PRBC aggregates, an effect that might limit the severity of cerebral pathology.
PATIENTS AND METHODS
Patients
We studied consecutive cases of uncomplicated malaria (UM), severe malarial anemia (SMA), and cerebral malaria (CM) admitted to the paediatric research ward of the Queen Elizabeth Central Hospital, Blantyre, Malawi, whose clinical data are detailed in Table 1. All patients with UM and SMA had a Plasmodium falciparum parasitaemia and were conscious (Blantyre coma score 5/5). These were considered as UM if the packed cell volume was above 25%, and SMA when it was below 12%. Patients with CM were admitted to the hospital in coma (Blantyre coma score 2/5 or less), had P. falciparum parasitaemia and had no other clinically evident cause of unconsciousness. All the CM patients included in this study showed evidence of malarial retinopathy, a recently described finding which improves the accuracy of the clinical diagnosis of CM (for review, see [8]). Patients’ relatives gave fully informed consent to participate in the study, and a 2.5 ml blood sample was taken for parasite culture and separation of plasma and platelets. The studies were approved by the ethical review committees of the College of Medicine, University of Malawi, the Liverpool School of Tropical Medicine, and Michigan State University.
Table 1. Clinical and laboratory characteristics in Malawian children with acute P falciparum malaria.
| malaria |
|||
|---|---|---|---|
| uncomplicated | severe anaemia | cerebral malaria | |
| Characteristic | n=12 | n=15 | n=22 |
| age, median (IQR), months | 55.5 (29-82.5) | 45 (30-82) | 36.5 (18-63) |
| Blantyre coma score, median (range) | 5 | 5 | 1 (0-3) |
| parasitaemia, geometric mean (95% CI), parasites/μL | 23,617 (8,910-62,560) | 99,550 (63,400-156,300) | 68,700 (27,750-171,300) |
| platelet count, median (IQR), x103 cells/μL | 67 (37.5-232.5) | 97.2 (72-124) (a) | 58 (21-145.5) (a) |
| Spearman’s correlation test between platelet count and parasitaemia (p value) | 0.3916 (p>0.05) | 0.1055 (p>0.05) | -0.675 (p<0.001) |
Thrombocytopenic groups: a patient was considered thrombocytopenic when his/her platelet count was below 150 ×103 cells/μL.
Parasite culture
Blood samples from patients were collected in sodium citrate tubes and centrifuged at 250g for 10 min. Plasma was stored at 4°C, and pelleted RBC were washed 3 times in RPMI-1640, then resuspended in a standard malaria culture medium of RPMI-1640 supplemented with 25 mmol/L HEPES, 10% fetal calf serum, and 40 mg/mL gentamicin, to achieve a final haematocrit of 5%. After up to 48 h of cultivation at 37°C in 5% CO2, the parasite stages and numbers were adjusted to a 10% mature form (pigmented trophozoites) parasitaemia by gelatine flotation, in order to standardize the assays and compare the results. Blood smears were then prepared, and the stage of parasite maturation was examined by microscopy.
Preparation of Platelet-Rich Plasma (PRP) and Platelet-Poor Plasma (PPP)
PRP and PPP were extracted from 5 ml of whole blood when provided by malaria-naive donors, and from 2.5 ml when obtained from CM patients. Blood was collected in a sodium citrate tube and centrifuged at 250g for 10 min. PRP fraction was then transferred to a new tube and a platelet count was performed with a Neubauer’s hematocytometer before storage. PPP was obtained by further centrifugation at 1500g for 10 minutes to discard platelets, and both PRP and PPP were stored at 4°C and used within 4 days.
Clumping assays
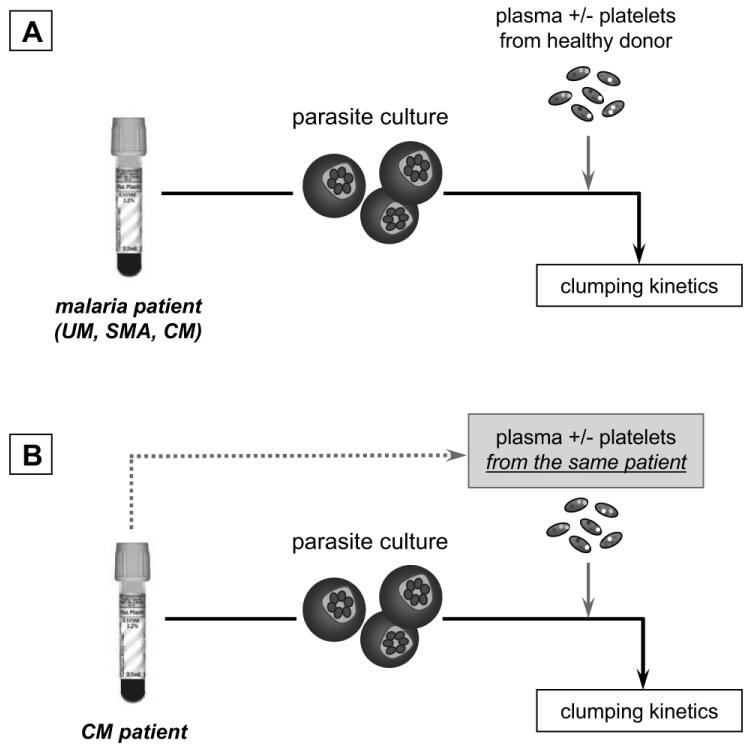
Clumping kinetics (Fig. 1A) were assessed when the parasites had grown to the stage of pigmented trophozoites, and were selected as described above. Parasite cultures labelled by addition of 20 mg/ml acridine orange were then rotated in 5% hematocrit in the presence of 20% PPP (platelet count <10 platelets/μL) or 20% PRP (platelet count >300 ×103 platelets/μL for healthy donors, and < 150 ×103 platelets/μL for CM patients). After 15 min rotation, 25μL of sample was taken and placed on a glass slide and examined by fluorescent microscopy. Further samples were taken at 30, 60, and 120 min, as described elsewhere [4]. A clump was defined as consisting of three or more infected erythrocytes and the frequency of the clumping phenotype in isolates was measured as the number of infected cells in clumps among 1,000 infected cells counted in duplicate assays. For the auto-assays (Fig. 1B), PRP, PPP and cultured parasites isolated from the same CM patient were used (in vivo platelet count < 150 ×103 platelets/μL). Platelet numbers were concentred 5 times in plasma samples used for auto-assays, so that when mixed with parasite suspensions, the final concentration of platelets would be representative of the in vivo conditions. Platelet concentration was performed by gentle centrifugation, removal of a calculated portion of plasma, and resuspension of platelets. To assess the potential influence of plasma factors on the clump formation, the same technique was used to adjust several platelet suspensions to a final average count (> 300 ×103 platelets/μL). Clumping assays were then carried out according to the protocol for classic assays described above.
Figure 1. Schematic representation of clumping assays.
Classic clumping assays were performed as previously described [4, 5] with parasites isolated from infected patients, cultured and incubated with PPP and PRP from healthy donors with a normal platelet count (A). In auto-assays, parasites, PPP and PRP were all obtained from the same patient to assess the potential for occurrence of this phenomenon ex vivo (B).
Analysis of platelet activation in clumps
Variation of platelet membrane P-selectin expression was measured by immunofluorescence on slide and by flow cytometry. Both were performed on platelets alone in PRP from CM patients, and on clumps after 2 min of PRP co-incubation with PRBC. For the immunofluorescence studies, PRP alone or after clumping assays was incubated at RT for 20 min with FITC-coupled mouse anti-human CD62-P (P-selectin, clone JMCD62P, Orbigen, San Diego, CA) before observation with a fluorescence microscope. Flow cytometry analyses required clumps of small or medium size, and the antibody was added 5 min prior to PRP analysis, or after 2 min of PRP-PRBC co-incubation, for 5 min. In the latter case, the total of 7 min co-incubation led to the formation of clumps of desired dimension.
Inhibition of platelet-induced clumping by monoclonal antibodies
PRP samples were incubated at 37°C for 1 hour with several blocking antibodies against platelet membrane glycoproteins such as CD36 (GPIV, clone FA6-152, Beckman Coulter Immunotech), P-selectin (CD62-P, clone AK-6, Serotec, Raleigh, NC), GPIIb/IIIa (CD41/CD61 chimeric antibody, clone 7E3, Reopro, Centocor, a gift from Dr G. Wiemann), or IgG isotype control. Lamifiban (Ro44-9883), kindly provided by Dr Jürgen Fingerle (F. Hoffmann-La Roche), is a peptidomimetic synthetic GPIIb/IIIa inhibitor, and was also incubated with PRP for the same time. PRP were then used in clumping assays performed as previously described.
Statistical analysis
Statistical analyses were performed with Stata 8.1 software from Stata Corporation. Data were analysed by Mann-Whitney U test to compare pairs of groups. Results were expressed as means ± standard deviations of individual experimental groups, and the clumping data of the different patient groups were analysed by the Kruskall-Wallis and Dunn’s pairwise tests. The correlation between parasite density and platelet count in Table 1 was assessed by the Spearman’s correlation test. A p-value of p<0.05 was considered significant.
RESULTS
1. The platelet-induced PRBC clumping phenotype is associated with severe malaria in Malawian children
We assessed the occurrence of the previously described platelet-induced in vitro PRBC clumping phenomenon in Malawian children with P. falciparum malaria. Parasites isolated from 49 patients were studied: 12 with uncomplicated malaria (UM) and 37 with severe malaria, of whom 15 had SMA, and 22 CM (Table 1). In the presence of platelet-poor plasma (PPP) from healthy donors, no PRBC auto-agglutination was observed in any of the patient groups after a 120 min co-culture (Fig. 2A-C). When incubated with platelet-rich plasma (PRP) however, the platelet-induced clumping phenotype was observed in all groups. This phenomenon was time-dependent, and was associated with disease severity and type. Parasites from UM patients showed a weak ability to agglutinate in the presence of platelets, with 27.1±12.0% (mean ±SD) of clumped PRBC at the end of the kinetics studies. Microscopic analysis showed in this case a homogeneous suspension of PRBC, with some small clumps (8.6 ±3.0 PRBC/clump) randomly distributed (Fig. 2A). In the case of SMA or CM, the auto-agglutination was significantly higher than for UM: at 60 min and after for SMA (p<0.05 at 60 min, then p<0.001 for all the other times), and at 15 min and after for CM (p<0.001 for each kinetics point). In the case of SMA, clumped PRBC constituted (mean+SD) 59.1±14.1% of the total PRBC population at the end of the kinetic study, and compared to UM, significantly larger clumps were observed (42.9 ±9.5 and 6.5 ±4.3 PRBC/clump, respectively, p<0.001) (Fig. 2B). In the presence of platelets, parasites isolated from CM patients exhibited a statistically stronger pro-clumping phenotype than parasites from SMA patients (p<0.001 for each kinetics point), leading within the first 15 minutes of incubation to the formation of giant clumps (>50 PRBC/clump), and to the aggregation of all the PRBC in the suspension. The number of PRBC could not be counted precisely in giant clumps, but some contained up to 125 PRBC (Fig. 2C). A control was performed in each experiment with uninfected erythrocytes isolated from healthy and malaria-naïve donors, all of whom exhibited platelet counts within the normal range (150 ×103 to 450 ×103 platelets/μL). Cells were incubated with PPP or PRP obtained and processed as described in the experimental procedures, and aggregation was never observed (data not shown).
Figure 2. Platelet-induced PRBC clumping is a common parasite phenotype among Malawian children, and is associated with disease severity.
The ability to form clumps in the presence of platelets was assessed in parasite populations isolated from paediatric patients from Malawi. Parasites from patients with uncomplicated malaria (UM, n=12), severe malaria anemia (SMA, n=15), and cerebral malaria (CM, n=22) were incubated for different times with plasma with or without platelets. Results are expressed as mean ± SD of the percentage of PRBC that are associated with clumps. Assays were performed twice per patient sample.
2. Platelet activation and subsequent P-selectin up-regulation are involved in the clumping effect
To assess platelet activation during clumping, we used immunofluorescent staining to identify P-selectin on platelets before and after 10 min of co-incubation with PRBC. Samples were analysed by fluorescent microscopy and by flow cytometry. Before co-incubation with PRBC, platelets from CM patients did not show any sign of activation: they were of normal circular shape (Fig 3A). Flow cytometry analysis showed that the gated platelet population had low P-selectin surface expression, with 5.8% of mean fluorescence intensity (MFI, Fig 3C, E). Within 10 minutes of adding PRBC, platelets exhibited typical activation pseudopodia (Fig 3B), and a significant up-regulation of membrane P-selectin was observed for the platelet and clump populations, reaching up to 77.4% of MFI (Fig 3D, F). Platelets not associated with clumps (Fig 3D, gate [p]) exhibited an increased MFI of 49.9% (data not shown). A control was performed by mixing unparasitised erythrocytes with PRP, and no platelet changes or uninfected erythrocyte clumping were observed (data not shown). To investigate the potential role of platelet P-selectin as a receptor for PRBC in this aggregation phenomenon, PRP were then incubated in the presence of blocking anti-P-selectin, as well as several other antibodies or antagonists against platelet membrane glycoproteins listed above, before the incubation with PRBC isolated from CM patients. In the presence of anti-P-selectin, the platelet-induced PRBC clumping was significantly but partially decreased, with a 20.7 ±11.0% inhibition (p<0.001) compared to the controls (mean + SD) (Fig. 3E). Incubation with anti-CD36 led to a dramatic reduction of the aggregation (67.3 ±6.5%, p<0.01), as previously described [4], and a combination of both antibodies led to almost complete abrogation of clumping, with a greater inhibition than the additive effect of the antibodies taken separately (97.7 ±10.5%, p<0.01). By contrast, the blocking of the platelet surface glycoproteins GPIIb and IIIa by high concentrations of Reopro or Lamifiban (1 to 30 μg/ml and 50 to 100 ng/ml, respectively, agents which are both known to block platelet aggregation [9]) failed to induce a significant inhibition of PRBC aggregation (p>0.05, Fig. 3E). When platelets were inactivated by a pre-treatment with heparin to inhibit the upregulation of P-selectin on their surface (1000 units/ml), the same level of inhibition reported for anti-P-selectin treatment was observed (data not shown).
Figure 3. P-selectin expressed by activated platelets acts with CD36 as a major receptor for PRBC clumping.
Platelet activation was measured before and after co-incubation with PRBC. The level of membrane P-selectin expression was assessed by immunofluorescence on slide (A, B) and flow cytometry (C, D, E and F). Micrographs, dotplots and histograms presented here are representative of the results observed for 4 assays. The addition of PRBC resulted in the generation of a platelet-PRBC clump population observed in the dotplot D, and the platelet population non associated with PRBC was sub-gated in [p]. Inhibition assays were then performed to evaluate the role of P-selectin and other several platelet surface molecules in the parasite clumping phenomenon. PRP from healthy donors was incubated with either IgG [control] (10 μg/ml), anti-P-selectin (20μg/ml), anti-CD36 (10 μg/ml), a mixture of both anti-P-selectin and CD36 (stated as ‘mix’), Reopro (30 μg/ml) or Lamifiban (100 ng/ml) before the clumping assays with PRBC from CM patients. Histograms are expressed as mean ± SD of percentage of PRBC that are associated with clumps after 120 min. Mann-Whitney U test was used to compare pairs of groups, and a p value < 0.05 was considered significant. Assays were performed 2 times per CM patient sample.
3. Thrombocytopenia of CM patients restricts ex vivo clump formation
The occurrence of clump formation ex vivo in the presence of thrombocytopenia was then evaluated by using PRP and PRBC from the same CM patients. Healthy donors that provided PRP used for the previous assays exhibited an average platelet count >300 ×103 platelets/μL, whereas all the 22 CM cases were thrombocytopenic, with platelet counts <150 ×103 platelets/μL on admission (Table 1). When incubated with PRP from the same thrombocytopenic patient, PRBC showed a significantly reduced pro-clumping phenotype, at a mean of 39.6± 19.4% after a 120 min incubation step. To assess if this clumping restriction was only due to the low platelet count and not to other circulating plasma factor(s) in these CM patients, we adjusted the obtained PRP to a final average platelet count greater than 300 ×103 platelets/μL by a low centrifugation step as described above. When PRP with an adjusted platelet count was incubated with PRBC, there was a partial, but significant restoration of the clumping, reaching a mean of 82.6 ± 11.6% after 120 min (p<0.001, Fig. 4). A control performed with PRP from healthy donors with a platelet count adjusted to disease-type concentration by dilution (60×103 platelets/μL, as calculated in Table 1), led to a significant decrease of the clumping (data not shown).
Figure 4. CM patients’ thrombocytopenia restricts the formation of platelet-PRBC clumps in vitro.
To perform auto-assays, PRBC were incubated in the presence of control PPP, PRP from the same CM patient (final count <150 ×103 platelets/ml), or PRP from the same patient but with a platelet suspension adjusted to a normal platelet count (final count >300 ×103 platelets/ml). Results are expressed as mean ± SD of percentage of PRBC that are associated with clumps. Assays were performed 3 times per CM patient sample.
4. The degree of thrombocytopenia correlates with density of parasitaemia in Malawi CM patients
The relationship between platelet count and density of parasitaemia was investigated in the 3 patient categories (Table 1). A negative coefficient of correlation (-0.675) was found between these two variables in the CM group, with a statistically significant Spearman’s correlation test (p<0.001). This association was not observed in children with SMA or UM.
DISCUSSION
In this study we have demonstrated that platelet-mediated PRBC clumping is a stable and common in vitro phenotype of all the Plasmodium falciparum strains isolated from 49 infected Malawian children. We have also shown that this phenotype is quantitatively associated with the disease severity and type, that the platelet molecules responsible for this effect are CD36 and P-selectin, and that the degree of thrombocytopenia present in patients with CM is sufficient to limit the further formation of PRBC clumps in vitro.
The aims of the present study were first, to document the occurrence of the platelet-induced PRBC clumping phenomenon in parasites isolated from Malawian paediatric patients and, second, to assess its association with disease severity and type (UM versus severe malaria, and SMA versus CM). We demonstrated clumping of PRBC in the presence of PRP for all the isolates from malaria patients admitted to the ward, irrespective of their clinical syndrome. The ability to generate clumps in the presence of a standard preparation of platelets was significantly greater in P. falciparum isolates from patients with severe disease (SMA and CM) than in parasites from those with uncomplicated illness. These results are consistent with those from previous studies on Kenyan children [4] and Thai adults [5]. In the present study, the tendency to generate clumps was also shown to be significantly greater with CM isolates then with SMA isolates, indicating a relationship between clumping phenotype and disease syndrome. Whether this relationship is causal - i.e., whether clumping contributes directly to pathogenesis - remains to be studied. In CM, addition of PRP to parasite cultures led to the formation of large clumps within only 20 min of incubation. The giant clump formation we have observed in vitro as a consequence of platelet and PRBC adhesion in CM patients may, if it occurs in vivo, result in the increased accumulation of PRBC within the microvasculature of CM patients, compared to patients with other malarial syndromes [10]. The recently described accumulation of platelets within cerebral venules of CM patients from Malawi supports this hypothesis. Several patterns of platelet distribution have been demonstrated by peroxidase immunostaining of cerebral tissue, some of which include platelets clustered between parasite pigment in the whole length of visible vessels [3], suggesting a clumping between platelets and PRBC. Platelets may also directly contribute to the adhesion of PRBC to activated endothelium [11]. Uneven deposition of platelets may contribute to the observed disparate distribution of sequestration in the microvasculature of CM patients [12].
We then further analysed the role of platelet activation in the formation of clumps. Previous studies have shown that PRBC are able to activate platelets [13, 14], leading to the immediate transfer of the internal P-selectin (stocked in the platelet alpha-granules) to its surface [15]. As the presence of P-selectin has been described as a synergistic factor for the binding of PRBC on endothelial CD36 [16], we hypothesized that platelet P-selectin was likely to be involved in the clump formation. Its expression on platelet membranes increased dramatically during clumping, confirming that platelets are activated by PRBC. We also demonstrated by inhibition assays that P-selectin was involved as a PRBC receptor in clump formation. The other molecule shown to be involved in PRBC-platelet adherence in the present study was CD36. Both P-selectin and CD36 are expressed on platelets, and both are known to be receptors for PfEMP-1, the parasite protein expressed on the surface of PRBCs [17-20]. These findings are consistent with previous studies [4] for CD36, but P-selectin has not previously been identified as a receptor mediating platelet-PRBC clumping. When both CD36 and P-selectin were blocked by antibodies, the clumping phenomenon was abrogated, and our results suggest a synergism between the two receptors during clump formation. P-selectin has been reported to increase the adherence of PRBC to CD36 on endothelium [16], and its substantial but transient expression on PRBC-activated platelet surfaces may act as a trigger for the amplification of the clumping phenomenon. Such a ‘clumping-activation-clumping’ loop might explain the massive aggregation observed in the early minutes of our assays with CM-derived PRBC. Platelet activation as a consequence of clumping formation in vivo would lead to a massive release of TGF-β1, contained in the platelet α-granules. This pleiotropic cytokine has recently been described to act as an inducer of apoptosis in endothelium in inflammatory conditions; platelet activation in sequestration sites in vivo may therefore make additional indirect contributions to pathogenesis [14].
We finally investigated the possibility of a natural restriction of clumping by thrombocytopenia in malaria patients. The formation of giant clumps in vivo could be expected to lead to important rheological disturbances and to microvessel occlusion, yet in vivo studies have suggested that sequestration of PRBC is not sufficient to block brain microcirculation in CM patients [1, 21]. As platelets seem to be a crucial element in clump generation, we investigated the effect of thrombocytopenia, exhibited by all our CM patients (see Table 1), on clump formation ex vivo. We developed a new method of ex vivo co-culture of different cell types isolated from the same patient, i.e. platelets and PRBC, and we compared the effect of the PRP from healthy individuals (average platelet count >300×103/μL) to the effect of PRP from the same patient (<150×103/μL) on clump formation. Plasma from thrombocytopenic patients failed to generate giant clumps, and an adjustment of the platelet count to within the normal range in these plasma samples led to the restoration of the phenomenon. These results suggest that in patients with malaria, thrombocytopenia may have the potentially beneficial effect of restricting clump formation in vivo, leading to less platelet activation and thereby modulating pathogenesis.
The degree of thrombocytopenia in paediatric CM patients correlated significantly with parasitaemia (Spearman’s correlation test -0.675, p<0.001, Table 1). A possible explanation of this correlation would be that thrombocytopenia results from widespread clumping in sequestration sites, the degree of which is directly linked to the patient’s parasite burden. However, this hypothesis is not supported by experiments in mice in which abrogation of the platelet trapping in cerebral microvasculature had no effect on the low platelet count of the animals [22]. Whatever the mechanism of thrombocytopenia, it appears that in human CM the invariably low platelet count may function as a protective mechanism for the host.
ACKNOWLEDGEMENTS
We wish to thank warmly the staff of the Paediatric Research Ward, Queen Elizabeth Central Hospital, Blantyre, Malawi, for collecting samples, Dr David Roberts for his helpful comments, and Dr Jacqui Montgomery for her logistic help during the study. We also thank Dr Gundula Wiemann for her generous gift of Reopro, and Dr Jürgen Fingerle (F. Hoffmann-La Roche) for providing us with Lamifiban.
Financial support: This work was funded by grants from the Wellcome Trust, UK (to M.E.M., D.J.R., G.E.G, and C.M.), and from the National Institutes of Health, USA (to T.E.T., Grant AI R01-34969).
Footnotes
Conflict of interest statement: none of the authors listed above has a financial interest related to this work
REFERENCES
- 1.Rogerson SJ, Grau GE, Hunt NH. The microcirculation in severe malaria. Microcirculation. 2004;11:559–76. doi: 10.1080/10739680490503311. [DOI] [PubMed] [Google Scholar]
- 2.Lewallen S, White VA, Whitten RO, et al. Clinical-histopathological correlation of the abnormal retinal vessels in cerebral malaria. Arch Ophthalmol. 2000;118:924–8. [PubMed] [Google Scholar]
- 3.Grau GE, Mackenzie CD, Carr RA, et al. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis. 2003;187:461–6. doi: 10.1086/367960. [DOI] [PubMed] [Google Scholar]
- 4.Pain A, Ferguson DJ, Kai O, et al. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc Natl Acad Sci U S A. 2001;98:1805–10. doi: 10.1073/pnas.98.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chotivanich K, Sritabal J, Udomsangpetch R, et al. Platelet-induced autoagglutination of Plasmodium falciparum-infected red blood cells and disease severity in Thailand. J Infect Dis. 2004;189:1052–5. doi: 10.1086/381900. [DOI] [PubMed] [Google Scholar]
- 6.Gerardin P, Rogier C, Ka AS, Jouvencel P, Brousse V, Imbert P. Prognostic value of thrombocytopenia in African children with falciparum malaria. Am J Trop Med Hyg. 2002;66:686–91. doi: 10.4269/ajtmh.2002.66.686. [DOI] [PubMed] [Google Scholar]
- 7.Ladhani S, Lowe B, Cole AO, Kowuondo K, Newton CR. Changes in white blood cells and platelets in children with falciparum malaria: relationship to disease outcome. Br J Haematol. 2002;119:839–47. doi: 10.1046/j.1365-2141.2002.03904.x. [DOI] [PubMed] [Google Scholar]
- 8.Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett JS. Novel platelet inhibitors. Annu Rev Med. 2001;52:161–84. doi: 10.1146/annurev.med.52.1.161. [DOI] [PubMed] [Google Scholar]
- 10.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 11.Wassmer SC, Lepolard C, Traore B, Pouvelle B, Gysin J, Grau GE. Platelets reorient Plasmodium falciparum-infected erythrocyte cytoadhesion to activated endothelial cells. J Infect Dis. 2004;189:180–9. doi: 10.1086/380761. [DOI] [PubMed] [Google Scholar]
- 12.Silamut K, Phu NH, Whitty C, et al. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol. 1999;155:395–410. doi: 10.1016/S0002-9440(10)65136-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polack B, Peyron F, Sheick Zadiuddin I, Kolodie L, Ambroise-Thomas P. Erythrocytes infected by Plasmodium falciparum activate human platelets. C R Acad Sci III. 1990;310:577–82. [PubMed] [Google Scholar]
- 14.Wassmer SC, de Souza JB, Frere C, Candal FJ, Juhan-Vague I, Grau GE. TGF-{beta}1 Released from Activated Platelets Can Induce TNF-Stimulated Human Brain Endothelium Apoptosis: A New Mechanism for Microvascular Lesion during Cerebral Malaria. J Immunol. 2006;176:1180–4. doi: 10.4049/jimmunol.176.2.1180. [DOI] [PubMed] [Google Scholar]
- 15.Israels SJ, Gerrard JM, Jacques YV, et al. Platelet dense granule membranes contain both granulophysin and P-selectin (GMP-140) Blood. 1992;80:143–52. [PubMed] [Google Scholar]
- 16.Yipp BG, Anand S, Schollaardt T, Patel KD, Looareesuwan S, Ho M. Synergism of multiple adhesion molecules in mediating cytoadherence of Plasmodium falciparum-infected erythrocytes to microvascular endothelial cells under flow. Blood. 2000;96:2292–8. [PubMed] [Google Scholar]
- 17.Barnwell JW, Ockenhouse CF, Knowles DM. Monoclonal antibody OKM5 inhibits in vitro binding of Plasmodium falciparum infected erythrocytes to monocytes, endothelial, and C32 melanoma cells. J.Immunol. 1985;135:3494–3497. [PubMed] [Google Scholar]
- 18.Ockenhouse CF, Magowan C, Chulay JD. Activation of monocytes and platelets by monoclonal antibodies or malaria-infected erythrocytes binding to the CD36 surface receptor in vitro. J Clin Invest. 1989;84:468–75. doi: 10.1172/JCI114188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udomsangpetch R, Reinhardt PH, Schollaardt T, Elliott JF, Kubes P, Ho M. Promiscuity of clinical Plasmodium falciparum isolates for multiple adhesion molecules under flow conditions. J.Immunol. 1997;158:4358–4364. [PubMed] [Google Scholar]
- 20.Thomas S. Platelet membrane glycoproteins in haemostasis. Clin Lab. 2002;48:247–62. [PubMed] [Google Scholar]
- 21.Newton CR, Marsh K, Peshu N, Kirkham FJ. Perturbations of cerebral hemodynamics in Kenyans with cerebral malaria. Pediatr Neurol. 1996;15:41–9. doi: 10.1016/0887-8994(96)00115-4. [DOI] [PubMed] [Google Scholar]
- 22.Sun G, Chang WL, Li J, Berney SM, Kimpel D, van der Heyde HC. Inhibition of platelet adherence to brain microvasculature protects against severe Plasmodium berghei malaria. Infect Immun. 2003;71:6553–61. doi: 10.1128/IAI.71.11.6553-6561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]