Abstract
The oncogenic process leading to nasopharyngeal carcinoma (NPC) requires the combination of genetic and epigenetic alterations, latent infection by the Epstein-Barr virus and local inflammation. A transcriptome analysis of NPC xenografts identified the gene encoding the cellular inhibitor of apoptosis protein 2 (c-IAP2) among the top five most intensely expressed. Consistently, the very high levels of the c-IAP2 protein were detected in 11 of 13 NPC biopsies. RMT 5265, a structural analog of second mitochondria-derived activator of caspase (SMAC), induced the rapid degradation of c-IAP2 in nasopharyngeal epithelial cells, whether malignant or not, but blocked clonal cell growth in NPC cells only. In short-term experiments, RMT 5265 induced apoptosis in a fraction of NPC cells, and this apoptosis was dramatically enhanced when RMT 5265 was combined with Toll-like receptor 3 (TLR3) stimulation. By contrast, the cooperative effect with tumor necrosis factor α was only marginal. The apoptosis induced by the combination of RMT 5265 and TLR3 stimulation was mediated by caspase-8 and associated with a decrease in the cellular content of the long isoform of FLICE-like inhibitory protein. Similar caspase-8 activation was obtained when siRNA knockdown of c-IAP2 was combined with TLR3 stimulation. In conclusion, c-IAP2 has a specific protective function in NPC cells challenged by TLR3 agonists. This protective function is probably important to make NPC cells tolerant to their own production of small viral RNAs, which are potential agonists of TLR3. Our data will help to design a rational use of IAP inhibitors in NPC patients.
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor arising from the epithelial lining of the nasopharynx. Consistently associated with the Epstein-Barr virus (EBV), NPC is the third most frequent virus-associated malignancy in humans [1,2]. Foci of high incidence (25 cases per 100,000 individuals per year) are localized in Southeast Asia, particularly in southern China. There are also large areas of intermediate incidence (three to eight cases), for example, Indonesia and northern Africa [3,4]. The multifactorial pathogenesis of NPC relies on germ line genetic susceptibility, acquired cellular genetic and epigenetic alterations, especially under the influence of diet carcinogens, and EBV infection [1,4,5]. Although most viral genes are silent in NPC cells, some are consistently expressed including those encoding the nuclear protein Epstein-Barr nuclear antigen 1, two membrane proteins latent membrane proteins 1 and 2 (LMP1 and LMP2), and the short untranslated EBV-encoded RNA (EBER) and EBER2. Of the very large amounts of EBERs produced and present in the nuclei of NPC cells, some are exported in the cytoplasm and possibly in the extracellular space [6].
The treatment of NPC has improved significantly in recent years, but its prognosis remains serious because of the frequency of distant metastases—even when the primary tumor is small—and because of long-term secondary effects of radiotherapy and chemotherapy [7]. The malignant phenotype of NPC cells is supported by a network of altered biologic pathways resulting from cellular genetic and epigenetic alterations combined with latent EBV infection. Local inflammation is also suspected to play a major role because of the consistent presence of a massive lymphoid infiltrate in the primary tumor and the intense local production of inflammatory cytokines [8,9]. To better characterize the cellular part in the NPC oncogenic mechanisms, we took advantage of our cytogenetically characterized NPC xenografts to carry out a transcriptome analysis that identified the cellular inhibitor of apoptosis protein 2 (c-IAP2) gene as consistently overexpressed in NPC cells.
The IAPs constitute a family of evolutionarily conserved antiapoptotic proteins that are thought to contribute to a variety of human cancers [10,11]. They all contain one to three copies of the characteristic baculovirus IAP repeat (BIR). One IAP protein, X-linked IAP (XIAP), is produced in large amounts in all cell types and is often regarded as a housekeeping protein. Another one, survivin, present in very small amounts in most adult tissues, is overproduced in all human cancers [12]. Recent studies have highlighted the role of two other IAPs, c-IAP1 and c-IAP2, as ubiquitin ligases and regulators of nuclear factor κB activation and tumor necrosis factor (TNF) production [13,14]. There is also direct genetic evidence that c-IAP1 and c-IAP2 can function as oncogenes. An amplification of the 11q21-23 chromosomal region, which encompasses the c-IAP1 and c-IAP2 genes, is observed in a variety of malignancies including medulloblastomas, renal cell carcinomas, glioblastomas, gastric carcinomas, and both small and non-small cell lung carcinomas [10]. Furthermore, both c-IAP1 and c-IAP2 are overproduced and contribute to rapid tumor growth in human hepatocellular carcinomas, whereas in esophageal carcinomas, it is only c-IAP1 that is overproduced [15,16]. In this light, it seemed of interest to further investigate the role of c-IAP2 in NPC cells.
Macrophages from c-IAP2 knockout mice show a high sensitivity to apoptosis in a lipopolysaccharide (LPS)-induced proinflamatory environment, which stimulates the Toll-like receptor 4 (TLR4) [17]. c-IAP2 could thus play a key role in response to TLR stimulation. The TLRs are type I transmembrane proteins thought to be critically involved in the detection of pathogens and in triggering inflammation and immune response to microbial infections [18]. The stimulation of TLRs by their respective ligands initiates well-characterized signaling cascades that enhance cellular resistance against pathogens. Occasionally, it also induces cell death, one of the mechanisms that limit virus diffusion in the host. Toll-like receptors are expressed not only in immune cells, but also in some cancer cells, opening the way for TLR-based cancer therapy [19,20]. Among TLRs, TLR3 is specifically involved in antiviral responses triggered by the binding of double-stranded RNA of viral origin. This binding leads in turn to mitogen-activated protein kinases, nuclear factor κB, and interferon regulatory factor 3 activation and to interferon I induction through the adaptor proteins TIR domain-containing adapter-inducing IFN-β (TRIF) and receptor-interacting protein (RIP) [18,21]. Moreover, a direct proapoptotic effect of TLR3 agonists has been reported in several tumor cells [22,23].
This work aimed to answer the following questions: Is c-IAP2 consistently overexpressed in NPC cells? Are NPC cells permanently dependent on c-IAP2 overexpression? What are the specific functions of c-IAP2 in NPC cells? We report that c-IAP2 plays a major role in the resistance of NPC cells to apoptosis induced by TLR3 stimulation, whereas it is not involved, or marginally involved, in NPC cell response to other proapoptotic factors like TNF-α or cisplatin. Because NPC cells are known to produce large amounts of viral EBER RNA, which are potential agonists of TLR3, our data will have important implications for our understanding of virus-cell interactions and design of novel therapeutic strategies.
Materials and Methods
Clinical Specimens of NPCs and Immunohistology
Nasopharyngeal carcinoma biopsy fragments were obtained, before treatment, from 13 NPC patients referred to the Institut Gustave Roussy (9 patients; Villejuif, France) or the Sfax University hospital (4 patients; Sfax, Tunisia). The clinical and pathologic characteristics of these tumors are summarized in Table W1. Tumor pieces were fixed in PBS-formaldehyde 4% for 16 hours and were paraffin-embedded. Tissue sections were microwaved at 98°C for 30 minutes in citrate buffer (10 mM, pH 7.3) and were incubated with an antihuman c-IAP2 mouse monoclonal antibody (clone F30-2285; BD Biosciences, San Jose, CA). Binding of the primary antibody was detected with the CSA II kit from Dako (based on a tyramide amplification system; DakoCytomation, Glostrup, Denmark).
Xenografts and Cell Lines
C15 and C17 are EBV-positive NPC xenografts routinely propagated by subcutaneous passages into nude mice. Some data presented in this report were obtained from C19, another NPC xenograft that is no longer propagated into nude mice, for which frozen tumor pieces and DMSO-frozen cells were available [24]. So far, it has not been possible to establish long-term in vitro cultures from the C15 and C17 xenografts. They can only be handled in short-term in vitro cultures after mouse sacrifice, tumor dissection, and collagenase dispersion [8,25]. In contrast, C666-1, another EBV-positive NPC cell line, can be propagated either as xenografts or by long-term in vitro cultures [25,26]. In the rest of this report, C15, C17, and C666-1 are often referred as “NPC tumor lines.” Persistence of latent EBV infection in the C15, C17, and C666-1 xenografts was checked at regular time intervals by EBER hybridization (Figure W1). Primary cultures of nasopharyngeal epithelial cells were established in Hong Kong from nonmalignant biopsies from the nasopharyngeal region as previously described [27]. Nontumorigenic immortalized epithelial cell lines were obtained from such primary cultures by SV-40 infection (NP69 cells) or stable transfection of the gene encoding the telomerase catalytic subunit hTert (NP460 cells) [27,28]. CNE2 is an atypical EBV-negative NPC cell line [24]. Non-NPC malignant cell lines were also used: HeLa (epithelial, cervix carcinoma), A431 (epithelial, vulvar epidermoid carcinoma), and Jurkat derived from a T-cell leukemia. MRC5 cells are human untransformed fibroblasts purchased from Biomerieux (Marcy l'Etoile, France) [29]. Before in vitro experiments, C17 xenografted tumors were minced and treated with type II collagenase for cell dispersion as previously reported [8,24,25]. C666-1 cells were continuously grown in vitro in RPMI 1640 medium (Gibco-Invitrogen, Carlsbad, CA) supplemented with 25 mM HEPES and 7.5% fetal calf serum (FCS), in plastic flasks coated with collagen I (Biocoat; Becton-Dickinson, Franklin Lakes, NJ). NP69 and NP460 were grown in keratinocyte serum-free medium (Gibco) supplemented with 10% FCS and 50 µg/ml bovine pituitary extract for NP460 (Gibco). In some experiments requiring precise comparisons with C15 and C17 xenografts, CNE2, HeLa, and A431 cells were grown as xenografts into nude mice (subcutaneous injections of 2 to 5 million cells and tumor collection 3 to 4 weeks later).
RNA Extraction and Transcriptome Analysis
Detailed protocols for RNA extraction and transcription profiling on microarrays are available from the ArrayExpress Repository (http://www.ebi.ac.uk/microarray-as/) under the accession number E-TABM-382. Briefly, total RNA was extracted from xenografted tumors and cultured cells with the TriReagent method (Euromedex, Souffelweyersheim, France) and further purified on RNeasy columns (Qiagen, Valencia, CA). For transcription profiling on microarrays, target cDNA were synthesized by incubating total RNA (25 µg) with a cocktail containing Cy3 or Cy5-dUTP and SuperScript II reverse transcriptase (Life Technologies, Gibco-Invitrogen). The microarray slides used were pan-genomic oligo-arrays containing 22,000 60-mer oligomers representative of 14,217 genes and were manufactured by Agilent (Santa Clare, CA; ref. G 4110A). Labeled targets were hybridized with these slides for 16 hours at 42°C. The slides were washed and scanned with an Agilent 2565AA DNA array scanner with a resolution of 10 µm. Data were analyzed with the Rosetta Resolver system for gene expression analysis (Rosetta Inpharmatics LLC, Seattle, WA).
Reverse Transcription-Polymerase Chain Reaction Analysis
Total RNA (1 µg) was reverse-transcribed using the Protoscript First Strand cDNA Synthesis Kit (New England BioLabs, Ipswich, MA). Real-time polymerase chain reaction (PCR) was performed in a 25-µl reaction volume, containing 25 ng of cDNA template, 10 pmol of each primer, and 12.5 µl of SYBR-Green Master Mix (Applied Biosystems, Foster City, CA) or TaqMan Universal PCR Master Mix (Roche Molecular, Neuilly sur Seine, France). Baculoviral IAP repeat-containing 3 (BIRC3) mRNA was amplified with the following oligonucleotides: BIRC3-sense: 5′-ATCTGGAGATGATCCATGGG-3′ and BIRC3-antisense: 5′-TGTTCAAGTAGATGAGGG-3′. Amplified GAPDH RNA was used as an endogenous control. Amplification reactions were performed in an Applied Biosystems ABI Prism 7000 Sequence Detection System. Conventional reverse transcription-PCR (RT-PCR) was used for the detection of TLR3, 4, and 9 transcripts, using specific primer pairs from R&D Systems (Minneapolis, MN; RDP 266, 268, and 274, respectively).
Fluorescent In Situ Hybridization Analysis
Interphase nuclei and metaphase spreads were prepared according to standard procedures from the C666-1 NPC cell line and, after collagenase dispersion, from the C15, C17, and C19 NPC xenografts. The BIRC3 DNA probe was derived from the BAC clone RP11-659L4, starting at nucleotide 101,609,259 and ending at nucleotide 101,837,762 (BacPac Resources, Children's Hospital, Oakland, CA). This BAC clone contains the entire BIRC3 gene, running from nucleotide 101,693,404 to 101,713,658 on 11q22.2 (http://www.genome.ucsc.edu/). This probe was labeled by random priming with fluorescein 5-dUTP (Abbot-Vysis, Downers Grove, IL). It was then tested for fluorescent in situ hybridization on normal metaphases to ensure that it hybridized specifically with the target chromosome. The same BAC was then hybridized with interphase nuclei and metaphases from the four NPC tumor lines, according to a previously published protocol [30].
Treatments of Cells with Pharmacological Reagents and siRNA
The polycyclic C2-symmetric (40 carbon atoms) compound RMT 5265, which mimics the three-dimensional structure of the second mitochondria-derived activator of caspases (Smac)/Diablo N-terminal tetrapeptide, has been described elsewhere [31]. HS4044 has a similar structure but is acetylated at a critical alanine group; it was used as a negative control [31]. Both reagents were dissolved in DMSO. Caspase-8 was inhibited with Z-IETD-FMK (N-1830; Bachem, Bubendorf, Switzerland) at a concentration of 50 µM. The proteasome was neutralized by incubation with 10 µM MG132 (Calbiochem, San Diego, CA) or 1 µM epoxomicin (Calbiochem) for 15 minutes. As a positive control for apoptosis induction, cells were treated with a Fas agonist, the monoclonal antibody 7C11, at a concentration of 100 ng/ml for 6 hours (Immunotech, Beckman-Coulter, Fullerton, CA). Agonists of TLR3 (poly(I:C)) and TLR9 (type C CpG) were obtained from InvivoGen (San Diego, CA). TNF-α (Peprotech, Rocky Hill, NJ) toxicity was verified on MCF7 cells. cis-Platinum(II)diammine dichloride (cisplatin) was purchased from Sigma (St. Quentin Fallavier, France). C666-1 cells at 30% to 50% confluence in six-well plates were transiently transfected with siRNA. For RNA interference, double-stranded RNA oligonucleotides directed against c-IAP2 (a: HSS100562, b: HSS100561), XIAP (a: HSS100566, b: HSS100565), survivin (a: HSS141243, b: HSS141245), and a negative control (1390109) were purchased from Invitrogen. Transfections were carried out using Oligofectamine (Invitrogen). Two successive rounds of siRNA transfection at 48-hour intervals were required for the efficient depletion of c-IAP2.
Cell Growth and Viability Assays
The clonal growth of C666-1 NPC cells was assessed using a feeder layer of Swiss 3T3 murine fibroblasts as previously described [32]. Briefly, irradiated Swiss 3T3 cells kindly provided by T. Magnaldo (Institut Gustave-Roussy, Villejuif, France) were plated in six-well plates (1.5 x 105 per well). Once the fibroblasts had adhered to the plate, epithelial cells were added at a density of 500 (immortalized NP69 cells) to 5000 cells per well (C666-1 NPC cells). After 2 to 4 weeks of culture, cell colonies were stained with a solution of crystal violet in methanol. Alternatively, the viability of C666-1 cells was determined in a short-term assay based on the reduction of WST (a soluble form of MTT; Roche Molecular), as previously described [25]. For this assay, cells were seeded in 96-well plates at a density of 3 x 104 cells per well. The WST reaction was performed after 72 hours of culture. The growth of C17 NPC cells in vitro was evaluated with a short-term colony assay. Dispersed cells derived from xenografted tumors were seeded in 24-well plates at a density of 106 cells per well in 1.5 ml in RPMI 1640 medium (Gibco-Invitrogen) supplemented with 25 mM HEPES and 20% FCS. The culture medium was changed twice, 16 and 72 hours later. At the second medium change, the concentration of FCS was decreased to 7.5%. During the next 3 days, scattered colonies of epithelial cells became apparent on a layer of murine fibroblasts. These colonies were stained with rhodamine in 4% formaldehyde in PBS and counted under an inverted microscope.
Assessment of Apoptosis and Caspase Activation
Apoptosis was assessed quantitatively by determining the sub-G1 DNA content in ethanol-fixed cells, stained with propidium iodide and analyzed using a Becton Dickinson FACScalibur flow cytometer and the CellQuest Pro software. Alternatively, apoptosis was evaluated by the detection of the cleavage of poly(ADP-ribose)polymerase (PARP) by Western blot analysis performed on total cell protein extracts (see next paragraph). The activities of caspases-3/7 and caspase-8 were measured with the Caspases-Glo 3/7 and Caspases-Glo 8 Assay kits, respectively (Promega, Madison, WI). These assays are based on the cleavage of luminogenic substrates containing the amino acid sequences Z-DEVD and Z-LETD, respectively.
Cell Protein Extraction and Western Blot Analysis
Proteins from cultured cells or xenografts were extracted by lysis in RIPA buffer (50 mM Tris, 150 mM NaCl, 5 mM EDTA, 0.5% sodium deoxycholic acid, 0.5% NP-40, 0.1% SDS) supplemented with a protease inhibitor cocktail (Complete; Roche Molecular). They were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Immobilon, Millipore, Billerica, CA) by electroblot analysis at 4°C for 90 minutes at 90 V or overnight at 45 V. The antibodies used for Western blot analysis were mouse monoclonal antibodies directed against human c-IAP2, XIAP, and c-IAP1, obtained from BD Biosciences (refs. 552782, 610763, and 556533, respectively). The other mouse monoclonal antibodies used were specific for Smac/Diablo (ref. 9494; Cell Signaling, Danvers, MA), survivin (ref. 17779; Santa Cruz Biotechnology, Santa Cruz, CA), PARP (clone C-2-10, ref. 53643; Santa Cruz Biotechnology), and FLICE-like inhibitory protein (FLIP, ref. 804-428; Alexis Biochemical, San Diego, CA). Blots were incubated with a secondary peroxidase-conjugated antibody, and chemiluminescent detection was done using the Immobilon Western Chemiluminescent HRP Substrate (Millipore). When required, the probe was removed from the blot by incubating in Re-Blot Plus Mild Antibody Stripping Solution (Chemicon, Millipore) for 20 minutes at room temperature. In one instance, c-IAP2 was immunoprecipitated before Western blot analysis using magnetic beads, according to our previously described protocol [33].
Statistics
Results of functional tests—colony, viability, and caspase assays—were given as the means ± SD. Statistical significance was assessed using a 2-tailed Student's t test for comparison of two experimental conditions. When making comparisons involving multiple experimental conditions, we used the analysis of variance test completed by the Tukey test (bilateral) in the XLSTAT software (95% confidence interval).
Results
Abundant Transcription of the BIRC3 Gene in NPC Cells, Even in the Presence of Hemizygous Deletions
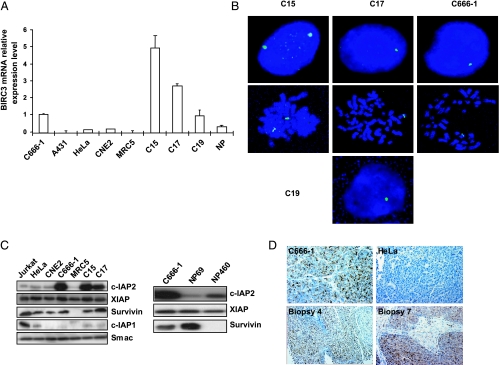
To analyze the cellular part of NPC oncogenesis, we assessed gene expression profiling on two NPC xenografts (C15 and C17) on Agilent microarrays containing 22,000 oligonucleotides representative of 14,217 genes using untransformed quiescent human fibroblasts (MRC5) as a reference. All data are available from the ArrayExpress Repository (http://www.ebi.ac.uk/microarray-as/; under the accession number: E-TABM-382). We focused subsequent investigations on genes transcribed at very high levels in each xenograft. The main characteristics of the top 13 most transcribed genes, including their chromosomal location, are provided in Table W2. BIRC3, encoding the c-IAP2 protein, was one of these genes. Its consistent overexpression was confirmed by quantitative RT-PCR (Figure 1A). BIRC3 transcripts were much more abundant in EBV-positive NPC tumor lines (either xenografted or propagated in vitro)—C15, C17, C19, and C666-1—than in any other cell type, including untransformed MRC5 fibroblasts, non-NPC malignant epithelial cells (HeLa, A431), atypical EBV-negative NPC cells (CNE2), and a primary culture of nasopharyngeal epithelial cells (NP) from normal mucosa. Meanwhile, we noticed the BIRC3 gene maps to a chromosomal band (11q22) frequently affected by hemizygous deletions in NPCs and in certain other tumor types [26,34,35]. From our previous studies, it is known that the 11q22 band is affected by hemizygous losses in C17, C19, and C666-1 but not in C15 [26,34]. Accordingly, BIRC3 was expected to be reduced to one copy in C17, C19, and C666-1 but not in C15 cells. This was verified by fluorescent in situ hybridization analysis using a BAC probe encompassing the BIRC3 gene (Figure 1B). Thus, the reduction of the BIRC3 gene to a single copy was not accompanied by a significant decrease in its level of transcription, which remained in the same order of magnitude as for the C15 xenograft. This was particularly striking because most genes mapping to chromosome 11q22 displayed a marked decrease in expression (complete data provided in the ArrayExpress repository, accession number: E-TABM-382). These observations suggested that BIRC3 overexpression could provide a selective advantage on NPC cells even at a late stage of tumor progression (e.g., in C17 and C19 xenografts that had been derived from metastatic cells).
Figure 1.
Recurrent overproduction of the c-IAP2 protein in NPC tumor lines and clinical specimens despite hemizygous losses of BIRC3. (A) Assessment of BIRC3 gene expression by real-time RT-PCR. Amounts of BIRC3 transcripts were deduced from Ct values and normalized with values obtained from GAPDH RNA amplifications. Relative levels of expression are given, with the value obtained for C666-1 NPC cells arbitrarily set at 1. For accurate comparisons with NPC xenografts, CNE2, HeLa, and A431 cells were grown as xenografts before RNA extraction. Human untransformed MRC5 fibroblasts, which are sensitive to contact inhibition, were collected as quiescent cells after 3 days of growth arrest resulting from cell confluence. (B) Fluorescent in situ hybridization detection of the BIRC3 gene on interphase nuclei and metaphases of NPC cells. The images were acquired with a color CCD camera using the Smart Capture program (Digital Scientific, UK). Three of four NPC tumor lines (C666-1, C17, and C19) displayed alterations to chromosome 11q resulting in the deletion of one copy of the BIRC3 gene as shown by the detection of a single spot on interphase nuclei. By contrast, the cells from the C15 xenograft retained two copies of the BIRC3 gene. Hybridizations on chromosome spreads were obtained for three tumor lines. Consistent with observations on interphase nuclei, C666-1 and C17 cells displayed BIRC3 spots linked only to one chromosome 11 (with a double spot due to the presence of two chromatides after completion of the “S” phase). By contrast, C15 cells contained BIRC3 spots linked to two chromosomes 11 (each chromosome containing a double spot). (C) Left panel, Western blot analysis of c-IAP2, XIAP, c-IAP1, survivin, and Smac in NPC and non-NPC cell lines. Right panel, Western blot analysis of IAP proteins in immortalized nontumorigenic nasopharyngeal epithelial cells: NP69 (SV40 infection) and NP460 (telomerase transfection). (D) Detection of c-IAP2 by immunohistochemistry on tissue sections of NPC specimens using the F30-2285 monoclonal antibody. Panel 1 (original magnification, x400): C666-1 cells grown as a xenografted tumor showing intense cytoplasmic expression consistent with the Western blot analysis data displayed in panel (C). Panel 2 (original magnification, x400): HeLa cells grown as a xenografted tumor; no c-IAP2 staining is visible, although a faint band was detected by Western blot analysis (panel C), which is more sensitive than immunohistochemistry. Panels 3 (original magnification, x200) and 4 (original magnification, x400): cytoplasmic staining of c-IAP2 in biopsies 4 and 7, respectively; note the intense expression in malignant cells, contrasting with the very weak expression in stromal cells.
Recurrent Overproduction of the c-IAP2 Protein in NPC Tumor Lines and Clinical Specimens
We next investigated whether the overexpression of BIRC3 at the mRNA level was accompanied by a high level of c-IAP2 protein in NPC cells. Western blot analysis was performed on three NPC tumor lines and several reference cells, including non-NPC malignant epithelial cells (xenografted HeLa cells), atypical EBV-negative NPC cells (xenografted CNE2 cells), nonmalignant human fibroblasts grown in vitro (MRC5), and nonmalignant nasopharyngeal epithelial cells immortalized by SV40 infection (NP69) or telomerase gene transfection (NP460) [27,28]. As shown in Figure 1C, the c-IAP2 protein levels were consistent with those obtained at mRNA level. A very high concentration of c-IAP2 protein was found specifically in NPC tumor lines, in which this protein was approximately 10-fold more abundant than in non-NPC xenografts, MRC5 cells, or immortalized nasopharyngeal cells. In the subsequent stages of our study, we never found a cell type with a c-IAP2 content higher than those of NPC cells. In contrast, in Figure 1C, the expression level of XIAP was equally abundant in all cell types, including non-malignant MRC5 cells. Survivin was present in similar large amounts in NPC and non-NPC tumor lines but in only small amounts in MRC5 cells. c-IAP1 was abundant in the Jurkat T-cell line used as a positive control, whereas it was barely detectable in other cell types, including the NPC tumor lines. Importantly, those results were confirmed in tissue sections from clinical NPC specimens. High levels of c-IAP2 expression were found in 11 of 13 biopsies (Figure 1D and Table W1). Its distribution was essentially cytoplasmic with only rare examples of nuclear localization. c-IAP2 expression was consistently more intense in malignant NPC cells than in the lymphoid stroma or in adjacent residual non malignant mucosa. The abundance of c-IAP2 in patient biopsies was in favor of its important role in NPC tumor growth in situ.
RMT 5265 Induces the Proteasome-Mediated Degradation of c-IAP2
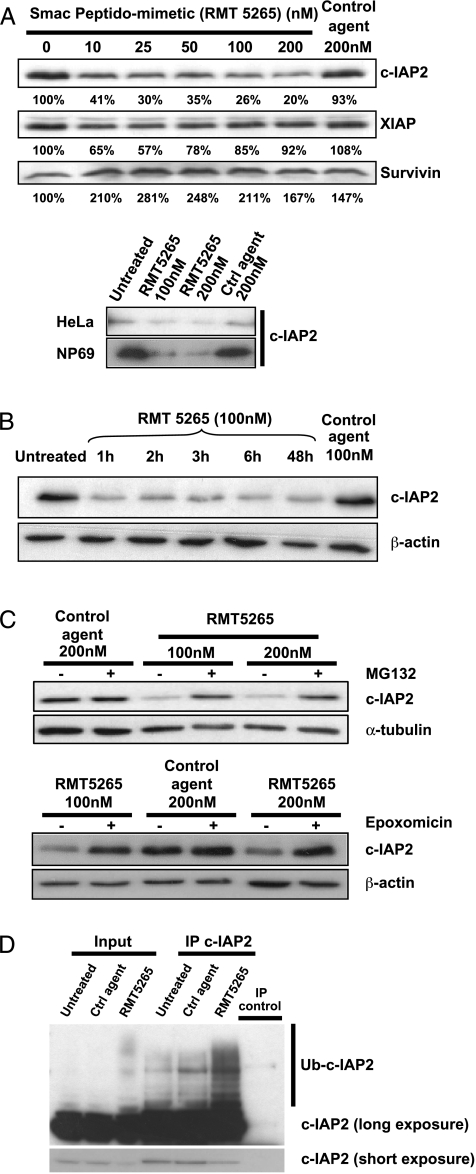
Data from previous experiments supported the hypothesis of a critical role of c-IAP2 in NPC cell growth. To test this hypothesis by functional inhibition in vitro, we first resort to RMT 5265, a pharmacological inhibitor of IAP proteins. This compound is a molecular mimic of the N-terminal AVPI sequence of the mature Smac-Diablo protein. It is known to bind c-IAP1, c-IAP2, and XIAP [14,31]. To get formal evidence of its inhibitory effect on c-IAP2, we monitored c-IAP2, XIAP, and survivin content of cells treated with RMT 5265. A marked, dose-dependent decrease in c-IAP2 levels—but not in those of XIAP and survivin—was observed in all cell types tested, including C666-1 NPC cells, HeLa cells, and NP69 nonmalignant nasopharyngeal cells (Figure 2A). c-IAP2 levels decreased dramatically and very rapidly, reaching a minimum in less than 1 hour (Figure 2B). This decrease was prevented by prior treatment with two proteasome inhibitors, MG132 and epoxomicin (Figure 2C). A ladder highly suggestive of the presence of ubiquitinated forms of c-IAP2 was visualized by immunoprecipitation and Western blot analysis of c-IAP2 from C666-1 cell extracts. The amount of proteins present in this ladder increased substantially if C666-1 cells were treated with RMT 5265 for 15 minutes, suggesting that the proteasome-mediated degradation induced by RMT 5265 resulted from the enhanced polyubiquitination of c-IAP2 (Figure 2D). The above experiments demonstrated that in NPC cells c-IAP2 was a major target of RMT 5265 and justified the subsequent use of this compound for pharmacological inhibition of c-IAP2 functions in these cells.
Figure 2.
Proteasome-dependent degradation of c-IAP2 induced by RMT 5265. (A) Upper panel, selective and dose-dependent decrease in c-IAP2 concentration in C666-1 cells treated with RMT 5265 or control compound (HS4044). Cells were collected after 48 hours of treatment. Specific protein bands were quantified by film densitometry using a GS-710 calibrated imaging densitometer with Quantity One software (Biorad, Marnes la Coquette, France). The decrease in c-IAP2 concentration is marked and dose-dependent. Survivin is not affected. The concentration of XIAP was apparently modified for low but not for high concentrations of RMT 5265. These variations were not regarded as significant. Lower panel, a similar decrease in c-IAP2 concentration was induced by RMT 5265 in HeLa and NP69 cells. (B) Kinetics of the decrease in c-IAP2 concentration induced by RMT 5265 in C666-1 cells. (C) The decrease in c-IAP2 concentration induced by RMT 5265 was prevented by prior incubation (15 minutes) and concomitant incubation with the proteasome inhibitors MG132 or epoxomicin. (D) RMT 5265 increased the amount of ubiquitinated forms of c-IAP2 detected in C666-1 cells. Immunoprecipitation was carried out before Western blot analysis to increase the signal-to-noise ratio for the detection of all forms of c-IAP2. Treatment by RMT 5265 enhanced the detection of a ladder of minor c-IAP2 species with a spacing of approximately 8 kDa, highly suggestive of a polyubiquitination process. Control agent and RMT 5265 were used at a 100-nM concentration.
RMT 5265 Selectively Blocks the Clonogenic Growth of NPC Cells
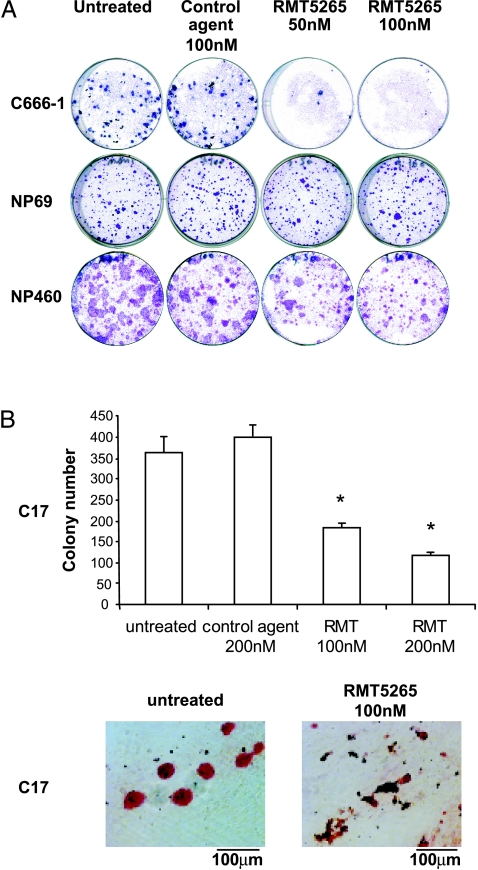
On the basis of our previous results, we used RMT 5265 to assess the influence of c-IAP2 inhibition on NPC cell growth. Although c-IAP2 was degraded by RMT 5265 in all cell typese, the inhibition of clonogenic growth was observed only in NPC cells. A concentration of 50 nM RMT 5265 was sufficient to abolish the growth of C666-1 cells with little or no effect on nonmalignant nasopharyngeal cells (NP69 and NP460; Figure 3A). To confirm the inhibition of NPC cell growth by RMT 5265, using cells distinct from the C666-1 model, we resort to the C17 NPC xenograft. Although C17 cells cannot be permanently propagated in vitro, we were able for the first time to perform a short-term colony assay using this material. We could show that the viability and/or proliferation of C17 NPC cells were significantly decreased by RMT 5265 (Figure 3B). Altogether, these results suggested that NPC cells were dependent on high expression of c-IAP2, whereas c-IAP2 expression in nonmalignant nasopharyngeal cells was both consistently low and dispensable for clonogenic growth.
Figure 3.
Selective inhibitory effects of RMT 5265 on NPC cell growth in vitro. (A) Clonogenic growth of NPC cells (C666-1) and immortalized nasopharyngeal epithelial cells (NP69 and NP460) treated with RMT 5265. Cells were seeded at low density in six-well plates, on a feeder layer of irradiated Swiss 3T3 fibroblasts, as described in the Materials and Methods section. Initial cell density: C666-1 cells: 5000 cells per well; NP69: 500 cells per well; NP460: 2500 cells per well. Culture medium and treatment were renewed twice a week. Colonies were stained with crystal violet after two to four weeks of growth. (B) Short-term colony assay performed on C17 NPC cells treated with RMT 5265, control agent (HS4044), or left untreated. Cells obtained by dispersion of C17 xenografts were seeded on day 1 at high density (1.2 x 106 cells per well in 24-well plates) and treated with pharmacological agents from days 4 to 7. During this period, dead cells were progressively removed through partial medium changes. At the completion of this period, cell colonies were stained with rhodamine and numbered under an inverted microscope. Colony numbers were the mean of three counts made for three separate wells on 24-well plates. For star-marked conditions, the differences in colony numbers with the control condition were statistically significant (P < .05).
RMT 5265 Induces Apoptosis in a Fraction of NPC Cells
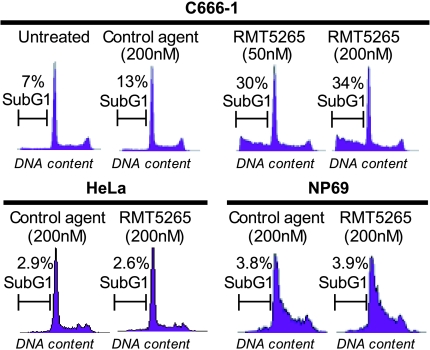
Because IAP functions are mainly related to apoptosis control we then investigated whether the toxicity of RMT 5265 to NPC cells involved apoptosis. C666-1 cells were treated in short-term experiments (48 hours) with two concentrations of RMT 5265 (50 or 200 nM), and the rate of apoptosis was assessed by flow cytometry measurements of the sub-G1 fraction. As shown in Figure 4, approximately 30% of C666-1 cells became apoptotic with a concentration of RMT 5265 as low as 50 nM. In contrast, there was no apoptosis induction in HeLa cells and nonmalignant nasopharyngeal epithelial cells (NP69) with concentration of RMT 5265 as high as 200 nM. This absence of apoptotic response was observed despite the fact that c-IAP2 was almost completely degraded under treatment by RMT 5265 (Figure 2A). This was one additional observation supporting the hypothesis that permanent high expression of c-IAP2 was obligatory in NPC cells, whereas its low-level expression was dispensable in nonmalignant nasopharyngeal epithelial cells (NP69) or non-NPC carcinoma cells (HeLa cells).
Figure 4.
Short-term effects of RMT 5265 treatment in C666-1 NPC cells. Flow cytometry assessment of DNA content and counting of cells with sub-G1 characteristics. Treatment with 50 nM RMT 5265 for 48 hours increased the fraction of sub-G1 cells to 30% with no substantial change at a higher concentration of RMT 5265 (200 nM). In contrast, the fraction of sub-G1 cells was not increased by RMT 52656 in non-NPC carcinoma cells (HeLa) or in nonmalignant nasopharyngeal epithelial cells (NP69).
RMT 5265 Unmasks a High Sensitivity of NPC Cells to TLR3-Mediated Apoptosis
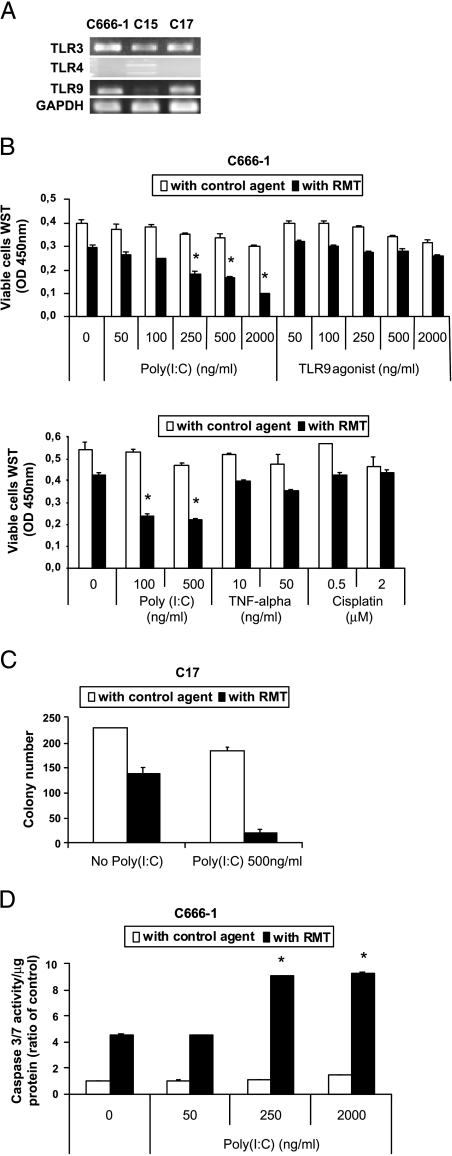
To obtain more information about the antiapoptotic functions of c-IAP2 in the context of NPC cells, treatment by RMT 5265 was combined with various potential apoptotic stimuli, especially some likely to occur in a context of local inflammation. A previous study showed that c-IAP2 knock-out in mice results in the complete inversion of the monocyte response to LPS, a specific agonist of the TLR4 receptor. In mice with a KO for c-IAP2, LPS induces monocyte apoptosis instead of monocyte activation [17]. In humans like in other mammals, TLRs are key effectors of tissue inflammation, and several of them, including TLR3, 4, and 9, are frequently expressed by carcinoma cells [20,22]. Therefore, we considered the hypothesis that c-IAP2 could modulate the response of NPC cells to TLR stimulation. To address this hypothesis, TLR expression was characterized in three NPC tumor lines (C15, C17, and C666-1). TLR3 and TLR9 transcripts were detected by RT-PCR in all three specimens, whereas TLR4 was detected only in C15 (Figure 5A). Consequently, we focused our study on TLR3 and 9 and checked the impact of their activation on NPC cells. In short-term growth assays, C666-1 viability and proliferation were decreased at best marginally by TLR9 agonists, TNF-α and cisplatin, either alone or in combination with RMT 5265 (Figure 5B). By contrast, treatment with the TLR3 agonist (poly(I:C)) combined with RMT 5265 had a highly cytotoxic effect on C666-1 cells, even at concentrations of 100 to 500 ng/ml, which are very small by comparison with concentrations routinely used in most other studies dealing with TLR3 (usually in the range of 5–10 µg/ml; Figure 5B) [23]. The same growth-inhibitory effect was obtained in a short-term colony assay performed on cells from the C17 xenograft (Figure 5C). The number of colony was reduced by 11-fold in the presence of RMT 5265 combined with poly(I:C). The cytotoxic effect of the combined treatment on NPC cells was accounted for by apoptosis, at least to a large extent, as indicated by its impact on caspase-3/7 activation in C666-1 cells (data obtained by caspase chemiluminescence assays are presented in Figure 5D). Caspase-3/7 activity was increased fourfold in the presence of RMT 5265, whereas it was increased ninefold with the combined treatment. By contrast, no induction of caspase-3/7 was obtained in nonmalignant nasopharyngeal cell lines (NP69 and NP460) treated by RMT 5265 or the combined treatment (data not shown).
Figure 5.
RMT 5265 unmasks the sensitivity of NPC cells to TLR3-mediated apoptosis. (A) Transcription of TLR genes assessed by RT-PCR in three NPC tumor lines. Toll-like receptor 3 (601 bp) and TLR9 (451 bp) amplicons are detected in all three tumor lines. Two TLR4-specific amplicons (409 and 242 bp) are detected in C15 but not in C666-1 and C17. (B) Short-term growth assay for C666-1 cells treated for 72 hours with various concentrations of TLR3 (poly(I:C)) or TLR9 (CpG) agonists (upper panel) or TNF-α or cisplatin (lower panel) combined with RMT 5265 (100 nM) or control agent (100 nM). Numbers of viable cells were evaluated based on the reduction of WST, a soluble form of MTT, as explained in the Materials and Methods section. Each measurement was done in triplicate. (C) Counting of C17 NPC cell colonies after 3 days of treatment with poly(I:C) combined with RMT 5265 (100 nM) or control agent (100 nM). The procedure used for this short-termcolony assay was as described in the legend of Figure 3B. Colony numbers were the mean of three counts made for three separate wells on 24-well plates. (D) Chemiluminescence assay of caspases-3/7 activity in C666-1 cells after 48 hours of treatment with various concentrations of poly(I:C) combined with RMT 5265 or control agent (100 nM). Each measurement was done in duplicate. All data in this figure are representative of at least three similar experiments. The stars indicate statistical differences from respective controls or other experimental conditions (P < .05).
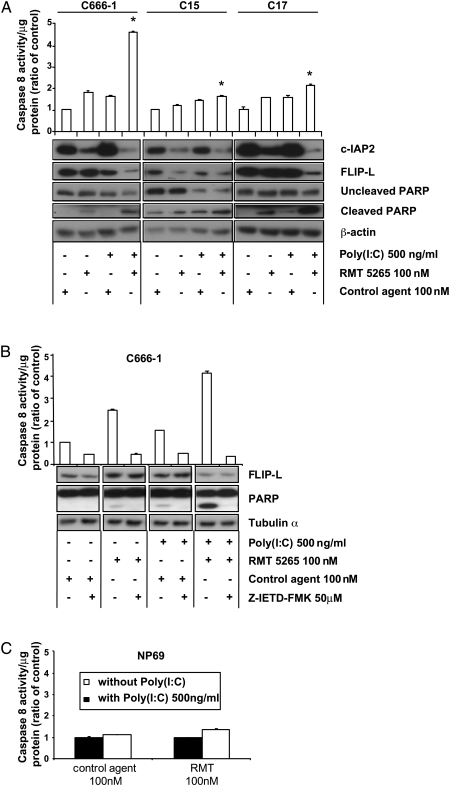
Apoptosis Induced by RMT 5265 Combined with TLR3 Stimulation Is Dependent on Caspase-8 Activation
Previous studies on other tumor models have shown that RMT 5265 induces apoptosis by RIPK1-dependent caspase-8 activation in several host cells [13,14]. Conversely, TLR3 is known to activate caspase-8 through a TRIF-RIPK1-FADD pathway in 293 cells [21]. We therefore investigated the contribution of caspase-8 to the apoptotic process induced in the three NPC tumor lines—C666-1, C15, and C17—by RMT 5265 combined with TLR3 stimulation (Figure 6A). Caspase-8 activation and PARP cleavage were monitored as well as c-IAP2 and FLIP-L cellular concentrations (FLIP is an important regulator of caspase-8 activity with predominant inhibitory effects of the long isoform FLIP-L) [36]. In the absence of any treatment, low levels of constitutive caspase-8 activity were detected in all three NPC tumor lines. By itself, RMT 5265 treatment led to a substantial decrease in c-IAP2 concentration accompanied by a mild increase in caspase-8 activity and a small increase in PARP cleavage in two of the three NPC tumor lines (C666-1 and C17). By itself, treatment with poly(I:C) induced a mild increase in caspase-8 activity without significant changes regarding other molecules. Finally, combination of both treatment induced a dramatic decrease in c-IAP2 and FLIP-L concentrations combined with a strong increase in caspase-8 activity and PARP cleavage in all three tumor lines, although this cooperative effect was at its highest magnitude in C666-1 cells. In the next experiment, using a membrane-permeable inhibitor of caspase-8 (Z-IETD-FMK), we intended to determine whether the cleavage of PARP and the decrease in FLIP-L concentration were dependent on caspase-8 activation (Figure 6B). Again, a strong increase in caspase-8 activity and a marked decrease in FLIP-L concentration was achieved by the combined treatment (RMT 5265 + poly(I:C)). Remarkably, caspase-8 inhibition completely abrogated PARP cleavage but did not restore the normal level of FLIP-L, indicating that the low level of FLIP-L is not a consequence of its degradation by caspase-8 and suggesting a change in its regulation occurring upstream of caspase-8 activation. Conversely, experiments displayed in Figure 6C showed that a treatment combining RMT 5265 with poly(I:C) had no significant effects on caspase-8 activation in nonmalignant nasopharyngeal epithelial cells (NP69).
Figure 6.
Role of caspase-8 activation in apoptosis induced by TLR3 stimulation combined with RMT 5265. (A) Assay of caspase-8 activity (chemiluminescence) in three NPC tumor lines C666-1, C15, C17, treated for 16 hours with poly(I:C) (500 ng/ml) combined with RMT 5265 or control agent (100 nM). Each measurement was done in duplicate. Cells not used in the caspase-8 assay were saved for protein extraction in RIPA and Western blot detections of c-IAP2, FLIP-L, and PARP. (B) Caspase-8 assay, FLIP-L detection (Western blot), and PARP cleavage assay (Western blot) for C666-1 cells treated with various combinations of the following reagents: RMT 5265, poly(I:C), and a caspase-8 inhibitory peptide (Z-IETD-FMK) (50 µM). The caspase-8-inhibitory peptide was added 1 hour before poly(I:C) and/or RMT 5265. Cells were collected for protein extraction 16 hours later. (C) Caspase-8 assay in NP69 cells treated for 16 hours with poly(I:C) (500 ng/ml) combined with RMT 5265 or control agent. All data in this figure are representative of at least two similar experiments. The stars indicate a statistical difference from respective controls (P < .05).
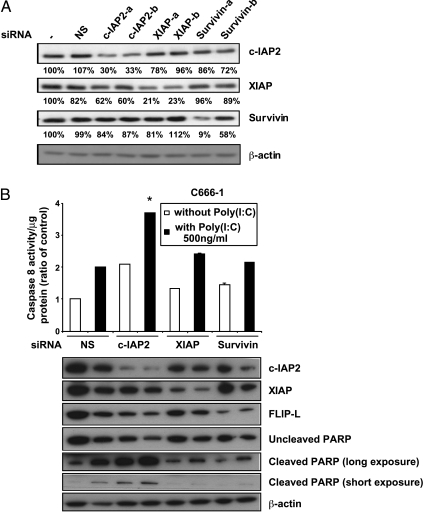
Cooperation Between siRNA Inhibition of c-IAP2 and TLR3 Stimulation in Caspase-8 Activation in NPC Cells
In our previous experiments, despite the absence or the low level of XIAP protein degradation induced by RMT 5265, functional interference with XIAP could not be excluded. Therefore, to confirm a specific role of c-IAP2 in blocking caspase-8 activation induced by TLR3 stimulation in NPC cells, we resorted to a series of siRNA specific of various IAPs. These siRNA were selected to minimize cross-inhibition (Figure 7A). As shown in Figure 7B, a mild increase in caspase-8 activity was observed when nonspecific siRNA as well as XIAP and survivin siRNA were combined with TLR3 stimulation. However, the greater cooperative effect was obtained with the c-IAP2 siRNA, and the differences with the other combinations were statistically significant. In addition, a stronger PARP cleavage was detected when the c-IAP2 siRNA was associated to TLR3 stimulation (notice the decrease in the amount of the uncleaved form in addition to the increase in the cleaved form). A concomitant decrease in FLIP-L concentration was also recorded. Overall, these data are similar to the data obtained with the combination of RMT 5265 and poly(I:C) and confirm the specific role of c-IAP2 in preventing caspase-8 activation by TLR3 agonists in NPC cells.
Figure 7.
Caspase-8 activation induced by TLR3 stimulation combined with IAP RNA interference. (A) Impact of siRNA on the cellular concentration of c-IAP2, XIAP, and survivin assessed by Western blot detection (specific protein bands were quantified by film densitometry using a GS-710 calibrated imaging densitometer with the Quantity One software; Biorad). (-) indicates no siRNA; NS: non-specific siRNA. (B) Caspase-8 assay (chemiluminescence), c-IAP2, XIAP, and FLIP-L detection (Western blot) and PARP cleavage assay (Western blot) in C666-1 undergoing knockdown of various IAPs with or without poly(I:C) treatment. Cells underwent two rounds of siRNA transfection at 48-hour intervals. NS indicates nonspecific siRNA. Treatment with poly(I:C) was started 32 hours after the second transfection, and cells were collected 16 hours later for protein extraction and caspase-8 assay. Each measurement was done in duplicate. The star indicates a statistical difference from nonspecific siRNA combined with poly(I:C) (P < .05).
Discussion
There is ample evidence to suggest that antiapoptotic proteins are essential for cancer cell proliferation and development [37]. This is largely due to the frequent association of cell transformation by viral or cellular oncogenes with the permanent activation of proapoptotic pathways, which must be balanced by antiapoptotic mechanisms supported by proteins such as those of the IAP family [11,38]. One IAP protein—survivin—is consistently overexpressed in most of human malignancies. Nasopharyngeal carcinoma does not make an exception to this rule as shown by previous publications and our own results in this study [12,39] (Figure 1). By contrast, the distribution of c-IAP1 and c-IAP2 is highly variable depending on the tumor types. Here, we have revealed a specific pattern of IAP expression in NPC cells. c-IAP2 is consistently produced in very large amounts, whereas c-IAP1 is barely detectable. This pattern is the reverse of that reported in certain other tumors, such as esophageal carcinomas, in which c-IAP1 is overproduced in a context of low c-IAP2 expression [16]. It means that specific functions of c-IAP 1 and 2 should be considered depending on the tumor type. Elucidation of these functions is expected to be important to understand which pathways could provide the best targets for pharmacological action.
After showing that c-IAP2 is consistently overexpressed in NPC specimens, we intended to demonstrate that NPC cell growth is dependent on its presence at a high concentration. This aim was achieved in a large extent by the use of the RMT 5265 inhibitor, which blocked clonogenic growth of the C666-1 and C17 tumor lines but not the growth of the nonmalignant nasopharyngeal epithelial cells NP69 and NP460. Although we cannot exclude a contribution of XIAP inhibition to the interruption of NPC cell growth mediated by RMT 5265, two lines of evidence strongly suggest that c-IAP2 is the major target of this drug in NPC cells: 1) regardless of the cellular background, RMT 5265 induced a very rapid and strong decrease in c-IAP2 levels (Figure 2); 2) although NP69 and NP460 cells contain XIAP levels comparable to NPC cells, they are not sensitive to RMT 5265 (Figures 1 and 3).
Regarding our third question about a specific antiapoptotic function of c-IAP2 in NPC cells, we have shown its role in preventing TLR3-mediated apoptosis. This conclusion is supported both by pharmacological and siRNA inhibition of c-IAP2. For caspase-8 activation, siRNA specific of c-IAP2 are much more efficient than those directed to XIAP or survivin when combined with TLR3 stimulation. This finding opens interesting perspectives for a better understanding of virus-cell interactions in NPC. As pointed out above, NPC cells produce large amounts of small EBV-encoded RNA with partially double-stranded structures named EBERs [1]. Most EBERs are contained in the nucleus but some are transported to the cytoplasm and, probably, to the extracellular medium. EBERs have been shown to have signaling activity outside the nucleus of EBV-infected cells [6]. For example, they induce insulin-like growth factor 1, possibly by stimulating TLR3 receptors [40]. This secreted insulin-like growth factor 1 has autocrine growth-promoting activities [40]. Our data show that TLR3 stimulation in NPC cells also has apoptosis-inducing effects that are antagonized by c-IAP2. This may account for the susceptibility of NPC cells to the long-term effects of RMT- 5265 when it is used alone. This compound may render NPC cells sensitive to the cytotoxic effects of a permanent, low-level stimulation of TLR3 receptors by endogenous EBERs, a point that will deserve further investigation.
The mechanism of caspase-8 activation by c-IAP2 inhibition and much more strikingly by the combination of c-IAP2 inhibition and TLR3 stimulation is not yet elucidated. Two recent publications based on other tumor models have recently reported that caspase-8 activation by c-IAP2 inhibition is mediated by an increase in the secretion of endogenous TNF-α and the autocrine stimulation of the TNF-R1 receptor [13,14]. Experiments made in our NPC tumor lines suggest that the same mechanism is at least in part applicable to our model (data not shown). Nevertheless, we noticed a decrease in FLIP-L cellular content triggered by the combined treatments (Figures 6 and 7). This change occurring upstream of caspase-8 activation might also have a causative role in apoptosis induction by the combined treatment. A marked decrease in FLIP-L content was also observed using survivin siRNA; there are other examples in the literature of parallel changes in FLIP-L and survivin cellular contents [41].
The mechanism of c-IAP2 overexpression in NPC cells has not been addressed in this study. c-IAP2 gene transcription is known to be transactivated in certain cell types by the EBV oncoprotein LMP1 [42]. However, c-IAP2 is strongly expressed in the C17 and C666-1 tumor lines that lack LMP1 expression ([43] and data not shown). We are currently investigating possible transactivation mechanisms by other EBV latent products contained in NPC cells.
Finally, various types of pharmacological c-IAP2 inhibitors and TLR3 agonists are currently in clinical trials [11,19]. Combinations of both types of agents might be beneficial for NPC patients. The benefits of this strategy may even extend to patients experiencing other malignancies.
Supplementary Material
Acknowledgments
The authors thank Xiaodong Wang, Lin Li, and Patrick Harran for generously providing RMT 5265 and HS 4404; Thierry Magnaldo and his group for technical assistance with clonogenic assays; Rajiv Khanna, Naoufal Zamzani, Marc Lipinski, and Alain Bernheim for helpful discussions.
Abbreviations
- BIRC3
baculoviral IAP repeat-containing 3
- c-IAP
cellular inhibitor of apoptosis protein
- NPC
nasopharyngeal carcinoma
- TLR3
Toll-like receptor 3
- Smac
second mitochondria-derived activator of caspases
- XIAP
X-linked inhibitor of apoptosis protein
Footnotes
This study was supported by grants from the Ligue Nationale contre le Cancer (comité du Val de Marne) and the Agence Nationale de la Recherche (EBV-inter). Luc Friboulet was supported by a fellowship from the Ligue Nationale contre le Cancer.
This article refers to supplementary materials, which are designated by Tables W1 and W2 and Figure W1 and are available online at www.neoplasia.com.
References
- 1.Busson P, Keryer C, Ooka T, Corbex M. V-associated nasopharyngeal carcinomas: from epidemiology to virus-targeting strategies. Trends Microbiol. 2004;12(8):356–360. doi: 10.1016/j.tim.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 3.Fachiroh J, Schouten T, Hariwiyanto B, Paramita DK, Harijadi A, Haryana SM, Ng MH, Middeldorp JM. Molecular diversity of Epstein-Barr virus IgG and IgA antibody responses in nasopharyngeal carcinoma: a comparison of Indonesian, Chinese, and European subjects. J Infect Dis. 2004;190(1):53–62. doi: 10.1086/421245. [DOI] [PubMed] [Google Scholar]
- 4.Feng BJ, Jalbout M, Ayoub WB, Khyatti M, Dahmoul S, Ayad M, Maachi F, Bedadra W, Abdoun M, Mesli S, et al. Dietary risk factors for nasopharyngeal carcinoma in Maghrebian countries. Int J Cancer. 2007;121(7):1550–1555. doi: 10.1002/ijc.22813. [DOI] [PubMed] [Google Scholar]
- 5.Lu SJ, Day NE, Degos L, Lepage V, Wang PC, Chan SH, Simons M, McKnight B, Easton D, Zeng Y, et al. Linkage of a nasopharyngeal carcinoma susceptibility locus to the HLA region. Nature. 1990;346(6283):470–471. doi: 10.1038/346470a0. [DOI] [PubMed] [Google Scholar]
- 6.Samanta M, Iwakiri D, Kanda T, Imaizumi T, Takada K. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 2006;25(18):4207–4214. doi: 10.1038/sj.emboj.7601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma BB, Chan AT. Systemic treatment strategies and therapeutic monitoring for advanced nasopharyngeal carcinoma. Exp Rev Anticancer Ther. 2006;6(3):383–394. doi: 10.1586/14737140.6.3.383. [DOI] [PubMed] [Google Scholar]
- 8.Sbih-Lammali F, Clausse B, Ardila-Osorio H, Guerry R, Talbot M, Havouis S, Ferradini L, Bosq J, Tursz T, Busson P. Control of apoptosis in Epstein Barr virus-positive nasopharyngeal carcinoma cells: opposite effects of CD95 and CD40 stimulation. Cancer Res. 1999;59(4):924–930. [PubMed] [Google Scholar]
- 9.Tang KF, Tan SY, Chan SH, Chong SM, Loh KS, Tan LK, Hu H. A distinct expression of CC chemokines by macrophages in nasopharyngeal carcinoma: implication for the intense tumor infiltration by T lymphocytes and macrophages. Hum Pathol. 2001;32(1):42–49. doi: 10.1053/hupa.2001.20886. [DOI] [PubMed] [Google Scholar]
- 10.Hunter AM, LaCasse EC, Korneluk RG. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12(9):1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- 11.Vucic D, Fairbrother WJ. The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin Cancer Res. 2007;13(20):5995–6000. doi: 10.1158/1078-0432.CCR-07-0729. [DOI] [PubMed] [Google Scholar]
- 12.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8(1):61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 13.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131(4):669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12(5):445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125(7):1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imoto I, Yang ZQ, Pimkhaokham A, Tsuda H, Shimada Y, Imamura M, Ohki M, Inazawa J. Identification of cIAP1 as a candidate target gene within an amplicon at 11q22 in esophageal squamous cell carcinomas. Cancer Res. 2001;61(18):6629–6634. [PubMed] [Google Scholar]
- 17.Conte D, Holcik M, Lefebvre CA, Lacasse E, Picketts DJ, Wright KE, Korneluk RG. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol Cell Biol. 2006;26(2):699–708. doi: 10.1128/MCB.26.2.699-708.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13(5):552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 19.Salaun B, Romero P, Lebecque S. Toll-like receptors' two-edged sword: when immunity meets apoptosis. Eur J Immunol. 2007;37(12):3311–3318. doi: 10.1002/eji.200737744. [DOI] [PubMed] [Google Scholar]
- 20.Huang B, Zhao J, Unkeless JC, Feng ZH, Xiong H. TLR signaling by tumor and immune cells: a double-edged sword. Oncogene. 2008;27(2):218–224. doi: 10.1038/sj.onc.1210904. [DOI] [PubMed] [Google Scholar]
- 21.Han KJ, Su X, Xu LG, Bin LH, Zhang J, Shu HB. Mechanisms of the TRIF-induced interferon-stimulated response element and NF-kappaB activation and apoptosis pathways. J Biol Chem. 2004;279(15):15652–15661. doi: 10.1074/jbc.M311629200. [DOI] [PubMed] [Google Scholar]
- 22.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176(8):4894–4901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 23.Salaun B, Lebecque S, Matikainen S, Rimoldi D, Romero P. Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clin Cancer Res. 2007;13(15 Pt. 1):4565–4574. doi: 10.1158/1078-0432.CCR-07-0274. [DOI] [PubMed] [Google Scholar]
- 24.Busson P, Zhang Q, Guillon JM, Gregory CD, Young LS, Clausse B, Lipinski M, Rickinson AB, Tursz T. Elevated expression of ICAM1 (CD54) and minimal expression of LFA3 (CD58) in Epstein-Barr-virus-positive nasopharyngeal carcinoma cells. Int J Cancer. 1992;50(6):863–867. doi: 10.1002/ijc.2910500605. [DOI] [PubMed] [Google Scholar]
- 25.Vicat JM, Ardila-Osorio H, Khabir A, Brezak MC, Viossat I, Kasprzyk P, Jlidi R, Opolon P, Ooka T, Prevost G, et al. Apoptosis and TRAF-1 cleavage in Epstein-Barr virus-positive nasopharyngeal carcinoma cells treated with doxorubicin combined with a farnesyl-transferase inhibitor. Biochem Pharmacol. 2003;65(3):423–433. doi: 10.1016/s0006-2952(02)01449-1. [DOI] [PubMed] [Google Scholar]
- 26.Hui AB, Cheung ST, Fong Y, Lo KW, Huang DP. Characterization of a new EBV-associated nasopharyngeal carcinoma cell line. Cancer Genet Cytogenet. 1998;101(2):83–88. doi: 10.1016/s0165-4608(97)00231-8. [DOI] [PubMed] [Google Scholar]
- 27.Tsao SW, Wang X, Liu Y, Cheung YC, Feng H, Zheng Z, Wong N, Yuen PW, Lo AK, Wong YC, et al. Establishment of two immortalized nasopharyngeal epithelial cell lines using SV40 large T and HPV16E6/E7 viral oncogenes. Biochim Biophys Acta. 2002;1590(1–3):150–158. doi: 10.1016/s0167-4889(02)00208-2. [DOI] [PubMed] [Google Scholar]
- 28.Li HM, Man C, Jin Y, Deng W, Yip YL, Feng HC, Cheung YC, Lo KW, Meltzer PS, Wu ZG, et al. Molecular and cytogenetic changes involved in the immortalization of nasopharyngeal epithelial cells by telomerase. Int J Cancer. 2006;119(7):1567–1576. doi: 10.1002/ijc.22032. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs JP, Jones CM, Baille JP. Characteristics of a human diploid cell designated MRC-5. Nature. 1970;227(5254):168–170. doi: 10.1038/227168a0. [DOI] [PubMed] [Google Scholar]
- 30.Valent A, Benard J, Clausse B, Barrois M, Valteau-Couanet D, Terrier-Lacombe MJ, Spengler B, Bernheim A. In vivo elimination of acentric double minutes containing amplified MYCN from neuroblastoma tumor cells through the formation of micronuclei. Am J Pathol. 2001;158(5):1579–1584. doi: 10.1016/S0002-9440(10)64112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalphamediated cell death. Science. 2004;305(5689):1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 32.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 33.Pioche-Durieu C, Keryer C, Souquere S, Bosq J, Faigle W, Loew D, Hirashima M, Nishi N, Middeldorp J, Busson P. In nasopharyngeal carcinoma cells, Epstein-Barr virus LMP1 interacts with galectin 9 in membrane raft elements resistant to simvastatin. J Virol. 2005;79(21):13326–13337. doi: 10.1128/JVI.79.21.13326-13337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez SK, Keryer A, Drira C, Ghorbel M, Jlidi A, Bernehiem R, Valent A, Busson P. Conventional and array-based CGH analysis of nasopharyngeal carcinomas from the Mediterranean area: high frequency of gains at 1q and 12p and losses at 11q and 13q. Cancer Genet Cytogenet. 2005;157:343–347. doi: 10.1016/j.cancergencyto.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Hui AB, Lo KW, Leung SF, Choi PH, Fong Y, Lee JC, Huang DP. Loss of heterozygosity on the long arm of chromosome 11 in nasopharyngeal carcinoma. Cancer Res. 1996;56(14):3225–3229. [PubMed] [Google Scholar]
- 36.Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124(3):601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8(2):121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 38.Weinstein IB, Joe AK. Mechanisms of disease: oncogene addiction—a rationale for molecular targeting in cancer therapy. Nat Clin Pract Oncol. 2006;3(8):448–457. doi: 10.1038/ncponc0558. [DOI] [PubMed] [Google Scholar]
- 39.Yip KW, Shi W, Pintilie M, Martin JD, Mocanu JD, Wong D, MacMillan C, Gullane P, O'Sullivan B, Bastianutto C, et al. Prognostic significance of the Epstein-Barr virus, p53, Bcl-2, and survivin in nasopharyngeal cancer. Clin Cancer Res. 2006;12(19):5726–5732. doi: 10.1158/1078-0432.CCR-06-0571. [DOI] [PubMed] [Google Scholar]
- 40.Iwakiri D, Sheen TS, Chen JY, Huang DP, Takada K. Epstein-Barr virus-encoded smallRNA induces insulin-like growth factor 1 and supports growth of nasopharyngeal carcinoma-derived cell lines. Oncogene. 2005;24(10):1767–1773. doi: 10.1038/sj.onc.1208357. [DOI] [PubMed] [Google Scholar]
- 41.Son YG, Kim EH, Kim JY, Kim SU, Kwon TK, Yoon AR, Yun CO, Choi KS. Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5 up-regulation and down-regulation of c-FLIP and survivin. Cancer Res. 2007;67(17):8274–8284. doi: 10.1158/0008-5472.CAN-07-0407. [DOI] [PubMed] [Google Scholar]
- 42.Hong SY, Yoon WH, Park JH, Kang SG, Ahn JH, Lee TH. Involvement of two NF-kappa B binding elements in tumor necrosis factor alpha-, CD40-, and Epstein-Barr virus latent membrane protein 1-mediated induction of the cellular inhibitor of apoptosis protein 2 gene. J Biol Chem. 2000;275(24):18022–18028. doi: 10.1074/jbc.M001202200. [DOI] [PubMed] [Google Scholar]
- 43.Khabir A, Karray H, Rodriguez S, Rose M, Daoud J, Frikha M, Boudarawa T, Middeldorp J, Jlidi R, Busson P. EBV latent membrane protein 1 abundance correlates with patient age but not with metastatic behavior in North African nasopharyngeal carcinomas. Virol J. 2005;2(1):39. doi: 10.1186/1743-422X-2-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.