Abstract
Signaling events leading to Schwann cell tumor initiation have been extensively characterized in the context of neurofibromatosis (NF). Similar tumors are also observed in patients with the endocrine neoplasia syndrome Carney complex, which results from inactivating mutations in PRKAR1A. Loss of PRKAR1A causes enhanced protein kinase A activity, although the pathways leading to tumorigenesis are not well characterized. Tissue-specific ablation of Prkar1a in neural crest precursor cells (TEC3KO mice) causes schwannomas with nearly 80% penetrance by 10 months. These heterogeneous neoplasms were clinically characterized as genetically engineered mouse schwannomas, grades II and III. At the molecular level, analysis of the tumors revealed almost complete loss of both NF proteins, despite the fact that transcript levels were increased, implying posttranscriptional regulation. Although Erk and Akt signaling are typically enhanced in NF-associated tumors, we observed no activation of either of these pathways in TEC3KO tumors. Furthermore, the small G proteins Ras, Rac1, and RhoA are all known to be involved with NF signaling. In TEC3KO tumors, all three molecules showed modest increases in total protein, but only Rac1 showed significant activation. These data suggest that dysregulated protein kinase A activation causes tumorigenesis through pathways that overlap but are distinct from those described in NF tumorigenesis.
Introduction
There are four human diseases associated with the formation of Schwann cell tumors. These lesions are the hallmarks of the neurofibromatosis (NF) syndromes, NF1 [von Recklinghausen disease, Online Mendelian Inheritance in Man (OMIM) #162200] and NF2 (OMIM #101000), as well as familial schwannomatosis (OMIM #162091) and Carney complex (CNC, OMIM #160980). NF1 is caused by mutations in the NF1 tumor suppressor (encoding neurofibromin), and patients may clinically manifest Schwann cell neoplasia as neurofibromas or as malignant peripheral nerve sheath tumors (MPNSTs). NF2 is also caused by mutations in a tumor suppressor gene (NF2, which encodes the protein merlin). Patients with NF2 develop multiple benign schwannomas, including both the pathognomonic vestibular schwannomas as well as nonvestibular schwannomas. Familial schwannomatosis, which has recently been suggested to be caused by mutations in the SMARCB1 gene [1], seems only to involve benign, nonvestibular schwannomas.
Schwannomas are also a component of CNC, an autosomal dominant neoplasia syndrome characterized as the complex of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas [2,3]. Schwannomas are observed in approximately 14% of CNC patients and have been designated histopathologically as psammomatous melanotic schwannomas for their histopathologic appearance and high degree of pigmentation. Because of their location in and around the spinal column, these tumors are a significant cause of morbidity and mortality for CNC patients [4]. At the genetic level, CNC is caused by inactivating mutations in PRKAR1A, the gene that encodes the type 1A regulatory subunit for the cAMP-dependent protein kinase A (PKA), and patient tumors exhibit enhanced PKA activity [5]. In a Prkar1a+/- mouse model, schwannomas were observed in approximately 33% of animals, and facial neural crest-specific knockout (KO) of the Prkar1a gene recapitulated Schwann cell tumorigenesis [6].
Mouse models of NF1 and NF2 have also been generated by creating the appropriate KO alleles. In contrast to the observations in the Prkar1a+/- model, neither Nf1+/- nor Nf2+/- mice develop Schwann cell tumors [7]. However, tissue-specific KO of these genes does recapitulate neoplasia in Schwann cells and other tissues [8–11].
Because the genetics of NF have been well described, this information has been used to study signaling pathways that may contribute to Schwann cell tumorigenesis. Neurofibromin contains a GTPase-activating protein (GAP) domain, which promotes the return of Ras to its inactive guanosine 5c-diphosphate-bound state. When neurofibromin is lost, Ras signaling is up-regulated, thereby causing activation of downstream effectors such as extracellular signal-regulated kinase (ERK) and Akt [12–16]. In contrast, merlin is a member of the ezrin-radixin-moesin family of proteins that links the cytoskeleton to membrane signaling complexes. Although merlin does not have a GAP motif, nor does it directly interact with Ras, it can interfere with the complex of ezrin-radixin-moesin family proteins that couples Ras signaling to cytoskeletal changes that occur during cell division [17]. As a result, alterations in ERK and Akt signaling may also occur [17,18]. Loss of merlin can also cause activation of the small G proteins Rac and Rho and their downstream effectors [19]. These pathways are also activated indirectly by loss of neurofibromin in tumors through the downstream effectors of Ras signaling, including the PI3K pathway [20].
In contrast to studies showing the effects of mutations in the NF genes, activation of the PKA pathway does not have a well-established role in Schwann cell tumorigenesis, despite the fact that both humans and mice with PRKAR1A/Prkar1a mutations develop these neoplasms. In this study, we sought to characterize, in detail, the Schwann cell tumors arising in our tissue-specific KO model of Prkar1a and to study the effects of this genetic manipulation on the function of neurofibromin and merlin. We report that ablation of Prkar1a in Schwann cells leads to posttranscriptional loss of both the Nf1 and Nf2 gene products. Despite these observations, this unique model of Schwann cell tumorigenesis occurs in the absence of Ras (and therefore ERK or Akt) pathway activation. However, expression of the small G proteins Rac and Rho is increased in the tumors, and Rac activity is significantly elevated. Collectively, these data indicate that PKA dysregulation triggered by loss of Prkar1a causes Schwann cell tumorigenesis through pathways that overlap, but are distinct from, those that cause NF1 and NF2.
Materials and Methods
Mouse Experiments
All mice were maintained in a sterile environment under 12-hour light/dark cycles. All animal experiments were carried out in accordance with the highest standards of animal care under an Institutional Animal Care and Use Committee-approved protocol. The generation of Prkar1aloxP/loxP and TEC3 mice has been previously described [6,21]. For this study, TEC3KO (TEC3;Prkar1aloxP/loxP) animals were monitored weekly for tumor onset, which was defined as the age at which a tumor of 0.5-cm linear dimension (as measured by calipers) was first detected. Mice with bilateral tumors were designated as having tumor onset at the time the first tumor reached 0.5 cm.
Immunohistochemistry
Tissue samples were fixed overnight in 10% formalin, processed, and embedded in paraffin. Immunohistochemistry was performed on 8-µm sections after antigen retrieval (Vector Laboratories, Burlin-game, CA) with the following antibodies: phospho-Akt, Akt, phospho-ERK, ERK (Cell Signaling Technology, Danvers, MA). Antigens were incubated with the appropriate secondary antibodies, and color was developed by adding DAB chromagen reagent (Vector Laboratories) to each section for 1 to 2 minutes before counterstaining with hematoxylin, dehydrating, and coverslipping. Samples were analyzed on a microscope (Model BX50; Olympus, Center Valley, PA), and images were captured using Spot Basic v4.1 software. LacZ staining of frozen tissues was performed as described [22] and visualized as above.
Immunofluorescence
Freshly dissected tissue samples were frozen in Tissue Tek Optimal Cutting Temperature Compound (Sakura Finetek USA, Inc., Torrence, CA). Sections, 8 µm in sizes, were fixed using cold acetone and permeabilized in 0.1% sodium citrate with 0.1% Triton X-100 detergent (Sigma, St. Louis, MO). The following primary antibodies were prepared as per the manufacturers' recommendations in 3% BSA in PBS: neurofibromin (sc-67), merlin (sc-331; Santa Cruz Biotechnology, Santa Cruz, CA), and Prkar1a (#610610; BD Biosciences, San Jose, CA). Slides were incubated with primary antibodies at room temperature for 45 minutes, washed, and incubated with Alexa Fluor 488-conjugated secondary antibodies (Invitrogen, Carlsbad, CA) for 25 minutes in the dark. Samples were then washed, mounted with DAPI mounting medium (Vector Laboratories), and visualized using an Axioskop 40 microscope and AxioVision software (Carl Zeiss, Inc., Thornwood, NY).
Western Blot Analysis
Primary Schwann cells were isolated and maintained as previously described [23]. Wild type cells were harvested for protein using Mammalian Protein Extraction Reagent (M-PER) with protease inhibitors (Pierce Biotechnology, Rockford, IL). Primary tumor samples were dissected from TEC3KO animals and homogenized in protein lysis buffer containing 20 mM HEPES buffer, 20 mM NaCl, 5 mM EDTA, 5 mM EGTA, 0.5% Triton X-100, 1 mM DTT, and protease inhibitors. Samples were resolved by SDS-PAGE before being transferred onto nitrocellulose membranes (Pall Corporation, East Hills, NY). Antibodies were obtained from the following sources and used according to the manufacturers' recommendations: Nf1 and Nf2 (Santa Cruz Biotechnology); actin (20–33; Sigma); phospho-ERK (Thr202/Tyr204; #9101), ERK (#9102), phospho-Akt (Ser473; #9271), and Akt (#9272; Cell Signaling Technology). Blots were developed using Western Lightning Chemiluminescence reagents (PerkinElmer, Waltham, MA) exposed to Blue Lite Autorad film (ISC BioExpress, Kaysville, UT), and images were captured using the GeneLine imaging system (Spectronics Corporation, Westbury, NY). Quantitation of blots was determined by first normalizing all the samples to the actin loading control using Genetools imaging software (Spectronics Corporation, Westbury, NY). Band intensities of the tumor samples were expressed as percentages in comparison to the control Schwann cells (set to 1.0 arbitrary units). Statistics were calculated using 2-tailed t test to generate P values.
Real-time Polymerase Chain Reaction
Primary murine Schwann cells and tumor samples (n = 6) were harvested, and mRNA was prepared as described [24]. Wild type Schwann cells were collected from one litter of embryos (n = 6) pooled together to comprise the control sample. cDNA was prepared using the iScript cDNA Synthesis kit (BioRad, Hercules, CA) and analyzed by quantitative real-time polymerase chain reaction (PCR) using iQ SybrGreen Supermix (BioRad). Each sample was run in triplicate, and the values for all six tumors were averaged and compared with those of the wild type Schwann cells. The following primer sets were used for this study: Nf1 (forward: CACGGTGACCCCAGCTAT, reverse: TCCCTGATTCCATTTCTTGTC); Nf2 (forward: GGGATTTTTAGCCCAAGAGG, reverse: ATCCACTCCAAGCAGCAACT); Prkar1a (forward: AGATCGTGGTGCAAGGAGAG, reverse: CGGTCCAACTTAACGCA CTT); and Gapdh (forward: GCAAATTCAACGGCACAGTCAAG, reverse: GTTC ACACCCATCACAAACATGG). Additional primers sets are included as supplemental information.
G Protein Activation Assays
Ras, Rac, and Rho activity assays were performed on 18 TEC3KO tumors using wild type rat Schwann cells (n = 3) as the comparative control. Tumors and Schwann cells were lysed in buffer containing 1% NP-40, 10% glycerol, 20 mM Tris (pH 8.0), 137 mM NaCl, 1 mM MgCl2, 0.5 mM EDTA, and 10 mM sodium pyrophosphate. The lysis buffer also contained the following protease inhibitors: 1 µg/ml aprotinin, 1 µg/ml leupeptin, 100 µg/ml PMSF, 1 mM sodium vanadate, and 5 mM sodium fluoride. For each sample, 0.5 mg (Ras and Rac) and 1 mg (Rho) of protein was kept for 1 hour at 4°C, rocking, with agarose beads conjugated to the substrate for the appropriate activated G proteins: Raf1-RBD (Ras), PAK1-PBD (Rac), and Rhotekin-RBD (Rho; Upstate, Charlottesville, VA). After incubation, all samples were washed three times with lysis buffer, resuspended in Laemmli buffer, and run on 12% SDS-PAGE for Western blot using the following primary antibodies: anti-Ras (clone RAS10; #05-516), anti-Rac1 (clone 23A8; #05-389; Upstate), and RhoA (26C4; sc-418; Santa Cruz Biotechnology). Separate Western blots were performed to test for the total amount of each G protein, using actin (Sigma) as a loading control.
Results
Characterization of TEC3;Prkar1aloxP/loxP (TEC3KO) Mice
The TEC3 line was developed to express cre under the control of the enhancer-less Tyrosinase promoter, which enables transgene expression in a limited subset of facial neural crest derivatives [21]. This cre line was crossed with mice carrying a conditional null allele of Prkar1a [6] to generate tissue-specific KO mice (TEC3;Prkar1aloxP/loxP, hence-forth called TEC3KO animals). These mice were born at expected Mendelian frequencies (data not shown) but developed unilateral or bilateral tumors on the face (Figure 1, A and B) within a few months. Observation of a cohort of 35 animals revealed that the penetrance of the tumor phenotype was approximately 50% by 18 weeks of age and nearly 80% by 40 weeks (Figure 1C). Although most mice manifested the phenotype early in life, tumors could develop as late as 1 year.
Figure 1.
Tumor incidence and anatomic localization of tumors in TEC3KO mice. (A, B) A typical schwannoma is shown in a TEC3KO mouse. Note the gelatinous consistency of the tumor and its localization to the facial nerve lateral to the orbit. (C) Kaplan-Meier plot of tumor onset in a cohort of TEC3KO animals (n = 35). Animals were monitored for 40 weeks of life, and tumor onset was considered when a tumor reached 0.5 cm in diameter.
To identify the tissue of origin for the schwannomas, TEC3KO and control mice were studied by magnetic resonance imaging at a time before palpable tumor development (Figure W1). Coronal and axial scanning revealed small masses on the side of the face and lateral to the orbit in TEC3KO animals. Although they frequently caused extrusion of the orbit when larger (data not shown), these studies did not indicate an origin from the optic nerve. Based on these data and careful anatomic observation, we determined that the tumors originated from Schwann cells originating in the trigeminal ganglion and supporting the fifth cranial nerve, also referred to as the trigeminal nerve. Because the TEC3 transgene was not initially reported to be expressed in this location, we reanalyzed the expression of the cre by crossing it to mice carrying the Rosa26lacZ reporter allele [25]. This analysis revealed robust expression of cre in Schwann cells of the trigeminal ganglion (Figure W2), further supporting our observations that TEC3KO tumors arise from progeny of these cells.
Histopathology of TEC3KO Tumors
We have previously described the TEC3KO tumors as schwannomas, although detailed histopathologic characterization was not performed [6]. To better understand the biology of these lesions, they were analyzed according to the recently published guidelines described for the Genetically Engineered Mouse classification of PNSTs [26]. TEC3KO tumors were very myxoid (Figures 1, A and B, and W3), and consisted mainly of spindle cells with many mitotic figures and elongated nuclei (Figure W3, A and B). There were areas of marked cellularity and cellular atypia, and in some cases, inflammatory cells were present. According to the diagnostic criteria for PNSTs, the TEC3KO tumors were classified as Genetically Engineered Mouse schwannoma, grades II and III [26]. This classification signified that, similar to the schwannomas in CNC patients, these tumors may behave as malignant schwannomas. Additionally, nearby tissue was impacted such that the bones of the skull underwent remodeling to accommodate larger tumors. Although there was no actual invasion of the skull cavity and the mice showed no signs of neurologic problems, the tumors would often obstruct the eye causing visual impairment. Interestingly, there have been no signs of metastases with these schwannomas, even as they grew to nearly 1.5 cm in diameter (data not shown).
A previous report from our laboratory showed that cyclin D1 was up-regulated in Prkar1a null mouse embryonic fibroblasts (MEFs) [24]. In line with these data, TEC3KO tumors revealed heavy staining for this marker by immunohistochemistry, even in areas where cell proliferation was not obvious (Figure W3C). The heterogeneity of these tumors was further observed by LacZ staining of frozen tissue sections taken from TEC3KO animals carrying the Rosa26lacZ allele [22]. Analysis of this staining revealed that both cre-positive and cre-negative cells were present in the tumors, further confirming their heterogeneity (Figure W3D).
Effects of Prkar1a Ablation on the NF Proteins in TEC3KO Schwannomas
As described above, mutations in NF1 or NF2 cause human schwannomas and tissue-specific KO of these genes in mice can also cause tumorigenesis [8–11]. To determine the effects of Prkar1a KO on the NF proteins, the expression pattern of each of these gene products was tested in TEC3KO schwannomas. Immunofluorescence experiments confirmed the loss of Prkar1a within the tumors (Figure 2, top) and, interestingly, demonstrated loss of both Nf1 and Nf2 proteins as well (Figure 2, middle and bottom). These data were further confirmed by Western blot analysis of tumor lysates, which revealed almost complete lack of both Nf1 and Nf2 when compared with wild type murine Schwann cells (Figure 3A). Additionally, similar loss of Nf2 was observed in primary TEC3KO tumor cultures (data not shown).
Figure 2.
Decreased expression of the NF proteins in TEC3KO schwannomas. Frozen tissue sections from the same tumor were stained by immunofluorescence for Prkar1a, neurofibromin, and merlin proteins. Tumor stroma (S) stained positively and was therefore used as a control for staining. The dashed line marks the interface between stromal and tumor tissues. As expected, Prkar1a was not present in the tumor tissue (T), but surprisingly both neurofibromin and merlin were absent as well.
Figure 3.
Down-regulation of Nf1 and Nf2 occurs at the posttranscriptional level. (A) Protein lysates of WT Schwann cells and six different TEC3KO schwannomas were analyzed for Nf1 and Nf2 proteins. Note the marked down-regulation of both proteins in comparison to the actin control. (B) Real-time PCR analysis of mRNA for Prkar1a, Nf1, and Nf2 from WT Schwann cells and TEC3KO tumors. Results are shown as averaged expression among all six tumors compared with WT Schwann cells. ΔΔCt was calculated compared with a Gadph standard, and error bars represent the relative SD.
To determine whether Prkar1a ablation caused down-regulation of the NF genes at the transcriptional level, we performed quantitative real-time PCR on tumor samples (Figure 3B). As a control, primary murine embryonic Schwann cells were obtained from the dorsal root ganglia of e12.5 embryos [23]. Because these cells are slow growing and have a short life span, all cells obtained from one litter of wild type animals (n = 6) were pooled and used for analysis. As expected, Prkar1a levels were significantly down-regulated in the tumors compared with WT Schwann cells. Residual Prkar1a message is likely detected due to the heterogeneity of the tumors and the presence of non-Schwann cells (e.g., endothelial cells and immune cells). Intriguingly, transcript levels for both Nf1 and Nf2 were elevated at least 10-fold (corresponding to a ΔΔCt value of 3.32) in TEC3KO schwannomas compared with wild type Schwann cells. To rule out somatic mutation as a mechanism by which the protein can be down-regulated in the presence of normal levels of mRNA, we sequenced the Nf2 cDNA from three independent TEC3KO tumors and found no evidence of transcript alterations. These data suggest that there may be posttranscriptional regulation of NF gene expression after Prkar1a mutation, although the mechanism of these alterations is not yet known.
Signaling Pathways in TEC3KO Schwannomas
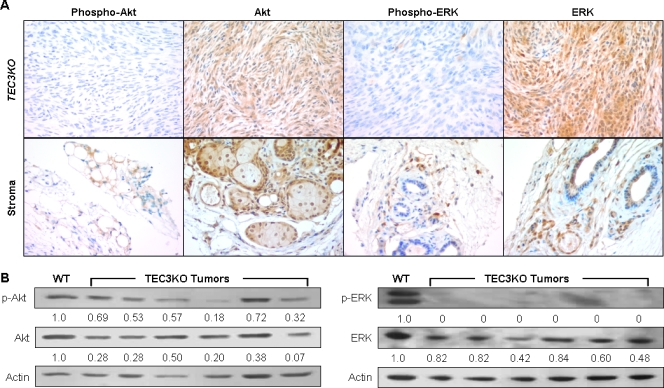
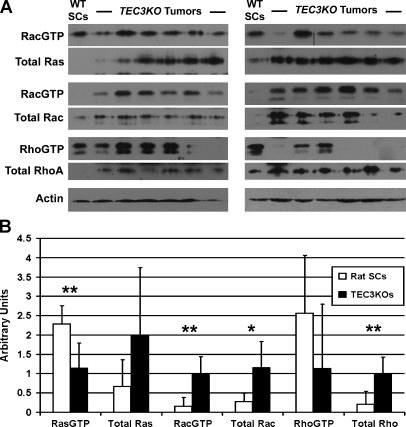
Previous work has shown that both neurofibromin and merlin can inhibit proliferation by down-regulating Ras signaling and therefore its downstream effectors, Akt and ERK [17,27,28]. To examine the extent of activation of these pathways in the TEC3KO tumors, we analyzed tumors for total and activated (phosphorylated) Akt and ERK. Surprisingly, immunohistochemistry staining (Figure 4A) revealed a complete lack of activation of either of these pathways, which was confirmed by Western blot analysis of tumor lysates (Figure 4B). Quantitative analysis of the Western blot data revealed that there were significant decreases in total Akt (P < .0001), phospho-Akt (P = .0022), and total ERK (P = .0072) in tumors. Phospho-ERK expression was essentially unmeasurable in the tumors, making statistical analysis impractical. Furthermore, although the levels of Ras expression seemed to be elevated in the TEC3KO tumors, the active (GTP-bound) form of the protein was significantly decreased compared with levels in normal rat Schwann cells (P = .0096; Figure 5, A and B).
Figure 4.
Loss of Akt and ERK pathway activation in TEC3KO schwannomas. (A) Paraffin-embedded sections of TEC3KO tumors were probed for the phosphorylated (activated) and unphosphorylated (total) forms of Akt and ERK. Stromal tissue stained positively for each protein and was therefore used as a control for staining. (B) Western blot analysis of the same lysates as in Figure 3A for p-Akt, Akt, p-ERK, and ERK. Actin is shown as a loading control. Note that ERK was probed from the same blot as in Figure 3A, and thus, the actin control lanes are the same. Quantitation of band intensities was performed, and tumor samples were compared with Schwann cells. Values shown below the blots represent the relative band intensity compared with the Schwann cell sample (set to 1.0) after normalization to actin.
Figure 5.
Signaling analysis in TEC3KO tumors. (A) Representative blots from small G protein activity assays performed on 18 TEC3KO tumors. Samples were probed for the proteins indicated at left, and actin was used as a loading control. (B) Quantification of results from panel (A). Note the up-regulation of total forms of each protein; however, significant increases in activity levels were seen only with Rac1. *P < .05, **P < .01.
Additional molecules thought to be important downstream effectors of the NF proteins include the small G proteins Rac, Cdc42, and Rho. Although neurofibromin may affect these signaling molecules as downstream targets from Ras, merlin has been shown to play a direct role in the inhibition of Rac/Cdc42, Rho, and PAK1 signaling [17,29–31]. In TEC3KO tumors, not only the expression of Rac1 (P = .0439) but also its activity (P = .0098) was significantly increased compared with normal rat Schwann cells (Figure 5, A and B). Rho expression was increased overall in the tumors (P = .0092); however, its activation was highly variable, failing to produce a significant difference between levels of Rho-GTP in normal Schwann cells compared with the panel of 18 tumors taken as a whole (P = .1789; Figure 5, A and B; and data not shown). Cdc42, which is highly homologous to Rac1, was observed in wild type Schwann cells and was essentially unchanged in the tumors (data not shown). Finally, real-time PCR was performed to check the transcript levels of each G protein (Ras, Rac1, and RhoA); similarly to the protein expression, mRNA expression was elevated for each gene (Figure W4).
Discussion
As in many biochemical processes, the study of inherited syndromes has contributed substantially to our knowledge of functional interactions. For Schwann cell tumorigenesis, the major syndromes that are involved are NF1, NF2, schwannomatosis, and the CNC, all multiple neoplasia syndromes associated with Schwann cell tumors.
At the biochemical level, it was previously reported that there are interactions between PKA and the NF proteins, as PKA is known to phosphorylate neurofibromin, although the functional effects of this reversible modification are unclear [32,33]. Additionally, loss of neurofibromin has been shown to up-regulate cAMP levels, which would presumably increase PKA activity [34]. PKA has also been shown to phosphorylate merlin at Serine-518, the same site that is phosphorylated by the p21-activated kinase [35]. At the functional level, this phosphorylation reduces the ability of merlin to suppress cell growth, potentially through heterodimerization with ezrin. Moreover, merlin has been shown to function as an A-kinase anchoring protein by binding directly to the Prkar1b subunit, although no binding of Prkar1a was observed [36].
In TEC3KO schwannomas, the most striking observation was the marked down-regulation of both neurofibromin and merlin in the tumors, shown both by immunofluorescence (Figure 2) and by Western blot analysis (Figure 3A). Furthermore, this alteration occurs at the posttranscriptional level, because mRNA levels of both genes were significantly elevated when compared with normal Schwann cells (Figure 3B). Moreover, at least in the case of Nf2, there were no somatic mutations found in the gene that would account for its down-regulation. These findings suggest that PKA may play a role in regulating the stability of the NF proteins. Such a role for PKA has previously been described, because phosphorylation by PKA has been shown to promote the degradation of proteins such as GRIP1 and Matrin 3 by triggering ubiquitin-dependent proteolysis [37,38]. The converse can also occur, because PKA causes cellular redistribution of RhoA and, through phosphorylation, reduces its degradation rate [39]. We have observed the same phenomenon in Prkar1a KO MEFs, in which an enhanced stability of the cell cycle progression marker cyclin D1 was observed [24]. These observations are all consistent with the recently proposed role of Prkar1a in modulating autophagy and the mTOR pathway [40]. Further studies are required to elucidate the role of autophagy or proteasomal-mediated degradation in the present observations.
From prior studies of the function of the NF genes, it has been proposed that there are three major downstream pathways that contribute to the Schwann cell tumorigenesis phenotype. Ablation of neurofibromin causes activation of the Ras, ERK, and Akt pathways, whereas loss of merlin causes increases in small G protein (Rac/Cdc42/Rho) signaling [19,41]. This latter pathway is also activated by mutations in Nf1, because Rac and Rho may be activated by pathways downstream of Ras [42,43].
Because we observed striking down-regulation of both neurofibromin and merlin in the TEC3KO model, we expected to find increases in the activation of each of the three pathways (ERK, Akt, and small G proteins). Surprisingly, Ras activity was significantly decreased in TEC3KO tumors compared with wild type Schwann cells, and furthermore, there was a significant decrease in the activation of both Akt and ERK in the tumors. These results mimic earlier findings from our laboratory in Prkar1a KO MEFs, which also showed that immortalization occurred independently of ERK or Akt activation [24]. Interestingly, previous studies on neurofibromin have indicated that Akt signaling, particularly through mTOR, is most important for tumor growth [12,13], whereas loss of merlin seems to up-regulate signaling primarily through ERK [17]. Data from the TEC3KO model indicate that there may be an alternative pathway, mediated by PKA activation, that can effectively promote Schwann cell hyperplasia as well. In addition, we observed a marked up-regulation of Rac activity, whereas Rho was not consistently activated. Rac and Rho are small G proteins of the Rho family, and although they have different functions, they are both shown to be important for normal Schwann cell function. Rac seems to be involved in membrane ruffling and establishment of Schwann cell-neuron interactions during myelin formation [41,44]. Conversely, Rho, which is activated by integrin signaling, is required for stress fiber formation and cell motility [45]. Nonetheless, despite their differences in function, both proteins are known to be dysregulated in Schwann cell neoplasias [19].
There is good evidence that PKA can specifically affect the activity of Rac and Rho, although clearly those effects are cell type- and condition-specific [46]. The interaction between PKA and Rho is better defined, such that PKA directly phosphorylates Rho at S188 and negatively regulates its activity [47]. The mechanism of this regulation entails PKA phosphorylation leading to enhanced binding of Rho to the cytosolic Rho GTP-dissociation inhibitor (Rho-GDI) protein, which sequesters Rho from its active location at the cell membrane. However, relocation from the membrane is not solely associated with enhanced GTPase activity. It seems that PKA phosphorylation can also cause dissociation between the total GTP-loaded form of Rho and its intracellular activity [47–49].
Unlike Rho, Rac lacks a direct PKA phosphorylation site, although enhanced PKA activity increases GTP-loaded Rac and seems to also enhance its activity. The mechanism by which this occurs has yet to be elucidated, but it has been proposed that alterations in Rac-GTP exchange factors may be involved [50].
For both Rho and Rac, PKA activation has generally been associated with alterations in protein activity without changes in protein level. The exception to this is the decreased degradation of Rho, where GDI binding S188 phospho-Rho enhances its stability. Thus, although we observe significantly increased expression levels of both Rho and Rac in our tumors, the effect on signaling through these pathways is not so straightforward now.
In summary, studies to date of pathways leading to schwannoma and PNST formation have revolved around Nf1 and Nf2 signaling through Ras, PI3K, Akt, and ERK, as well as the downstream effectors Rac, Cdc42, and Rho. Although PKA is known to be important for Schwann cell growth, its role in this process has not yet been elucidated. In this article, we present data indicating that PKA may have multiple roles in promoting Schwann cell tumorigenesis. First, it seems to modulate the stability of the NF proteins, such that dysregulation of PKA leads to posttranscriptional loss of these proteins during tumorigenesis. Secondly, PKA seems to signal, either directly or indirectly, to the same downstream effectors activated by mutations in the NF genes. However, unlike activation of Schwann cell tumorigenesis by NF mutations, PKA seems to promote tumorigenesis by a mechanism that excludes activation of Ras, ERK, and Akt. Thus, PKA promotes Schwann cell tumors by mechanisms that overlap but are distinct from those seen in NFassociated tumorigenesis.
Supplemental Methods
Magnetic Resonance Imaging
Prkar1aloxP/loxP and TEC3KO mice were imaged under avertin anesthesia by a 4.7-T Bruker MRI system using 120-cm ID, a 400-mT/M gradient insert, a 72-cm ID volume RF transmit, and a 5-cm diameter surface receiver coil. Sequences were captured with the T2-weighted, 137 x 137 x 1000 µm resolution, RARE sequence at 2579-ms repetition time and 47.4-ms effective echo time, and then averaged four times. Scans were 5 minutes 30 seconds long, with a 256 x 256-matrix size, 3.5-cm field of view, and 1-mm slice thickness. Both coronal and axial views were obtained for each mouse.
LacZ Enzymatic Stain
LacZ staining of frozen tissues was performed as described (Yin et al., 2008). Images were visualized on an Olympus BX50 microscope and captured using Spot Basic v4.1 software.
Primers
The following primers were used for real-time PCR amplification:
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
| H-Ras | gaaagaggcgggaaggaag | actgagaggggtggaggact |
| K-Ras | tctgttcgtgcaaactgtca | tcaactgcatgcaccaaatc |
| N-Ras | gttctgacatccctggagga | agctggaggctgtgtctgtt |
| RhoA | agcctcatgcggttaatttg | ctggtcagacaggttggaca |
| Rac1 | tgcagacccttccagagttc | caaaagctagtcggctggtc |
Acknowledgments
The authors thank Nancy Ratner (Cincinnati Children's Hospital Medical Center, Cincinnati, OH) and her laboratory for technical guidance with murine Schwann cell preparation.
Abbreviations
- CNC
Carney complex
- GAP
GTPase-activating protein
- KO
knock (ed) out
- NF
neurofibromatosis
- PKA
protein kinase A
- PNST
peripheral nerve sheath tumor
- TEC
tyrosinase-enhanced Cre
- TEC3KO
TEC3;Prkar1aloxP/loxP
Footnotes
This work was supported by Children's Tumor Foundation Young Investigator Award 2006-01-026 (to G.N.J.) and by the National Institutes of Health grants HD01323 and CA112268-02 (to L.S.K.) and CA16058 (to the OSU Comprehensive Cancer Center).
This article refers to supplementary materials, which are designated by Figures W1 to W4 and are available online at www.neoplasia.com.
References
- 1.Hulsebos TJ, Plomp AS, Wolterman RA, Robanus-Maandag EC, Baas F, Wesseling P. Germline mutation of INI1/SMARCB1 in familial schwannomatosis. Am J Hum Genet. 2007;80:805–810. doi: 10.1086/513207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Carney JA, Hruska LS, Beauchamp GD, Gordon H. Dominant inheritance of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Mayo Clin Proc. 1986;61:165–172. doi: 10.1016/s0025-6196(12)61843-6. [DOI] [PubMed] [Google Scholar]
- 4.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 5.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 6.Kirschner LS, Kusewitt DF, Matyakhina L, Towns WH, II, Carney JA, Westphal H, Stratakis CA. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65:4506–4514. doi: 10.1158/0008-5472.CAN-05-0580. [DOI] [PubMed] [Google Scholar]
- 7.Gutmann DH, Giovannini M. Mouse models of neurofibromatosis 1 and 2. Neoplasia. 2002;4:279–290. doi: 10.1038/sj.neo.7900249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su W, Xing R, Guha A, Gutmann DH, Sherman LS. Mice with GFAP-targeted loss of neurofibromin demonstrate increased axonal MET expression with aging. Glia. 2007;55:723–733. doi: 10.1002/glia.20501. [DOI] [PubMed] [Google Scholar]
- 10.Wu M, Wallace MR, Muir D. Tumorigenic properties of neurofibromin-deficient Schwann cells in culture and as syngrafts in Nf1 knockout mice. J Neurosci Res. 2005;82:357–367. doi: 10.1002/jnr.20646. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y, Harada T, Liu L, Lush ME, Guignard F, Harada C, Burns DK, Bajenaru ML, Gutmann DH, Parada LF. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development. 2005;132:5577–5588. doi: 10.1242/dev.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci USA. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johannessen CM, Johnson BW, Williams SM, Chan AW, Reczek EE, Lynch RC, Rioth MJ, McClatchey A, Ryeom S, Cichowski K. TORC1 is essential for NF1-associated malignancies. Curr Biol. 2008;18:56–62. doi: 10.1016/j.cub.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 14.Guilding C, McNair K, Stone TW, Morris BJ. Restored plasticity in a mouse model of neurofibromatosis type 1 via inhibition of hyperactive ERK and CREB. Eur J Neurosci. 2007;25:99–105. doi: 10.1111/j.1460-9568.2006.05238.x. [DOI] [PubMed] [Google Scholar]
- 15.Dasgupta B, Gutmann DH. Neurofibromin regulates neural stem cell proliferation, survival, and astroglial differentiation in vitro and in vivo. J Neurosci. 2005;25:5584–5594. doi: 10.1523/JNEUROSCI.4693-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cichowski K, Santiago S, Jardim M, Johnson BW, Jacks T. Dynamic regulation of the Ras pathway via proteolysis of the NF1 tumor suppressor. Genes Dev. 2003;17:449–454. doi: 10.1101/gad.1054703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison H, Sperka T, Manent J, Giovannini M, Ponta H, Herrlich P. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res. 2007;67:520–527. doi: 10.1158/0008-5472.CAN-06-1608. [DOI] [PubMed] [Google Scholar]
- 18.Fraenzer JT, Pan H, Minimo L, Jr, Smith GM, Knauer D, Hung G. Overexpression of the NF2 gene inhibits schwannoma cell proliferation through promoting PDGFR degradation. Int J Oncol. 2003;23:1493–1500. [PubMed] [Google Scholar]
- 19.Pelton PD, Sherman LS, Rizvi TA, Marchionni MA, Wood P, Friedman RA, Ratner N. Ruffling membrane, stress fiber, cell spreading and proliferation abnormalities in human Schwannoma cells. Oncogene. 1998;17:2195–2209. doi: 10.1038/sj.onc.1202141. [DOI] [PubMed] [Google Scholar]
- 20.Yohay KH. The genetic and molecular pathogenesis of NF1 and NF2. Semin Pediatr Neurol. 2006;13:21–26. doi: 10.1016/j.spen.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Tonks ID, Nurcombe V, Paterson C, Zournazi A, Prather C, Mould AW, Kay GF. Tyrosinase-Cre mice for tissue-specific gene ablation in neural crest and neuroepithelial-derived tissues. Genesis. 2003;37:131–138. doi: 10.1002/gene.10242. [DOI] [PubMed] [Google Scholar]
- 22.Yin Z, Williams-Simons L, Rawahneh L, Asa S, Kirschner LS. Development of a pituitary-specific cre line targeted to the Pit-1 lineage. Genesis. 2008;46:37–42. doi: 10.1002/dvg.20362. [DOI] [PubMed] [Google Scholar]
- 23.Ratner N, Williams JP, Kordich JJ, Kim HA. Schwann cell preparation from single mouse embryos: analyses of neurofibromin function in Schwann cells. Methods Enzymol. 2005;407:22–33. doi: 10.1016/S0076-6879(05)07003-5. [DOI] [PubMed] [Google Scholar]
- 24.Nadella KS, Kirschner LS. Disruption of protein kinase a regulation causes immortalization and dysregulation of D-type cyclins. Cancer Res. 2005;65:10307–10315. doi: 10.1158/0008-5472.CAN-05-3183. [DOI] [PubMed] [Google Scholar]
- 25.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 26.Stemmer-Rachamimov AO, Louis DN, Nielsen GP, Antonescu CR, Borowsky AD, Bronson RT, Burns DK, Cervera P, McLaughlin ME, Reifenberger G, et al. Comparative pathology of nerve sheath tumors in mouse models and humans. Cancer Res. 2004;64:3718–3724. doi: 10.1158/0008-5472.CAN-03-4079. [DOI] [PubMed] [Google Scholar]
- 27.Martin GA, Viskochil D, Bollag G, McCabe PC, Crosier WJ, Haubruck H, Conroy L, Clark R, O'Connell P, Cawthon RM, et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63:843–849. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- 28.Xu GF, Lin B, Tanaka K, Dunn D, Wood D, Gesteland R, White R, Weiss R, Tamanoi F. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell. 1990;63:835–841. doi: 10.1016/0092-8674(90)90149-9. [DOI] [PubMed] [Google Scholar]
- 29.Xiao GH, Beeser A, Chernoff J, Testa JR. p21-Activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem. 2002;277:883–886. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- 30.Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, O'Bryan JP, Gupta V, Ratner N, Der CJ, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 31.Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12:841–849. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- 32.Tokuo H, Yunoue S, Feng L, Kimoto M, Tsuji H, Ono T, Saya H, Araki N. Phosphorylation of neurofibromin by cAMP-dependent protein kinase is regulated via a cellular association of N(G),N(G)-dimethylarginine dimethylaminohydrolase. FEBS Lett. 2001;494:48–53. doi: 10.1016/s0014-5793(01)02309-2. [DOI] [PubMed] [Google Scholar]
- 33.Izawa I, Tamaki N, Saya H. Phosphorylation of neurofibromatosis type 1 gene product (neurofibromin) by cAMP-dependent protein kinase. FEBS Lett. 1996;382:53–59. doi: 10.1016/0014-5793(96)00137-8. [DOI] [PubMed] [Google Scholar]
- 34.Kim HA, Ratner N, Roberts TM, Stiles CD. Schwann cell proliferative responses to cAMP and Nf1 are mediated by cyclin D1. J Neurosci. 2001;21:1110–1116. doi: 10.1523/JNEUROSCI.21-04-01110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfthan K, Heiska L, Gronholm M, Renkema GH, Carpen O. Cyclic AMP-dependent protein kinase phosphorylates merlin at serine 518 independently of p21-activated kinase and promotes merlin-ezrin heterodimerization. J Biol Chem. 2004;279:18559–18566. doi: 10.1074/jbc.M313916200. [DOI] [PubMed] [Google Scholar]
- 36.Gronholm M, Vossebein L, Carlson CR, Kuja-Panula J, Teesalu T, Alfthan K, Vaheri A, Rauvala H, Herberg FW, Tasken K, et al. Merlin links to the cAMP neuronal signaling pathway by anchoring the RIbeta subunit of protein kinase A. J Biol Chem. 2003;278:41167–41172. doi: 10.1074/jbc.M306149200. [DOI] [PubMed] [Google Scholar]
- 37.Giordano G, Sanchez-Perez AM, Montoliu C, Berezney R, Malyavantham K, Costa LG, Calvete JJ, Felipo V. Activation of NMDA receptors induces protein kinase A-mediated phosphorylation and degradation of matrin 3. Blocking these effects prevents NMDA-induced neuronal death. J Neurochem. 2005;94:808–818. doi: 10.1111/j.1471-4159.2005.03235.x. [DOI] [PubMed] [Google Scholar]
- 38.Hoang T, Fenne IS, Cook C, Borud B, Bakke M, Lien EA, Mellgren G. cAMP-dependent protein kinase regulates ubiquitin-proteasome-mediated degradation and subcellular localization of the nuclear receptor coactivator GRIP1. J Biol Chem. 2004;279:49120–49130. doi: 10.1074/jbc.M409746200. [DOI] [PubMed] [Google Scholar]
- 39.Rolli-Derkinderen M, Sauzeau V, Boyer L, Lemichez E, Baron C, Henrion D, Loirand G, Pacaud P. Phosphorylation of serine 188 protects RhoA from ubiquitin/proteasome-mediated degradation in vascular smooth muscle cells. Circ Res. 2005;96:1152–1160. doi: 10.1161/01.RES.0000170084.88780.ea. [DOI] [PubMed] [Google Scholar]
- 40.Mavrakis M, Lippincott-Schwartz J, Stratakis CA, Bossis I. Depletion of type IA regulatory subunit (RIalpha) of protein kinase A (PKA) in mammalian cells and tissues activates mTOR and causes autophagic deficiency. Hum Mol Genet. 2006;15:2962–2971. doi: 10.1093/hmg/ddl239. [DOI] [PubMed] [Google Scholar]
- 41.Nakai Y, Zheng Y, MacCollin M, Ratner N. Temporal control of Rac in Schwann cell-axon interaction is disrupted in NF2-mutant schwannoma cells. J Neurosci. 2006;26:3390–3395. doi: 10.1523/JNEUROSCI.4865-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ingram DA, Hiatt K, King AJ, Fisher L, Shivakumar R, Derstine C, Wenning MJ, Diaz B, Travers JB, Hood A, et al. Hyperactivation of p21(ras) and the hematopoietic-specific Rho GTPase, Rac2, cooperate to alter the proliferation of neurofibromin-deficient mast cells in vivo and in vitro. J Exp Med. 2001;194:57–69. doi: 10.1084/jem.194.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ozawa T, Araki N, Yunoue S, Tokuo H, Feng L, Patrakitkomjorn S, Hara T, Ichikawa Y, Matsumoto K, Fujii K, et al. The neurofibromatosis type 1 gene product neurofibromin enhances cell motility by regulating actin filament dynamics via the Rho-ROCK-LIMK2-cofilin pathway. J Biol Chem. 2005;280:39524–39533. doi: 10.1074/jbc.M503707200. [DOI] [PubMed] [Google Scholar]
- 44.Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Nave KA, Franklin RJ, Meijer D, et al. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J Cell Biol. 2007;177:1051–1061. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Connor KL, Nguyen BK, Mercurio AM. RhoA function in lamellae formation and migration is regulated by the alpha6beta4 integrin and cAMP metabolism. J Cell Biol. 2000;148:253–258. doi: 10.1083/jcb.148.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Howe AK. Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta. 2004;1692:159–174. doi: 10.1016/j.bbamcr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Ellerbroek SM, Wennerberg K, Burridge K. Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem. 2003;278:19023–19031. doi: 10.1074/jbc.M213066200. [DOI] [PubMed] [Google Scholar]
- 48.Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996;15:510–519. [PMC free article] [PubMed] [Google Scholar]
- 49.Diviani D, Abuin L, Cotecchia S, Pansier L. Anchoring of both PKA and 14-3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO J. 2004;23:2811–2820. doi: 10.1038/sj.emboj.7600287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waschke J, Drenckhahn D, Adamson RH, Barth H, Curry FE. cAMP protects endothelial barrier functions by preventing Rac-1 inhibition. Am J Physiol Heart Circ Physiol. 2004;287:H2427–H2433. doi: 10.1152/ajpheart.00556.2004. [DOI] [PubMed] [Google Scholar]
Supplemental References
- Yin Z, Williams-Simons L, Rawahneh L, Asa S, Kirschner LS. Development of a pituitary-specific cre line targeted to the Pit-1 lineage. Genesis. 2008;46:37–42. doi: 10.1002/dvg.20362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.