Abstract
Chronic alcohol consumption causes pathological changes in the brain and neuronal loss. Ethanol toxicity may partially result from the perturbation of microtubule associated proteins, like tau. Tau dysfunction is well known for its involvement in certain neurodegenerative diseases, such as Alzheimer's disease. In the present study, the effect of ethanol on tau was examined using differentiated human neuroblastoma cells that inducibly express the 4R0N isoform of tau via a tetracycline-off expression system. During tau induction, ethanol exposure (1.25-5 mg/ml) dose-dependently increased tau protein levels and reduced cell viability. The increase in cell death likely resulted from tau accumulation since increased levels of tau were sufficient to reduce cell viability and ethanol was toxic to cells expressing tau but not to non-induced controls. Tau accumulation did not result from greater tetracycline-off induction since ethanol neither increased tau mRNA expression nor the expression of the tetracycline-controlled transactivator. Additionally, ethanol increased endogenous tau protein levels in neuroblastoma cells lacking the tetracycline-off induction system for tau. Ethanol delayed tau clearance suggesting ethanol impedes its degradation. Though ethanol inhibited neither cathepsin B, cathepsin D, nor chymotrypsin-like activity, it did significantly reduce calpain 1 expression and activity. Calpain I knockdown by shRNA increased tau levels indicating that calpain participates in tau degradation in this model. Moreover, the activation of calpain, by the calcium ionophore A23187, partially reversed the accumulation of tau resulting from ethanol exposure. Impaired calpain-mediated degradation may thus contribute to the increased accumulation of tau caused by ethanol.
Keywords: microtubule associated protein tau, ethanol, human neuroblastoma cells, calpain
Introduction
Chronic alcohol consumption causes pathological changes in the brain. Oxidative damage [29] and reduced protein degradation [6] are among the culprits implicated in ethanol toxicity. Ethanol may also exert damaging effects by perturbing the microtubule system [15, 25, 32] which plays an important role in intracellular trafficking. Microtubule associated proteins, like MAP2 and tau, regulate microtubule assembly and stability. Ethanol alters MAP2 and tau phosphorylation [1, 3, 30] and MAP2 expression [19]. Furthermore, cellular filamentous inclusions immunopositive for tau are detected in hepatocytes of alcoholics [22] and tau-positive inclusions are observed in the brain of alcoholics with thiamine-deficiency [4]. Tau-containing inclusions, in the form of neurofibrillary tangles (NFT), are a hallmark feature of Alzheimer's disease and related tauopathies. Yet, whether NFT are neurotoxic or a consequence of prior tau dysfunction, is under debate. For instance, tau-mediated neuronal death, in the absence of tau filaments, is observed in Drosophila and mice overexpressing human tau [23, 28, 33], suggesting tau toxicity results from altered tau activity. It is proposed that an acceptable range of tau must be maintained in order to sustain neuronal viability; either too much or too little would hinder tau function [8]. Indeed, tau overexpression in hippocampal cells leads to the improper distribution of tau, microtubule bundling, transport inhibition and cell death [31].
Since ethanol alters the activity or expression of proteases and regulators of protein degradation [9, 16], ethanol may impede the turnover of tau, thus increasing tau levels beyond the tolerable window of activity. The aim of the present study was to examine the effect of ethanol on tau accumulation in differentiated human neuroblastoma cells that inducibly express tau. The ability to conditionally control the expression of tau in response to tetracycline makes this system ideal to study the involvement of tau in ethanol-induced cell death.
Materials and Methods
Cell Culture
M1C cells, from the human neuroblastoma BE2-M17D cell line, express the 4R0N isoform of human tau upon TetOff induction [17]. The day following seeding, DMEM was replaced with tetracycline-containing (2000 ng/ml; Tet-Plus) Neurobasal Media supplemented with B-27 containing antioxidants and 0.01 mM retinoic acid to promote neuronal differentiation. At 10 days of differentiation, tau induction was initiated by reducing the concentration of tetracycline to 1 ng/ml (TetOff). Ethanol was added to the media to yield concentrations of 1.25, 2.5 and 5 mg/ml corresponding to 27.2, 54.4 and 108.7 mM, respectively.
Viability Assay
To assess cell viability, cells grown in 12-well plates were incubated with 2 μM calcein AM (Invitrogen) in balanced salt solution for 30 min at room temperature in the dark [27]. In live cells, calcein AM is metabolized by esterases to green-fluorescent calcein. The fluorescence (Ex: 495 nm, Em: 530nm) was measured with Spectra Max M2 and Soft Max Protein 4.6 software (Molecular Devices, Sunnyvale, CA). Twenty-one locations per well (4 wells per condition for each independent experiment) were scanned and transformed to an average signal which was then normalized to the signal present in non-induced cells not exposed to ethanol.
Western Blotting Analysis
Protein lysates were harvested and the protein concentration was determined using the bicinchoninic acid assay (Pierce). Since TetOff induction leads to the progressive expression of tau, the concentration of protein loaded per lane depended on the duration of induction. Thus, 25, 17.5 and 3.5 μg of protein were loaded per lane for cells exposed to 1, 2 and 4 days of induction, respectively. Samples were electrophoresed on 10% sodium dodecyl sulfatepolyacrylamide gels and transferred to nitrocellulose membranes. To examine tau expression following induction, blots were probed with the antibodies P44 (1:5000), as well as Tau46 (1:5000) and Tau12 (1:10,000) which were gifts from V. M. Lee (University of Pennsylvania, Philadelphia, PA) and L. Binder (Northwestern University, Chicago, IL, USA), respectively. Anti-VP16 and anti-calpastatin (Sigma), as well as anti-calpain-1 (Santa Cruz Biotechnology, Inc.) immunoreactivity were also studied. Anti-α-tubulin (Epitomics, Inc.), -β-tubulin (Sigma) or -GAPDH (Covance Research Products, Inc.) were used as loading controls. Immunoreactivity was visualized using Enhanced Chemiluminesence Plus (Amersham Bioscience, Buckinghamshire, UK) and analyzed densitometrically using MCID software (Imaging Research Inc., Ontario, Canada). Note that, to examine endogenous tau expression, 20-25 μg of protein was loaded per lane, blots were probed with P44 at a dilution of 1:1000, and the film exposure duration was increased in order to detect a signal.
Reverse transcription polymerase chain reaction
To study tau mRNA expression, total RNA was extracted from cells using TRIzol® Reagent (Invitrogen) and converted to cDNA for PCR amplification. PCR cycling conditions: 94 °C, 5 min (1 cycle); 94 °C, 30 s; 50 °C, 30 s; 72 °C, 20 s (30 cycles); 72 °C, 15 min. Primer sequences for tau were: 5′TGAGCCCCGCCAGGAGTTC and 5′TTGGAGCGGGCGGGGTTTTTG and result in product sizes of 446 or 359 bp for tau isoforms containing or lacking exon 2 (like 4R0N), respectively [17]. Primer sequences for GAPDH were 5′TTGATTTTGGAGGGATCTCG and 5′GAGTCAACGGATTTGGTCGT (product size = 238 bp).
Protease Activity Assays
To measure protease activity, protein lysates were incubated with specific fluorogenic substrates in 96-well plates. Enzyme activity was assessed by measuring the fluorescence emitted following substrate cleavage using Spectra Max M2 and Soft Max Protein 4.6 software. Calpain and cathepsin B (Biovision) as well as cathepsin D (Sigma) activity were measured using activity assay kits, as per the manufacturer's instructions. The fluorogenic substrate, Suc-LLVY-AMC (Biomol International, LP), was used to assess chymotrypsin-like activity.
Calpain I lentiviral shRNA knockdown
Four shRNA MISSION™ RNA interference vectors targeting calpain I, and one non-targeted control vector, were obtained from the Sigma and Mayo Clinic RNA Interference Technology Resource. Lentiviral stocks, produced in 293FT cells with the Virapower lentiviral expression kit (Invitrogen), were used to transduce non-differentiated M1C cells seeded in 24-well plates. The virus-containing medium was diluted 1:1 in DMEM containing 10% fetal bovine serum (FBS) and 6 μg/ml of Polybrene (Sigma) in a volume of 250 μl per well. After overnight incubation, medium was replaced with DMEM containing 10% FBS and 3 ng/ml tetracycline. Tetracycline was not completely removed in order to avoid expressing toxic levels of tau. Two days later protein was collected for analysis.
Statistical analysis
Data is presented as the mean + SEM from a minimum of 3 experiments. Statistical comparisons were made by Student's t-test or ANOVA. Statistical significance was inferred at P < 0.05.
Results
Ethanol enhances tau accumulation and cell death
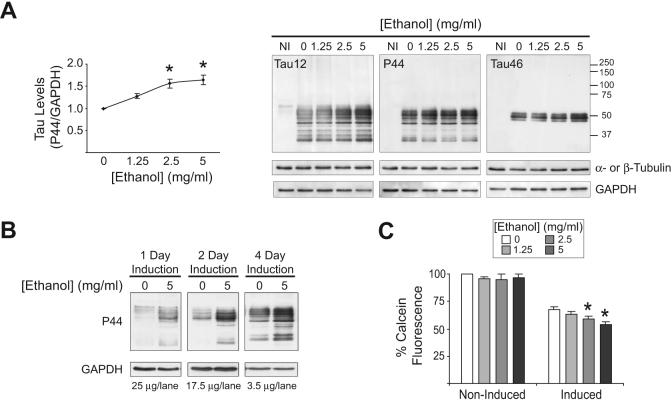
To study the effect of ethanol exposure on tau, differentiated M1C cells were induced to express tau for 4 days in the absence or presence of ethanol (1.25-5 mg/ml) and tau levels were examined by Western blot. Samples from non-induced (Tet-Plus) cells were marginally, if at all, immunopositive for the Tau12, P44 and Tau46 antibodies, which recognize epitopes near the N-terminal, middle and C-terminal of tau, respectively (Fig 1A, NI). However, the 4-day induction of tau led to the production of full-length tau (50-62 kDa) immunopositive for all three antibodies. Also observed were tau fragments (<50 kDa) immunopositive for Tau12 and P44 but not Tau46, suggesting C-terminal truncation. In the presence of ethanol, a dose-dependent increase in tau was observed (Fig. 1A). Indeed, ethanol increased tau as early as 1 day of treatment (Fig. 1B). Ethanol exposure during 4 days of induction dose-dependently reduced cell viability despite the presence of antioxidants from the B27 supplementing the media. The increase in cell death likely resulted from tau accumulation since tau induction was sufficient to reduce cell viability and ethanol was toxic to cells expressing tau but not to non-induced controls (Fig. 1C).
Figure 1. Ethanol leads to tau accumulation and cell death.
(A) M1C cells were induced to express tau by TetOff induction in the absence or presence of ethanol (1.25-5 mg/ml) for 4 days. Tau expression was examined by western blot using the antibodies Tau12, P44 and Tau46. GAPDH (all blots), β-tubulin (P44 blot) and α-tubulin (Tau12 and Tau46 blots) were used to verify sample loading. Ethanol significantly increased tau immunoreactivity. *P<0.01 compared to ethanol-free control as assessed by One-way ANOVA (n=3). NI=non-induced. (B) Cells were induced to express tau for 1, 2 or 4 days in the absence or presence of 5 mg/ml ethanol. P44-immunopositive tau was increased by ethanol exposure. Note the difference in the amount of protein loaded per lane for 1, 2 and 4 day samples. (C) Ethanol reduced cell viability in cells induced to express tau but not in non-induced cells, as assessed using calcein AM which is metabolized to fluorescent calcein in live cells. The average fluorescence per condition was normalized to ethanol-free, non-induced controls. *P<0.01 compared to ethanol-free induced control, as assessed by One-way ANOVA (n=5).
Ethanol increases endogenous tau protein
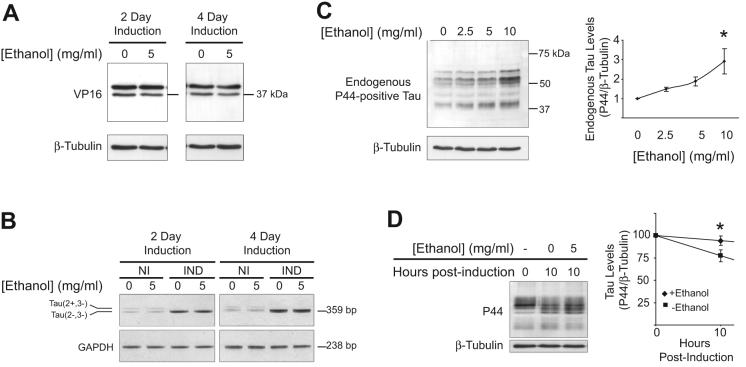
To establish that ethanol was not increasing tau by enhancing TetOff induction, the effect of ethanol on tetracycline-controlled transactivator (tTa) expression was examined. The tTa is a fusion protein composed of the Tetracycline Repressor and a C-terminal fragment of the Herpes Simplex Virus VP16 activation domain. The immunoreactivity for anti-VP16 was not increased following ethanol treatment compared to controls (Fig. 2A), suggesting ethanol does not up-regulate TetOff induction. This is supported by the finding that ethanol did not increase tau mRNA expression when present during TetOff induction of tau (Fig. 2B, IND). Additionally, ethanol did not increase endogenous tau mRNA in non-induced cells (Fig. 2B, NI) though it did increase endogenous tau protein in cells lacking the expression system for tau induction (Fig. 2C). To test if ethanol alters tau clearance, cells were subjected to TetOff induction for 3 days. Tau expression was then terminated by returning cells to media containing tetracycline (2000 ng/ml) and cycloheximide (30 μg/ml), either in the presence or absence of 5 mg/ml ethanol. Ten hours following the cessation of tau induction, tau levels were reduced by approximately 23% in the absence of ethanol, but only by 6% in cells exposed to ethanol. This suggests that delayed tau degradation may contribute to tau accumulation during ethanol treatment (Fig 2D).
Figure 2. Ethanol increases endogenous tau levels.
(A-C) To determine if ethanol increases tau by acting on the TetOff system, the effect of ethanol on the expression of the tetracycline-controlled transactivator (tTa), tau mRNA and endogenous tau protein, were examined. (A) The tTa is a fusion protein of the Tetracycline Repressor and the C-terminal of the Herpes Simplex Virus VP16 activation domain. Exposure of cells to 5 mg/ml ethanol during tau induction did not increase VP16 immunoreactivity. (B) Ethanol did not increase tau mRNA expression. Note that non-induced cells endogenously express low levels of tau isoforms containing or lacking exon 2, resulting in products of 446 bp and 359 bp, respectively. TetOff leads to the induction of 4R0N tau which lacks exon 2. NI= non-induced; IND=induced. (C) Ethanol (2.5-10 mg/ml, 2 d) increased endogenous tau protein levels in BE2-M17D neuroblastoma cells that do not contain the machinery for tau induction. Twenty-five micrograms of protein were loaded per lane and the blot was probed with P44 at a dilution of 1:1000 instead of the usual 1:5000. * P<0.05, as assessed by One-way ANOVA (n=3). (D) To determine if ethanol delays tau clearance, cells were subjected to TetOff induction for 3 days. Induction was then terminated by returning cells to media containing tetracycline (2000 ng/ml) and cycloheximide (30 μg/ml), either in the presence or absence of 5 mg/ml ethanol. Ten hours following the cessation of induction, tau levels were assessed by Western Blot. A significant difference in tau was observed between ethanol-free and ethanol-treated cells (P<0.01 by t-test, n=3).
Ethanol attenuates calpain expression and activity
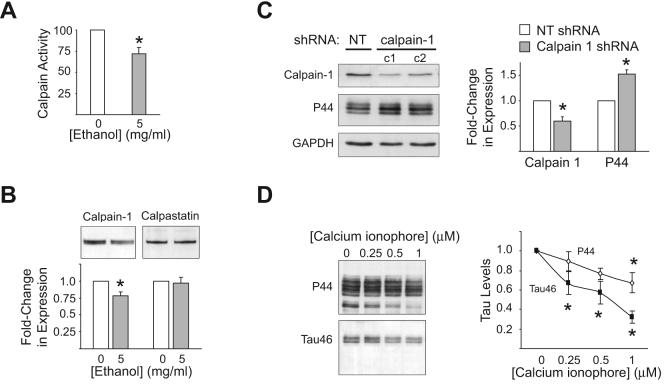
Next, the effect of ethanol on the activity of various proteolytic enzymes was examined. Neither cathepsin D and B activity, nor chymotrypsin-like activity, were reduced in ethanol-treated samples compared to controls (data not shown). However, ethanol (5 mg/ml, 1 d) did significantly decrease calpain activity (Fig. 3A) and calpain 1 expression (Fig 3B), though it did not alter the expression of the endogenous calpain inhibitor, calpastatin (Fig. 3B). To establish if reducing calpain can increase tau, cells were treated with four different shRNA constructs targeting calpain I. All four constructs reduced calpain I expression, with the average reduction being approximately 40% of the calpain I expression observed in non-targeted controls. In contrast, levels of P44-positive tau were increased by approximately 50% following calpain I knockdown (Fig. 3C). Finally, calpain activity was stimulated to see if it would reverse ethanol-enhanced tau accumulation. Cells exposed to ethanol were treated with the calcium ionophore A23187 (0.25-1 μM), which activates calpain in neuroblastoma cells [26]. A23187 partially, yet significantly, attenuated the accumulation of tau produced by ethanol (Fig. 3D).
Figure 3. Ethanol decreases calpain expression and activity.
(A) To examine whether ethanol reduces calpain activity, cells were induced to express tau for 1 day in the absence or presence of 5 mg/ml ethanol and lysates containing activated calpain were collected. Ethanol significantly decreased calpain activity, as assessed by measuring the fluorescence emitted following the cleavage of a fluorogenic calpain substrate added to lysates. (B) Western blot analysis of calpain-1 and calpastatin expression following a 1 day induction of tau in the absence or presence of 5 mg/ml ethanol. Ethanol significantly reduced calpain-1 expression but did not effect the expression of calpastatin. For (A-B), *=P<0.05 compared to ethanol-free control (t-test; n=4). (C) To ascertain if reduced calpain expression can increase tau, cells were treated with four different shRNA constructs targeting calpain I, two of which are shown in panel C. Calpain I and tau expression levels for each of the four constructs were normalized to those observed in cells exposed to non-targeted shRNA and the average change in expression +/− SEM was calculated. NT= non-targeted. c1 and c2 = construct 1 and 2, respectively. *=P<0.05 compared to non-targeted control as assessed by t-test. (D) To determine if stimulating calpain activity would reverse ethanol-enhanced tau accumulation, cells were induced to express tau for 1 day in the presence of 5 mg/ml ethanol and in the absence or presence of the calcium ionophore A23187 (0.25-1 μM). Protein lysates were examined by Western blot using the tau antibodies, P44 and Tau46. A23187 significantly attenuated ethanol-enhanced tau accumulation. *=P<0.05, compared to A23187-free control as assessed by One-way ANOVA (n=4).
Discussion
The present study shows that ethanol exposure leads to the accumulation of tau in differentiated M1C cells induced to express human 4R0N tau, with a concomitant dose- and tau-dependent decrease in cell viability. Indeed, tau accumulation is reported to cause cell death in hippocampal neurons [31], cerebellar granule cells and cortical cultures [2]. Tau overexpression alters tau distribution and the bundling of microtubules, thereby impeding their proper functioning [31]. Of interest, exposing PC12 cells to 4.6 mg/ml ethanol for 4 days, a treatment similar to the one used in the present study, enhances microtubule polymerization [25].
The increase in tau caused by ethanol is not likely the result of increased TetOff induction since ethanol: (1) did not increase tTa protein expression which regulates tau induction, (2) did not increase tau mRNA expression, and (3) led to a significant increase in endogenous tau protein.
That tau clearance was reduced, or at least delayed, by ethanol treatment suggests that ethanol either stabilizes tau or inhibits its degradation. There is evidence that tau is degraded by the autophagic-lysosomal system [11], the proteasome [7], and by caspases and calpains [13]. Although neither cathepsin B, cathepsin D nor chymotrypsin-like activity were inhibited by ethanol (data not shown), ethanol did reduce calpain activity consistent with the finding that ethanol inhibits calpain activity in PC12 cells [5]. Previous reports show that calpain degrades tau in vitro [14, 18, 35] and within cells [10, 34]. Similarly, in the present study, calpain I knockdown increased tau levels, indicating that calpain I plays a role in tau clearance in M1C cells. Thus, the decrease in calpain expression and activity produced by ethanol could result in tau accumulation. This is supported by the fact that activation of calpain, using a calcium ionophore, partially reversed the accumulation of tau caused by ethanol.
In addition to reduced calpain activity, other mechanisms may play a role in ethanol-induced tau accumulation. Ethanol decreases the formation of autophagic vacuoles in the perfused rat liver [21] and suppresses autophagy in cerebral cortical progenitor cells [24]. Ethanol could also alter the interaction between tau and chaperones that stabilize tau or target tau to the proteasome. For example, ethanol leads to tyrosine hydroxylase (TH) accumulation in neuroblastoma cells by increasing the association between TH and HSP90, thus stabilization TH [12].
It is important to highlight that ethanol led to the accumulation of tau not only in an inducible model of tau expression, which allows the easy detection and monitoring of tau, but also in cells that express only endogenous tau. The duration of treatment used to study the effect of ethanol on endogenous tau was relatively short. Nonetheless, it is expected that longer durations of ethanol exposure would further increase tau expression such that tau levels would exceed the acceptable range for normal tau activity. It should be kept in mind that very low molar ratios of tau/tubulin are necessary for tau to alter microtubule dynamics [20].
Future studies will investigate the long-term effect of ethanol on tau in animal models. Ethanol-induced tau disruption may render neurons more vulnerable to insults or may exacerbate physiological changes in the brain due to aging. By providing insight on the intracellular events involved in ethanol-induced tau accumulation, these studies may shed light on some of the mechanisms involved in tau toxicity.
Acknowledgements
This study was supported by the NIH Grants AG 17216 and NS 48052, the Mayo Foundation, and by philanthropy from Robert H. and Clarice Smith and the M.L. Simpson Foundation Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahluwalia B, Ahmad S, Adeyiga O, Wesley B, Rajguru S. Low levels of ethanol stimulate and high levels decrease phosphorylation in microtubule-associated proteins in rat brain: an in vitro study. Alcohol Alcohol. 2000;35:452–457. doi: 10.1093/alcalc/35.5.452. [DOI] [PubMed] [Google Scholar]
- 2.Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A. 2006;103:2892–2897. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakraborty G, Saito M, Mao RF, Wang R, Vadasz C, Saito M. Lithium blocks ethanol-induced modulation of protein kinases in the developing brain. Biochem Biophys Res Commun. 2008;367:597–602. doi: 10.1016/j.bbrc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cullen KM, Halliday GM. Mechanisms of cell death in cholinergic basal forebrain neurons in chronic alcoholics. Metab Brain Dis. 1995;10:81–91. doi: 10.1007/BF01991785. [DOI] [PubMed] [Google Scholar]
- 5.DePetrillo PB. Calcium-activated neutral protease activity is decreased in PC12 cells after ethanol exposure. J Neurochem. 1997;68:1863–1869. doi: 10.1046/j.1471-4159.1997.68051863.x. [DOI] [PubMed] [Google Scholar]
- 6.Donohue TM. The ubiquitin-proteasome system and its role in ethanol-induced disorders. Addict Biol. 2002;7:15–28. doi: 10.1080/135562101200100562. [DOI] [PubMed] [Google Scholar]
- 7.Elliott E, Tsvetkov P, Ginzburg I. BAG-1 associates with Hsc70.Tau complex and regulates the proteasomal degradation of Tau protein. J Biol Chem. 2007;282:37276–37284. doi: 10.1074/jbc.M706379200. [DOI] [PubMed] [Google Scholar]
- 8.Feinstein SC, Wilson L. Inability of tau to properly regulate neuronal microtubule dynamics: a loss-of-function mechanism by which tau might mediate neuronal cell death. Biochim Biophys Acta. 2005;1739:268–279. doi: 10.1016/j.bbadis.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Gutala R, Wang J, Kadapakkam S, Hwang Y, Ticku M, Li MD. Microarray analysis of ethanol-treated cortical neurons reveals disruption of genes related to the ubiquitin-proteasome pathway and protein synthesis. Alcohol Clin Exp Res. 2004;28:1779–1788. doi: 10.1097/01.alc.0000148117.17707.b4. [DOI] [PubMed] [Google Scholar]
- 10.Guttmann RP, Johnson GV. Oxidative stress inhibits calpain activity in situ. J Biol Chem. 1998;273:13331–13338. doi: 10.1074/jbc.273.21.13331. [DOI] [PubMed] [Google Scholar]
- 11.Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, Ko LW. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci. 2008;22:22. doi: 10.1111/j.1460-9568.2008.06084.x. [DOI] [PubMed] [Google Scholar]
- 12.He DY, Ron D. GDNF reverses ethanol-mediated increases in tyrosine hydroxylase immunoreactivity via altering the activity of HSP90. J Biol Chem. 2008;14:14. doi: 10.1074/jbc.M706216200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson GV. Tau phosphorylation and proteolysis: insights and perspectives. J Alzheimers Dis. 2006;9:243–250. doi: 10.3233/jad-2006-9s326. [DOI] [PubMed] [Google Scholar]
- 14.Johnson GV, Jope RS, Binder LI. Proteolysis of tau by calpain. Biochem Biophys Res Commun. 1989;163:1505–1511. doi: 10.1016/0006-291x(89)91150-9. [DOI] [PubMed] [Google Scholar]
- 15.Kannarkat GT, Tuma DJ, Tuma PL. Microtubules are more stable and more highly acetylated in ethanol-treated hepatic cells. J Hepatol. 2006;44:963–970. doi: 10.1016/j.jhep.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Kharbanda KK, McVicker DL, Zetterman RK, Donohue TM., Jr. Ethanol consumption reduces the proteolytic capacity and protease activities of hepatic lysosomes. Biochim Biophys Acta. 1995;1245:421–429. doi: 10.1016/0304-4165(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 17.Ko LW, Rush T, Sahara N, Kersh JS, Easson C, Deture M, Lin WL, Connor YD, Yen SH. Assembly of filamentous tau aggregates in human neuronal cells. J Alzheimers Dis. 2004;6:605–622. doi: 10.3233/jad-2004-6605. discussion 673-681. [DOI] [PubMed] [Google Scholar]
- 18.Mercken M, Grynspan F, Nixon RA. Differential sensitivity to proteolysis by brain calpain of adult human tau, fetal human tau and PHF-tau. FEBS Lett. 1995;368:10–14. doi: 10.1016/0014-5793(95)00590-6. [DOI] [PubMed] [Google Scholar]
- 19.Noraberg J, Zimmer J. Ethanol induces MAP2 changes in organotypic hippocampal slice cultures. Neuroreport. 1998;9:3177–3182. doi: 10.1097/00001756-199810050-00010. [DOI] [PubMed] [Google Scholar]
- 20.Panda D, Samuel JC, Massie M, Feinstein SC, Wilson L. Differential regulation of microtubule dynamics by three- and four-repeat tau: implications for the onset of neurodegenerative disease. Proc Natl Acad Sci U S A. 2003;100:9548–9553. doi: 10.1073/pnas.1633508100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poso AR, Surmacz CA, Mortimore GE. Inhibition of intracellular protein degradation by ethanol in perfused rat liver. Biochem J. 1987;242:459–464. doi: 10.1042/bj2420459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preisegger KH, Zatloukal K, Spurej G, Riegelnegg D, Denk H. Common epitopes of human and murine Mallory bodies and Lewy bodies as revealed by a neurofilament antibody. Lab Invest. 1992;66:193–199. [PubMed] [Google Scholar]
- 23.Probst A, Gotz J, Wiederhold KH, Tolnay M, Mistl C, Jaton AL, Hong M, Ishihara T, Lee VM, Trojanowski JQ, Jakes R, Crowther RA, Spillantini MG, Burki K, Goedert M. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol. 2000;99:469–481. doi: 10.1007/s004010051148. [DOI] [PubMed] [Google Scholar]
- 24.Prock TL, Miranda RC. Embryonic cerebral cortical progenitors are resistant to apoptosis, but increase expression of suicide receptor DISC-complex genes and suppress autophagy following ethanol exposure. Alcohol Clin Exp Res. 2007;31:694–703. doi: 10.1111/j.1530-0277.2007.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiter-Funk CK, Dohrman DP. Chronic ethanol exposure increases microtubule content in PC12 cells. BMC Neurosci. 2005;6:16. doi: 10.1186/1471-2202-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shea TB. Restriction of microM-calcium-requiring calpain activation to the plasma membrane in human neuroblastoma cells: evidence for regionalized influence of a calpain activator protein. J Neurosci Res. 1997;48:543–550. [PubMed] [Google Scholar]
- 27.Spencer JP, Rice-Evans C, Williams RJ. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J Biol Chem. 2003;278:34783–34793. doi: 10.1074/jbc.M305063200. [DOI] [PubMed] [Google Scholar]
- 28.Spittaels K, Van den Haute C, Van Dorpe J, Bruynseels K, Vandezande K, Laenen I, Geerts H, Mercken M, Sciot R, Van Lommel A, Loos R, Van Leuven F. Prominent axonopathy in the brain and spinal cord of transgenic mice overexpressing four-repeat human tau protein. Am J Pathol. 1999;155:2153–2165. doi: 10.1016/S0002-9440(10)65533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun AY, Sun GY. Ethanol and oxidative mechanisms in the brain. J Biomed Sci. 2001;8:37–43. doi: 10.1007/BF02255969. [DOI] [PubMed] [Google Scholar]
- 30.Tan SE, Abel EL, Berman RF. Brain MAP-2 phosphorylation is decreased following prenatal alcohol exposure in rats. Alcohol. 1993;10:391–396. doi: 10.1016/0741-8329(93)90026-k. [DOI] [PubMed] [Google Scholar]
- 31.Thies E, Mandelkow EM. Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. J Neurosci. 2007;27:2896–2907. doi: 10.1523/JNEUROSCI.4674-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whatley VJ, Brozowski SJ, Hadingham KL, Whiting PJ, Harris RA. Microtubule depolymerization inhibits ethanol-induced enhancement of GABAA responses in stably transfected cells. J Neurochem. 1996;66:1318–1321. doi: 10.1046/j.1471-4159.1996.66031318.x. [DOI] [PubMed] [Google Scholar]
- 33.Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- 34.Xie HQ, Johnson GV. Calcineurin inhibition prevents calpain-mediated proteolysis of tau in differentiated PC12 cells. J Neurosci Res. 1998;53:153–164. doi: 10.1002/(SICI)1097-4547(19980715)53:2<153::AID-JNR4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Yen S, Easson C, Nacharaju P, Hutton M, Yen SH. FTDP-17 tau mutations decrease the susceptibility of tau to calpain I digestion. FEBS Lett. 1999;461:91–95. doi: 10.1016/s0014-5793(99)01427-1. [DOI] [PubMed] [Google Scholar]