Abstract
Ultrasmooth nanostructured diamond (USND) films were synthesized on Ti–6Al–4V medical grade substrates by adding helium in H2/CH4/N2 plasma and changing the N2/CH4 gas flow from 0 to 0.6. We were able to deposit diamond films as smooth as 6 nm (root-mean-square), as measured by an atomic force microscopy (AFM) scan area of 2 μm2. Grain size was 4–5 nm at 71% He in (H2 + He) and N2/CH4 gas flow ratio of 0.4 without deteriorating the hardness (~50–60 GPa). The characterization of the films was performed with AFM, scanning electron microscopy, x-ray diffraction (XRD), Raman spectroscopy, and nanoindentation techniques. XRD and Raman results showed the nanocrystalline nature of the diamond films. The plasma species during deposition were monitored by optical emission spectroscopy. With increasing N2/CH4 feedgas ratio (CH4 was fixed) in He/H2/CH4/N2 plasma, a substantial increase of CN radical (normalized by Balmer Hα line) was observed along with a drop in surface roughness up to a critical N2/CH4 ratio of 0.4. The CN radical concentration in the plasma was thus correlated to the formation of ultrasmooth nanostructured diamond films.
I. INTRODUCTION
In the United States, the need for long service life medical implants such as artificial joints is increasing rapidly. Wear of articulating surfaces is a major concern in orthopedic and dental implant devices, including the temporo-mandibular joint (TMJ), which resides in very close proximity to the eye, the ear, various nerves, and the brain.1,2 The National Institute of Dental and Craniofacial Research (NIDCR) of the National Institutes of Health (NIH) states that over 10 million people in the United States suffer from TMJ problems at any given time.3 In some cases, TMJ implant devices are needed, and their success is greatly influenced by the degree of wear at articulating surfaces. Complications arising from wear include component loosening, deleterious biological responses, osteolysis, mechanical instability, decreased joint mobility, increased pain, and ultimately implant failure.4 A major goal is to develop smooth and wear-resistant films on the articulation surfaces to reduce the friction and wear in mating total joint replacement components. Hard, ultrasmooth, and wear resistance diamond films on metal surface can serve this purpose very well.
Chemical vapor deposited (CVD) diamond films grown using gas mixtures such as hydrogen, nitrogen, and methane have been investigated primarily to obtain smooth nanocrystalline diamond film.5–8 The surface roughness value of 15–20 nm (root-mean square; rms) and grain size of 13–15 nm were achieved. The film grown without nitrogen addition shows large, well-defined crystalline facets indicative of high-phase-purity diamond.9 In contrast, the films grown with added nitrogen exhibit a nanocrystalline appearance with weak agglomeration into rounded nodules of submicron size. It has also been observed that the transformation from microcrystalline to nanocrystalline diamond structure can occur by adding Ar in H2/CH4 feed gases with a total transformation observed at Ar/H2 volume ratio of 9. 10,11 Surface roughness as low as 18 nm and grain size of 3–50 nm were demonstrated on highly polished (area rms ~1 nm) silicon (111) wafers.10 We have recently reported the effect of helium addition to H2/CH4/N2 feedgas mixtures on growth of high-quality ultrasmooth nanostructured diamond films on Ti–6Al–4V.12 The addition of He significantly reduced film roughness to 9–10 nm and grain size of diamond nanocrystals to 5–6 nm without deterioration of film hardness or adhesive properties.12 In this article, we describe the synthesis of ultrasmooth nanostructured diamond films by using He/H2/CH4/N2 plasma with different N2/CH4 volume ratio (CH4 is fixed) in a microwave plasma chemical vapor deposition (MPCVD) reactor. Structural and mechanical properties were evaluated by x-ray diffraction (XRD), Raman spectroscopy, atomic force microscopy (AFM), scanning electron microscopy (SEM), and nanoindentation techniques.
II. EXPERIMENTAL DETAILS
Ti–6Al–4V alloy disks with 25.4 mm diameter and 3.4 mm thickness were punched from Ti–6Al–4V sheets supplied by Robin Materials (Mountain View, CA). They were polished to a rms roughness of 3–4 nm using a mechanical polisher with SiC paper, followed by a chemical–mechanical polish with a 0.06-μm colloidal silica solution containing 10% hydrogen peroxide. The polished disks were cleaned by ultrasonic agitation in a 1-μm diamond powder/water solution after a series of detergent solution, methanol, acetone, and finally, deionized water. Cleaned substrates were placed in a Wave-mat MPCVD reactor (Plymouth, MI), equipped with a 6 kW, 2.4 GHz microwave generator, as shown in Fig. 1. Chamber pressure was 65 Torr, and the substrate temperature, as measured by a Mikron M77LS Infraducer two-color infrared (IR) pyrometer, (Oakland, NJ) was kept in the range 690–720 °C by adjusting microwave power in the range 0.93–1.1 kW. This two-color pyrometer provided accurate measurement of the substrate temperature without requiring correction of the emissivity of the surface during growth. Total flow rate of He, H2, and CH4 gases was fixed at 336 sccm (71% He in He + H2, 36 sccm of CH4), and the ratio of N2/CH4 gas flow changed from 0 to 0.6.
FIG. 1.
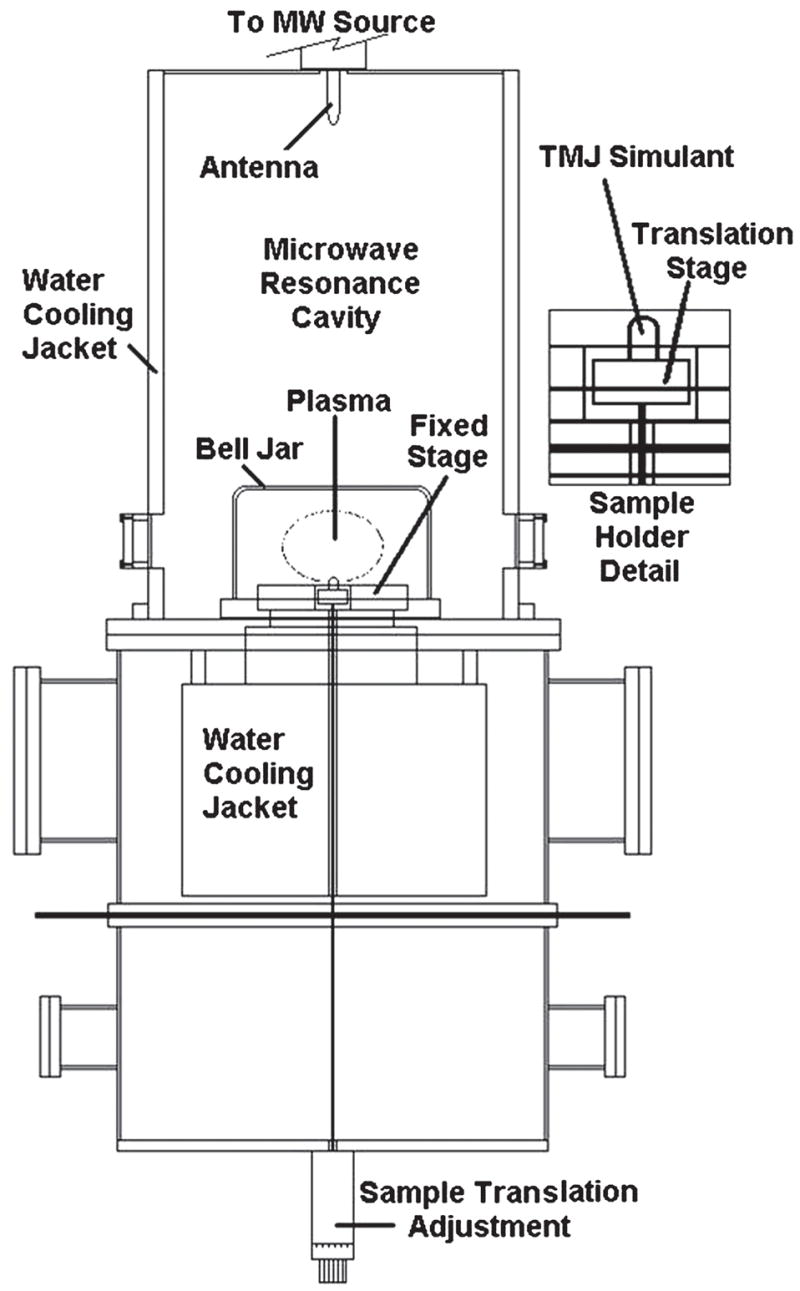
Schematic illustration of the MPCVD reactor.
Optical emission spectroscopy (OES) was performed to qualitatively determine the activated species present in the plasma. All the measurements were taken with 3000 points in the range of 350–700 nm wavelength and integration time of 250 ms. The crystallinity of the diamond films was analyzed by micro-Raman spectroscopy. The Raman spectra were taken using the 514.5 nm line of an argonion laser focused onto the film at a laser power of 100 mW. The Raman scattered signal was analyzed by a high-resolution spectrometer (1 cm−1 resolution) coupled to a charge-coupled device (CCD) system. XRD patterns on the diamond sample were examined using glancing angle XRD (X’pert MPD, Philips, Eindhoven, The Netherlands). XRD was performed using a glancing angle of 3° incident beam directed at the topmost surface of the coating surface. Spectra were taken from 30 to 90 (2θ) at a scan speed of 0.012° min−1 and a step size of 0.005°, as well as from 40 to 47 (2θ) to clearly document the intensity and full width at half-maximum (FWHM) of the diamond (111) diffraction peak.
Structure and surface morphology of the diamond surfaces was imaged by a TopoMetrix Explorer AFM (Santa Clara, CA). The images were collected in non-contact imaging mode. The cantilevers used were high resonance frequency (HRF) silicon I-shaped cantilevers with a frequency range of 279–318 kHz. The images obtained were processed by TopoMetrix SPM Lab NT Version 5.0 software supplied with the microscope. The processing consists of a second order leveling of the surface and a left shading of the image. Roughness was measured from a 2 μm2 scan area consistently for all samples. Surfaces of the diamond film were also imaged by FEI Nova NanoSEM (Hillsboro, OR).
The hardness and elastic modulus of the diamond films was measured using a Nanoindenter XP (MTS Systems, Oak Ridge, TN) system with a continuous stiffness attachment such that the loading and unloading displacement rates were constant. This provided continuous hardness/modulus measurements with increasing depth into the film.13,14 The system was calibrated using silica samples for a range of operating conditions. Silica modulus and hardness were calculated as 70 GPa and 9.1 GPa and 69.6 and 9.4, respectively, before and after indentation on diamond samples. The tip functions before and after the indentation were held constant. A Berkovich diamond indenter with total included angle of 142.3° was used, and the maximum indentation depth of 150 nm was maintained for all the measurements. The data were processed using proprietary software to produce load–displacement curves, and the mechanical properties were calculated using the Oliver and Pharr method.15
III. RESULTS AND DISCUSSION
Previously, it was found that with the introduction of helium gas in H2/CH4/N2 plasma, the transformation from microcrystalline to nanocrystalline occurred, and roughness decreased dramatically.12 The roughness decreased from 19–20 nm to 9–10 nm with the introduction of helium up to 71% (in He/H2 with fixed N2 and CH4 ratio 0.1). Helium gas was also introduced in H2/CH4 feedgas without N2, and interestingly it was found that the roughness and grain size of the diamond films also decreased with increase of helium flow rate. The rms roughness was as low as 20 nm, and grain size was 16–18 nm at helium flow up to 71% (in He/H2 with fixed CH4 content), as shown in Figs. 2(a) and 2(b). It was found that the combined effect of He and N2 played the vital role of decreasing the roughness and grain size and producing ultrasmooth nanocrystalline diamond films.12 In this previous study, N2/CH4 ratio was fixed at 0.1, and the role of varying nitrogen on structure and morphology of diamond films was not investigated. In this study, we determined the effect of N2 (N2/CH4 ratios 0.05–0.6) with fixed He, CH4, and H2 feedgases. There is a substantial difference between our growth chemistry and the chemistry used by the researchers from Argonne National Laboratory, Argonne, IL.10,11,16 We have introduced much higher CH4 feedgas (over 10%) compared to that typically used in the Argonne group (1%). Helium was introduced as a noble gas in our growth recipes, replacing argon. We used nitrogen feedgas addition, which is critical for achieving the smoothest nanophase diamond films. The Argonne group does not require nitrogen in their feedgas to produce their smooth diamond films which are characterized as “phase pure” nanocrystalline diamond. However, they have used nitrogen for doping, thus creating field-emission properties.17
FIG. 2.
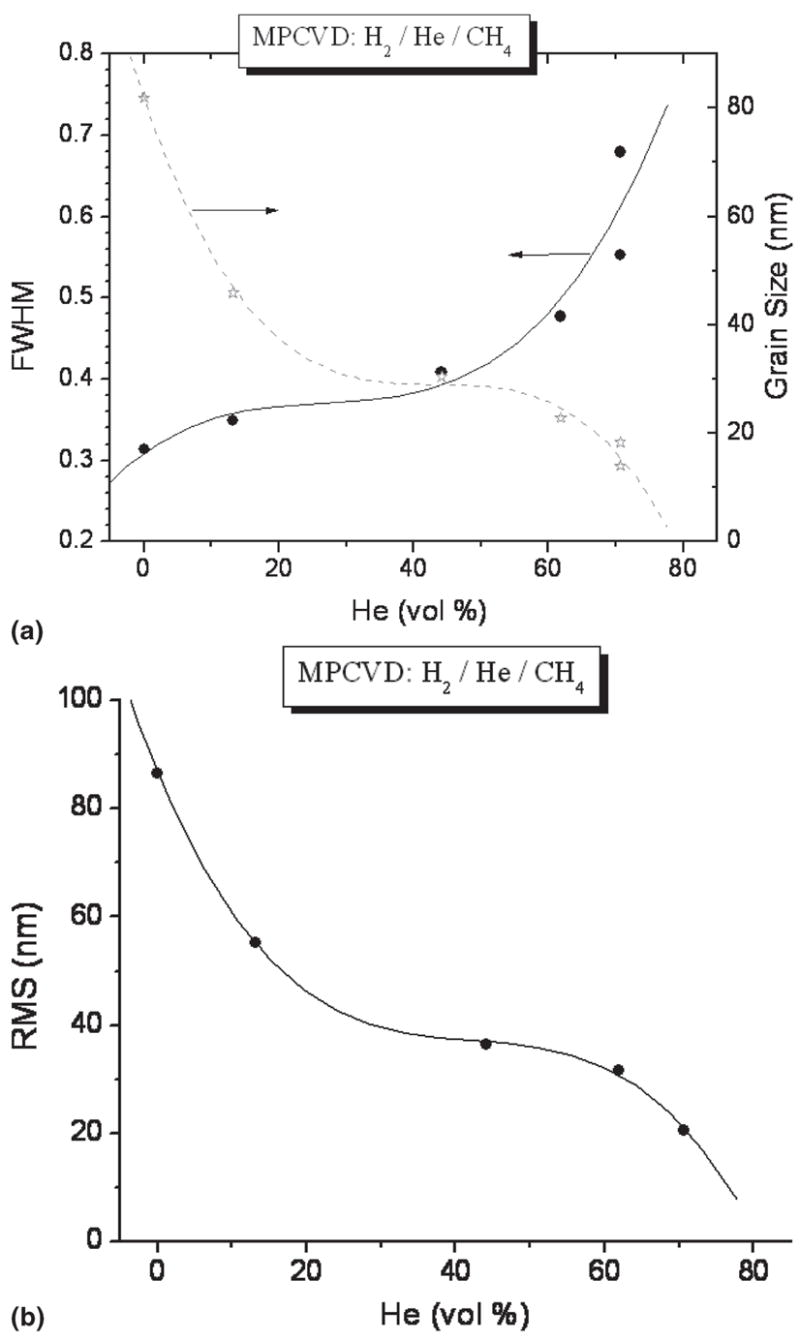
Diamond films grown in He/H2/CH4 plasma at different He contents and no N2: (a) FWHM of the (111) diamond XRD peak and calculated average diamond grain size and (b) rms surface roughness of the films calculated from 2 × 2 μm AFM images.
According to Afzal et al.,7 higher levels of gas phase CN radicals reduce the CH3 concentration and thus reduce growth rate. Figure 3 shows the drop of the growth rate from 0.37 to 0.22 μm/h by changing N2/CH4 ratios from 0.05 to 0.6. In the inset of Fig. 3, an optical interference pattern collected from the interferometer is shown for the film deposited at N2/CH4 of 0.4. Optical emission spectroscopy was used to monitor the changes in plasma chemistry upon N2 addition during diamond growth. The optical emission spectra of the He/H2/CH4/N2 microwave plasmas with N2/CH4 ratios of 0.05 and 0.4 are shown in Fig. 4. The ratios of OES intensities of plasma species (CN/Hα, C2/Hα and Hβ/Hα) as a function of N2/CH4 ratio are also shown in Fig. 5. It was found that upon an increase of N2/CH4 ratio, the Hβ/Hα remained practically constant, indicating only minor changes in plasma temperature. The ratio C2/Hα remained almost constant, as well. Interestingly, the CN/Hα ratio increased from 1 to 5 up to the N2/CH4 ratio of 0.4 and then decreased afterwards. Previous results indicated that with an increase of He addition up to 71% (in He + H2) and N2/CH4 gas concentration ratio of 0.1, there was a 3-fold increase of CN/Hα.12 With the same 71% of He (in He + H2 gas mixture) addition but N2/CH4 gas concentration ratio of 0.4, we had increase of CN/Hα of 2.5 times. It decreased beyond a N2/CH4 ratio of 0.4.
FIG. 3.
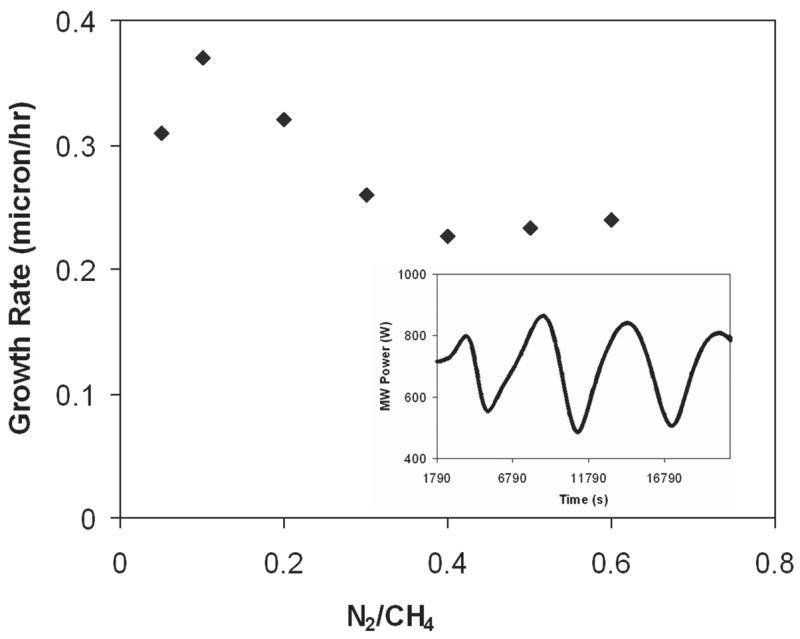
Growth rate of diamond films at different N2/CH4 volume ratio in He/H2/CH4/N2 plasma. In the inset, the optical interference pattern collected from interferometer is shown for film deposited at N2/CH4 = 0.4.
FIG. 4.
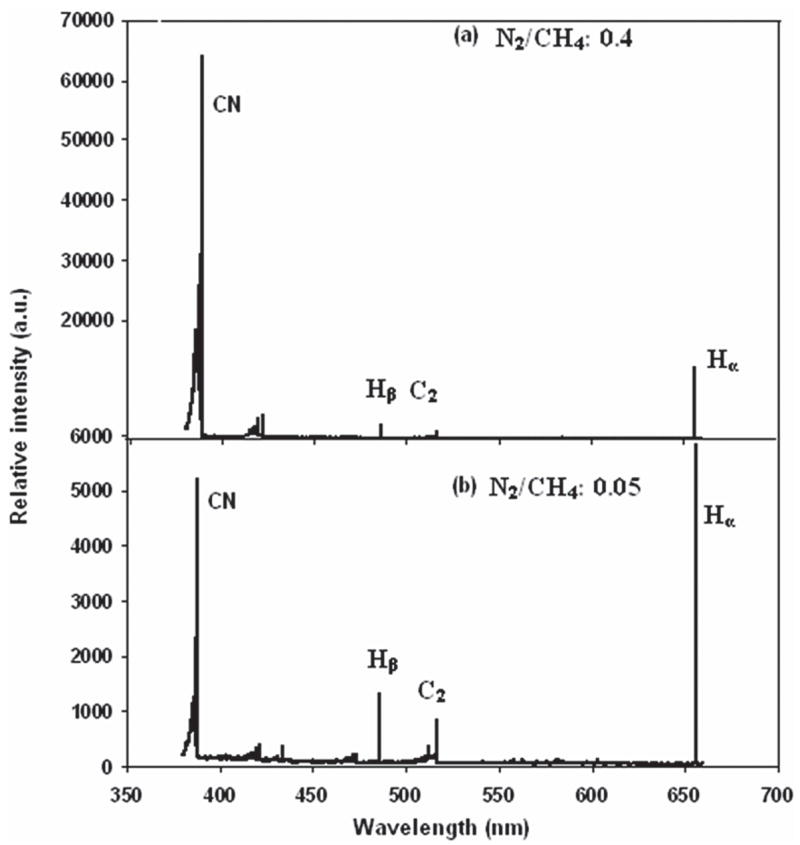
Optical emission spectra of the He/H2/CH4/N2 microwave plasmas with different ratios of N2/CH4: (a) N2/CH4 = 0.4 and (b) N2/CH4 = 0.05. It is to be noted that the intensity scales in the two spectra are different; CN peaks increase in intensity by a factor of ten in (a).
FIG. 5.
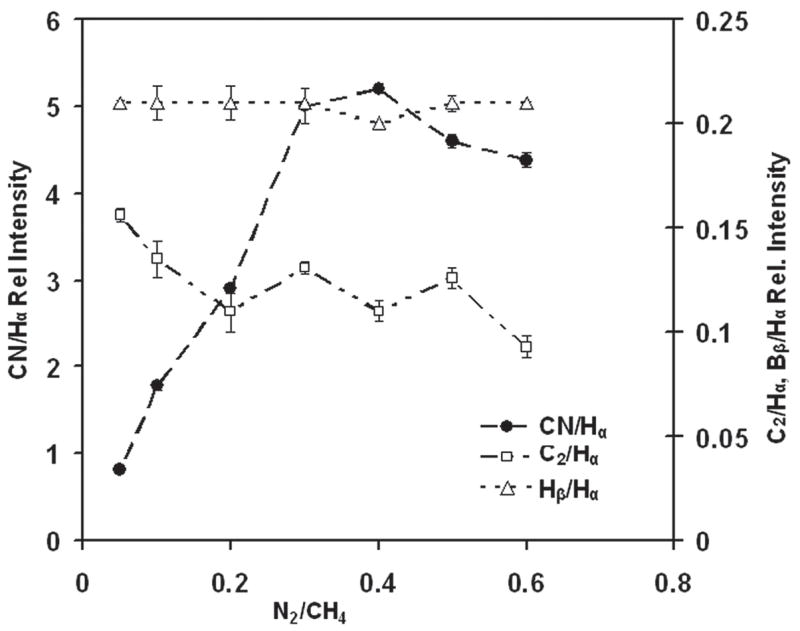
Normalized optical emission intensities of Balmer Hβ (486.14 nm), C2 (516.5 nm), and CN (386 nm) lines versus N2/CH4 content in He/H2/CH4/N2 plasma. Lines were normalized to Balmer Hα line (656.3 nm) intensity.
Figure 6 shows the glancing angle XRD patterns for the nanostructured diamond films grown on Ti–6Al–4V alloy using the He/CH4/H2/N2 feedgas mixture with different N2/CH4 ratios. Characteristic of these patterns are the cubic diamond (111) and (220) reflections as well as several peaks attributed to interfacial titanium carbide phases. The diamond peaks (shown in the inset) were significantly broadened as compared to those obtained from the conventional CVD process. The average grain size as calculated from the diamond (111) peak width using the Scherrer formula was between 4 and 8 nm. The grain size decreased to around 4–5 nm as N2/CH4 ratio increased up to 0.4 and then increased again. It was also found that as the N2 content increased, the intensity of the (200) diffraction peak from the TiC phase decreased. At N2/CH4 of 0.4, there was no TiC peak, and the intensity again increased as the ratio N2/CH4 increased. The average grain size of diamond films with different N2/CH4 concentration ratios was estimated from the FWHM of the (111) diamond peak using the Scherrer equation (after correction for instrumental broadening) and is presented in Fig. 7. A SEM image at 300,000× on the surface of diamond sample grown in He/H2/CH4/N2 plasma at N2/CH4 ratio of 0.4 is shown in Fig. 8. The grain size is as low as 5.7 nm and confirms the diamond nanostructure.
FIG. 6.
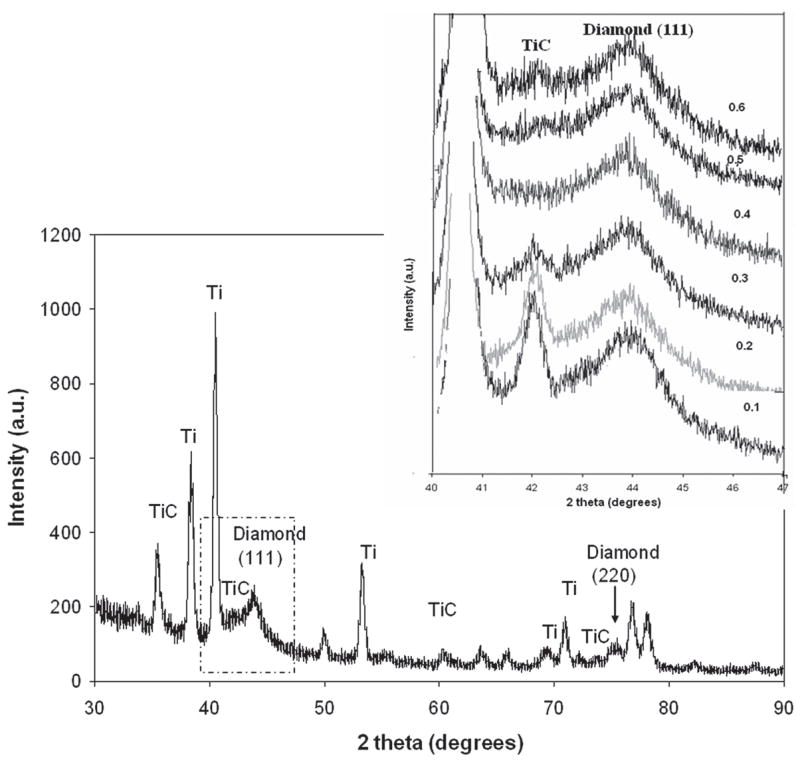
X-ray diffraction patterns of the He/H2/CH4/N2 microwave plasmas with different ratio of N2/CH4. The inset shows the close-up view of TiC and diamond (111) peaks showing the change due to the change of N2/CH4 volume ratio.
FIG. 7.
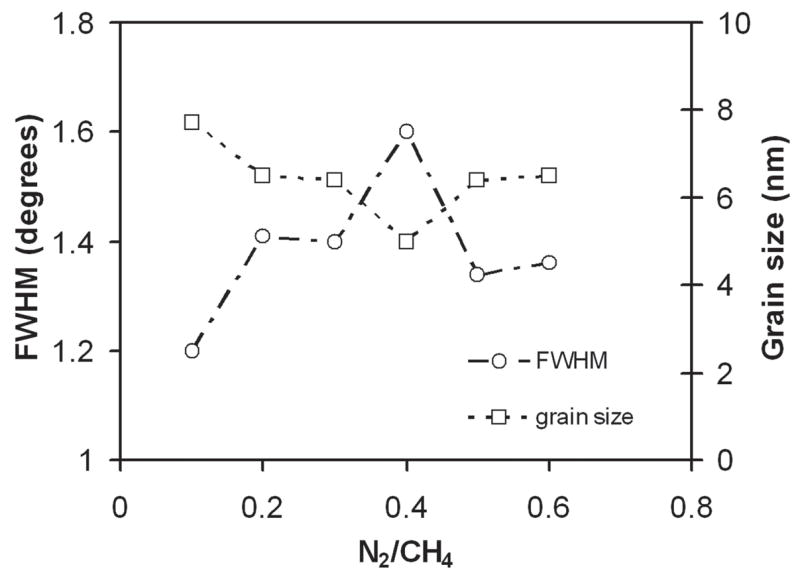
FWHM of the diamond (111) XRD peak and calculated average diamond grain size of diamond films grown in He/H2/CH4/N2 plasma at different N2/CH4 flow ratio.
FIG. 8.
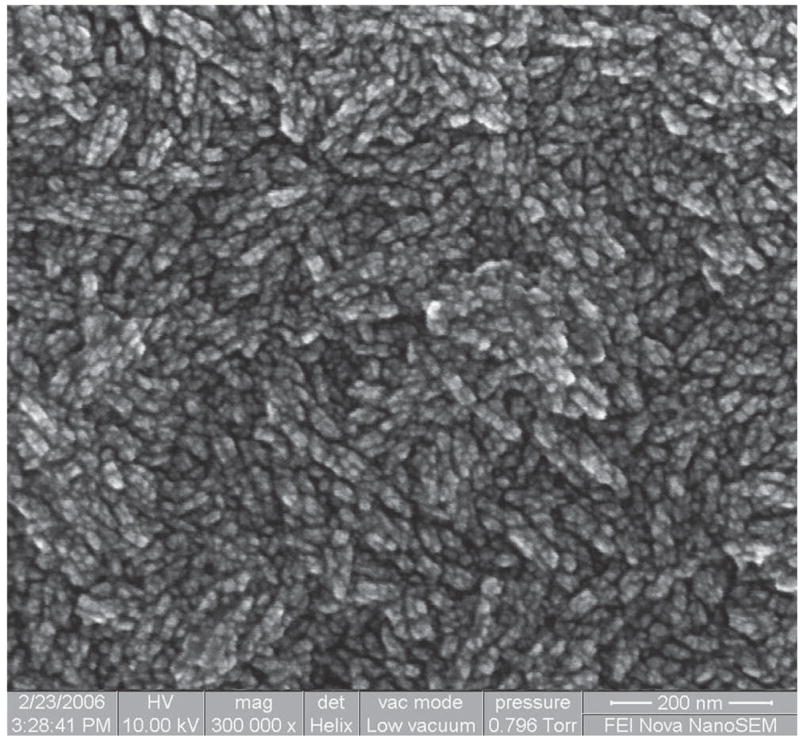
SEM image at 300,000× of the diamond film surface grown in He/H2/CH4/N2 plasma at N2/CH4 flow ratio of 0.4.
The micro-Raman spectra for each of the nanostructured diamond films on Ti–6Al–4V alloy are shown in Fig. 9. The diamond band at 1332 cm−1 is significantly broadened, and Raman scattering intensity in the 1400–1600 cm−1 region is pronounced. This band is usually associated with the “G-band” of disordered graphite which is downshifted from 1580 cm−1 and therefore likely involves scattering from amorphous sp2 and sp3 bonded carbon domains.18 The Raman spectra have another peak at 1150 cm−1 in addition to the main diamond and graphite bands. It is suggested that this band is due to the nanocrystalline nature of the diamond films.19 The films produced consist of diamond nanocrystallites imbedded in amorphous carbon matrix with a relatively small amount of graphitic carbon.
FIG. 9.
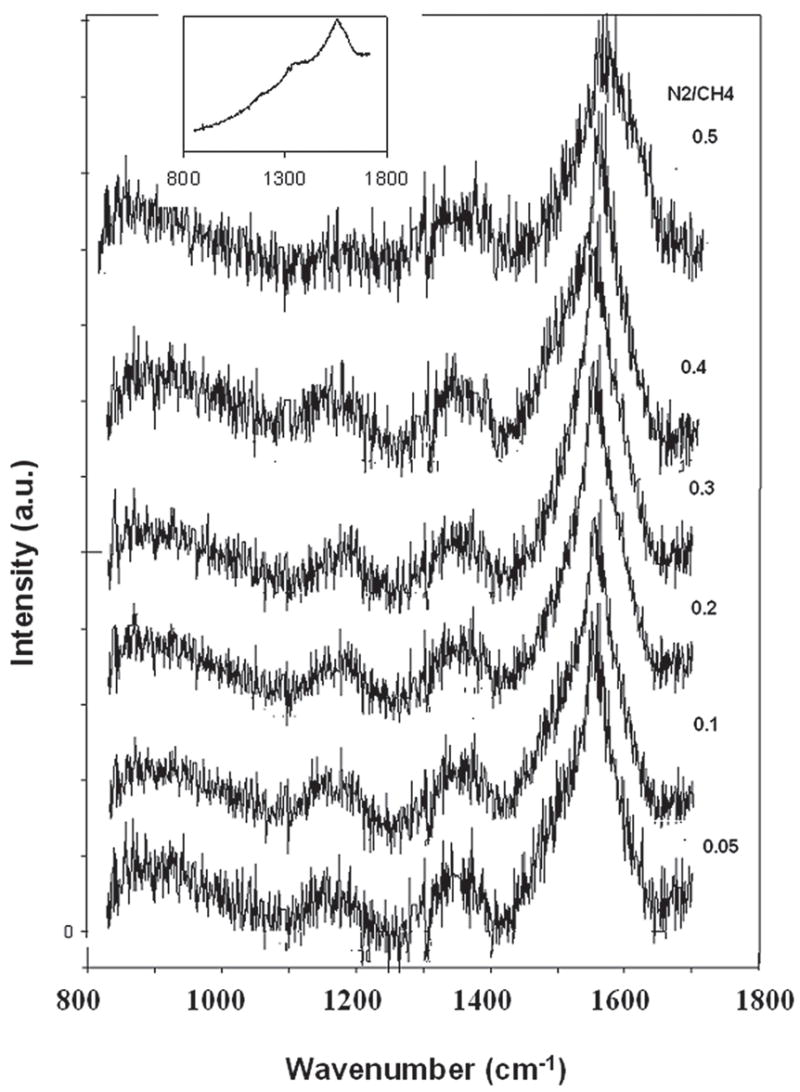
Micro-Raman spectra for high-density plasma processed nanostructured diamond films on Ti–6Al–4V alloy at different N2/CH4 feed gas fractions. The spectra were background subtracted from the as-received one shown in the inset and are displaced along vertical axis for visual clarity.
The plan-view AFM images in Fig. 10 of the as-grown diamond films show the morphological change due to change in different deposition conditions. Roughness measurements by AFM in the 2-μm2 scan area for diamond films deposited in CH4/H2 plasma without N2 [Fig. 10(a)] and with N2 addition [Fig. 10(b)] were found to be 41 and 17 nm, respectively. The AFM images of diamond films with different N2/CH4 ratios in He/H2/CH4/N2 plasma are shown in Figs. 10(c)–10(h). We could clearly observe the morphological change with change in the N2 concentration in the feed gases. Earlier roughness measurement showed that by adding He in CH4/H2 feedgas (with no N2) changed the roughness of the diamond films dramatically [shown in Fig. 2(b)]. We also reported 9–10-nm rms roughness value from diamond films deposited in He/H2/CH4/N2 with 71% He in He + H2 and a N2/CH4 ratio of 0.1.12 When the N2/CH4 ratio was increased to 0.4 in the same gas mixture, roughness decreased even further to 6 nm (rms) and produced ultrasmooth diamond surfaces. These roughness values increased again with the increase of N2/CH4 ratios, as shown in Fig. 11. The nanoindentation load–displacement curve and hardness values for N2/CH4 ratio of 0.4 are given in Fig. 12. Nanoindentation measurement revealed that the hardness (H) and modulus (E) of diamond films with different N2/CH4 ratios were in the range of H = 50 to 60 GPa and E = 330 to 380 GPa, respectively.
FIG. 10.
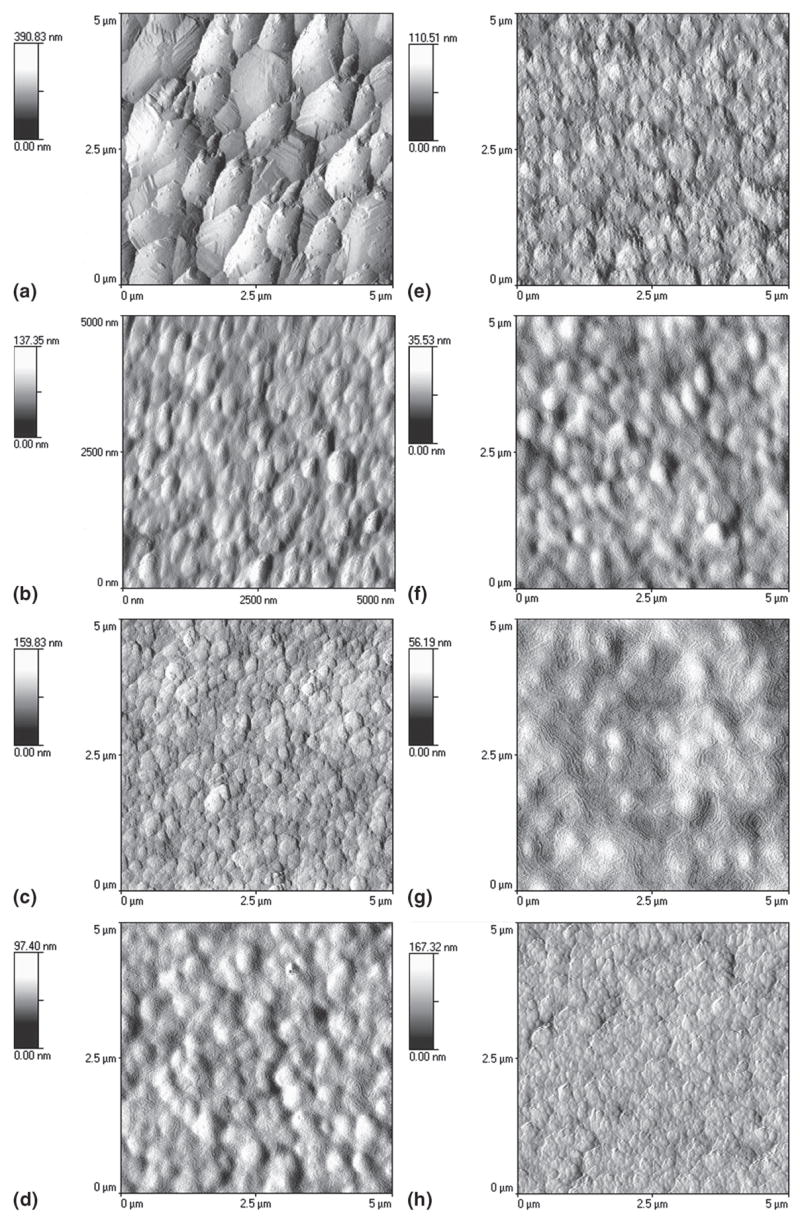
Plan-view AFM images of the as-grown diamond films prepared from microwave plasma showing the morphological change due to change in different deposition conditions: (a) CH4/H2 plasma without N2, (b) CH4/H2 plasma with N2, (c–h) He/H2/CH4/N2 plasma with different N2/CH4 ratios: N2/CH4 = (c) 0.05, (d) 0.1, (e) 0.2, (f) 0.3, (g) 0.4, and (h) 0.5.
FIG. 11.
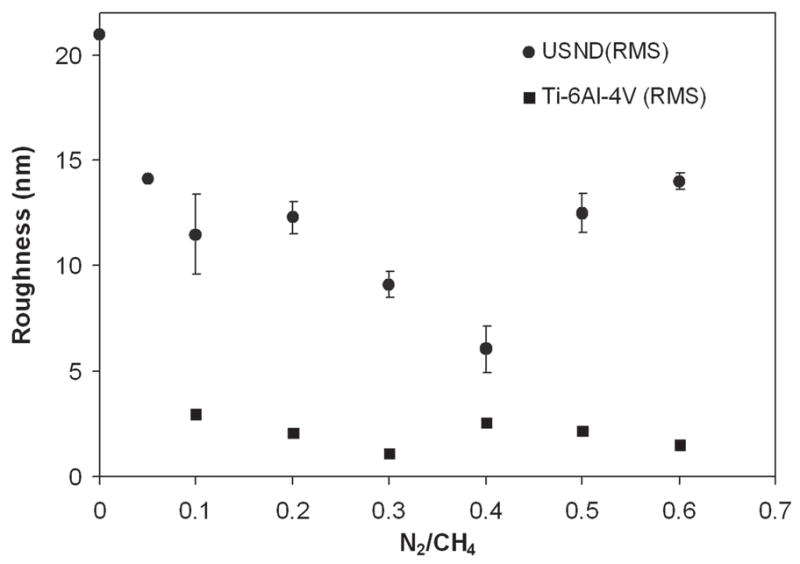
A plot of the surface roughness measured in 2 × 2 μm area of different as-grown diamond films with different N2/CH4 ratios in He/H2/CH4/N2 plasma. Roughness values are also given for uncoated polished Ti-6Al-4V substrates.
FIG. 12.
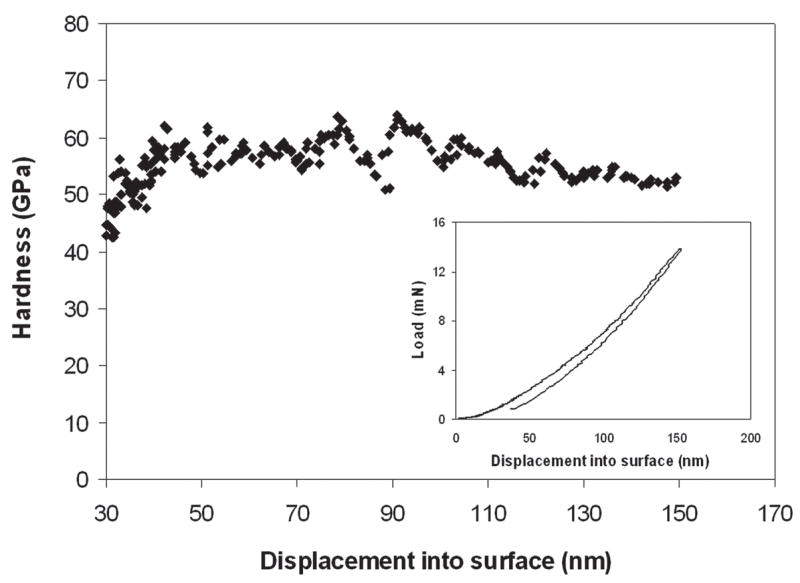
Nanoindentation hardness versus depth for high density plasma processed ultra smooth nanostructured diamond coating at N2/CH4 ratio 0.3 in He/H2/CH4/N2 plasma. Nanoindentation load-displacement curve for same sample is shown in the inset.
The effect of He addition is not a simple effect of plasma dilution but is based on a more complex mechanism. Diamond films grown in H2/CH4/N2 plasma without He at a corresponding high CH4/H2 ratio of 0.6 produced poor-quality films with a high content of the graphitic phase.20 Helium addition reduced the diamond grain size, and this suggests that the rate of secondary nucleation/renucleation increases in He/H2/CH4/N2 plasma, terminating the growth of large diamond nanocrystals.12 The effect of He addition was simply to increase the effective CH4/H2 ratio in He/H2/CH4/N2 plasma, which should reduce the effect of hydrogen on suppressing secondary nucleation by regasifying nondiamond carbon. Helium has also a strong influence on the CN radical on the degree of diamond nanocrystallinity.6–8 Small amounts of CN and HCN in conventional mixtures (CH4/H2) effectively abstract adsorbed H atoms, creating vacant growth sites, thereby reducing the carbon supersaturation.21–23 The use of large N2 additions (N2/CH4 ratios greater than 0.05) resulted in a reduction of diamond phase purity, a more nanocrystalline structure, and a smoother film surface. The larger amounts of CN and HCN resulted in excessive abstraction of adsorbed H, which leaves the surface open to further adsorption by CN or other nitrogen species that are not able to stabilize the diamond structure efficiently.23 Therefore, higher CN species promote higher nanocrystallinity and more CN species form in N2 and He gas mixture. Apart from causing the nanocrystallinity of the diamond component in the film, the addition of nitrogen in the gas phase also resulted in amorphous carbon content in the film with a corresponding increase in the Raman 1550 cm−1 peak intensity.24 Higher CN levels also induced increased twinning, and stacking faults resulting in the nanocrystalline structure.7 Our previous OES measurements taken from He/He/CH4/N2 plasma reflected that, at 71% He content and N2/CH4 ratio of 0.1, both the CN/Hα and C2/Hα were maximized. The present results indicated that at the same 71% He content and 0.4 N2/CH4 gas flow ratio there was an increase of 2.5 the CN/Hα ratio. There was no significant change in C2/Hα values. The lowest roughness and smaller grain size values were achieved in the diamond films at the N2/CH4 ratio of 0.4. Thus, CN has a strong influence on the formation of smooth nanocrystalline diamond films, and the activity of CN radical is highly affected by the addition of He.
IV. CONCLUSION
In summary, we have synthesized ultrasmooth nanostructured diamond films on Ti–6Al–4V medical grade substrates by adding helium in H2/CH4/N2 plasma and by changing the N2/CH4 gas flow from 0 to 0.6. We were able to deposit diamond films with 6 nm (rms) roughness in 2 μm2 area and grain size of 4–5 nm. Roughness decreased from rms 22 to 6 nm from N2/CH4 gas flow ratio of 0.05 to 0.4 (CH4 is fixed) and then increased again up to 13 nm at N2/CH4 ratio of 0.6. Raman spectra were typical for nanostructured diamond films and did not show significant changes with varying N2/CH4 ratio. Nanoindentation demonstrated that the hardness and Young’s modulus of the films are in the range of 50–60 GPa and 330–380 GPa, respectively. X-ray diffraction showed that all the spectra have broad diamond (111) peaks characteristic of nanostructure diamond, and the grain size was calculated between 4 and 8 nm. The grain size decreased and drop to around 4–5 nm as the N 2/CH4 ratio increased up to 0.4 and then again increased. The surface morphology imaged by nano SEM at 300,000× also confirmed the nanocrystalinity of the diamond films. It was also found that as the N2 content increased, the intensity of the TiC peak decreased. At a N2/CH4 ratio of 0.4, there was no (200) TiC peak, and the intensity again increased as the N2/CH4 ratio increased beyond 0.4. It was previously described that reducing diamond grain size and film surface roughness by He addition is attributed to plasma dilution, enhanced fragmentation of carbon containing species, and enhanced formation of CN radical. From optical emission data, we found that CN/Hα relative intensity was highest at a N2/CH4 gas concentration ratio of 0.4, which resulted in the smoothest nanostructured hard diamond films. Therefore, it can be concluded that CN radical has a strong influence in formation of smooth nanocrystalline diamond films.
Acknowledgments
We acknowledge support from the National Institute of Dental and Craniofacial Research (NIDCR), National Institutes of Health (NIH) under Grant No. R01 DE013952.
References
- 1.Saha S, Campbell C, Sarma A, Christiansen R. A biomechanical evaluation of the christensen temporomandibular joint implant. Crit Rev Biomed Eng. 2000;28:399. doi: 10.1615/critrevbiomedeng.v28.i34.90. [DOI] [PubMed] [Google Scholar]
- 2.Shankland WE. TMJ: Its Many Faces. 2. Anadem Publishing; Columbus, OH: 1998. p. 15. [Google Scholar]
- 3.National Center for Health Statistics. Vital Health Statistics, 1994 Report. Hyattsville, MD: 1994. [Google Scholar]
- 4.Praemer S, Furner D. Musculoskeletal conditions in the United States. AAOS; Rosemont, IL: 1992. p. 125. [Google Scholar]
- 5.Catledge SA, Vohra YK. High-density plasma processing of nanostructured diamond films on metals. J Appl Phys. 1998;84:6469. [Google Scholar]
- 6.Catledge SA, Borham J, Vohra YK, Lacefield WR, Lemons JE. Nanoindentation hardness and adhesion investigations of vapor deposited nanostructured diamond films. J Appl Phys. 2002;91:5347. [Google Scholar]
- 7.Afzal A, Rego CA, Ahmed W, Cherry RI. HFCVD diamond grown with added nitrogen: Film characterization and gas-phase composition studies. Diamond Relat Mater. 1998;7:1033. [Google Scholar]
- 8.Corvin RB, Harrison JG, Catledge SA, Vohra YK. Gas-phase thermodynamic models of nitrogen-induced nanocrystallinity in chemical vapor-deposited diamond. Appl Phys Lett. 2002;80:2550. [Google Scholar]
- 9.Catledge SA, Vohra YK. Mechanical properties and quality of diamond films synthesized on Ti–6Al–4V alloy using the microwave plasmas of CH4/H2 and CO/H2 systems. J Appl Phys. 1998;83:198. [Google Scholar]
- 10.Zhou D, Gruen DM, Qin LC, McCauley TG, Krauss AR. Control of diamond film microstructure by Ar additions to CH4/H2 microwave plasmas. J Appl Phys. 1998;84:1981. [Google Scholar]
- 11.Gruen DM. Nanocrystalline diamond films. Ann Rev Mater Sci. 1999;29:211. [Google Scholar]
- 12.Konovalov VV, Melo A, Catledge SA, Chowdhury S, Vohra YK. Ultra-smooth nanostructured diamond films deposited from He/H2/CH4/N2 microwave plasmas. J Nanosci Nano-technol. 2006;6:258. doi: 10.1166/jnn.2006.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabes BD, Oliver WC, McKee RA, Walker FJ. The determination of film hardness from the composite response of film and substrate to nanometer scale indentations. J Mater Res. 1992;7:3056. [Google Scholar]
- 14.McHargue J. Mechanical properties and applications of diamond. In: Tzeng Y, Yoshikawa M, Murakawa M, Feldman A, editors. Applications of Diamond Films and Related Materials. Elsevier; Amsterdam, The Netherlands: 1991. p. 113. [Google Scholar]
- 15.Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564. [Google Scholar]
- 16.Zhou D, McCauley TG, Qin LC, Krauss AR, Gruen DM. Synthesis of nanocrystalline diamond thin films from an Ar–CH4 microwave plasma. J Appl Phys. 1997;83:540. [Google Scholar]
- 17.Zhou D, Krauss AR, Qin LC, McCauley TG, Gruen DM, Corrigan TD, Chang RPH, Gnaser H. Synthesis and electron field emission of nanocrystalline diamond thin films grown from N2/CH4 microwave plasmas. J Appl Phys. 1997;82:4546. [Google Scholar]
- 18.Tamor MA, Vassell WC. Raman “fingerprinting” of amorphous carbon films. J Appl Phys. 1994;76:3823. [Google Scholar]
- 19.Nemanich J, Glass JT, Lucovsky G, Shroder RE. Raman scattering characterization of carbon bonding in diamond and diamondlike thin films. J Vac Sci Technol A. 1988;6:1783. [Google Scholar]
- 20.Catledge SA, Vohra YK. Mechanical Properties of Structural Films. ASTM STP1413, ASTM; West Conshohocken, PA: 2001. Effect of nitrogen feedgas addition on the mechanical properties of nano-structured carbon coatings; p. 5. [Google Scholar]
- 21.Jin S, Moustakas TD. Effect of nitrogen on the growth of diamond films. Appl Phys Lett. 1994;65:403. [Google Scholar]
- 22.Cao GZ, Schermer JJ, van Enckevort WJP, Elst WALM, Giling LJ. Growth of {100} textured diamond films by the addition of nitrogen. J Appl Phys. 1996;79:1357. [Google Scholar]
- 23.Bohr S, Haubner R, Lux B. Influence of nitrogen additions on hot-filament chemical vapor deposition of diamond. Appl Phys Lett. 1996;68:1075. [Google Scholar]
- 24.Catledge SA, Vohra YK. Effect of nitrogen addition on the microstructure and mechanical properties of diamond films grown using high-methane concentrations. J Appl Phys. 1999;86:698. [Google Scholar]


