Abstract
This study reports extensive characterization of the silicone gel (3−4680, Dow Corning, Midland, MI), for potential use as an artificial dural sealant in long-term electrophysiological experiments in neurophysiology. Dural sealants are important to preserve the integrity of the intra-cranial space after a craniotomy and in prolonging the lifetime and functionality of implanted brain probes. In this study, we report results of our tests on a commercially available silicone gel with unique properties that make it an ideal dural substitute. The substitute is transparent, elastic, easy to apply, and has resealing capabilities, which makes it desirable for applications where multiple penetrations by the brain probe is desirable over an extended period of time. Cytotoxicity tests (for up to 10 days) with fibroblasts and in vivo tests (for 12 weeks) show that the gel is non-toxic and does not produce any significant neuronal degeneration when applied to the rodent cortex even after 12 weeks. In-vivo humidity testing showed no sign of CSF leakage for up to 6 weeks. The gel also allows silicon microprobes to penetrate with forces less than 0.5 mN, and a 200 μm diameter stainless steel microprobe with a blunt tip to penetrate with a force less than 2.5 mN. The force dependency on the velocity of penetration and thickness of the gel was also quantified and empirically modeled. . The above results demonstrate that the silicone gel (3−4680) can be a viable dural substitute in long-term electrophysiology of the brain.
Keywords: Microelectrodes, chronic brain implants, Dural substitute, neurosurgery
1. Introduction
It has been over 45 years since the first metal microelectrode was implanted to record single unit activity in awake behaving animals (Green, 1958; Hubel, 1959). There have been many advances in electrode design over the past years that have allowed for longer lasting devices and better recordings (Moxon et al., 2004; Muthuswamy et al., 2005b; Nicolelis et al., 2003; Nordhausen et al., 1996; Rousche et al., 2001; Vetter et al., 2004). In all of these cases, the dura mater has to be removed or incised in order to penetrate the brain. A dural substitute or sealant is thus required primarily to prevent infection. However, dural substitutes have also been used to help keep the implanted electrodes from moving (Vetter et al., 2004) and for optical imaging purposes (Arieli et al., 2002). Improvements in the design and material selection of microelectrode devices have greatly improved the recording capabilities and helped prolong the lifetime of the electrodes. The surgical techniques and advances in dural substitute material are believed to be critical for improving the lifetime and functionality of the devices (Vetter et al., 2003).
The following criteria are of importance for an optimal dura sealant for microelectrodes: (1) the material must be biocompatible (2) protect the cortex from inflammation (3) prevent cerebro-spinal fluid (CSF) leakage and prevent humidity from escaping the craniotomy (4) easy to apply (5) the material should be durable, so that it does not degrade overtime (6) thin and elastic enough to allow microelectrodes to penetrate with minimal force (7) should not cause damage to the microelectrode upon penetration (8) the material should not adhere to the brain or the electrode, since sealants that adhere to the recording site of microelectrodes could cause recording failures (9) the material should help prevent dural regrowth, which forms a thick fibrous layer, that prevents further penetration of microelectrodes after a couple of weeks (Baker et al., 1999.) (10) The material should also be transparent, if brain imaging is desired. The prevention of humidity is crucial for future recording devices that are incorporating wireless technology and advanced electronics on the headstage chip (Wise et al., 2004), or for advanced MEMS based moveable microelectrodes (Muthuswamy et al., 2005a; Muthuswamy et al., 2005c).
Conventional material that has been used to close a craniotomy is Gelfoam® (Pharmacia & Upjohn Co.) Gelfoam is porous and does not produce a tight seal, which results in humidity or CSF leakage. Gelfoam also does not prevent dural regrowth, which is a prime failure mode of chronically implanted microelectrodes (Maynard et al., 2000). Gelfoam also does not help to prevent swelling which causes herniation of the brain. Over the years, there have been many new advances in dural substitutes including the use of novel hydrogels (Becker and Kipke, 2003; Preul et al., 2003), cast molded silicone substitutes (Arieli et al., 2002), silicone gel Kwik-Sil™ (World Precision Instruments). Graft based dural substitutes have also been used (Maurer and McDonald, 1985; San-Galli et al., 1992; San-Galli et al., 1996; Terasaka et al., 2006) and PRECLUDE (W.L. Gore and Associates, Inc., Flagstaff, AZ, USA), which is a polytetrafluoroethylene material. All of these dural substitutes are biocompatible and are efficient to use for most cases. However, one problem with all of the above materials is that they require significant forces to penetrate with an electrode or implant, and after penetration, the electrodes are difficult or impossible to move without compromising the sealing capabilities of the gel. Most of the materials are therefore not suited for applications requiring multiple penetrations. Therefore, there is a need for a soft dural sealant, which will allow multiple penetrations of implants, microelectrodes, or other devices while preserving its dimensional integrity and sealing properties during every recording session.
This study tests a commercially available silicone gel (3−4680, Dow Corning, Midland, MI) that is soft and elastic, and requires low penetration force. The gel is a polydimethylsiloxane (PDMS) based silicone gel. We have successfully used the silicone gel in extracellular recordings applications involving our MEMS based polysilicon electrodes in a rodent model for a period of over 12 weeks (Jackson et al., 2007). This dural sealant still offers most of the same desirable properties as previous dural substitutes described above. Currently there are no proven dural sealants that allow microelectrodes or brain implants to penetrate or move after the dural sealants are cured. Such a dural sealant will be beneficial in applications with (a) moveable implants (b) implants that need to be removed and re-inserted or repositioned multiple times (c) probes that need to be inserted post-surgery. In this paper we investigate the biocompatibility of this silicone gel, along with CSF leakage tests, and penetration force tests.
2. Material and methods
2.1 Dural substitute
The silicone gel that is being tested in this paper as a dural sealant is a two-part silicone gel that is commercially available (3−4680, Dow Corning, Midland, MI). The gel is primarily used for hydrophobic encapsulation of electronic chips. The gel was designed to be soft, to allow test probes to penetrate the encapsulation without loss in watertight sealing. The gel has a tacky property allowing it to re-seal if penetrated or cut and preserving its dimensional integrity, which helps to prevent humidity from entering the chip even after penetration. The above properties make 3−4680 silicone gel an attractive candidate as a dural sealant for moveable or re-implantable microelectrodes in long-term animal experiments. The gel has two parts before mixing - part A, which is clear, and part B, which is bluish in color. The exact compositions of part A and B are proprietary. Once the two-part silicone gel is mixed it takes 15−30 minutes to cure at room temperature. After mixing and curing, the gel is transparent with a bluish tint. Adding a higher concentration of part A causes the gel to be softer, while a higher concentration of part B causes the gel to be harder. The mixtures were stirred thoroughly for 1−3 minutes then applied using a micropipette. All of the concentrations used in the experiments below are reported as a volume ratio.
2.2 Cytotoxicity tests
Cytotoxicity tests were conducted for three different gel compositions (1:1, 1:2, and 2:1 volume ratio) of the gel. The tests were performed using ISO 10993−5 standards (Wallin and Arscott, 1998). NIH 3T3 fibroblast cell lines were cultured in Eagle's minimum essential medium (EMEM, Sigma), on n=2 samples of each concentration of the gel along with 2 samples of control (no gel) in separate standard polystyrene wells. The fibroblasts were then added on top of the gel after the silicone gels had cured. The cells were then placed back in an incubator at 37°C with 5% CO2. After 3 days the cells were examined using a lightfield microscope (Leica Microsystems), and the images were digitally captured using a CCD camera.
A second test was performed to test the cell viability. The fibroblasts were allowed to sit in a well containing a 1:1 mix ratio of gel for 10 days, after which a live/dead cell assay (Molecular Probes, USA) consisting of 2 μM Calcerin AM and 4 μM Ethidium Homodimer-I in 200 μL PBS was added to the well (Jain and Muthuswamy, 2007). Fluorescent imaging was performed using a fluorescent stereomicroscope (MZFLIII, Leica Microsystems). Images were digitally captured using a CCD camera.
2.3 Force Measurements during microelectrode penetration
Force measurement tests were performed on the silicone gel by using a precision load cell (MTC 10/30 ZER, Wipotec, Germany) with a resolution of 0.1 mg. The load cell was placed on a floating vibration free table (model 65−531, Technical Manufacturing Corporation, Peabody, MA) to help prevent any undesired mechanical noise. Glass wells (n=3), which were 9 mm in diameter, were filled with Jell-O™, in order to resemble the mechanical properties of the brain. The silicone gel was added on top of the Jell-O™ in all 3 wells and allowed to cure for 30 minutes. The wells were then placed on the load cell by using double-sided carbon tape to prevent any movement.
To study the effects of different microelectrodes two types of electrodes were used in the following experiments: (1) a blunt tip 200 μm diameter stainless steel probe (26002−20, Fine Science Tools) and (2) a silicon-based “Michigan” Probe. The stainless steel probe was soldered to a 24AWG wire, while the Michigan probe was bonded to a printed circuit board by using epoxy to enable them to be fastened securely to a motorized microdrive (FHC Inc., Bowdoinham, ME) for gel penetration. The motorized microdrive was used to control the velocity and displacement of the probes.
In the experiments below, the electrodes were moved down and allowed to penetrate the gel by using the motorized microdrive. After the electrodes penetrated the gel the direction was reversed, in order to determine the force required to extract the electrode from the silicone gel. At the end of each measurement, the gel was removed and replaced with a new layer of gel, in order to account for any variability in a batch of gel. Each well was thus tested two times for each type of electrode, for a total of n=6 measurements for each electrode. After the gel was removed, the thickness was verified by using Vernier calipers. If the thickness was different from the expected value by more than 300 μm then the results were discarded and the experiment repeated.
2.3.1 Effect of gel concentration
In order to determine which concentration of the two-part silicone gel was optimal for penetration, 3 different gel compositions were tested (1:1, 1:2, 2:1 ratios by volume of part A to part B). The gels were prepared as described earlier, and the ratios were mixed based on a volume ratio. Each electrode was tested n=6 times for each concentration. The electrodes were moved down with a velocity of 6.7 μm/s using the motorized microdrive. The average thickness of the gels was approximately 2.3 mm. The gel thickness is calculated by dividing the volume of gel injected by the cross-sectional area of the well.
2.3.2 Effect of velocity of penetration
To determine the effects of varying velocity on the penetration and extraction force from the silicone gel three different velocities were tested (3.1 μm/s, 5 μm/s, and 12.5 μm/s). The composition of the gel used for these measurements had a 1:1 ratio with a thickness of 2.5 mm. The experiment was conducted using the same setup described above. The velocities were chosen based on the minimum, maximum and mean rate using a 1μm step, of the motorized microdrive machine.
2.3.3 Effect of gel thickness
Determining the effects of varying gel thickness on force requirements is useful since the thickness of the skull varies across species and among animals of the same species. Therefore, different animals may require different thicknesses of gel for optimal sealing. Three different gel thicknesses (0.9 mm, 1.3 mm, and 2.5 mm) were used to determine the effects of thickness on the penetration and extraction force of the microelectrodes. The concentration of the gel used in this experiment had a 1:1 ratio, and a velocity of 5 μm/s. The experiment was conducted using the same setup as described earlier.
2.4 In vivo tests
To determine biocompatibility of the silicone gel in vivo, three adult Wistar rats (250 mg) were used. All procedures were carried out with the approval of the Institute Animal Care and Use Committee (IACUC) of Arizona State University, Tempe. The experiments were performed in accordance with the National Institute of Health (NIH) guide for the care and use of laboratory animals (NIH publications no. 80−23) revised 1996. All efforts were made to minimize animal suffering and to use only the number of animals necessary to produce reliable scientific data, and to utilize alternatives to in vivo techniques, if available. The animals were administered a general anesthesia of rat cocktail (50 mg/ml ketamine, 5 mg/ml xylazine, and 1 mg/ml acepromazine) intramuscularly with an initial dosage of 0.1 ml/100g body weight. After the animals were prepared and mounted on a stereotaxic frame (Kopf Instruments, Tujunga, CA) two circular craniotomies were performed (one on each side of the midline). The centers of the craniotomies were 3 mm posterior to bregma and 2.5 mm lateral to the midline, with a diameter of around 3−4 mm. The dura was then incised and removed in both craniotomies. One of the craniotomies was then packed with Gelfoam (Pharmacia & Upjohn Co.), while the other was injected with the 3−4680 gel (1:1 volume ratio of part A to part B). The gel was injected by using a micropipette, the volume of injected gel was determined based on an estimation of the skull thickness and size of craniotomy. The gel was applied until it filled the entire craniotomy then it was subsequently allowed to cure for 30 minutes. A micrograph of the cured gel is shown in Fig. 1.
Figure 1.
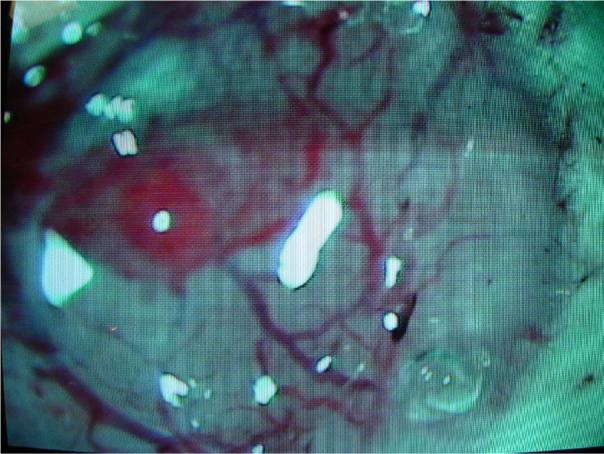
Micrograph of the silicone gel on a rodent brain after the dura was incised and removed.
A 9 mm diameter well made of Pyrex glass filled with a different biocompatible silicone (Sylgard 184, Dow Corning, Midland, MI) was placed to cover the craniotomies. The Sylgard gel was used because of its excellent transparency properties, but it did not contact the brain. Once the wells were in place dental acrylic was used to secure the wells onto the skull. Two rats were sacrificed at the end of 5 weeks and one at the end of 12 weeks of implantation and the brains perfused for histology. Each animal was perfused with 4% paraformaldehyde in 0.1 M phosphate buffer saline (PBS) pH 7.4 following a standard protocol (Szarowski et al., 2003). After perfusion, the brain was placed in 4% paraformaldehyde for an additional 48 hr. Then the brains were removed and placed in a 30% sucrose solution for an additional 48 hr prior to slicing. The glass wells and the gel covering the brain were removed prior to putting the brains in sucrose. Both hemispheres were sectioned using a vibratome into 75-μm thick coronal sections and stained using a silver impregnation protocol (FD NeuroSilver kit-I, FD Neurotechnologies, Baltimore, MD) (Beltramino et al., 1993). Silver staining was used to detect any neuronal degeneration and necrosis underneath the dural sealant.
2.5 Testing sealing capability of gel in vivo
To determine CSF leakage n=2 adult Wistar rats were used. The surgical techniques and gel implantation were carried out as described above in section 2.4, with the addition of a humidity sensor (HIH-3605, Honeywell, IL) placed inside the chamber, and sealed using epoxy from the top of the device. The humidity sensor was placed directly over the gel/craniotomy. Readings were measured every couple of days for a period of 3 weeks and 6 weeks for the two devices. In each session five readings were taken every 12 minutes to account for any variability. Room humidity levels were also measured during the recording sessions as control.
3. Results
3.1 Cytotoxicity and biocompatiblity
Results from the cytotoxicity test shown in Fig. 2 confirm that the 3−4680 gel is not toxic. The density of cells on the gel was comparable to the density of cells in the control well. Results in Fig. 2 are for the gel with a 1:1 concentration, however 2:1 and 1:2 concentrations also showed similar results but are not shown. The results of the live-dead assay test in Fig. 2(c) show that there was no cell death due to the gel even after 10 days of being on the gel.
Figure 2.
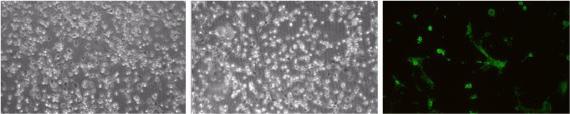
Results of cytotoxicity tests using mouse fibroblasts (3T3) show (left) cells in a standard well after 3 days in a control experiment, (middle) cells seeded on a 1:1 volume ratio of part A to part B composition of the gel after 3 days (right) live-dead cell assay of cells seeded on the gel where green represents live cells after 10 days.
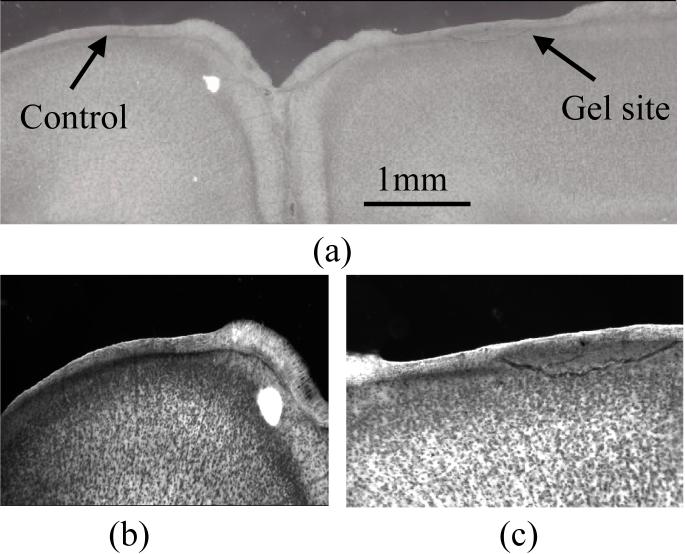
Of the three rats that were tested for biocompatibility, none of them showed any signs of behavioral deficits or infection over the period of the tests. During the removal of the brain, we visually confirmed that the gel was still in place on the brain. The bottom layer of the gel had a slight discoloration due to blood, but showed minimal signs of degradation. The gel was easily removed from the brain and showed no signs of adhesion to the brain tissue. The brain was then perfused, sliced, and stained according to the methods described in the previous section. The slices were visually inspected for noticeable differences in the two hemispheres. The results of the silver staining procedure are shown in Fig. 3. Visual analysis of the figures show no large-scale neuronal degeneration in the brain hemisphere covered with gel. Images from the hemisphere covered with the gel are comparable to the images from the control hemisphere, which was covered with gelfoam. The cells were stained with a high intensity of silver in order to detect any neuronal degeneration, which might explain the presence of dark neural cells in the background in both hemispheres.
Figure 3.
Histology (silver stain) results from in vivo tests of the gel after 12 weeks in adult rodent brains are shown. In (a) a coronal section showing both sides of the brain where the silicone gel was applied on right side and the gelfoam on the left show no significant differences between the silver stains of either side and negligible neuronal degeneration. The scale bar represents 1 mm. In (b) a close-up of the control or gel foam side is shown and in (c) a close up of the silicone gel side is shown indicating no significant neuronal degeneration in either side and no significant differences between the two sides. Magnification in (b) and (c) was 40X.
3.2 Force Measurements
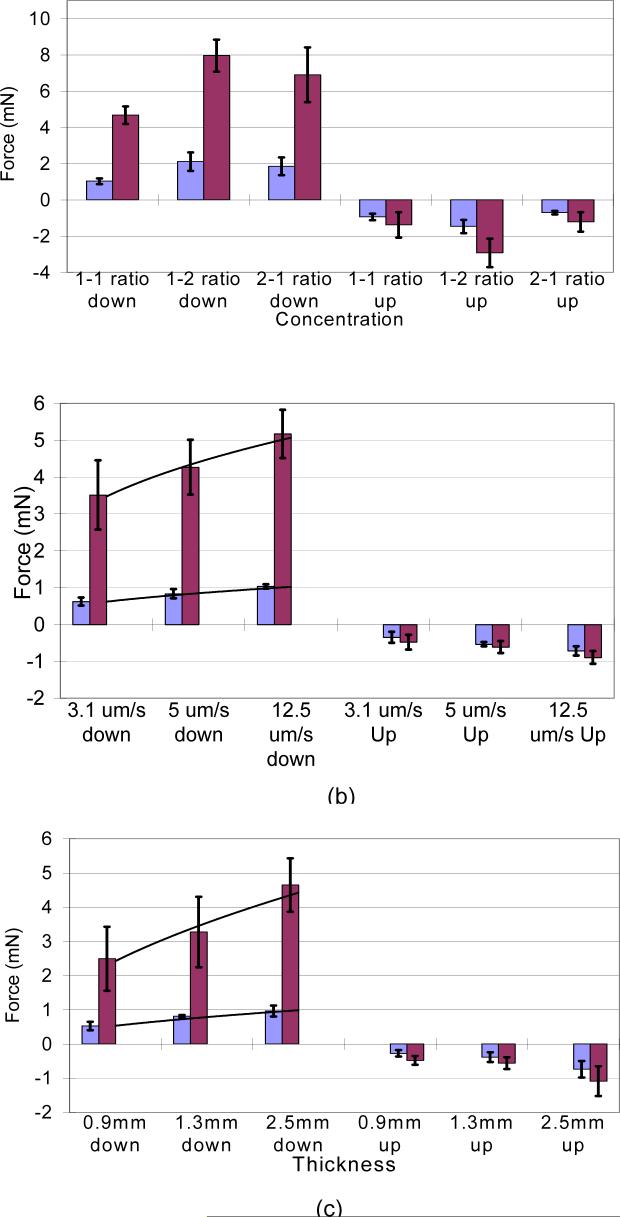
The penetration force measurements for the two types of microelectrodes tested are shown in Fig. 4. The results from (Fig. 4(a)) show that a gel composition of 1:1 requires the least force for penetration and extraction of microelectrodes amongst the three different concentrations. A student t-test showed that the penetration force for the 1:1 concentration was significantly different (p<0.05) when compared separately to both the 2:1 and 1:2 concentrations. However, there was no significant difference in penetration forces for 1:2 and 2:1 gel compositions (p>0.05). The extraction forces were significantly different between any two of the three gel compositions taken two at a time. The silicon probe (SP) required forces of 1.04±0.16 mN and −0.92±0.18 mN and the stainless steel (SS) probe 4.68±0.48 mN and −1.37±0.70 mN for penetrating and subsequently extracting from the gel. The 1:2 composition gel was the hardest to penetrate with forces of 2.12±0.51 mN and 7.96±0.88 mN for SP and SS probes respectively and the 2:1 ratio composition required penetration forces of 1.85±0.49 mN and 6.9±1.5 mN for SP and SS respectively even though it was the softest material. The 2:1 ratio gel was more difficult to penetrate when compared to the 1:1 concentration because it had more slippage and dimpling. However, because of the soft nature of the 2:1 gel composition, it required the least amount of force to extract the electrodes (SP: − 0.70±0.10 mN, SS: −1.21±0.54 mN).
Figure 4.
Force measurement plots, showing the force to penetrate the gel and the force to extract the electrodes at (a) different volume compositions of the gel (b) different penetration velocities of the probe and (c) different thicknesses of the gel. Red bars represent data for the 200-μm diameter stainless steel probe (SS), blue bars represent data for the silicon probe (SP) and error bars represent±1 S.D.
Force requirements under varying velocities of penetration are shown in Fig. 4(b). The results predictably verify that it requires less force to penetrate and extract from the gel at lower velocities (SP, p<0.02). Empirical models were fitted to relate the forces required to penetrate with the velocity of penetration through the gel. The model for the stainless steel probe is shown in (eqn. 1) and that for the silicon probe is shown in (eqn. 2) where F is the force in mN and v is the velocity in μm/s.
| (1) |
| (2) |
Equations (1) and (2) are only valid when the average thickness of the gel is 2.5 mm.
Force requirements under different thicknesses of gel are shown in Fig. 4(c). The results verify that with decreasing thickness, the force to penetrate or extract also decreases for both the silicon probe and the stainless steel probe. A student t-test showed that the penetration force for the 1.3mm thick silicone gel was significantly different (SP,p<0.01 and SS, p<0.05) when compared separately with the penetration forces for the 0.9mm and 2.5mm thick silicone gel. A thin, 0.9 mm thick layer of silicone gel takes 0.52 ± 0.13 mN of force using the silicon probe and 2.49 ± 0.93 mN for the stainless steel probe Using data form the three different gel thicknesses empirical models relating the thickness of the gel and penetration force for both stainless steel probe and silicon probe are shown in eqns. 3 & 4 below.
| (3) |
| (4) |
Using equation (5) we can determine that for any gel thickness less than 2.4 mm will require less than 1 mN of force to penetrate using a silicon probe, which is smaller than the minimal force required to cause buckling along the length of the probe (Moon et al., 2003).
3.3 Sealing capabilities of the gel in vivo
The results of the in vivo CSF leakage test shown in Fig. 5 demonstrate that the silicone gel prevents CSF leakage. Device 1 showed some fluctuations in humidity levels that correspond to changes in room humidity indicating the possibility of a leak in the epoxy sealing on top of the chamber housing the humidity sensor. However, device 2 shows no such fluctuations and does not show similar patterns when compared to the room humidity sensor readings. Data from both devices in Fig. 5 indicate no significant leakage of CSF from the craniotomy.
Figure 5.
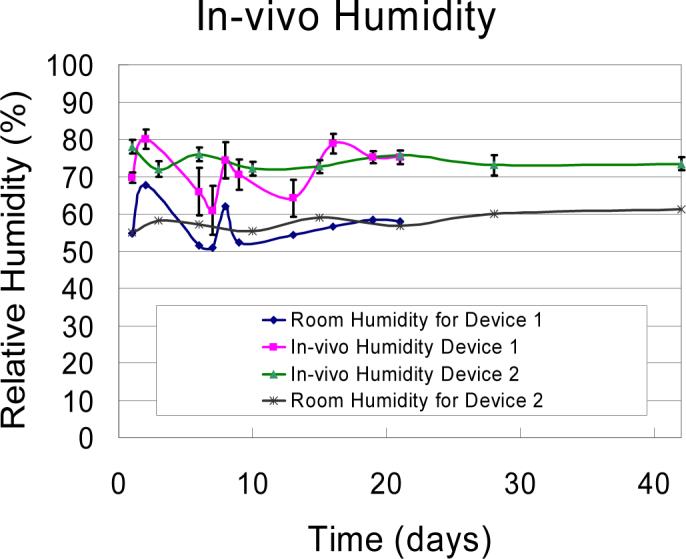
Results of humidity tests to assess the sealing capabilities of gel are shown. Four traces showing in vivo humidity levels and room humidity levels. Device 1, humidity was measured for 3 weeks, where device 2 was measured for 6 weeks. No CSF leakage was seen in either device.
4. Discussion
A novel soft silicone dural sealant is presented in this paper, which can be used for implanting moveable microelectrodes or in applications that require multiple penetrations with the implantable probes after the initial surgery. In this study, we have shown that the two-part silicone gel is non-toxic to cells, and that it causes little or no difference in neuronal degeneration in adult rodent models, compared to biocompatible gelfoam. Therefore, this new silicone gel can be used as a dural substitute without risking any tissue or neuronal damage.
The humidity tests demonstrate that the gel is able to preserve its dimensional integrity, hydrophobicity and sealing properties by maintaining the humidity at baseline values. The normal physiological intracranial pressure (ICP) for humans range from 6.5 to 18 cm H2O (Mokri et al., 1998; Preul et al., 2003); rats also have similar intracranial pressure (ICP) 5 cm H2O (Lorenzl et al., 1996). In vitro studies (not shown here) showed that a 1 mm thick layer of the silicon gel could withstand pressures as high as 55.88 cm H20 and hence be able to prevent CSF leakage due to normal physiological changes in intracranial pressure. This was further confirmed by the results in Fig. 5 from in vivo testing of this gel that demonstrated the sealing capabilities of the gel over 6-week duration.
The results of the force measurement tests show that both silicon probes and stainless steel probes have similar relationships between penetration and extraction forces for the microelectrode and (a) the gel composition, (b) penetration velocity of the microelectrode and (c) gel thickness. To reduce the force required to penetrate the gel one should use a concentration of 1:1, apply a thin layer, and penetrate at the lowest velocity. Most combinations used in this experiment, still allows us to penetrate the gel by using a force less than 1 mN for a silicon probe, which should not cause any significant buckling (Moon et al., 2003), and under 5 mN for a blunt tip stainless steel probe. The low force required to penetrate the gel will allow users to apply the gel to the brain before electrode penetration and implantation. The low extraction force also allows the user to easily remove the electrodes if necessary.
Although, some of the silicone gel adhered to the silicon probe there did not appear to be enough that could significantly affect the recording properties of the electrode. Successful neural recording after gel penetration have been previously demonstrated using a polysilicon probe for over 12 weeks (Jackson et al., 2007). Since little damage is associated with penetration, it is recommended that the gel be applied after removal of the dura to allow time for the gel to cure before penetrating the gel with the electrodes. Little to no degradation of the silicone gel was seen throughout these experiments, however if degradation does occur another layer of gel can be easily applied to fill any voids at a later time. The silicone dural sealant that was tested in this study appears to be ideal for moveable or re-implantable electrodes in vivo. The dural substitute appears to meet most of the needs for an ideal dural sealant for a moveable electrode discussed earlier, with the exception of preventing dural regrowth, which was not tested in this study. However, no visible dural regrowth has been seen in any of our experiments. The silicone gel is easy to apply using a syringe or pipette and has a short curing time (15−30 min) at room temperature, which makes it convenient for use during surgery.
Acknowledgments
The authors wish to thank Paula Stice for her help with surgeries and histology and Massoud Khraiche for his help with the cell culture. This study was supported by NIH grants NS051773 and the Arizona Biomedical Research Commission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arieli A, Grinvald A, Slovin H. Dural substitute for long-term imaging of cortical activity in behaving monkeys and its clinical implications. J Neurosci Meth. 2002;114:119–33. doi: 10.1016/s0165-0270(01)00507-6. [DOI] [PubMed] [Google Scholar]
- Baker SN, Philbin N, Spinks R, Pinches EM, Wolpert DM, MacManus DG, Pauluis Q, Lemon RN. Multiple single unit recording in the cortex of monkeys using independently moveable microelectrodes. J Neurosci Meth. 1999;94:5–17. doi: 10.1016/s0165-0270(99)00121-1. [DOI] [PubMed] [Google Scholar]
- Becker TA, Kipke DR. Algel as dural sealant, determination of effects on the sensorimotor cortex in rats.. 25th Annual International Conference of the IEEE EMBS: Cancun; Mexico. 2003: 1972−5. [Google Scholar]
- Beltramino C, de Olmos J, Gallyas F, Heimer L, Zabroszky L. Silver staining as a tool for neurotoxic assessment. NIDA Res Monogr. 1993;136:101–25. doi: 10.1037/e495922006-007. [DOI] [PubMed] [Google Scholar]
- Green J. A simple microelectrode for recording from the central nervous system. Nature. 1958;182:962. doi: 10.1038/182962a0. [DOI] [PubMed] [Google Scholar]
- Hubel D. Single Unit Activity in Striate Cortex of Unrestrained Cats. J Physiol (Lond) 1959;147:226–38. doi: 10.1113/jphysiol.1959.sp006238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson N, Stice P, Okandan M, Muthuswamy J. Long-term cortical recordings with microactuated microelectrodes.. 3rd International IEEE-EMBS Neural Engineering: Kohala Coast; Hawaii. 2007. [Google Scholar]
- Jain T, Muthuswamy J. Microsystem for transfection of exogenous molecules with spatio-temporal control into adherent cells. Biosens Bioelectron. 2007;22:863–70. doi: 10.1016/j.bios.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Lorenzl S, Koedel U, Pfister H-W. Mannitol, but not allopurinol, moduates changes in cerebral blood flow, intracranial pressure, and brain water content during pneumococcal meningitis in the rat. Crit Care Med. 1996;24:1874–80. doi: 10.1097/00003246-199611000-00018. [DOI] [PubMed] [Google Scholar]
- Maurer P, McDonald J. Vicryl (polyglactin910) mesh as a dural substitute. J Neurosurg. 1985;63:448–52. doi: 10.3171/jns.1985.63.3.0448. [DOI] [PubMed] [Google Scholar]
- Maynard E, Fernandez E, Normann R. A technique to prevent dural adhesions to chronically implanted microelectrode arrays. J Neurosci Meth. 2000;97:93–101. doi: 10.1016/s0165-0270(00)00159-x. [DOI] [PubMed] [Google Scholar]
- Mokri B, Hunter SF, Atkinson JLD, Piepgras DG. Orthostatic headaches caused by CSF leak but with normal CSF pressures. Neurology. 1998;51:786–90. doi: 10.1212/wnl.51.3.786. [DOI] [PubMed] [Google Scholar]
- Moon T, Ghovanloo M, Kipke D. Buckling Strength of Coated and Uncoated Silicon Microelectrodes.. Proceedings of the 25th Annual International Conference of the IEEE EMBS: Cancun; Mexico. 2003. [Google Scholar]
- Moxon K, Leiser S, Gerhardt G, Barbee K, Chapin J. Ceramic-Based Multisite Electrode Arrays for Chronic Single-Neuron Recording. IEEE T Bio-Med Eng. 2004;51:647–56. doi: 10.1109/TBME.2003.821037. [DOI] [PubMed] [Google Scholar]
- Muthuswamy J, Okandan M, Gilletti A, Baker MS, Jain T. An array of microactuated microelectrodes for monitoring single-neuronal activity in rodents. IEEE T Bio-Med Eng. 2005a;52:1470–7. doi: 10.1109/TBME.2005.851478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy J, Okandan M, Jackson N. Single neuronal recordings using surface micromachined polysilicon microelectrodes. J Neurosci Meth. 2005b;142:45–54. doi: 10.1016/j.jneumeth.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Muthuswamy J, Okandan M, Jain T, Gilletti A. Electrostatic microactuators for precise positioning of neural microelectrodes. IEEE T Bio-Med Eng. 2005c;52:1748–55. doi: 10.1109/TBME.2005.855712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis M, Dimitrov D, Carmena J, Crist R, Lehew G, Kralik J, Wise S. Chronic, multisite, multielectrode recordings in macaque monkeys. PNAS. 2003;100:11041–6. doi: 10.1073/pnas.1934665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordhausen C, Maynard E, Normann R. Single unit recording capabilities of a 100 microelectrode array. Brain Res. 1996;726:129–40. [PubMed] [Google Scholar]
- Preul M, Bichard W, Muench T, Spetzler R. Toward Optimal Tissue Sealants for Neurosurgery: Use of a Novel Hydrogel Sealant in a Canine Durotomy Repair Model. Neurosurgery. 2003;53:1189–97. doi: 10.1227/01.neu.0000089481.87226.f7. [DOI] [PubMed] [Google Scholar]
- Rousche P, Pellinen D, Pivin D, Williams J, Vetter R, Kipke DR. Flexible Polyimide-Based Intracortical Electrode Arrays with Bioactive Capability. IEEE T Bio-Med Eng. 2001;48:361–71. doi: 10.1109/10.914800. [DOI] [PubMed] [Google Scholar]
- San-Galli F, Darrouzet V, Rivel J, Baquey C, Ducassou D, Guerin J. Experimental evaluation of a collagen-coated vicryl mesh as a dural substitute. Neurosurgery. 1992;30:396–401. doi: 10.1227/00006123-199203000-00014. [DOI] [PubMed] [Google Scholar]
- San-Galli F, Deminiere C, Guerin J, Rabaud M. Use of biodegradable elastin-fibrin material, Neuroplast, as a dural substitute. Biomaterials. 1996;17:1081–5. doi: 10.1016/0142-9612(96)85908-4. [DOI] [PubMed] [Google Scholar]
- Szarowski D, Andersen M, Retterer S, Spence A, Isaacson M, Craighead H, Turner J, Shain W. Brain responses to micro-machined silicon devices. Brain Res. 2003;983:23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- Terasaka S, Iwasaki Y, Shinya N, Uchida T. Fibrin Glue and Polyglycolic Acid Nonwoven Fabric as a Biocompatible Dural Substitute. Neurosurgery. 2006;58:134–9. doi: 10.1227/01.NEU.0000193515.95039.49. [DOI] [PubMed] [Google Scholar]
- Vetter R, Becker TA, Williams J, Kipke DR. The Use of ALGEL as an Artificial Dura for Chronic Cortical Implant Neuroprosthetics.. 1st International IEEE EMBS: Capri Island; Italy. 2003: 633−6. [Google Scholar]
- Vetter R, Williams J, Hetke J, Nunamaker E, Kipke D. Chronic Neural Recording Using Silicon-Substrate Microelectrode Arrays Implanted in Cerebral Cortex. IEEE T Bio-Med Eng. 2004;51:896–904. doi: 10.1109/TBME.2004.826680. [DOI] [PubMed] [Google Scholar]
- Wallin R, Arscott E. A Practical Guide to ISO 10993−5: Cytoxicity. Medical Device & Diagnositc Industry Magazine. 1998 [Google Scholar]
- Wise KD, Andersen DJ, Hetke JF, Kipke DR, Najarfi K. Wireless Implantable Microsystems: High-Density Electronic Interfaces to the Nervous System. Proceedings of the IEEE. 2004;92:76–97. [Google Scholar]