Abstract
Highly substituted, tethered alkyne dipolarophiles participate in the internal 2 + 3 cycloaddition with azomethine ylides generated by treatment of oxazolium salts with cyanide ion. Starting from oxazole 26, a sequence of N-methylation, cyanide addition, and electrocyclic ring opening of a 4-oxazoline intermediate affords the indoloquinone 31 in a one-pot process. A similar reaction from the protected alkynol derivative 25 affords the sensitive, but isolable enone 32, and subsequent oxidation affords 31 and the deprotected quinine alcohol 34. Related azomethine cycloaddition methodology via intramolecular oxazolium salt formation from 43 or 46 is also demonstrated, and allows the synthesis of quinone 45 and derived structures having the substitution pattern of aziridinomitosene A. Removal of the N-trityl protecting group could not be achieved without aziridine cleavage.
Introduction
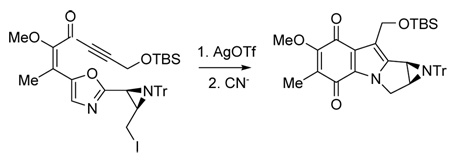
Enantiocontrolled synthesis of aziridinomitosenes has been a challenging problem over many years.1–7 Prior reports from our laboratory describe a potential solution, illustrated by the synthesis of non-racemic quinones 1–3 related to aziridinomitosene A (4), but lacking the C(7) methoxy group (Scheme 1).5c The approach uses an intramolecular azomethine ylide cycloaddition to assemble the tetracyclic aziridinomitosene core in a sequence that begins with conversion of an oxazole 5 into the oxazolium salt 6. Cyanide addition then forms a labile 4-oxazoline intermediate 7 and electrocyclic ring opening occurs to generate the transient azomethine ylide 8. Intramolecular cycloaddition to 9 proceeds with yields of ca. 60% or better when R1 = H or Me and R2 = trityl, but becomes less efficient when R2 = Me (30–40% yield). Our continuing efforts to prepare aziridinomitosene A (4) using similar methodology have therefore focused on the N-trityl series to learn whether a late stage detritylation may be possible in the highly sensitive environment. Although detritylation could not be accomplished without aziridine cleavage, the studies described below demonstrate access to relevant methoxy-substituted indoloquinones and reveal reaction pathways not encountered previously. The total synthesis of racemic 4 by Jiminez and Dong in 1999 remains the only route to the fully functionalized molecule.8
Scheme 1.
Methods and Results
Our azomethine ylide cycloaddition approach to 4 requires a 2,5-disubstituted oxazole intermediate 10 (Y, Z= O, H or Y=Z=O). Introduction of the acetylenic dipolarophile subunit via alkynyl anion addition to the aldehyde 11 borrows from prior work, but the current route features a more direct method for sidechain assembly from 12 based on lithiation at the oxazole C(5) position, metal exchange via 13, and palladium catalyzed coupling with enol triflate 14-E. The latter was easily prepared by deprotonation of the β-keto ester 159 with various bases and enolate quenching with N-phenylbistriflimide (Table 1).10 As expected, the E:Z ratio of 14 increased with the coordinating ability of the enolate counterion (Li>Na<K, entries 1–3), but enolate reactivity decreased markedly. The best compromise of yield and E:Z ratio was obtained using LiH as the base (entry 6; 65% yield, 93:7 E:Z), although the reaction was very slow and required seven days at rt for good conversion. The isomers were assigned from the methyl 1H NMR chemical shifts, assuming a greater downfield shift for 14-Z (δ = 2.36 ppm) compared to 14-E (δ = 2.18 ppm) due to proximity of the methyl and ester groups.11
Table 1.
Conversion of 15 to enol triflate 14.a
| entry | base | Time | Yield | E:Z |
|---|---|---|---|---|
| 1 | KHMDS | 15 h | 63% | 25:75 |
| 2 | NaHMDS | 15 h | 61% | 40:60 |
| 3 | LiHMDS | 7 d | 28% | >95:5 |
| 4 | NaH | 15 h | 72% | 54:46 |
| 5 | MesLi | 4 d | 20% | >95:5 |
| 6 | LiH | 7 d | 65% | 93:7 |
Enolate generated in THF at −78 °C, N-phenylbistriflimide added and warmed to and stirred (time: see table).
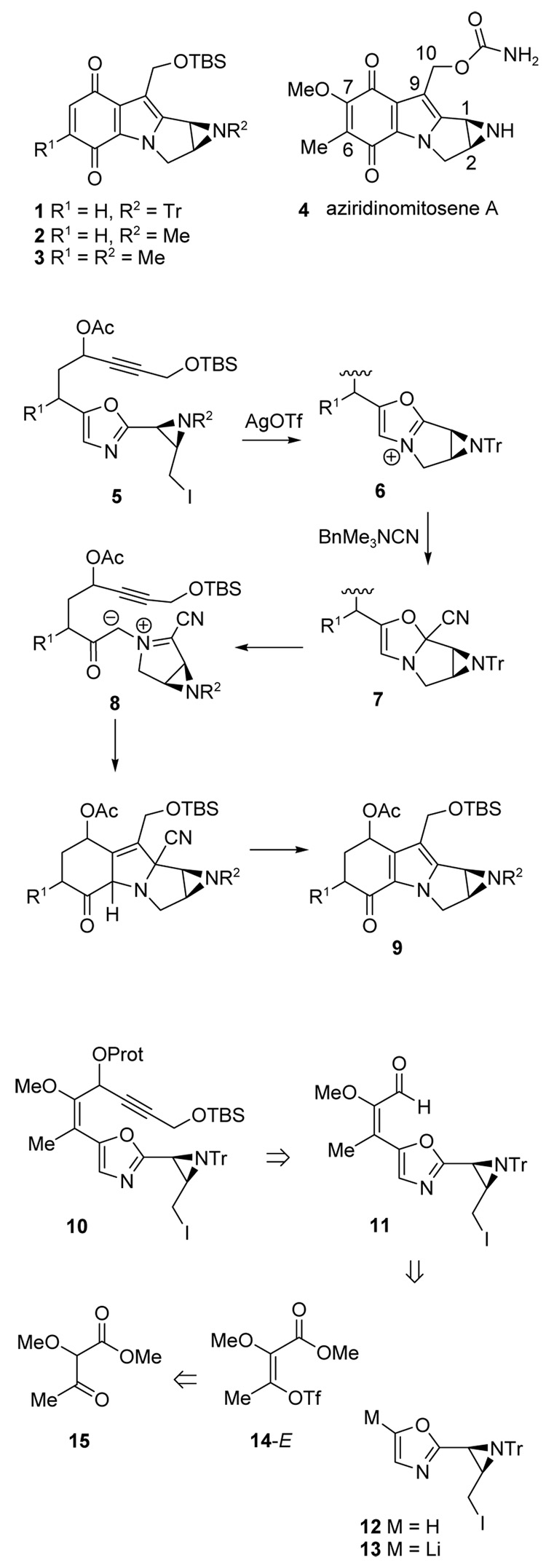
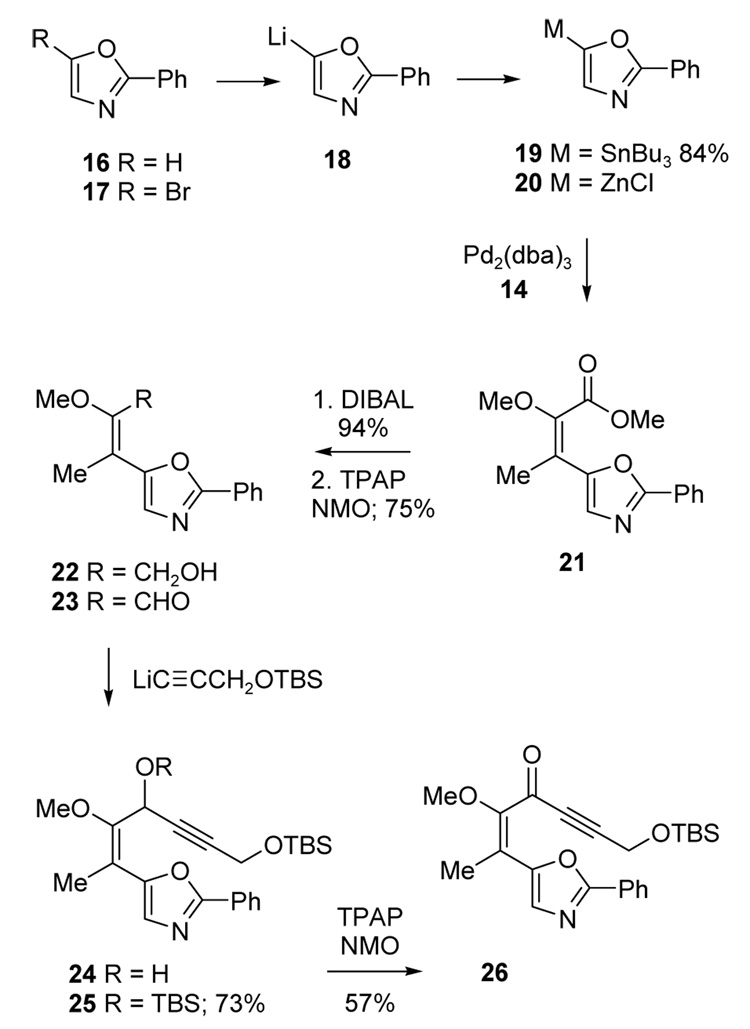
With access to the enol triflate 14-E established, the oxazole coupling and azomethine ylide cycloaddition were investigated in a model system (Scheme 2). Metallated oxazoles were obtained from 2-phenyloxazole 1612 via deprotonation (BuLi/TMEDA)13 or from the 5-bromo derivative 1714 by lithium halogen exchange. The intermediate lithiooxazole 18 was then quenched with Bu3SnCl to afford the stannane 19 (84% isolated),15 or with anhydrous ZnCl2 to generate 20 in situ. Starting from 16, a preliminary test of the Negishi coupling16 of 20 was performed using Pd2(dba)3/PPh3 with 1.1 equiv of a 1:1 mixture of the enol triflates 14-E and 14-Z. After 15h at rt, the enoate 21 was obtained in a modest 36% overall yield, but with a promising 9:1 ratio of E:Z isomers suggesting higher reactivity for the E-isomer. Enoate 21 was also prepared by Stille coupling.17 Thus, 20 was heated at 65 °C with 1.1 equiv of 14 (57:43 E:Z mixture) in the presence of Pd2(dba)3 and trifurylphosphine.18 This gave 21 in 84% yield, but with a lower E:Z ratio (3:1). On the other hand, when enriched 14-E (>95:5 E:Z, 1.3 equiv) was used, the desired enoate 21 was obtained in >95% yield as the sole product.
Scheme 2.
Next, the tethered alkyne required for the cycloaddition was installed. Enoate 21 was converted to the enal 23 (71% overall) by DIBAL reduction to the allylic alcohol 22 and oxidation with TPAP-NMO.19 Installation of the alkyne dipolarophile was then accomplished by addition of LiC≡CCH2OTBS to give an intermediate alcohol 24 and protection afforded the TBS ether 25 (73% from 23). Alternatively, oxidation of 24 gave the ynone 26 (57% from 23).
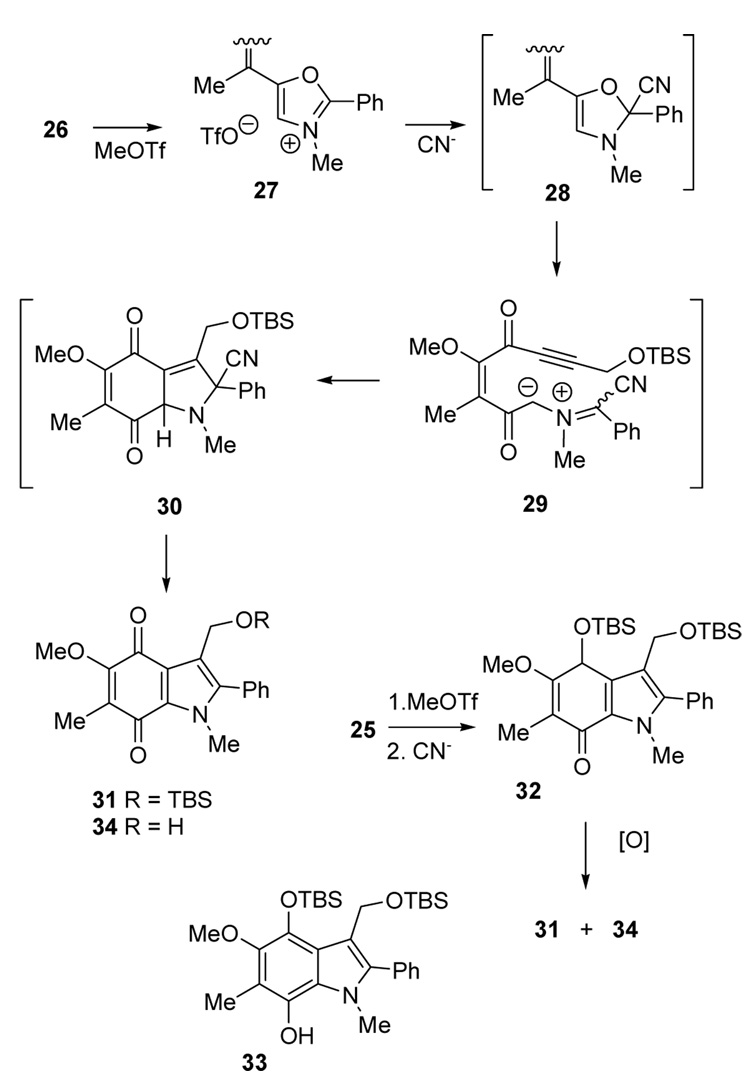
With cycloaddition precursors 25 and 26 in hand, generation of the azomethine ylide and subsequent [3 + 2] cycloaddition could be explored (Scheme 3). Enone 26 was studied first because the expected cycloadduct would be at the desired quinone oxidation state. Thus, the oxazole nitrogen of 26 was alkylated selectively with MeOTf (CH3CN, 48 h). The resulting oxazolium salt 27 was added to excess BnMe3N+ CN− at 0 °C to form the transient 4-oxazoline 28, followed by electrocyclic ring opening to the azomethine ylide 29 and subsequent [3 + 2] cycloaddition to 30.5a,5c,15 Spontaneous elimination of HCN then produced the indoloquinone 31, isolated in 40% overall yield from the oxazole 26.
Scheme 3.
Focus then turned to the protected diol oxazole 25 as the cycloaddition precursor (Scheme 3). Alkylation of 25 with MeOTf as before and the usual treatment with cyanide ion initiated the cycloaddition sequence. Despite initial concerns about the stability of the desired cycloadduct 32, the substance could be purified by chromatography on silica gel (47% yield), although some decomposition was noted. Structure 32 was confirmed, and the aromatic tautomer 33 was ruled out by 1H and 13C NMR evidence, including the characteristic enone 13C signal at δ 179.5 ppm.
Oxidation of 32 proceeded smoothly using 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ),15 and allowed conversion of the crude cycloaddition product to the more stable quinone oxidation state. Partial deprotection occurred as well, and the product was obtained as a 7:1 mixture of 31:34 (95% combined from oxazole 25 without purification of 32). Several other oxidations20 were also effective (Table 2), although partial deprotection of the primary OTBS ether occurred in entries 1–3. On the other hand, the combination of various oxidants with stronger bases for the oxidation step5c resulted in conversion to the protected quinone 31, although in somewhat lower yield. Presumably, these reactions proceed via the phenoxide anion derived from 33.
Table 2.
Oxidation of 32 to quinones 31 and 34.
| Entry | [O] | Temp | time | yield | Products |
|---|---|---|---|---|---|
| 1 | DDQ | rt | 2 h | 95%a | 7:1 31:34 |
| 2 | TBAF, O2, Pd/C | rt | 15 h | 88%a | 4:1 31:34 |
| 3 | HF-pyr, O2, NEt3 | rt | 15 h | 99%b | 3:2 31:34 |
| 4 | KHMDS; then NCS | −78 ° C | 10 min | 72%b | 31 |
| 5 | DBU and NCS | 0–20 ° C | 1 h | 84%b | 31 |
| 6 | Phosphazene,c NCS | rt | 15 h | 56%b | 31 |
| 7 | KHMDS; PhSeCl | −78 ° C to rt | 10 min | 74%b | 31 |
| 8 | KHMDS; PhI(OAc) | −78−20 ° C | 1 h | 69%b | 31 |
Isolated combined yield of 31 and 34 from crude 32
Purified 32 used.
Phosphazene base P1-t-Bu.
As shown in Scheme 3, the methoxy enone substitution pattern corresponding to the aziridinomitosene A quinone is fully compatible with the azomethine ylide cycloaddition step. Indeed, better yields were obtained from 25 and from 26 compared to related cycloadditions studied previously in our laboratory. Furthermore, the potentially troublesome non-aromatized intermediate 32 could be handled with minimal complications using the in situ oxidation approach. With these encouraging results in hand, we sought to apply similar methodology to the synthesis of aziridinomitosene A (4) according to the retrosynthesis shown in Scheme 1.
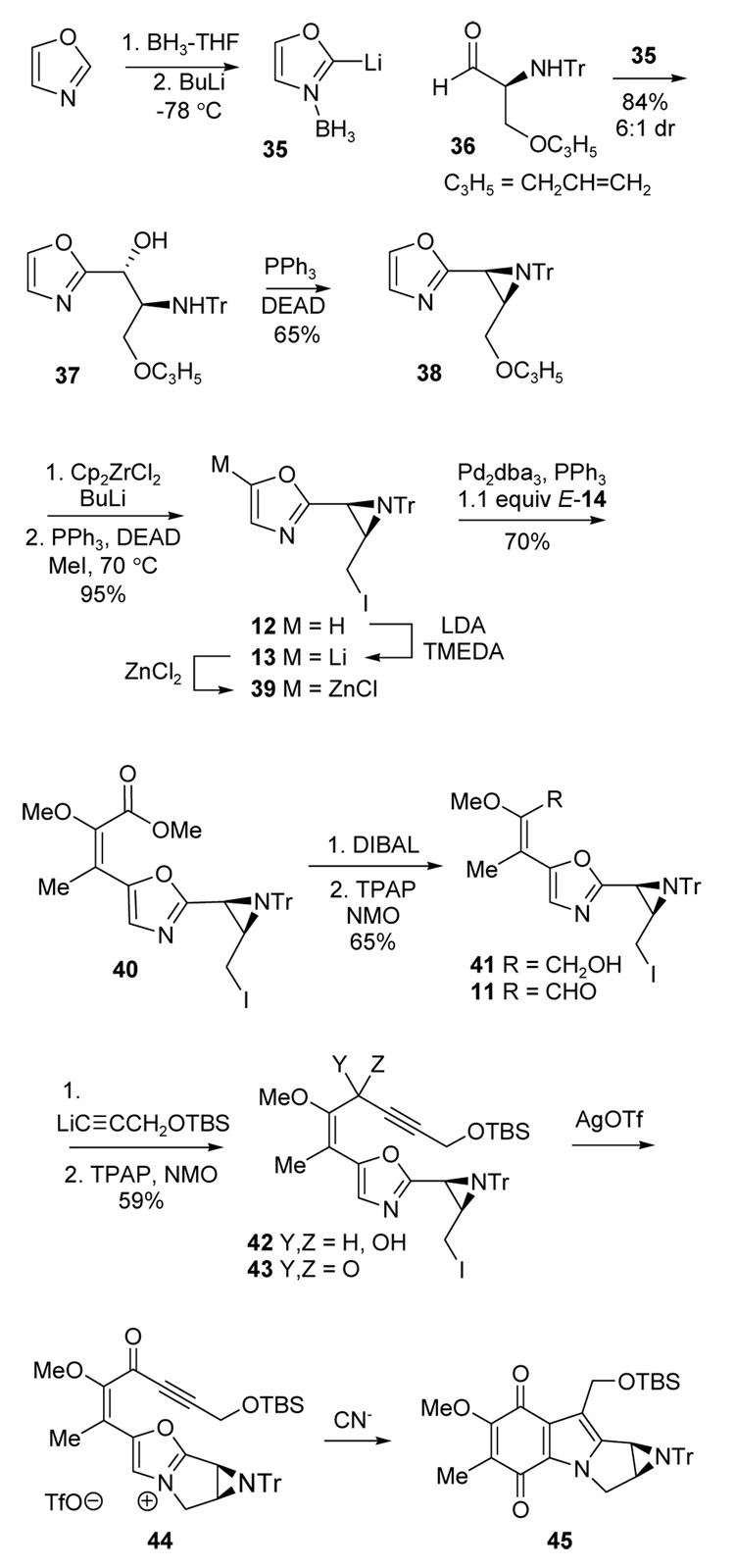
The aziridinyl oxazole 12 was prepared starting with the addition of lithiated oxazole borane 3521 to N-trityl-O-allylserinal 365c (Scheme 4). This gave 37 as an inseparable 6:1 diastereomer mixture (84%), but the major isomer could be purified after aziridine formation under Mitsunobu conditions. Based on the vicinal coupling constant J= 6.1 Hz for the aziridine methine protons, the major aziridine (65%) was assigned the cis stereochemistry 38. This result was expected from previous studies using 5-substituted oxazoles,5c and provides the basis for assigning the stereochemistry of 37. Allyl deprotection using zirconocene generated in situ22 followed by iodide formation following a modified Mitsunobu procedure23 then gave the desired 12.
Scheme 4.
Compared to the lithiation of oxazole 16 used in the model system (Scheme 2), the corresponding reaction of 12 must contend with the potentially sensitive iodomethylaziridine subunit. Although lithium-iodine exchange or oligomerization of 16 were potential concerns, it was anticipated that steric shielding in the cis-fused, trityl substituted aziridine would discourage intermolecular events involving either the primary iodide or the aziridine moieties, and that sp2 hybridization and adjacent heteroatoms would favor the lithiated oxazole. Fortunately, 12 was deprotonated cleanly with LDA/TMEDA13 at −78 °C (Scheme 4) without any additional precautions compared to the model system. The resulting anion 13 was stirred with freshly fused ZnCl2 to generate 39, followed by Negishi coupling16 with triflate 14 (1.1 equiv of E-14) to give ester 40 in 70% overall yield from oxazole 12. The corresponding Stille coupling17 was investigated briefly, but 40 was obtained in a lower 56% yield from 12.
To install the alkyne necessary for [3 + 2] cycloaddition, ester 40 was converted to aldehyde 11 by the usual two-step reduction/oxidation procedure via alcohol 41 (65%). Addition of LiC≡CCH2OTBS to 11 then gave an intermediate alcohol 42 that was oxidized to ketone 43 in 70% overall yield.
The key [3+2] cycloaddition could now be explored starting with ketone 43. This approach is the most direct route to the tetracyclic aziridinomitosene core, and it is also the safest route because it avoids the hydroquinone oxidation state corresponding to solvolytically reactive leucoaziridino-mitosenes. Best results were obtained using the crystallized benzene complex of AgOTf24 for iodine activation and intramolecular N-alkylation. Conversion of 43 to the oxazolium salt 44 was monitored by 1H NMR spectroscopy in C6D6 and was complete after 3 h. Subsequent azomethine ylide generation was then performed by addition of 44 to excess BnMe3N+ CN− in CH3CN at rt, the same procedure used in the model study. A transient yellow color was observed that faded within seconds, presumably due to the formation and subsequent reactions of the azomethine ylide. After 2 h at rt, the desired tetracyclic aziridinomitosene 45 was indeed isolated. A single experiment gave 45 in 33% yield, but this result was never reproduced despite much effort. The crucial variables could not be identified and yields in the 10–27% range were more typical, and unknown byproducts were formed that could not be isolated.
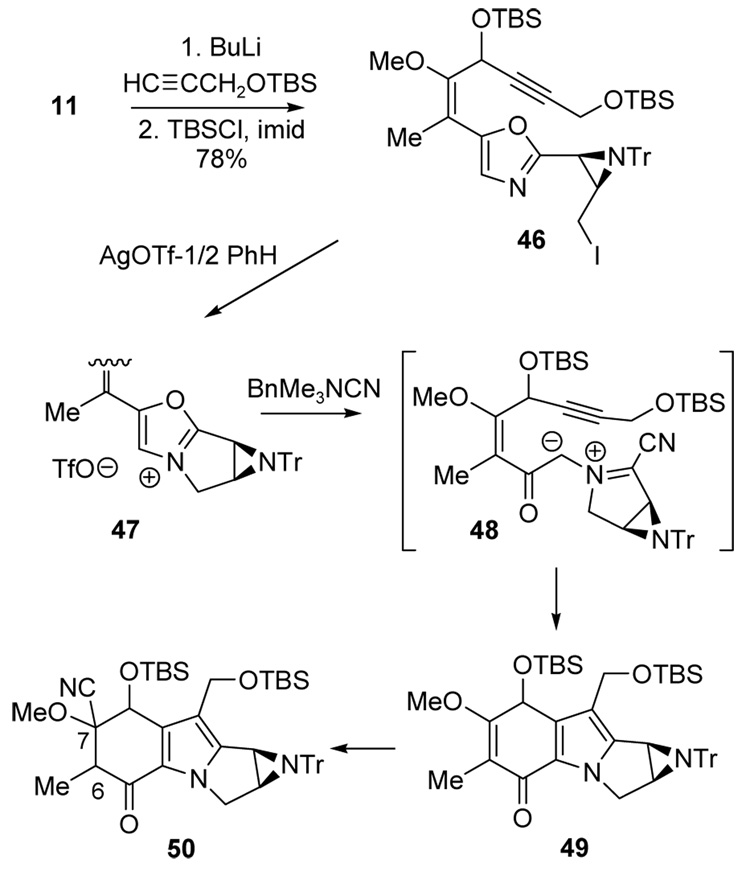
Because the cycloaddition from ketone 43 was too unpredictable for scaleup, an alternative route from alcohol 42 was explored after protection as the TBS ether 46 (78% over the two steps from 11; Scheme 5). Intramolecular alkylation and ylide generation were then performed as before using the purified AgOTf-benzene complex.24 Following the same procedure used in Scheme 4 and in prior studies, the intermediate oxazolium salt 47 was added to BnMe3N+ CN− (4 equiv) in CH3CN at rt and the transient color of ylide 48 appeared as usual. However, the isolated product proved to be 50 (33%, 2:1 dr) and not the expected enone 49. The formula of 50 was deduced using ESMS (m/z= 49 + HCN), and the connectivity was established by NMR spectroscopy. Furthermore, the 13C NMR spectrum showed CN peaks at δ = 117.0 and 117.1 ppm for the two diastereomers and the 1H NMR spectrum contained two signals for the C(6) methine hydrogens at δ = 3.30 ppm and δ = 3.26 ppm as quartets, consistent with structure 50. Analogous cyanide adducts were not observed starting from the ketone 44, nor in the model study from either 25 or 26. Furthermore, nucleophilic addition at C(7) in mitomycin-derived quinones is reported to follow an addition/elimination pathway.25 On the other hand, simple addition of cyanide to a vinylogous ester has been reported in an unrelated substrate.26
Scheme 5.
Even though the product was not the desired vinylogous ester 49, oxidation of the cycloaddition product mixture containing 50 was nonetheless attempted in the hope that elimination of HCN might generate 49 in situ and that enolization and subsequent oxidation would afford the desired quinone 45. However, treatment with KHMDS/NCS at −78 °C gave 45 in a marginal 17% yield from 46.
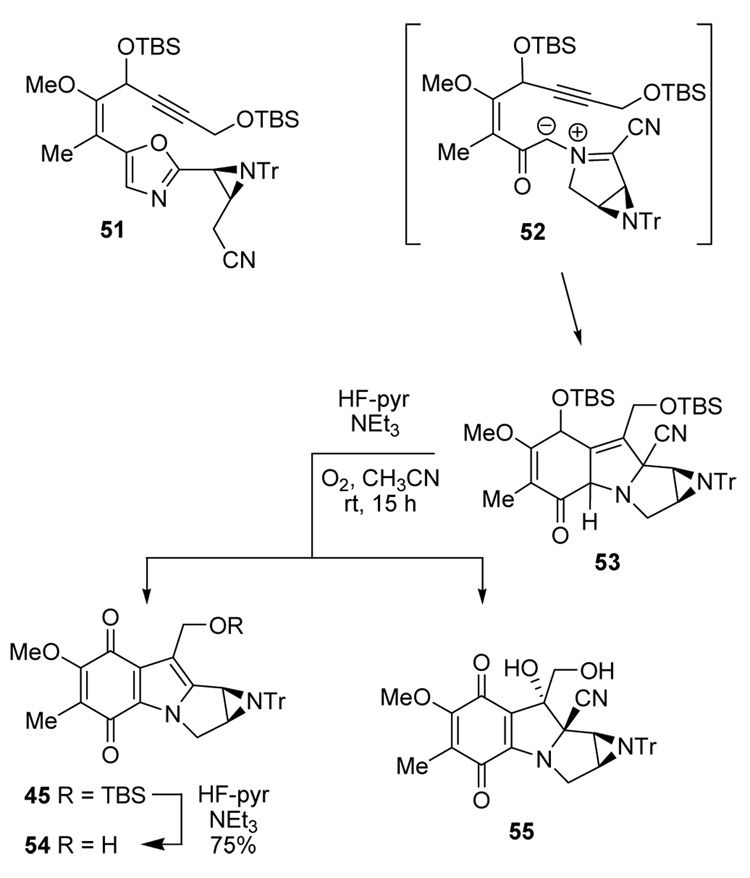
In an attempt to prevent formation of the cyanide adduct 50, azomethine ylide generation was performed with 1.0 equiv of BnMe3N+ CN− instead of the excess used earlier (Scheme 6). This experiment gave a low yield (<10%) of the quinone 45, but neither 49 nor 50 was detected by NMR. On the other hand, a mass corresponding to 50 (m/z= 49 + HCN) did appear in the electrospray mass spectrum of the crude product, suggesting that isomers of 50 had been formed. Structure 51 resulting from simple iodide-cyanide exchange was ruled out based on disappearance of the oxazole proton in the NMR spectrum, but further characterization of the sensitive product mixture was difficult. Eventually, partially enriched fractions of two new products were obtained by chromatography on NEt3 buffered silica gel that proved to be two diastereomers 53 resulting from the desired [2+3] cycloaddition of azomethine ylide 52, but without the usual elimination of HCN. Varying amounts of decomposition products were also observed during attempts to purify 53 by chromatography, including a structure that is tentatively assigned as the expected adduct 49 from MS and NMR evidence. However, enriched samples of 49 were never obtained, nor could either isomer of 53 be isolated without contamination. Structure 53 is consistent with the upfield chemical shift of the aziridine protons (δ = 2.15, 2.59 ppm and δ = 2.34, 2.79 ppm) compared to δ = 2.89 and 3.03 ppm for the analogous protons of 45. However, decisive evidence for 53 proved elusive until yet another unexpected product was isolated during attempts to aromatize 53 to 45.
Scheme 6.
Oxidation of 53 using base/NCS, DDQ, or TBAF/Pd-C/O2 gave extensive degradation along with traces of 45. However, treatment of crude 53 with HF-pyridine/O220 and added NEt3 to prevent aziridine cleavage produced a separable mixture of highly colored quinones (Scheme 6), including quinone alcohol 54 as well as the silyl ether 45. A deep red quinone was also isolated, and was assigned structure 55, corresponding to oxidation without HCN elimination. The high field chemical shifts of the aziridine protons at δ = 2.64 and 2.96 ppm suggested the same sp3 environment at C(9a) as seen in 53. With a mass corresponding to 54 + CN + OH and strong NMR evidence for the HO and CH2OH subunits (one exchangeable proton, singlet at δ = 4.03 ppm; a second exchangeable proton at δ = 4.65 ppm as a doublet of doublets coupled to the C(10) methylene protons). Eventually, X-ray quality crystals were obtained and the structure was confirmed.
For preparative purposes, it was best to oxidize the crude cycloaddition mixture obtained from 46 by activation with AgOTf-benzene complex24 followed by 1.0 equiv of BnMe3N+ CN−. Without separation, the resulting mixture was treated with HF-pyridine/O220 and NEt3 to give 34% of 45 + 54 combined, together with 14% of 55. Similar experiments with partially purified 53 were less efficient overall, but did provide evidence that only one of the two diastereomers of 53 undergoes elimination of HCN while the other oxidizes to 55. The combined 48% yield of tetracyclic adducts is not far from the range of yields obtained using simpler cycloaddition substrates, but the 34% recovery of useful quinones (45+54) was less than desired. Some solace was taken by considering the dramatic change in structure and the number of transformations to 45 in the one pot procedure starting from oxazole 46.
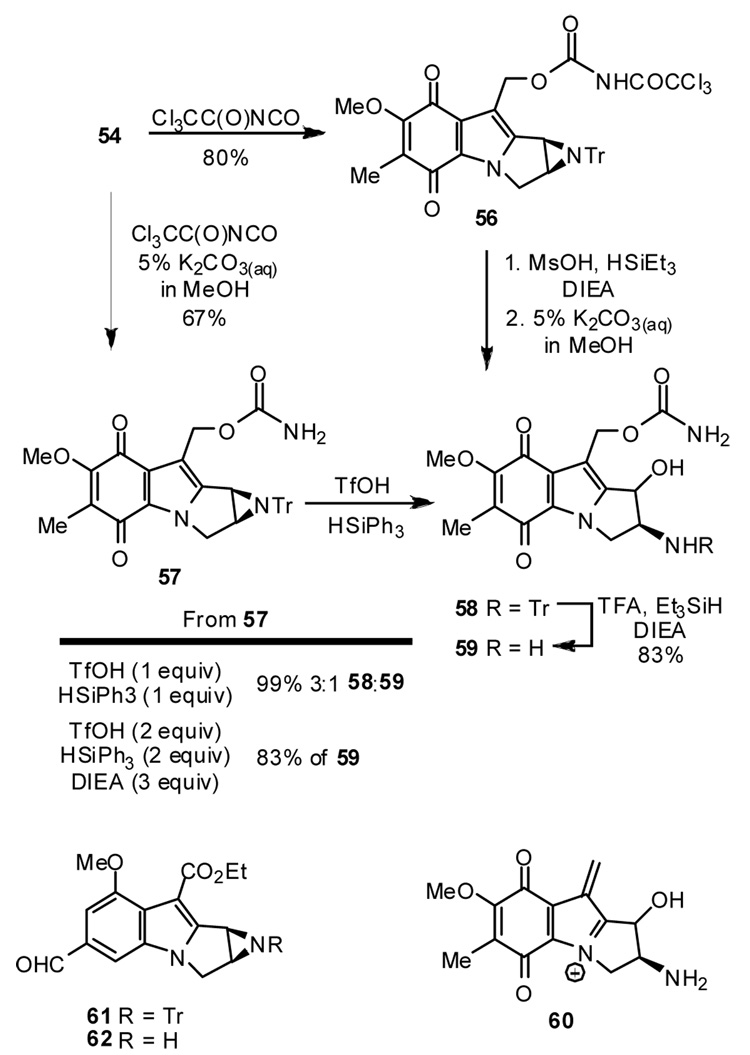
The last steps potentially leading to aziridinomitosene A require introduction of the carbamate and removal of the trityl protecting group. When tetracycle 45 was subjected to reductive detritylation conditions with Et3SiH/MsOH at −40 °C desilylation to 54 proved faster than detritylation.27 The TBS ether in 45 was therefore removed with NEt3/HF-pyr, and the alcohol 54 was converted to carbamates 56 and 57 using standard methods (Scheme 7).28 Treatment of 56 with Et3SiH/MsOH at 0 °C followed by cleavage of the trichloroacyl group with K2CO3 afforded a product mixture having a major ESMS peak at m/z= 358 amu corresponding to the ring opened amino alcohol 59 + Na. Another peak at m/z= 275 amu was assigned to the cation 60 resulting from heterolysis of the carbamate, identical to ESMS data reported previously for 59.29 A small peak at m/z= 340 amu, the mass expected for aziridinomitosene A (4) + Na, was also observed in samples of the crude product mixture. However, 4 was not detected by NMR comparison with a spectrum of authentic material, and was not present above the detection threshold level of ca. 2–3%. Treatment of the carbamate 57 with Et3SiH/MsOH also gave the amino alcohol 59 according to ESMS assay, although the mass corresponding to 4 was not detected in this case.29
Scheme 7.
In view of the above results, the reductive detritylation procedure was re-optimized in an attempt to lower the temperature threshold. Using the stronger acid TfOH together with the more potent hydride source triphenylsilane allowed detritylation of simpler model structures (for example, (R)-benzyl 1-tritylaziridine-2-carboxylate) as low as −40 °C with 1.0 equiv of each reagent over a time scale of 0.5–15 h. However, when the new procedure (15 h at −40 °C and workup with pH 10.3 buffer) was applied to 57, ESMS assay (m/z= 600 amu, 58 + Na) indicated formation of the N-trityl amino alcohol 58 as the main component in a 3:1 mixture with the amino alcohol 59. The N-trityl amino alcohol was not purified, but structure 58 is consistent with the ESMS data and 1H NMR evidence for the intact N-trityl group, new C(6) methyl signals at δ = 1.94 and δ = 1.90 ppm in a 3:1 ratio, as well as new downfield methine signals. Tentatively, we interpret the methyl signals as evidence for diastereomeric amino alcohols from aziridine cleavage as reported for 59. When 2 equiv of TfOH and 2 equiv of HSiPh3 were used for the deprotection of 57 at −40 °C, only the amino alcohol 59 was recovered (ca. 5:1 dr by NMR), and the structure was confirmed by comparison with literature MS and NMR data.29,30
The deprotection studies of 57 are consistent with rapid C(1)-N heterolysis due to the activating effect of the indoloquinone nitrogen. This was no surprise given the long history of DNA alkylation involving similar events, and in particular, the studies by Kohn et al with aziridinomitosenes B, C, and D.31 Nevertheless, there was reason to think that deprotection might be possible. Thus, earlier efforts in our laboratory had succeeded in the detritylation of 61 to 62 using iPr3SiH/MsOH.27b Evidently, the vinylogous carbamate C=O group of the ester substituent in 62 is an important stabilizing factor, but we had imagined (hoped) that the vinylogous amide (quinone) carbonyls of 57 would be at least as effective. This has proven not to be the case.
Conclusions
Indoloquinone 31 was obtained in excellent yield using internal azomethine ylide cycloaddition methodology starting from the oxazole 25 when the sensitive intermediate cycloadduct 32 was oxidized without isolation. The ketone substrate 26 gave lower yields of 31 using the analogous oxazolium salt activation with cyanide ion. Similar routes to the tetracyclic quinone 45 encountered complications due to cyanide addition at the stage of the vinylogous ketone 49. Furthermore, two unexpectedly stable cycloadduct diastereomers 53 were encountered, and only one diastereomer could be converted into the desired quinone 45 after oxidation. The presence of a sensitive aziridine in all of the tetracyclic intermediates raises complications throughout the late stages of this sequence, but the challenge was met until the very last stage, the reductive N-trityl cleavage. This transformation has been achieved in a related system (61), but could not be demonstrated on a preparative scale with 57 due to aziridine ring opening.
We were well aware that N-trityl cleavage as the last step would be exceptionally challenging. This risk was accepted for two reasons. First, one of the goals of the study was to better define the limits of what chemistry is possible in the presence of the solvolytically sensitive aziridines. Second, the bulky N-trityl group is helpful at the stage of oxazolium salt formation using AgOTf. When smaller substituents are present at the aziridine nitrogen, activation of the iodide results in competing internal alkylation of oxazole nitrogen and intermolecular displacement by the same cyanide ion that is necessary to generate the azomethine ylide for the cycloaddition. At this point, it is clear that a different protecting group for aziridine nitrogen will be needed that is compatible with the internal oxazole alkylation/cyanide activation sequence.
Experimental
(E)-Methyl 2-methoxy-3-(trifluoromethylsulfonyloxy)but-2-enoate (14)
To a suspension of LiH (81.0 mg, 10.2 mmol) in THF (28 mL) at −78 °C was added a solution of 159 in THF (1 mL) dropwise over 5 min. After stirring at −78 °C for 1 h, a solution of N-phenyl-bis(trifluoromethanesulfonimide) (2.43 g, 6.80 mmol) in THF (6 mL) was added dropwise over 5 min. The resulting solution was stirred at −78 °C for 1 h then warmed to rt and stirred for 7 d. The yellow solution was poured into H2O (100 mL) and extracted with Et2O. The combined organic extracts were washed with a 10% citric acid solution, dried (MgSO4), filtered, and solvents were removed (aspirator). The residue was purified by flash chromatography on silica gel (50 mm × 15 cm, 10% Et2O/hexanes, 50 mL fractions) to yield 1.23 g (65%) of triflate 14 as a volatile clear oil as an inseparable 93:7 ratio of E:Z isomers (1H NMR analysis), analytical TLC on silica gel 60 F254, 10% Et2O/hexanes, Rf = 0.3. Molecular ion (M+Na) calculated for C7H9F3NaO6S: 300.9970; found m/z = 300.9963, error = 2 ppm; IR (neat, cm−1) 1735, C=O, 1424; 500 MHz 1H NMR (CDCl3, ppm) major E-isomer: δ 3.87 (3H, s) 3.70 (3H, s) 2.18 (3H, s). The presence of the minor Z-isomer was deduced from methyl integrals at δ 3.88 (0.07H, s) 3.71 (0.07H, s) 2.36 (0.07H, s). 13C NMR (125 MHz, CDCl3, ppm) E-isomer δ 161.5, 146.8, 142.4, 118.5 (q, 318 Hz), 60.5, 52.5, 15.8. Z-isomer δ 163.2, 147.1, 142.5, 118.5 (q, 318 Hz), 60.4, 52.7, 17.2.
Preparation of (E)-methyl 2-methoxy-3-(2-phenyloxazol-5-yl)but-2-enoate (21) by Negishi coupling between 17 and 14
5-Bromo-2-phenyloxazole14 (17) (151 mg, 0.673 mmol) in THF (3 mL) was cooled to −78 °C and n-BuLi (1.67 M solution in hexanes, 0.50 mL, 0.84 mmol) was added dropwise over 2 min. After stirring at −78 °C for 10 min, ZnCl2 (1.0 M solution in THF, 2.7 mL, 2.7 mmol) was added and the yellow solution was allowed to warm to rt over 25 min. In a separate flask, Pd2dba3 (60.0 mg, 0.0655 mmol) and PPh3 (38.0 mg, 0.0145 mmol) were dissolved in THF (2 mL) and stirred at rt 20 min. Triflate 14 (205 mg, 0.737 mmol of a 1:1 ratio of E/Z-14) in THF (1 mL) was added dropwise at rt and the resulting green solution was stirred at rt for 10 min. The previously made organozinc was added via cannula dropwise over 5 min followed by a THF rinse (1 mL). After stirring at rt for 15 h, the solution was poured into saturated aqueous NH4Cl (10 mL) and extracted with Et2O. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator). The yellow oil was purified by flash column chromatography on silica gel (30 mm × 16 cm, 15% EtOAc/hexanes, 30 mL fractions) to yield 66 mg (36%) of ester 21 as a light yellow oil in an inseparable 93:7 ratio of E:Z isomers, analytical TLC on silica gel 60 F254, 15% EtOAc/hexanes, Rf = 0.2. Molecular ion (M+H) calculated for C15H16NO4: 274.1079; found m/z = 274.1070, error = 3 ppm; IR (neat, cm−1) 1729, C=O, 1644; for major E-21: 400 MHz 1H NMR (CDCl3, ppm) δ 8.01-7.95 (2H, m) 7.48-7.43 (3H, m) 7.18 (1H, s) 3.82 (3H, s) 3.71 (3H, s) 2.10 (3H, s). The presence of the minor Z-isomer was deduced from methyl integrals at δ 3.88 (0.24H, s) 3.72 (0.24H, s) 2.46 (0.24H, s). 13C NMR of the major E-21 (100 MHz, CDCl3, ppm) δ 165.1, 161.2, 149.1, 144.6, 130.6, 129.0, 127.4, 127.0, 126.4, 112.9, 58.6, 52.6, 13.7.
Preparation of 21 by Negishi coupling between 16 and 14
To a solution of 2-phenyloxazole 1612 (32.0 mg, 0.220 mmol) in THF (1.5 mL) at −78 °C was added TMEDA (0.33 mL, 2.20 mmol, distilled over solid Na) and n-BuLi (1.30 M solution in hexanes, 0.19 mL, 0.242 mmol) dropwise over 5 min. After stirring at −78 °C for 1 h, ZnCl2 (1.12 M in THF, 0.79 mL, 0.880 mmol) was added dropwise then the cooling bath was removed and the organozinc solution was allowed to warm to rt. In a separate flask, Pd2dba3 (20.1 mg, 0.0220 mmol) and PPh3 (23.0 mg, 0.0880 mmol) was dissolved in THF (0.5 mL) and stirred at rt 20 min. Triflate 14 (71.1 mg, 0.255 mmol of a >95:5 ratio of E/Z-14) in THF (0.5 mL) was added dropwise and the resulting green solution was stirred at rt for 25 min. The previously made organozinc was added via cannula dropwise over 5 min followed by a THF rinse (0.5mL). After stirring at rt for 15 h, the solution was poured into saturated aqueous NH4Cl and extracted with Et2O. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator). The yellow oil was purified by preparatory TLC on silica gel (20 cm × 20 cm × 1000 µm, 15% EtOAc/hexanes eluent) to give 28.3 mg (47%) of 21 as a light yellow oil in a >95:5 ratio of E:Z isomers.
Preparation of 21 by Stille coupling of 19 and 14
To a suspension of Pd2(dba)3 (123 mg, 0.134 mmol) and trifurylphosphine (125 mg, 0.537 mmol) in DMF (4 mL) that was stirred at rt 20 min was added a solution of triflate 14 (478 mg, 1.48 mmol of a >95:5 ratio of E/Z-14) in DMF (4 mL, including cannula and flask washings) via cannula dropwise. The dark purple solution was allowed to stir at rt 20 min then a solution of stannane 1915 (583 mg, 1.34 mmol) in DMF (5 mL, including cannula and flask washings) was added via cannula dropwise. The reaction was heated to 65 °C and stirred 15 h. After cooling to rt, the green solution was poured into saturated aqueous NH4Cl (30 mL) and extracted with Et2O. The combined organic extracts were washed with H2O and brine, dried (MgSO4), and the solvents were removed (aspirator). The yellow oil was purified by flash column chromatography on silica gel (40 mm × 15 cm, 15% EtOAc/hexanes, 40 mL fractions) to yield 0.367 g (>99%) of ester 21 as a light yellow oil in a >95:5 ratio of E:Z isomers identical to previously made material.
(E)-2-Methoxy-3-(2-phenyloxazol-5-yl)but-2-en-1-ol (22)
To a solution of ester 21 (158 mg, 0.578 mmol) in toluene (6 mL) at −78 °C was added DIBAL-H (20 wt % solution in toluene, 1.80 mL, 2.17 mmol) dropwise over 5 min. After stirring at −78 °C for 2 h, the cooling bath was removed and 6 mL of Et2O was added followed by 6 mL of saturated aqueous sodium potassium tartrate (Rochelle’s Salt). The biphasic solution was vigorously stirred at rt for 1 h, then the mixture was extracted with Et2O. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator). The off white solid was purified by flash column chromatography on silica gel (20 mm × 15 cm, 3:1 Et2O/hexanes, 11 mL fractions) to yield 0.134 g (94%) of alcohol 22 as white solid, analytical TLC on silica gel 60 F254, 3:1 Et2O/hexanes, Rf = 0.3. Pure material was obtained by crystallization in EtOAc/hexanes as white rods, mp = 97 – 99 °C. Molecular ion (M+H) calculated for C14H16NO3: 246.1130; found m/z = 246.1119, error = 4 ppm; IR (neat, cm−1) 3263, OH, 1642; 400 MHz 1H NMR (CDCl3, ppm) δ 7.98-7.93 (2H, m) 7.44-7.41 (3H, m) 6.97 (1H, s) 4.59 (2H, d, J=5.9 Hz) 3.83 (3H, s) 2.93 (1H, t, J=5.9 Hz) 1.93 (3H, s). 13C NMR (100 MHz, CDCl3, ppm) δ 160.8, 153.5, 151.1, 130.4, 129.0, 127.3, 126.2, 125.3, 107.8, 58.3, 56.9, 13.4.
(E)-2-Methoxy-3-(2-phenyloxazol-5-yl)but-2-enal (23)
A solution of alcohol 22 (268 mg, 1.09 mmol), N-methyl morpholine N-oxide (NMO) (269 mg, 2.30 mmol), and molecular sieves (activated powder, 4Å, 1.34 g) in CH2Cl2 (14 mL) was stirred at rt for 30 min. Tetrapropylammonium perruthenate (TPAP) (77.0 mg, 0.219 mmol) was then added and the resulting black solution was stirred at rt 1 h then filtered through a silica gel plug eluting with 250 mL of a 1:1 acetone/hexanes solution. Solvents were removed (aspirator) and the light yellow solid was purified by flash column chromatography on silica gel (30 mm × 16 cm, 40% Et2O/hexanes, 12 mL fractions) to yield 0.200 g (75%) of aldehyde 23 as a light yellow solid, analytical TLC on silica gel 60 F254, 40% Et2O/hexanes, Rf = 0.3. Pure material was obtained by crystallization in hexanes as light yellow needles, mp = 77 – 80 °C. Molecular ion (M+H) calculated for C14H14NO3: 244.0974; found m/z = 244.0966, error = 3 ppm; IR (neat, cm−1) 1665 C=O; 500 MHz 1H NMR (CDCl3, ppm) δ 10.19 (1H, s) 8.06-8.02 (2H, m) 7.52-7.47 (3H, m) 7.34 (1H, s) 3.83 (3H, s) 2.29 (3H, s). 13C NMR (125 MHz, CDCl3, ppm) δ 187.1, 163.6, 151.9, 148.2, 131.4, 131.3, 129.4, 129.2, 126.9, 126.8, 60.3, 13.4.
(E)-7-(tert-Butyldimethylsilyloxy)-3-methoxy-2-(2-phenyloxazol-5-yl)hept-2-en-5-yn-4-one (26)
To a solution of tert-butyldimethylsilyl propargyl ether32 (33.0 mg, 0.193 mmol) in THF (1.0 mL) at −40 °C was added n-BuLi (0.374 M solution in hexanes, 0.52 mL, 0.193 mmol) dropwise over 5 min. After stirring at −42 °C for 1 h, a solution of aldehyde 23 (26.0 mg, 0.107 mmol) in THF (1.0 mL, including cannula and flask washings) was added dropwise via cannula and the reaction was stirred an additional 2 h at −42 °C then the cooling bath was removed and 2 mL of saturated aqueous sodium bicarbonate was added. After stirring at rt 10 min, the solution was poured into brine and extracted with CH2Cl2. The combined organic extracts were dried (Na2SO4), filtered, and solvents were removed (aspirator). To the crude alcohol from above in CH2Cl2 (2 mL) was added NMO (26.0 mg, 0.225 mmol) and molecular sieves (activated powder, 4Å,120 mg) and the mixture was stirred at rt 20 min. TPAP (8.0 mg, 0.021 mmol) was added in one portion and the black solution was stirred an additional 1 h at rt then filtered through a silica gel plug (2 cm × 5 cm) and eluted with 50 mL of a 1:1 hexanes/acetone solution. Solvents were removed (aspirator) and the yellow foam was purified by preparatory TLC on silica gel (20 cm × 20 cm × 1000 µm, 20% EtOAc/hexanes eluent) to give 25.0 mg (57%) of 26 as a yellow foam, analytical TLC on silica gel 60 F254, 20% EtOAc/hexanes, Rf = 0.3. Molecular ion (M+H) calculated for C23H30NO4Si: 412.1944; found m/z = 412.1935, error = 2 ppm; IR (neat, cm−1) 1640 C=O; 500 MHz 1H NMR (CDCl3, ppm) δ 8.05-8.01 (2H, m) 7.49 (1H, s) 7.47-7.44 (3H, m) 4.33 (2H, s) 3.75 (3H, s) 2.22 (3H, s) 0.84 (9H, s) 0.03 (6H, s). 13C NMR (125 MHz, CDCl3, ppm) δ 174.8, 162.1, 151.0, 148.4, 130.9, 130.5, 129.1, 127.2, 126.8, 120.3, 92.4, 84.3, 59.9, 51.7, 25.9, 18.4, 15.4, −5.2.
(E)-5-(4,7-Bis(tert-butyldimethylsilyloxy)-3-methoxyhept-2-en-5-yn-2-yl)-2-phenyloxazole (25)
To a solution of tert-butyldimethylsilyl propargyl ether32 (252 mg, 1.48 mmol) in THF (1.5 mL) at −78 °C was added n-BuLi (1.47 M solution in hexanes, 1.01 mL, 1.48 mmol) dropwise over 5 min. The solution was allowed to stir at −78 °C for 1 h then a solution of aldehyde 23 (200 mg, 0.822 mmol) in THF (0.5 mL, including cannula and flask washings) was added dropwise via cannula and the reaction was allowed to slowly warm to −42 °C over 20 minutes. The light yellow solution was stirred at −42 °C for 2 h then the cooling bath was removed and 2 mL of saturated aqueous sodium bicarbonate was added. After stirring at rt 10 min, the solution was poured into brine and extracted with CH2Cl2. The combined organic extracts were dried (Na2SO4), filtered, and solvents were removed (aspirator). The crude alcohol from above was dissolved in CH2Cl2 (2 mL) and tert-butyldimethylsilyl chloride (151 mg, 1.00 mmol) was added followed by imidazole (84.0 mg, 1.23 mmol). The suspension was stirred for 15 h at rt and then poured into brine and extracted with CH2Cl2. The combined organic extracts were dried (Na2SO4), filtered, and solvents were removed (aspirator). The clear oil was purified by flash column chromatography on silica gel (40 mm × 15 cm, 20% Et2O/hexanes, 15 mL fractions) to yield 0.318 g (73%) of oxazole 23 as a clear oil, analytical TLC on silica gel 60 F254, 20% Et2O/hexanes, Rf = 0.5. Molecular ion (M+Na) calcd for C29H45NNaO4Si2: 550.2785; found m/z = 550.2777, error = 1 ppm; IR (neat, cm−1) 1638; 400 MHz 1H NMR (CDCl3, ppm) δ 8.09-8.02 (2H, m) 7.49-7.42 (3H, m) 7.07 (1H, s) 5.80 (1H, t, J=1.8 Hz) 4.38 (2H, d, J=1.8 Hz) 3.99 (3H, s) 2.03 (3H, s) 0.89 (9H, s) 0.88 (9H, s) 0.11 (6H, s) 0.09 (3H, s) 0.05 (3H, s). 13C NMR (100 MHz, CDCl3, ppm) δ 161.2, 152.7, 150.6, 130.4, 129.0, 127.7, 126.4, 126.3, 107.5, 84.3, 83.8, 61.2, 59.9, 52.0, 26.0, 25.9, 18.4, 14.1, −4.6, −4.8, −5.0.
Azomethine ylide cycloaddition to form 4-(tert-butyldimethylsilyloxy)-3-((tert-butyldimethylsilyloxy)methyl)-5-methoxy-1,6-dimethyl-2-phenyl-1H-indol-7(4H)-one (31)
To a solution of ynone oxazole 26 (10.0 mg, 0.0243 mmol) in freshly distilled CH3CH2CN (0.5 ml, stirred over activated 4 Å molecular sieves, distilled over CaH2, and distilled again over P2O5) and molecular sieves (activated 4 Å, 7 beads) was added MeOTf (4 µL, 0.0365 mmol). The clear solution was allowed to stir at rt 15 h. MeOTf (4 µL, 0.0592 mmol) was added and the light brown solution was stirred an additional 15 h at rt. Salt formation was indicated by TLC analysis of reaction mixture, Rf = 0.0 and by mass spectroscopy with molecular ion (M+) calculated for C24H32NO4Si: 426, found m/z = 426. The oxazolium salt was transferred to a solution of BnMe3N+ CN− (9.0 mg, 0.054 mmol) in freshly distilled CH3CH2CN (2.0 mL, including cannula and flask washings, stirred over activated 4 Å molecular sieves, distilled over CaH2, and distilled again over P2O5) at 0 °C via cannula dropwise over 5 minutes. The reaction was stirred at 0 °C for 10 min then the cooling bath was removed and the light orange solution was stirred at rt for 15 h. The orange solution was poured into brine and extracted with Et2O. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator) to yield a light orange oil. The orange oil was purified by preparative TLC on silica gel (20 cm × 20 cm × 1000 µm, 15% EtOAc/hexanes eluent) to give 4.0 mg (40%) of 31 as an orange oil. Molecular ion (M+Na) calculated for C24H31NNaO4Si: 448.1920; found m/z = 448.1922, error = 1 ppm; IR (neat, cm−1) 1661 C=O, 1640 C=O, 1609; UV (CH3OH, nm) 266 (ε 33000), 347 (ε 8500), 448 (ε 4400). 500 MHz 1H NMR (CDCl3, ppm) δ 7.50-7.42 (5H, m) 4.67 (2H, s) 4.00 (3H, s) 3.80 (3H, s) 2.00 (3H, s) 0.86 (9H, s) 0.05 (3H, s). 13C NMR (125 MHz, CDCl3, ppm) δ 179.9, 179.6, 156.9, 141.9, 130.8, 129.4, 129.3, 129.3, 129.3, 128.7, 122.5, 121.9, 61.3, 55.6, 34.4, 26.2, 18.8, 9.0, −5.1.
Cycloaddition from protected akynol 25 to pyrroloenone 32
A solution of oxazole 25 (48.0 mg, 0.0909 mmol) and molecular sieves (activated 4 Å, 200 mg) in CH3CN (2 mL, stirred over activated 4 Å molecular sieves, distilled over CaH2, and distilled again over P2O5) was stirred at rt for 15 min. MeOTf (15 µL, 0.136 mmol) was added and the solution was allowed to stir at rt for 15 h. MeOTf (15 µL, 0.136 mmol) again was added and the solution stirred at rt an addition 15 h. Salt formation was indicated by TLC analysis of reaction mixture, Rf = 0.0, and by mass spectroscopy with molecular ion (M+) calculated for C30H48NO4Si2: 542, found m/z = 542. The newly formed oxazolium salt was added to a solution of BnMe3N+ CN− (35.0 mg, 0.200 mmol) in CH3CN (2 mL, including cannula and flask washings), stirred over activated 4 Å molecular sieves, distilled over CaH2, and distilled again over P2O5) at 0 °C via cannula dropwise over 5 min. The reaction was stirred at 0 °C for 10 min then warmed to rt and stirred for 2 h. The orange solution was poured into pH 5.8 buffer (15 mL) and extracted with Et2O. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator). The orange oil was purified by preparative TLC on silica gel (20 cm × 20 cm × 1000 µm, 20% EtOAc/hexanes eluent) to give 23.0 mg (47%) of 32 as a white solid, analytical TLC on silica gel 60 F254, 20% EtOAc/hexanes, Rf = 0.6. Pure material was obtained by crystallization in hexanes as off-white rods, mp = 72 – 74 °C. Molecular ion (M+Na) calculated for C30H47NNaO4Si2: 564.2941; found m/z = 564.2939, error = 0 ppm; IR (neat, cm−1) 1638; 500 MHz 1H NMR (CDCl3, ppm) δ 7.48-7.40 (3H, m) 7.38-7.36 (2H, m) 5.81 (1H, s) 4.86 (1H, AB, J=11.2 Hz) 4.26 (1H, AB, J=11.2 Hz) 4.08 (3H, s) 3.84 (3H, s) 1.86 (3H, s) 0.90 (9H, s) 0.86 (9H, s) 0.05 (3H, s) 0.04 (3H, s) −0.08 (3H, s) −0.46 (3H, s). 13C NMR (125 MHz, CDCl3, ppm) δ 179.5, 165.9, 139.7, 130.7, 130.5, 128.7, 128.6, 128.5, 125.3, 120.4, 116.1, 61.7, 56.9, 56.4, 33.9, 26.2, 25.9, 18.7, 18.5, 7.9, −4.0, −4.1, −5.1, −5.2.
Preparation of 31 and 3-(hydroxymethyl)-5-methoxy-1,6-dimethyl-2-phenyl-1H-indole-4,7-dione (34) from 25
To a solution of oxazole 25 (25.0 mg, 0.0474 mmol) in CH3CN (0.6 ml, stirred over activated 4 Å molecular sieves, distilled over CaH2, and distilled again over P2O5) and molecular sieves (activated 4 Å, 150 mg) was added MeOTf (14 µL, 0.0592 mmol). The clear solution was allowed to stir at rt 15 h. MeOTf (7 µL, 0.0592 mmol) was added and the light brown solution was stirred an additional 15 h at rt. The oxazolium salt was transferred to a solution of BnMe3N+ CN− (18.0 mg, 0.104 mmol) in CH3CN (4.5 mL, including cannula and flask washings, stirred over activated 4 Å molecular sieves, distilled over CaH2, and distilled again over P2O5) at 0 °C via cannula dropwise over 5 minutes. The reaction was stirred at 0 °C for 10 min then the cooling bath was removed and the light orange solution was stirred at rt for 2 h. The orange solution was poured into pH 5.8 buffer (15 mL) and extracted with Et2O. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator) to yield a light orange oil. The crude oil from above was dissolved in benzene (1.0 mL) and DDQ (22.0 mg, 0.0948 mmol) was added in one portion. The dark black solution was stirred at rt for 2 h then poured into Et2O:H2O:1 M NaOH (10 mL: 10 mL: 1 mL) and extracted with Et2O. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator). The orange oil was purified by preparative TLC on silica gel (20 cm × 20 cm × 1000 µm, 15% EtOAc/hexanes eluent) to give 16.8 mg of 31 as an orange oil and 1.7 mg of 34 as an orange solid (95% combined yield), analytical TLC on silica gel 60 F254, 15% EtOAc/hexanes, Rf = 0.5 (silyl ether) and Rf = 0.2 (alcohol). For alcohol 34: Pure material was obtained by crystallization in EtOAc/hexanes as orange needles, mp = 119 – 121°C. Molecular ion (M+Na) calculated for C18H17NNaO4: 334.1055; found m/z = 334.1055, error = 0 ppm; IR (neat, cm−1) 3425 OH, 1731 C=O, 1638 C=O; 500 MHz 1H NMR (CDCl3, ppm) δ 7.52-7.46 (3H, m) 7.31-7.29 (2H, m) 4.51 (2H, d, J=7.1 Hz) 4.19 (1H, t, J=7.1 Hz) 4.03 (3H, s) 3.79 (3H, s) 2.02 (3H, s). 13C NMR (125 MHz, CDCl3, ppm) δ 181.2, 179.5, 156.6, 139.5, 130.7, 130.2, 129.9, 129.6, 129.1, 128.6, 124.1, 122.8, 61.4, 56.4, 34.3, 9.2.
Aminoalcohol (37)
To a solution of the oxazole (1.74 g, 25.2 mmol) in 100 mL of anhydrous THF at rt was added BH3-THF (1.0 M solution in THF, Aldrich, fresh bottle, 26.5 mL, 26.5 mmol). After stirring at rt for 1h, the colorless solution was cooled to −78 °C, and nBuLi (1.52 M solution in hexanes, 17.4 mL, 26.5 mmol) was added dropwise over 30 min. The colorless solution was stirred at −78 °C for 1.5 h, and then a solution of the aldehyde 365c (9.36 g, 25.2 mmol) in anhydrous THF (75 mL, including cannula and flask washings) was added via cannula dropwise over 30 min. The resulting pale yellow solution was stirred at −78 °C for 1.5 h, and then quenched with 200 mL of 5% AcOH in EtOH. The cooling bath was removed, and the reaction mixture was allowed to warm to rt. After stirring at rt for 24 h, the colorless solution was concentrated by rotary evaporation, poured into ether, and washed with saturated aqueous NaHCO3. The aqueous layer was extracted with ether, all organic extracts were combined, dried (Na2SO4), and concentrated by rotary evaporation. The crude material contained a 6:1 dr ratio, as determined by 1H NMR assay. The residue was purified by flash chromatography on silica gel (5.5×20 cm, 900 mL of 1:1 hexane/ether then 900 mL of 2:1 hexane/acetone eluent, 20 mL fractions). Fractions 52–67 gave 9.21 g (84%) of the alcohol 37 with 12:1 dr (NMR analysis) as a white foam, analytical TLC on silica gel 60 F254, 2:1 hexane/EtOAc, Rf= 0.2. Molecular ion (M+Na+) calcd for C28H28N2NaO3: 463.19980; found (electrospray) m/z= 463.2019, error= 5 ppm; base peak= 243 amu; IR (neat, cm−1) 3342, O-H; 1571; 400 MHz NMR (CDCl3, ppm), major diastereomer, δ 7.62 (1H, d, J= 0.7 Hz) 7.56-7.51 (6H, m) 7.30-7.24 (6H, m) 7.22-7.16 (3H, m) 7.07 (1H, s) 5.77-5.62 (1H, m) 5.16-5.03 (2H, m) 4.58 (2H, d, J= 5.1 Hz) 3.60-3.50 (2H, m) 3.36-3.29 (1H, m) 2.85 (1H, dd, J= 9.5, 2.9 Hz) 2.82-2.50 (1H, s) 2.41 (1H, dd, J= 9.5, 6.6 Hz). In addition, signals for the minor (8%) diastereomer were resolved at 7.62-7.58 (1H, m) 6.99 (1H, s), 4.02 (1H, dd, J= 8.8, 3.7 Hz) 3.91 (1H, d, J= 8.8 Hz) 3.68-3.65 (2H, m) 3.26-3.19 (1H, s) 3.18 (1H, dd, J= 9.5, 2.6 Hz); 13C NMR (100 MHz, CDCl3, ppm), major diastereomer, δ 164.3, 146.4, 138.8, 134.1, 128.6, 128.0, 126.9, 126.6, 117.0, 71.8, 70.9, 69.3, 69.1, 54.9.
2-[(2S,3R)-3-Allyloxymethyl-1-trityl-aziridin-2-yl] oxazole 38 from aminoalcohol 37
To a solution of alcohol 37 (9.21 g, 20.9 mmol) and Ph3P (8.22 g, 31.3 mmol) in 150 mL of anhydrous THF was added diethyl azodicarboxylate (4.8 mL, 30.7 mmol) dropwise over 10 min. The cooling bath was removed and the reaction mixture was allowed to warm to rt. After stirring at rt for 14 h, the yellow solution was concentrated to approximately 10 mL by rotary evaporation and then passed through a 5×10 cm2 plug of silica and the plug was washed with 1:1 hexane/EtOAc. The filtrate was concentrated by rotary evaporation and purified by flash chromatography on silica gel (5×20 cm, 2:1 hexane/Et2O eluent, 1×300 mL then 20 mL fractions). Fractions 14–24 were concentrated to yield product. Fractions 25–63 were concentrated by rotary evaporation and the residue was purified by another flash chromatography on silica gel under the same conditions. Fractions 18–36 were combined with the original 14–24 to give 5.7 g (65%) of 38 with >95% dr (NMR analysis) as a white foam, analytical TLC on silica gel 60 F254, 5:1 hexane/EtOAc, Rf= 0.1. Molecular ion (M+Na+) calcd for C28H26N2NaO2: 445.18920; found m/z= 445.1880, error= 3 ppm; base peak= 243 amu; IR (neat, cm−1) 1596; 1574; 300 MHz NMR (CDCl3, ppm) δ 7.66 (1H, d, J= 0.8 Hz) 7.56-7.48 (6H, m) 7.30-7.17 (9H, m) 7.15 (1H, d, J= 0.8 Hz) 5.71 (1H, dddd, J= 17.0, 10.4, 5.5, 5.5 Hz) 5.14-5.04 (2H, m) 4.01 (1H, dd, J= 10.4, 4.9 Hz) 3.78 (2H, ddd, J= 5.5, 1.4, 1.4 Hz) 3.72 (1H, dd, J= 10.4, 7.1 Hz) 2.56 (1H, d, J= 6.1 Hz) 1.98 (1H, ddd, J= 7.1, 6.0, 4.9 Hz). 13C NMR (100 MHz, CDCl3, ppm) δ 161.7, 143.5, 138.6, 134.4, 129.4, 127.7, 127.5, 126.9, 116.9, 75.0, 71.8, 68.1, 38.6, 32.2.
Allyl ether cleavage from 38; (2S,3R)-2-(oxazol-2-yl)-3-hydroxymethyl-1-tritylaziridine
A solution of zirconocene dichloride (Aldrich, 2.63 g, 8.99 mmol) in 40 mL of anhydrous THF was cooled to −78 °C, and nBuLi (1.56 M solution in hexanes, 11.5 mL, 17.9 mmol) was added dropwise over 30 min. After the resulting yellow solution was stirred at −78 °C for 1 h, a solution of the allyl ether 38 (2.48 g, 5.87 mmol) in anhydrous THF (30 mL, including cannula and flask washings) was added dropwise via cannula. The yellow solution was stirred at −78 °C for 5 min, the cooling bath was removed, and the reaction mixture was allowed to warm to rt. After 1 h at rt, the resulting dark brown solution was cooled to 0 °C, and 30 mL of saturated aqueous NH4Cl was added. The pale yellow suspension was stirred at rt for 3 h and then filtered through Celite (3□5 cm) with ether (200 mL). The filtrate was washed with brine, dried (Na2SO4), and concentrated by rotary evaporation. The residue was purified by flash chromatography on silica gel (5□17 cm, 3:2 hexane/EtOAc eluent, 20 mL fractions). Fractions 16–46 gave 2.22 g (98%) of the product as a colorless foam, analytical TLC on silica gel 60, 1:1 hexane/EtOAc, Rf= 0.3. Molecular ion (M+Na+) calcd for C25H22N2NaO2: 405.15790; found m/z= 405.1582, error= 1 ppm; IR (neat, cm−1) 3379, O-H; 1574; 300 MHz NMR (CDCl3, ppm) δ 7.67 (1H, d, J= 0.8 Hz) 7.58-7.51 (6H, m) 7.34-7.20 (9H, m) 7.15 (1H, d, J= 0.8 Hz) 4.13 (1H, ddd, J= 12.1, 6.3, 4.1 Hz) 4.01-3.83 (2H, m) 2.38 (1H, d, J= 6.0 Hz) 2.02-1.94 (1H, m). 13C NMR (100 MHz, CDCl3, ppm) δ 162.4, 143.6, 139.2, 129.3, 127.8, 127.0, 127.0, 74.9, 61.1, 39.4, 31.6.
(2S,3R)-2-(Oxazol-2-yl)-3-iodomethyl-1-tritylaziridine (12)
A solution of Ph3P (2.28 g, 8.7 mmol) in 40 mL of anhydrous toluene was cooled to 0 °C, and diethyl azodicarboxylate (Aldrich, 1.3 mL, 8.12 mmol) was added, followed by a solution of the alcohol prepared above (2.22 g, 5.8 mmol) in anhydrous toluene (40 mL, including cannula and flask washings), followed by MeI (0.54 mL, 8.7 mmol). After stirring the resulting white suspension at 0 °C for 5 min, the cooling bath was removed, and the reaction mixture was heated at 70 °C for 2 h. The precipitate gradually dissolved forming a mixture of a pale yellow solution and a few drops of brown oil, which was concentrated by rotary evaporation. The residue was dissolved in 5 mL of CH2Cl2 and purified by flash chromatography on silica gel (4.5 × 15 cm, 5:1 hexane/ethyl acetate, 20 mL fractions). Fractions 9–31 were concentrated by rotary evaporation and purified by another flash chromatography on silica gel (4.5 × 15 cm, 10:1 hexane/ethyl acetate, 20 mL fractions). Fractions 9–22 gave 2.78 g (97%, 95% for 2 steps) of the pure product as a colorless foam, analytical TLC on silica gel 60, 5:1 hexane/ethyl acetate eluent, Rf= 0.3. Molecular ion (M+Na+) calcd for C25H21IN2NaO: 515.05990; found m/z= 515.0600, error= 0 ppm; IR (neat, cm−1) 1574; 300 MHz NMR (CDCl3, ppm) δ 7.70 (1H, d, J= 0.8 Hz) 7.55-7.48 (6H, m) 7.32-7.20 (9H, m) 7.19 (1H, d, J= 0.8 Hz) 3.68 (1H, dd, J= 9.9, 4.7 Hz) 3.55 (1H, dd, J= 9.9, 9.4 Hz) 2.60 (1H, d, J= 6.0 Hz) 2.11 (1H, ddd, J= 9.4, 6.0, 4.7 Hz). 13C NMR (100 MHz, CDCl3, ppm) δ 160.5, 143.3, 139.0, 129.2, 127.8, 127.8, 127.1, 75.4, 41.5, 34.9, 3.2.
Preparation of (E)-methyl 3-(2-((2S,3R)-3-(iodomethyl)-1-tritylaziridin-2-yl)oxazol-5-yl)-2-methoxybut-2-enoate (40) from 12
To a solution of diisopropyl amine (153 µL, 1.09 mmol) in THF (3 mL) at 0 °C was added n-BuLi (1.53M solution in hexanes, 0.69 mL, 1.04 mmol) dropwise over 5 min. After stirring at 0 °C for 30 min, theLDA solution was transferred via cannula dropwise over 20 min to a stirred solution of oxazole 12 (0.466 g, 0.946 mmol) and TMEDA (1.43 mL, 9.46 mmol, distilled over solid Na) in THF (3 mL) at −78 °C. After stirring the resulting yellow solution at −78 °C for 1 h, ZnCl2 (1.5 M in THF, 2.52 mL, 3.78 mmol) was added dropwise then the cooling bath was removed and the organozinc solution was allowed to warm to rt.
In a separate flask, Pd2dba3 (87.0 mg, 0.0946 mmol) and PPh3 (99.0 mg, 0.378 mmol) was dissolved in THF (2.5 mL) and stirred at rt 25 min. Triflate 14 (305 mg, 1.10 mmol of a >95:5 ratio of E/Z-14) in THF (2.5 mL) was added dropwise and the resulting green solution was stirred at rt for 25 min. The previously made organozinc was added via cannula dropwise over 5 min. After stirring at rt for 15 h, the solution was poured into saturated aqueous NH4Cl (20 mL) and extracted with Et2O (3 × 40 mL). The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator). The yellow oil was purified by flash column chromatography on silica gel (40 mm × 16 cm, 5:1 hexanes/EtOAc, 30 mL fractions) to yield 0.408 g (70%) of ester 40 as a colorless solid, analytical TLC on silica gel 60 F254, 5:1 EtOAc/hexanes, Rf = 0.3. Pure material was obtained by crystallization in EtOAc/hexanes as clear rods, mp = 125 – 127 °C. Molecular ion (M+Na) calculated for C31H29IN2NaO4: 643.1070; found m/z = 643.1077, error = 1 ppm; IR (neat, cm−1) 1729 C=O; 400 MHz 1H NMR (CDCl3, ppm) δ 7.50-7.49 (6H, m) 7.30-7.21 (9H, m) 7.10 (1H, s) 3.75 (3H, s) 3.69 (3H, s) 3.70-3.66 (1H, m) 3.53 (1H, dd, J=9.9Hz, 9.2 Hz) 2.53 (1H, d, J=5.9 Hz) 2.13-2.07 (1H, m) 2.07 (3H, s). 13C NMR (125 MHz, CDCl3, ppm) δ 165.0, 160.0, 149.7, 144.6, 143.5, 129.5, 128.0, 127.4, 126.2, 112.9, 75.6, 58.6, 52.6, 41.9, 35.3, 13.8, 3.3.
(E)-3-(2-((2S,3R)-3-(Iodomethyl)-1-tritylaziridin-2-yl)oxazol-5-yl)-2-methoxybut-2-enal (11)
To a solution of ester 40 (0.958 g, 1.54 mmol) in toluene (25 mL) at −78 °C was added DIBAL-H (20% wt solution, 4.8 mL, 5.79 mmol) dropwise. After stirring the resulting yellow solution for 2 h at −78 °C, the cooling bath was removed and 25 mL of Et2O was added followed by 25 mL of saturated aqueous sodium potassium tartrate (Rochelle’s Salt). The mixture was vigorously stirred for 1 h, then the mixture was extracted with Et2O. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator) to give 0.719 g (79%) of 41 as a white foam that was sufficiently pure for the next reaction without further purification, analytical TLC on silica gel 60 F254, 30% EtOAc/hexanes, Rf = 0.2. A solution of the alcohol from above (185 mg, 0.312 mmol), N-methyl morpholine N-oxide (77.0 mg, 0.655 mmol), and molecular sieves (activated powder, 4Å, 360 mg) in CH2Cl2 (5 mL) was stirred at rt. After stirring at rt 30 min, TPAP (22.0 mg, 0.0624 mmol) was added in one portion. After stirring at rt 1 h, the black solution was filtered through a silica gel plug eluting with 150 mL of a 1:1 acetone/hexanes solution. Solvents were removed (aspirator) and the light yellow solid was purified by flash column chromatography on silica gel (30 mm × 15 cm, 2:1 hexanes/Et2O, 20 mL fractions) to yield 0.150 g (82%) of aldehyde 11 as a light yellow foam, analytical TLC on silica gel 60 F254, 2:1 hexanes/Et2O, Rf = 0.3. Molecular ion (M+Na) calculated for C30H27IN2NaO3: 613.0964; found m/z = 613.0959, error = 1 ppm; IR (neat, cm−1) 1673 C=O; 500 MHz 1H NMR (CDCl3, ppm) δ 9.99 (1H, s) 7.53-7.51 (6H, m) 7.30-7.27 (6H, m) 7.27 (1H, s) 7.25-7.22 (3H, m) 3.80 (3H, s) 3.68 (1H, dd, J=9.8 Hz, 4.4 Hz) 3.50 (1H, dd, J=9.8 Hz, 9.8 Hz) 2.57 (1H, d, J=5.9 Hz) 2.24 (3H, s) 2.19-2.15 (1H, m). 13C NMR (125 MHz, CDCl3, ppm) δ 187.0, 162.4, 152.2, 148.6, 143.3, 130.2, 129.5, 129.4, 128.1, 127.4, 75.7, 60.1, 42.0, 35.1, 15.3, 3.2.
(E)-7-(tert-Butyldimethylsilyloxy)-2-(2-((2S,3R)-3-(iodomethyl)-1-tritylaziridin-2-yl)oxazol-5-yl)-3-methoxyhept-2-en-5-yn-4-one (43)
To a solution of tert-butyldimethylsilyl propargyl ether32 (213 mg, 1.25 mmol) in THF (8 mL) at −78 °C was added n-BuLi (1.53 M solution in hexanes, 0.82 mL, 1.25 mmol) dropwise over 5 min. The clear solution was stirred for 1 h at −78 °C, then a solution of aldehyde 11 (370 mg, 0.627 mmol) in THF (5 mL including flask and cannula washings) was added via cannula dropwise over 5 min. The resulting yellow solution was warmed to −42 °C and stirred for 2 h at −42 °C. The cooling bath was then removed and 10 mL of saturated aqueous sodium bicarbonate was added. After stirring at rt 10 min, the solution was poured into H2O and extracted with Et2O. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator).
The crude alcohol 42 from above, N-methyl morpholine N-oxide (154 mg, 1.32 mmol), and molecular sieves (activated powder, 4 Å, 1.40 g) in CH2Cl2 (13 mL) was stirred at rt for 30 min. TPAP (44.0 mg, 0.125 mmol) was then added in one portion and the black solution was stirred at rt 1 h. The black solution was then filtered through a silica gel plug eluting with 100 mL of a 1:1 acetone/hexanes solution. Solvents were removed (aspirator) and the light yellow solid was purified by flash column chromatography on silica gel (40 mm × 17 cm, 20% EtOAc/hexanes, 25 mL fractions) to yield 0.335 g (70%) of ketone 43 as a light yellow foam, analytical TLC on silica gel 60 F254, 20% EtOAc/hexanes, Rf = 0.4. Molecular ion (M+Na) calculated for C39H43IN2NaO4Si: 781.1934; found m/z = 781.1932, error = 0 ppm; IR (neat, cm−1) 1648 C=O; 500 MHz 1H NMR (CDCl3, ppm) δ 7.57 (1H, s) 7.53-7.51 (6H, m) 7.29-7.26 (6H, m) 7.23-7.22 (3H, m) 4.38 (1H, AB, J=17.6 Hz) 4.36 (1H, AB, J=17.6 Hz) 3.75 (3H, s) 3.66 (1H, dd, J=9.8 Hz, 4.9 Hz) 3.50 (1H, dd, J=9.8 Hz, 9.0 Hz) 2.56 (1H, d, J=5.9 Hz) 2.19 (3H, s) 2.13-2.09 (1H, m) 0.89 (9H, s) 0.10 (6H, s). 13C NMR (125 MHz, CDCl3, ppm) δ 174.7, 161.1, 150.5, 148.9, 143.5, 129.9, 129.5, 128.0, 127.3, 120.0, 92.7, 84.3, 75.6, 60.1, 51.9, 41.9, 35.2, 25.9, 18.4, 15.3, 3.4, −5.0.
Cycloaddition from ketone 43; isolation of 45
To a nitrogen flushed dry NMR tube was added AgOTf·1/2 benzene24 (10.0 mg, 0.0343 mmol) and C6D6 (0.25 mL) and then ketone 43 (20.0 mg, 0.0264 mmol) in C6D6 (0.2 mL including cannula and flask washings) via cannula and set in the dark. After 135 min, 1H NMR showed no starting oxazole 43 and oxazolium salt formation was indicated by characteristic changes in the chemical shift of the oxazole hydrogen from δ = 7.67 ppm (C6D6) to 8.55 ppm (C6D6), and this solution was transferred via syringe equipped with a syringe filter to a solution of BnMe3N+ CN− (19.0 mg, 0.106 mmol) in freshly distilled CH3CN (1.0 mL including NMR tube washings, stirred over activated 4 Å molecular sieves, distilled over CaH2, and distilled again over P2O5) at rt over 5 min. The dark orange solution was stirred at rt for 2 h, then poured into H2O:brine and extracted with Et2O. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator). The orange oil was purified by preparative TLC on silica gel (20 cm × 20 cm × 1000 µm pretreated with NEt3 vapor for 30 min, 5:1 hexanes/EtOAc with 2% NEt3 eluent) to give 5.4 mg (33%) of tetracycle 45 as an orange oil, analytical TLC on silica gel 60 F254, 40% EtOAc/hexanes, Rf = 0.7; [α]D 23 −12.5 (c 0.04, EtOAc). Molecular ion (M+Na) calculated for C39H42N2NaO4Si: 653.2812; found m/z = 653.2808, error = 1 ppm; IR (neat, cm−1) 1657 C=O, 1642 C=O; UV (CH3OH, nm) 286 (ε 1100) 350 (ε 600) 433 (ε 450). 500 MHz 1H NMR (CDCl3, ppm) δ 7.45-7.44 (6H, m) 7.31-7.22 (9H, m) 5.14 (1H, AB, J=14.2 Hz) 4.92 (1H, AB, J=14.2 Hz) 4.55 (1H, d, J=13.8 Hz) 4.10 (1H, dd, J=13.8 Hz, 3.9 Hz) 4.00 (3H, s) 3.03 (1H, d, J=4.9 Hz) 2.89 (1H, dd, J=4.9 Hz, 3.9 Hz) 1.97 (3H, s) 0.81 (9H, s) 0.01 (3H, s) −0.05 (3H, s). 13C NMR (125 MHz, CDCl3, ppm) δ 179.9, 179.0, 157.4, 141.7, 129.4, 128.4, 128.1, 128.0, 127.3, 127.2, 123.0, 120.3, 74.4, 61.4, 58.9, 50.2, 41.9, 35.4, 26.3, 18.7, 8.7, −5.2, −5.2.
5-((E)-4,7-Bis(tert-butyldimethylsilyloxy)-3-methoxyhept-2-en-5-yn-2-yl)-2-((2S,3R)-3-(iodomethyl)-1-tritylaziridin-2-yl)oxazole (46)
To a solution of tert-butyldimethylsilyl propargyl ether32 (251 mg, 1.47 mmol) in THF (10 mL) at −78 °C was added n-BuLi (1.47 M solution in hexanes, 1.0 mL, 1.47 mmol) dropwise over 5 min. After stirring the clear solution for 1 h at −78 °C, a solution of aldehyde 11 (435 mg, 0.737 mmol) in THF (5 mL including cannula washings) was added via cannula dropwise over 5 min. The resulting yellow solution was warmed to −42 °C and stirred for 2 h. The cooling bath was then removed and 10 mL of saturated aqueous sodium bicarbonate was added and stirred at rt 10 min. The resulting light yellow solution was poured into brine and extracted with Et2O. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator). The crude alcohol from above was dissolved in CH2Cl2 (15 mL) and tert-butyldimethylsilyl chloride (222 mg, 1.47 mmol) was added followed by imidazole (125 mg, 1.84 mmol). After stirring for 15 h at rt, the reaction was poured into H2O (20 mL) and extracted wth CH2Cl2 (3 × 30 mL). The combined organic extracts were dried (Na2SO4), filtered, and solvents were removed (aspirator). The clear oil was purified by flash column chromatography on silica gel (50 mm × 16 cm, 10% EtOAc/hexanes, 40 mL fractions) to yield 0.500 g (78%) of oxazole 46 as an inseparable 1:1 mixture of diastereomers as a white foam, analytical TLC on silica gel 60 F254, 10% EtOAc/hexanes, Rf = 0.2. Molecular ion (M+Na) calculated for C45H59IN2NaO4Si2: 897.2956; found m/z = 897.2947, error = 1 ppm; IR (neat, cm−1) 1638; 400 MHz 1H NMR (CDCl3, ppm); overlapping diastereomer signals given as a sum of individual isomer values, δ 7.53-7.51 (12H, m) 7.29-7.19 (18H, m) 7.02 (1H, s) 7.00 (1H, s) 5.69 (1H, t, J=1.8 Hz) 5.67 (1H, t, J=1.8 Hz) 4.38 (2H, d, J=1.8 Hz) 4.34 (2H, 2, J=1.8 Hz) 3.98 (3H, s) 3.97 (3H, s) 3.70-3.66 (2H, m) 3.59-3.53 (2H, m) 2.57-2.54 (2H, m) 2.16-2.06 (2H, m) 2.00 (3H, s) 1.99 (3H, s) 0.90 (9H, s) 0.89 (9H, s) 0.88 (18H, s) 0.11 (3H, s) 0.11 (3H, s) 0.09 (6H, s) 0.08 (3H, s) 0.07 (3H, s) 0.03 (3H, s) 0.02 (3H, s). 13C NMR (100 MHz, CDCl3, ppm) δ 159.9, 159.7, 152.5, 152.5, 151.0, 151.0, 143.5, 143.5, 129.5, 129.5, 128.0, 128.0, 127.3, 127.3, 125.6, 125.5, 107.4, 107.2, 84.4, 84.4, 83.7, 83.7, 75.7, 75.6, 61.1, 61.0, 59.9, 59.8, 52.0, 52.0, 41.8, 41.7, 35.3, 35.2, 26.0, 26.0, 18.4, 18.4, 18.3, 14.2, 14.1, 3.5, 3.5, −4.4, −4.5, −4.7, −4.7, −4.9, −4.9.
Cyanide adduct 50 via azomethine ylide generation from 46
To a solution of AgOTf·1/2 benzene24 (8.0 mg, 0.026 mmol) in C6D6 (0.2 mL) in a dry, nitrogen flushed NMR tube was added a solution of oxazole 46 (24 mg, 0.027 mmol) in C6D6 (0.4 mL including cannula and flask washings) via cannula. After sitting in the dark for 3.5 h at rt, 1H NMR showed no starting oxazole 46 and oxazolium salt formation was indicated by characteristic changes in the chemical shift of the oxazole hydrogens from δ = 7.01 ppm and 6.99 ppm (CD2Cl2) to 7.77 ppm and 7.73 ppm (CD2Cl2), and the brown solution was transferred via syringe equipped with a syringe filter to a solution of BnMe3N+ CN− (19 mg, 0.11 mmol) in freshly distilled CH3CN (1.0 mL including NMR tube washings, stirred over activated 4 Å molecular sieves, distilled over CaH2, and distilled again over P2O5) at 0 °C over 5 min. After stirring the orange solution at 0 °C for 30 min the reaction was warmed to rt over 10 minutes and stirred for an additional 30 min. The dark solution was poured into H2O:brine and extracted with EtOAc. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator). The brown oil was purified by preparative TLC on silica gel (20 cm × 20 cm × 1000 µm pretreated with NEt3 vapor for 30 min, 5:1 hexanes/EtOAc with 2% NEt3 eluent) to give 6.9 mg (33%) of tetracycle 50 as a white solid in a 3:2 mixture (1H NMR analysis) of inseparable diastereomers, analytical TLC on silica gel 60 F254 pretreated with NEt3 vapor, 5:1 hexanes/EtOAc with 2% NEt3, Rf = 0.6. Molecular ion (M+Na) calculated for C46H59N3NaO4Si2: 796.3942; found m/z = 796.3962, error = 3 ppm; IR (neat, cm−1) 1659 C=O; 500 MHz 1H NMR (CDCl3, ppm) overlapping diastereomer signals given as a sum of individual isomer values, δ 7.48-7.44 (10H, m) 7.30-7.21 (15H, m) 5.36 (1H, s) 5.36 (0.6H, s) 4.77 (1H, AB, J=12.0 Hz) 4.74 (0.6H, AB, J=12.5 Hz) 4.68 (0.6H, AB, J=12.5 Hz) 4.64 (1H, AB, J=12.0 Hz) 4.63 (0.6H, d, J=13.2 Hz) 4.56 (1H, d, J=13.2 Hz) 4.18-4.13 (1.6H, m) 3.59 (3H, s) 3.57 (2H, s) 3.30 (0.6H, q, J=7.1 Hz) 3.26 (1H, q, J=6.8 Hz) 2.91-2.88 (2.6H, m) 2.81 (0.6H, d, J=4.9 Hz) 1.44 (3H, d, J=6.8 Hz) 1.42 (2H, d, J=7.1 Hz) 0.89 (6H, s) 0.86 (9H, s) 0.84 (6H, s) 0.80 (9H, s) 0.25 (1.8H, s) 0.17 (3H, s) 0.06 (1.8H, s) 0.05 (3H, s) −0.01 (1.8H, s) −0.05 (1.8H, s) −0.06 (3H, s) −0.16 (3H, s). 13C NMR (100 MHz, CDCl3, ppm) δ 184.6, 184.5, 144.1, 143.9, 132.3, 132.3, 129.4, 129.3, 128.0, 127.3, 127.3, 123.8, 123.3, 117.1, 117.0, 116.3, 116.3, 83.9, 83.8, 77.4, 74.5, 74.2, 63.0, 62.7, 57.5, 56.9, 53.1, 50.8, 50.6, 45.1, 45.0, 43.1, 41.9, 35.1, 34.7, 29.9, 29.9, 26.2, 26.1, 25.7, 25.7, 18.5, 18.4, 8.9, 8.8, −4.2, −4.3, −4.7, −4.8, −5.0, −5.1, −5.2, −5.4.
NCS oxidation of 50 to indoloquinone 45
To a solution of AgOTf·1/2 benzene24 (23.0 mg, 0.0778 mmol) in CH2Cl2 (0.25 mL) in a dry, nitrogen flushed NMR tube was added a solution of oxazole 46 (34.0 mg, 0.0389 mmol) in CD2Cl2 (0.45 mL including cannula and flask washings) via cannula. After 1 h at rt, shielded from light, 1H NMR showed no starting oxazole 46 and the brown solution was transferred via syringe equipped with a syringe filter to a solution of BnMe3N+ CN− (27.0 mg, 0.156 mmol) in CD3CN (2.0 mL including NMR tube washings) at 0 °C over 5 min. The orange solution was warmed to rt over 5 min then stirred an addition 1 h at rt. The dark solution was poured into H2O:brine (20 mL, 1:1 v:v) and extracted with EtOAc (3 × 15 mL). The combined organic extracts were dried (MgSO4), filtered, and solvents were removed (aspirator). To the crude product obtained above in THF (0.6 mL) at −78 °C was added KHMDS (0.30 mL of a 0.258 M solution in THF, 0.0778 mmol) and stirred at −78 °C for 30 min. NCS (0.34 mL of a 0.232 M solution in THF, 0.0778 mmol) was added and stirred an additional 25 min at −78 °C. After warming to rt over 15 min, the orange solution was poured into H2O and extracted with EtOAc. The combined organic extracts were dried (MgSO4), filtered, and solvents were removed. The orange oil was purified by preparative TLC on silica gel (20 cm × 20 cm × 1000 µm pretreated with NEt3 vapor for 30 min, 5:1 hexanes/EtOAc with 2% NEt3 eluent) to give 4.1 mg (17% over 2 steps) of tetracycle 45 as an orange oil identical to the material described above by 1H NMR.
Azomethine ylide cycloaddition from bis-TBS protected diol 46; isolation of 54 and 55 and detection of 53
To a solution of AgOTf·1/2 benzene24 (13.5 mg, 0.0457 mmol) in CD2Cl2 (0.2 mL) in a dry, nitrogen flushed NMR tube was added a solution of oxazole 46 (40.0 mg, 0.0457 mmol) in CD2Cl2 (0.4 mL including cannula and flask washings) via cannula. After sitting in the dark for 1 h at rt, 1H NMR showed no starting oxazole 46 and oxazolium salt formation was indicated by characteristic changes in the chemical shift of the oxazole hydrogens from δ = 7.01 ppm and 6.99 ppm (CD2Cl2) to 7.77 ppm and 7.73 ppm (CD2Cl2), and this solution was transferred via syringe equipped with a syringe filter to a solution of BnMe3N+ CN− (8.0 mg, 0.0457 mmol) in CH2Cl2 (2.0 mL including NMR tube washings) at 0 °C over 5 min. After stirring the orange solution at 0 °C for 1 h, H2O (2 mL) was added and the solution was poured into H2O and extracted with CH2Cl2. The combined organic extracts were dried (Na2SO4), filtered, and solvents were removed (aspirator) to yield crude 53 as a 1:1 ratio of diastereomers. In preparative work, this mixture was used without further purification to minimize decomposition, but chromatography allowed isolation of small amounts of partially purified material for spectroscopy. Preparative TLC on silica gel 60 F254, 5:1 hexanes/EtOAc 2% NEt3, Rf = 0.4 (20 cm × 20 cm × 1000 µm, 5:1 hexanes/EtOAc 2% NEt3 eluent) gave the less polar diastereomer of 53; ESMS molecular ion (M + Na) calculated for C46H59N3NaO4Si2: 796.4; found m/z = 796.4; partial 500 MHz 1H NMR (CDCl3, ppm) δ 7.41-7.40 (6H, m) and 7.31-7.20 (9H, m) for the N-Tr group, 5.77 (1H, s) for the CH-OTBS hydrogen, 4.60 (1H, dd, J=14.6 Hz, 2.0 Hz) and 4.48 (1H, dd, J=14.6 Hz, 2.7 Hz) for the CH2OTBS hydrogens, 3.98 (3H, s) for the OCH3 hydrogens, 3.60 (1H, d, J=13.4 Hz) and 3.49 (1H, dd, J=13.4 Hz, 2.2 Hz) for the N-CH2-CHNTr hydrogens, 2.59 (1H, d, J=4.6 Hz) and 2.15 (1H, dd, J=4.6 Hz, 2.2 Hz) for the aziridine hydrogens, 1.79 (3H, s) for the C-6 CH3 hydrogens. 13C NMR (125 MHz, CDCl3, ppm) δ 117.4 for the CN. For the more polar diastereomer, Rf = 0.2, partial purification by preparative TLC on buffered silica gel as above gave peaks in the 500 MHz 1H NMR (CDCl3, ppm) δ 7.59-7.57 (6H, m) and 7.31-7.19 (9H, m) for the N-Tr group, 5.31 (1H, s) for the CH-OTBS hydrogen, 4.50 (1H, d, J=13.2 Hz) and 4.38 (1H, dd, J=13.2 Hz, 2.2 Hz) for the CH2OTBS hydrogens, 3.92 (3H, s) for the OCH3 hydrogens, 2.79 (1H, d, J=5.4 Hz) and 2.35 (1H, dd, J=5.4 Hz, 3.4 Hz) for the aziridine hydrogens, 1.70 (3H, s) for the C-6 CH3 hydrogens. If the chromatography was attempted without NEt3 buffer in the eluent, then increased decomposition was observed, tentatively to give ca. 10% conversion to 49 according to ESMS data (m/z= 747 amu; 49 + H) and proton chemical shifts of δ 5.06 (s, CH adjacent to OTBS), 4.83 (AB q, J=11.9 Hz) and 4.69 (AB q, J=11.9 Hz for methylene adjacent to OTBS), 4.09 (s, OMe), 2.87 (d, J=4.9 Hz) and 2.83 (dd, J=4.9 Hz, 3.6 Hz) for the aziridine protons, and 1.84 (s, Me). For preparative purposes, NEt3 (0.16 mL, 1.14 mmol) was added to the crude cycloadduct from above in CH3CN (7 mL) and the mixture was placed under an atmosphere of O2 (balloon). The reaction was stirred at rt 10 min and HF-pyr (0.18 mL of a 3.9 M solution in CH3CN, 0.702 mmol) was then added. After stirring at rt 16 h under O2, the dark red solution was poured into NaHCO3 (20 mL) and extracted with CH2Cl2 (3 × 20 mL). The combined organic extracts were dried (Na2SO4), filtered, and solvents were removed (aspirator). The red oil was purified by preparative TLC on silica gel (20 cm × 20 cm × 1000 µm, 50% EtOAc/hexanes eluent) to give 3.8 mg (16%) of alcohol 54 as an orange solid, 5.2 mg (18%) of 45 as an orange oil, and 3.5 mg (14%) of 55 as a red solid (total of 48% cylclized material), analytical TLC on silica gel 60 F254, 40% EtOAc/hexanes, for 54 Rf = 0.4. Pure 54 obtained by crystallization in EtOAc/hexanes as orange needles, MP = >200 °C (decomposes). Molecular ion (M+) calculated for C33H28N2O4: 516.2049; found m/z = 516.2051, error = 0 ppm; IR (neat, cm−1) 3417 OH, 1640 C=O, 1600 C=O; 500 MHz 1H NMR (CDCl3, ppm) δ 7.43-7.42 (6H, m) 7.32-7.29 (6H, m) 7.27-7.24 (3H, m) 4.78 (1H, dd, J=13.9 Hz, 6.1 Hz) 4.61 (1H, dd, J=13.9 Hz, 8.1 Hz) 4.54 (1H, d, J=13.9 Hz) 4.15 (1H, dd, 13.9 Hz, 3.9 Hz) 4.03 (3H, s) 3.90 (1H, dd, J=8.1 Hz, 6.1 Hz) 2.89 (1H, dd, 4.9 Hz, 3.9 Hz) 2.84 (1H, d, J=4.9 Hz) 1.99 (3H, s). 13C NMR (125 MHz, CDCl3, ppm) δ 180.9, 178.9, 157.5, 144.1, 140.8, 129.3, 129.1, 128.3, 128.1, 127.5, 125.0, 119.5, 74.5, 61.5, 56.7, 50.5, 42.6, 33.9, 8.9. For 55, analytical TLC on silica gel 60 F254, 40% EtOAc/hexanes Rf = 0.2. Pure 55 was obtained by crystallization in EtOAc/hexanes as fine red needles, MP = 165-168 °C (dec). Molecular ion (M+Na) calculated for C34H29N3NaO5: 582.2005; found m/z = 582.2007, error = 0 ppm; IR (neat, cm−1) 3415 OH, 1650 C=O, 1574 C=O; 500 MHz 1H NMR (CDCl3, ppm) δ 7.58-7.57 (6H, m) 7.33-7.30 (6H, m) 7.27-7.23 (3H, m) 4.65 (1H, dd, J=11.7 Hz, 2.4 Hz) 4.27 (1H, dd, J=11.7 Hz, 2.4 Hz) 4.12 (1H, d, J=12.2 Hz) 4.05 (3H, s) 4.03 (1H, s) 3.84 (1H, dd, J=11.7 Hz, 11.7 Hz) 3.66 (1H, dd, J=12.2 Hz, 4.4 Hz) 2.96 (1H, d, J=5.4 Hz) 2.64 (1H, broad dd, J obsc) 1.89 (3H, s). 13C NMR (125 MHz, CDCl3, ppm) δ 182.3, 179.3, 158.3, 152.8, 143.5, 129.6, 128.1, 127.5, 126.0, 121.4, 116.7, 82.8, 75.9, 64.3, 61.8, 52.7, 45.6, 41.1, 8.6.
Preparation of (1S, 2,S)-9-hydroxymethyl-2,3-dihydro-7-methoxy-6-methyl-1,2-(N-tritylaziridino)-1H-pyrrolo[1,2-a]indole (54) from 45
To a solution of tetracycle 45 (8.0 mg, 0.013 mmol) in CH3CN (0.6 mL) at rt was added NEt3 (11 µL, 0.076 mmol) dropwise. HF·pyr (10 µL of a 3.9 M solution in CH3CN, 0.039 mmol) was added dropwise and stirred at rt for 15 h. The dark orange solution was poured into brine (20 mL) and extracted with CH2Cl2 (3 × 15 mL). The combined organic extracts were dried (Na2SO4), filtered, and solvents were removed (aspirator). The orange oil was was purified by preparative TLC on silica gel (20 cm × 20 cm × 1000 µm pretreated with NEt3 vapor for 30 min, 30% EtOAc/hexanes with 2% NEt3 eluent) to give 4.9 mg (75%) of alcohol 54 as an orange solid identical to the material described above by 1H NMR.
Carbamoylation of 54 and analytical scale detritylation
To a solution of alcohol 54 (3.8 mg, 0.0074 mmol) in CH2Cl2 (0.5 mL) cooled to −78 °C was added trichloroacetyl isocyanate (18 µL of a 0.42 M solution in CH2Cl2, 0.0076 mmol) dropwise. After stirring at −78 °C for 1 h, the reaction was warmed to rt over 20 min and then stirred an additional 1 h. The orange solution was poured into H2O (8 mL) and extracted with CH2Cl2 (3 × 10 mL). The combined organic extracts were dried (Na2SO4), filtered, and solvents were removed (aspirator). The unstable orange oil was purified by preparative TLC on silica gel (20 cm × 20 cm × 250 µm pretreated with NEt3 vapor for 30 min, 100% EtOAc with 2% NEt3 eluent) to give 4.0 mg (80%) of 56 as an orange oil that was quickly used in the following reaction to minimize decomposition; analytical TLC on silica gel 60 F254 pretreated with NEt3 vapor, 100% EtOAc with 2% NEt3 eluent, Rf = 0.2. Molecular ion (M+Na) m/z calculated for C36H28Cl3N3NaO6: 726.1; found 726.1. Partial 500 MHz 1H NMR (CDCl3, ppm) δ 7.44-7.43 (6H, m) and 7.31-7.23 (9H, m) for the N-Tr group, 5.47 (2H, s) for the CH2-O hydrogens, 4.58 (1H, d, J=13.9 Hz) and 4.15 (1H, dd, J=13.9 Hz, 3.9 Hz) for the N-CH2-NTr hydrogens, 4.05 (3H, s) for the OCH3 hydrogens, 3.14 (1H, d, J=6.1 Hz) and 2.90 (1H, dd, J=6.1 Hz, 3.9 Hz) for the aziridine hydrogens, 1.98 (3H, s) for the C-6 CH3 hydrogens. To a solution of crude 56 from above (1.0 mg, 0.0014 mmol) in CH2Cl2 (0.4 mL) at 0 °C was added triethylsilane (7 µL of a 0.629 M solution in CH2Cl2, 0.043 mmol) followed by methansulfonic acid (8 µL of a 0.514 M solution in CH2Cl2, 0.043 mmol). After stirring the orange solution at 0 °C for 25 min NEt3 (4 µL, 0.030 mmol) was added and stirred an addition 25 min at 0 °C. 0.2 mL of a 5% K2CO3 aqueous solution in MeOH (1:1, v:v) was added and the reaction was allowed to warm to rt and stir an addition 1 h. Formation of known amino alcohol 5929 was indicated by the molecular ion (M+Na) for C15H17N3NaO6: 358.1, together with a weak mass peak corresponding to aziridinomitosene A8 (4), molecular ion (M+Na) for C15H15N3NaO5: 340.1. Characteristic signals to support the formation of 4 could not be found in the NMR spectrum.
(1S,2,S)-9-Carbamoyloxymethyl-2,3-dihydro-7-methoxy-6-methyl-1,2-(N-tritylaziridino)-1H-pyrrolo[1,2-a]indole (57)
To the tetracyclic alcohol 54 (2.8 mg, 0.0050 mmol) in CH2Cl2 (0.5 mL) at −78 °C was added trichloroacetyl isocyante (13 µL of a 0.42 M solution in CH2Cl2, 0.054 mmol) and the reaction was stirred at −78 °C for 1 h. The reaction was allowed to warm to rt over 20 min then stirred an additional 1 h. Next, 0.5 mL of a 5% K2CO3 aqueous solution in MeOH (1:1, v:v) was added and the reaction was stirred vigorously at rt. After stirring vigorously at rt for 3 h, the biphasic solution was poured into H2O (5 mL) and extracted with CH2Cl2 (3 × 7 mL). The combined organic extracts were dried (Na2SO4), filtered, and solvents were removed (aspirator). The orange solid was purified by preparative TLC on silica gel (20 cm × 20 cm × 250 µm pretreated with NEt3 vapor for 30 min, 100% EtOAc with 2% NEt3 eluent) to give 2.0 mg (67%) of 57 as an orange solid, analytical TLC on silica gel 60 F254 pretreated with NEt3 vapor, 100% EtOAc with 2% NEt3 eluent, Rf = 0.6. Molecular ion (M+Na) calculated for C34H29N3NaO5: m/ z = 582.2; found 582.2; IR (neat, cm−1) 3465 NH, 3346 NH, 1725 C=O, 1659 C=O, 1642 C=O; 500 MHz 1H NMR (CDCl3, ppm) δ 7.46-7.45 (6H, m) 7.32-7.24 (9H, m) 5.36 (1H, AB, J=13.4 Hz) 5.31 (1H, AB, J=13.4 Hz) 4.57 (1H, d, J=13.7 Hz) 4.19 (2H, bs) 4.14 (1H, dd, J=13.7 Hz, 3.9 Hz) 4.03 (3H, s) 2.98 (1H, d, J=4.8 Hz) 2.88 (1H, dd, J=4.8 Hz, 3.9 Hz) 1.97 (3H, s). 13C NMR (125 MHz, CDCl3, ppm) δ 179.6, 179.0, 157.5, 156.7, 142.4, 129.4, 128.2, 128.1, 127.8, 127.5, 123.8, 113.7, 106.5, 74.6, 61.5, 59.1, 50.4, 42.3, 34.8, 8.8.
Detritylation of 57; detection of 58 and isolation of 59
To a solution of 57 (2.7 mg, 0.0048 mmol) and triphenylsilane (78 µL of a 0.0614 M solution in CH2Cl2, 0.0048 mmol) in CH2Cl2 (0.45 mL) cooled to −78 °C was added triflic acid (0.5 µL, 0.0048 mmol). The reaction was allowed to stir at −78 °C for 5 min then slowly warmed to −40 °C over 30 min and stirred an addition 15 h at −40 °C. To the orange solution was added pH 10.3 buffer (0.2 mL) cooled to 0 °C. The biphasic solution was warmed to rt and extracted with CH2Cl2 (5 × 1 mL). The combined organic extracts were dried (Na2SO4), filtered, and solvents were removed (aspirator). The orange solid was purified by preparative TLC on silica gel (20 cm × 20 cm × 250 µm, 10:1 CH2Cl2:MeOH eluent) to give 2.0 mg (72%) of 58 as a 3:1 mixture of diastereomers as an orange solid, analytical TLC on silica gel 60 F254, 10:1 CH2Cl2:MeOH, Rf = 0.63 for the less polar major diastereomer and Rf = 0.60 for the more polar minor diastereomer. Molecular ion (M+Na) calculated for C34H31N3NaO6: m/z= 600.2, found 600.2. Partial 500 MHz 1H NMR (CDCl3, ppm) δ 7.60 (6H, m) and 7.32-7.22 (9H, m) for the N-Tr group, 5.02 (1H, AB, J=12.2 Hz) and 5.01 (1H, AB, J=12.2 Hz) for the CH2OC(O)NH2 hydrogens, 4.60 (2H, br s) for the C(O)NH2 hydrogens, 4.52 (1H, dd, J=12.4 Hz, 7.7 Hz) for one of the N-CH2-CHNTr hydrogen, 4.00 (3H, s) for the OCH3 hydrogens, 3.25 (1H, d, J=5.1 Hz) for the CH-OH hydrogen, 1.94 (3H, s) for the C-6 CH3 hydrogens.
To a solution of amino alcohol 58 (2.0 mg, 0.0035 mmol) prepared above in CH2Cl2 (0.3 mL) at 0 °C was added triethylsilane (11 µL of a 0.628 M solution in CH2Cl2, 0.0069 mmol) and trifluoroacetic acid (12 µL of a 0.573 M solution in CH2Cl2, 0.0069 mmol). After stirring the orange solution at 0 °C for 30 min, diisopropylethylamine (2 µL, 0.010 mmol) was added and stirred at 0 °C for an additional 30 min. Solvents were removed (aspirator) and the orange solid was purified by preparative TLC on silica gel (20 cm × 20 cm × 250 µm, 10:1 CH2Cl2:MeOH eluent) to give 1.0 mg (83%) of amino alcohol 59 identical by 1H NMR and mass spectrometry comparisons with literature data.29,30
Alternatively, a solution of tetracycle 57 (2.0 mg, 0.0036 mmol) and triphenylsilane (109 µL of a 0.0653 M solution in CH2Cl2, 0.0071 mmol) in CH2Cl2 (0.40 mL) cooled to −78 °C was treated with triflic acid (0.6 µL, 0.0071 mmol). The reaction was allowed to stir at −78 °C for 5 min and then was slowly warmed to −40 °C over 30 min and stirred an addition 15 h at −40 °C. To the orange solution was added DIEA (2 µL, 0.011 mmol) and the mixture was stirred at −40 °C for an additional 30 min. Solvents were then removed (aspirator) and the orange solid was purified by preparative TLC on silica gel as above to give 1.0 mg (83%) of amino alcohol 59.29
Supplementary Material
NMR spectra of new compounds; X-ray data tables; procedures for oxidation of 32 to 31.
ACKNOWLEDGMENT
The authors thank Prof. L. Jimenez for kindly providing an NMR spectrum of synthetic aziridinomitosene A. This work was supported by the National Institutes of Health (CA17918).
References
- 1.Danishefsky SJ, Schkeryantz JM. Synlett. 1995:475. [Google Scholar]
- 2.Shaw KJ, Luly JR, Rapoport H. J. Org. Chem. 1985;50:4515. [Google Scholar]
- 3.Utsunomiya I, Fuji M, Sato T, Natsume M. Chem. Pharm. Bull. 1993;41:854. [Google Scholar]
- 4.Lee S, Lee WM, Sulikowski GA. J. Org. Chem. 1999;64:4224. [Google Scholar]
- 5.(a) Vedejs E, Piotrowski DW, Tucci FC. J. Org. Chem. 2000;65:5498. doi: 10.1021/jo0001277. [DOI] [PubMed] [Google Scholar]; (b) Vedejs E, Little J. J. Am. Chem. Soc. 2002;124:748. doi: 10.1021/ja0120835. [DOI] [PubMed] [Google Scholar]; (c) Vedejs E, Naidu BN, Klapars A, Warner DL, Li VS, Na Y, Kohn H. J. Am. Chem. Soc. 2003;125:15796. doi: 10.1021/ja030452m. [DOI] [PubMed] [Google Scholar]; (d) Vedejs E, Little JD, Seaney LM. J. Org. Chem. 2004;69:1788. doi: 10.1021/jo030224a. [DOI] [PubMed] [Google Scholar]; (e) Vedejs E, Little JD. J. Org. Chem. 2004;69:1794. doi: 10.1021/jo030223i. [DOI] [PubMed] [Google Scholar]; (f) Warner DL, Hibberd AM, Kalman M, Klapars A, Vedejs E. J. Org. Chem. 2007;73 doi: 10.1021/jo7013615. in press. [DOI] [PubMed] [Google Scholar]
- 6.Michael JP, De Koning CB, Petersen RL, Stanbury TV. Tetrahedron Lett. 2001;42:7513. [Google Scholar]
- 7.Tsuboike K, Guerin DJ, Mennen SM, Miller SJ. Tetrahedron. 2004;60:7367. [Google Scholar]
- 8.Dong W, Jimenez L. J. Org. Chem. 1999;64:2520. [Google Scholar]
- 9.(a) Brehm WJ, Levenson T. J. Am. Chem. Soc. 1954;76:5389. [Google Scholar]; (b) Moriarty RM, Vaid RK, Ravikumar VT, Vaid BK, Hopkins TE. Tetrahedron. 1988;44:1603. [Google Scholar]
- 10.(a) Crisp GT, Meyer AG. J. Org. Chem. 1992;57:6972. [Google Scholar]; (b) Gibbs RA, Krishnan U, Dolence JM, Poulter CD. J. Org. Chem. 1995;60:7821. [Google Scholar]; (c) Yokoyama H, Miyamoto K, Hirai Y, Takahashi T. Synlett. 1997:187. [Google Scholar]; (d) Brummond KM, Gesenberg KD, Kent JL, Kerekes AD. Tetrahedron Lett. 1998;39:8613. [Google Scholar]; (e) Furstner A, De Souza D, Parra-Rapada L, Jensen JT. Angew. Chem., Int. Ed. 2003;42:5358. doi: 10.1002/anie.200352413. [DOI] [PubMed] [Google Scholar]; (f) Movassaghi M, Ondrus AE. Org. Lett. 2005;7:4423. doi: 10.1021/ol051629f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Movassaghi M, Ondrus AE. J. Org. Chem. 2005;70:8638. doi: 10.1021/jo051450i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larock RC, Doty MJ, Han XJ. J. Org. Chem. 1999;64:8770. doi: 10.1021/jo9821628. [DOI] [PubMed] [Google Scholar]
- 12.Morwick T, Hrapchak M, Deturi M, Campbell S. Org. Lett. 2002;4:2665. doi: 10.1021/ol020092s. [DOI] [PubMed] [Google Scholar]
- 13.Shafer CM, Molinski TF. J. Org. Chem. 1998;63:551. doi: 10.1021/jo971410h. [DOI] [PubMed] [Google Scholar]
- 14.Kashima C, Arao H. Synthesis. 1989:873. [Google Scholar]
- 15.Vedejs E, Monahan SD. J. Org. Chem. 1997;62:4763. [Google Scholar]
- 16.(a) Negishi EI. Acc. Chem. Res. 1982;15:340. [Google Scholar]; (b) Russell CE, Hegedus LS. J. Am. Chem. Soc. 1983;105:943. [Google Scholar]; (c) Knochel P, Singer RD. Chem. Rev. 1993;93:2117. [Google Scholar]
- 17.Farina V, Krishnamurthy V, Scott W. J. Org. React. 1997;50:1. [Google Scholar]
- 18.Andersen NG, Keay BA. Chem. Rev. 2001;101:997. doi: 10.1021/cr000024o. [DOI] [PubMed] [Google Scholar]
- 19.Ley SV, Norman J, Griffith WP, Marsden SP. Synthesis. 1994:639. [Google Scholar]
- 20.Feigelson GB, Egbertson M, Danishefsky SJ, Schulte G. J. Org. Chem. 1988;53:3390. [Google Scholar]
- 21.Vedejs E, Monahan SD. J. Org. Chem. 1996;61:5192. [Google Scholar]
- 22.Ito H, Taguchi T, Hanzawa Y. J. Org. Chem. 1993;58:774. [Google Scholar]
- 23.Kunz H, Schmidt P. Liebigs Ann. Chem. 1982:1245. [Google Scholar]
- 24.Dines MB. J. Organomet. Chem. 1974;67:C55. [Google Scholar]
- 25.Iyengar BS, Remers WA, Bradner WT. J. Med. Chem. 1986;29:1864. doi: 10.1021/jm00160a012. [DOI] [PubMed] [Google Scholar]
- 26.Tatsuta K, Hayakawa J, Tatsuzawa Y. Bull. Chem. Soc. Jpn. 1989;62:490. [Google Scholar]
- 27.(a) Vedejs E, Klapars A, Warner DL, Weiss AH. J. Org. Chem. 2001;66:7542. doi: 10.1021/jo0106243. [DOI] [PubMed] [Google Scholar]; (b) Kim M, Vedejs E. J. Org. Chem. 2004;69:7262. doi: 10.1021/jo040211c. [DOI] [PubMed] [Google Scholar]
- 28.Kocovsky P. Tetrahedron Lett. 1986;27:5521. [Google Scholar]
- 29.Paz MM, Tomasz M. Org. Lett. 2001;3:2789. doi: 10.1021/ol015517+. [DOI] [PubMed] [Google Scholar]
- 30.Taylor WG, Remers WA. J. Med. Chem. 1975;18:307. doi: 10.1021/jm00237a020. [DOI] [PubMed] [Google Scholar]
- 31.(a) Han I, Kohn H. J. Org. Chem. 1991;56:4648. [Google Scholar]; (b) Schiltz P, Kohn H. J. Am. Chem. Soc. 1993;115:10510. [Google Scholar]; (c) Li VS, Choi D, Tang MS, Kohn H. J. Am. Chem. Soc. 1996;118:3765. [Google Scholar]
- 32.Araki Y, Konoike T. J. Org. Chem. 1997;62:5299. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NMR spectra of new compounds; X-ray data tables; procedures for oxidation of 32 to 31.