Abstract
The goal of this study was to identify alterations in mRNA and protein expression of various xenobiotic transport proteins in mouse kidney during cisplatin-induced acute renal failure. For this purpose, male C57BL/6J mice received a single dose of cisplatin (18 mg/kg, ip) or vehicle. Four days later, tissues were collected for assessment of plasma BUN, histopathological analysis of renal lesions, and mRNA and western blot analysis of renal transporters including organic anion and cation transporters (Oat, Oct), organic anion transporting polypeptides (Oatp), multidrug resistance-associated proteins (Mrp), multidrug resistance proteins (Mdr), breast cancer resistance protein (Bcrp) and multidrug and toxin extrusion proteins (Mate). Cisplatin treatment caused necrosis of renal proximal tubules along with elevated plasma BUN and renal kidney injury molecule-1 mRNA expression. Cisplatin-induced renal injury increased mRNA and protein levels of the efflux transporters Mrp2, Mrp4, Mrp5, Mdr1a and Mdr1b. Uptake transporters Oatp2a1 and Oatp2b1 mRNA were also up-regulated following cisplatin. By contrast, expression of Oat1, Oat2, Oct2 and Oatp1a1 mRNA was reduced in cisplatin-treated mice. Expression of several uptake and efflux transporters was unchanged in cisplatin-treated mice. Apical staining of Mrp2 and Mrp4 proteins was enhanced in proximal tubules from cisplatin-treated mice. Collectively, these expression patterns suggest coordinated regulation of uptake and efflux pathways during cisplatin-induced renal injury. Reduced expression of basolateral and apical uptake transporters along with enhanced transcription of export transporters likely represents an adaptation to lower intracellular accumulation of chemicals, prevent their reabsorption and enhance urinary clearance.
Keywords: Transporters, cisplatin, kidney, toxicity
INTRODUCTION
Proximal tubule cells are sites of active secretion and reabsorption of xenobiotics and endogenous by-products into the urine and back into the body, respectively. Uptake of chemicals across basolateral membranes into the kidneys is mediated by secondary active transport systems including organic anion and cation transporters (Oats, Octs). Chemicals are subsequently effluxed across the brush border membrane into the urine by primary active transporters including multidrug resistance-associated proteins (Mrp) 2 and 4, multidrug resistance proteins (Mdr), breast cancer resistance protein (Bcrp) and multidrug and toxin extrusion proteins (Mate). P-glycoprotein (Pgp) is the protein product of Mdr genes. Reabsorption of chemicals from the filtrate can be accomplished by uptake carriers on the apical membrane including the organic anion transporting polypeptides (Oatps) as well as Oat2 (Klaassen and Lu 2008). Retrograde transporters on the basolateral face of the plasma membrane, such as Mrp1, Mrp5 and Mrp6, reabsorb chemicals back into blood.
During periods of acute and chronic renal injury, glomerular filtration and renal excretion are reduced. This often necessitates reducing the dose and/or lengthening dosing intervals for pharmaceuticals that are cleared renally. While decreases in renal blood flow, metabolism and glomerular filtration are likely determinants of reduced renal filtration and consequently drug elimination, it is unknown whether the expression and/or function of drug transporters in the kidneys are altered during drug-induced renal damage.
Cisplatin is an effective antineoplastic drug for the treatment of solid tumors, although its clinical use is often limited because of adverse effects on renal function. Nephrotoxicity can be observed in as many as 38% of patients after a single dose of cisplatin (100 mg/m2) (Shord et al. 2006). This side effect often delays or precludes subsequent chemotherapy cycles, thereby reducing the overall antineoplastic efficacy of cisplatin. Rodents and humans develop renal injury at comparable doses (15–20 mg/kg in mice is equivalent to 45–60 mg/mm2 in humans).
The initiation and progression of kidney injury by cisplatin is multifactorial, including biotransformation, adduct formation, oxidative stress, inflammation and changes in cell cycle. Humans and rodents exhibit similar histopathological changes and time profile for toxicity of cisplatin (Dobyan et al. 1980). Following cisplatin exposure, kidney sections demonstrate necrosis as well as apoptosis of renal proximal tubule cells in the S3 segment of the nephron, as well as occasional damage to distal tubules. The end result is compromised renal function. Because of the similar pathological changes among species, mice are routinely used to investigate mechanisms of cisplatin toxicity.
Previous reports demonstrate the affinity of cisplatin for cellular uptake via rat and human Oct2 (Ciarimboli et al. 2005; Yonezawa et al. 2005). Oct2 is localized to the basolateral surface of rat proximal tubule cells, predominantly in the S2 and S3 segments of the nephron (Karbach et al. 2000). Interestingly, administration of a single toxic dose of cisplatin to rats reduces Oct2 mRNA levels at 7 days, suggesting a defense response against subsequent exposure and renal uptake of cisplatin (Huang et al. 2001). Cisplatin treatment also increases expression of renal Mrp2 and Pgp in rats (Demeule et al. 1999; Huang et al. 2001). Mrp2 is overexpressed in a number of cisplatin-resistant cancer cell lines and tumors possibly implicating this transporter in the removal of cisplatin from cells (Cui et al. 1999; Kool et al. 1997). By contrast, Pgp does not transport cisplatin (Ishikawa and Ali-Osman 1993). Therefore, induction of renal Pgp during cisplatin-induced nephrotoxicity suggests that the kidneys adapt to injury by up-regulating additional efflux transporters that aid in chemical elimination from the nephrotic kidney. Regulation of renal drug transporters in mice following cisplatin exposure has not been examined. Therefore, the purpose of this study was to investigate the effects of cisplatin on expression of uptake (Oat, Oct and Oatp) and export (Mrp, Mdr, Mate) xenobiotic transporters in mouse kidneys. Data generated from this study suggests that the kidney coordinately regulates the expression of xenobiotic transporters during drug-induced toxicity. Shifts in the expression of uptake and efflux transporters in the kidney may influence the pharmacokinetics and pharmacodynamics of co-administered pharmaceuticals.
METHODS
Treatment Regimen
Male C57BL/6J mice, aged 10–12 weeks old, were purchased from Jackson Laboratories (Bar Harbor, ME). Mice acclimated one week upon arrival. Animals were housed in a 12-h dark/light cycle, temperature- and humidity-controlled environment. The mice were fed laboratory rodent diet ad libitum. Cisplatin (Sigma Chemical Co., St. Louis, MO) was dissolved in saline (10 ml/kg). Groups of mice (4 control, 6 treated) were administered cisplatin (18 mg/kg, i.p.) or vehicle following overnight fasting. The dose of cisplatin was selected to achieve a moderate level of nephrotoxicity. Kidneys and plasma were collected at 4 days. Half of one kidney was placed in formalin. The remaining tissue was snap-frozen in liquid nitrogen. Frozen tissues were stored at −80°C until assayed. All animal studies were conducted in accordance with National Institutes of Health standards and the Guide for the Care and Use of Laboratory Animals.
Blood Urea Nitrogen (BUN) Levels
Plasma BUN levels were determined as a biochemical indicator of renal function. Infinity BUN Liquid Stable Reagent (Thermotrace, Melbourne, Australia) was used according to the manufacturer’s protocol.
Histopathology
Kidney samples were fixed in 10% neutral-buffered formalin prior to routine processing and paraffin embedding. Sections (5 µm in thickness) were stained with hematoxylin and eosin. Sections were examined by light microscopy for the presence of degeneration and necrosis.
RNA Extraction
Total tissue RNA was extracted using RNA-Bee reagent (Tel-Test Inc., Friendswood, TX) according to the manufacturer's protocol. RNA pellets were resuspended in diethyl pyrocarbonate-treated deionized water. RNA samples were analyzed by agarose gel electrophoresis and integrity was confirmed by visualization of intact 18S and 28S rRNA under ultraviolet light.
Branched DNA Signal Amplification Assay
Mouse Kidney injury molecule-1 (Kim-1), Oat1-3, Oct1-3, Oatp1a1, 1a4, 1a6, 2b1, 3a1, 2a1, Mrp1-6, Mdr1a, 1b, 2, Bcrp, Mate-1 and -2 mRNA levels were measured using the branched DNA signal amplification assay with published probe sets (QuantiGene® Reagent System, Panomics; Fremont, CA) (Aleksunes et al. 2005; Alnouti et al. 2006; Buist and Klaassen 2004; Cheng et al. 2005). A standard concentration (1 µg/ul) and amount of RNA (5 µg) was loaded into each well. Novel probe sets for Mdr1a, Mdr1b, Mdr2 and Kim-1 are provided in Supplementary Table 1. Data were collected as relative light units (RLU) per 5 µg total RNA and expressed as mean relative expression.
Preparation of Crude Kidney Membranes
Kidneys were homogenized in buffer (0.25 M sucrose, 10 mM Tris–HCl, pH 7.4) containing 50 µg/ml aprotonin and centrifuged at 100,000 × g for 1 h. Pellets were resuspended in homogenization buffer. Protein concentrations were determined using Bio-Rad protein assay reagents (Bio-Rad Laboratories, Hercules, CA).
Western Blot Analysis
Proteins were electrophoretically resolved using polyacrylamide gels (8–12% resolving, 4% stacking) and transblotted overnight at 4°C onto PVDF-Plus membrane (Micron Separations, Westboro, MA). Membranes were blocked for 2 h in blocking buffer (1% non-fat dry milk with 0.5% Tween 20 in phosphate-buffered saline). All primary and secondary antibodies were diluted in blocking buffer. Primary antibody dilutions are as follows: Oct2 (OCT-21A, 1:1000, Alpha Diagnostics, San Antonio, TX), Mrp1 (MRPr1, 1:2000), Mrp2 (M2III-5, 1:600), Mrp4 (M4I-10, 1:2000), Mrp5 (M5I-10, 1:100), Mrp6 (M6II-68, 1:1000), Pgp (C219, 1:1000, Abcam, Cambridge, MA) and Bcrp (BXP-53, 1:5000). Mrp1-6 and Bcrp antibodies were obtained from George Scheffer (VU Medical Center, Amsterdam). Of note, the M3II-2 antibody did not detect Mrp3 protein in kidneys. Blots were subsequently incubated with a species-appropriate horseradish peroxidase-conjugated secondary antibody for 1 h. Blots were stripped and reprobed with a dilution of 1:2500 β-actin antibody (ab8227, Abcam, Cambridge, MA) to confirm equal protein loading. Protein-antibody complexes were detected using an enhanced chemiluminescent kit (Amersham Life Science, Arlington Heights, IL) and exposed to Fuji Medical X-ray film (Fisher Scientific, Springfield, NJ). Intensity of protein bands was determined using the Discovery Series Quantity One 1-D Analysis software (Bio-Rad Laboratories, Hercules, CA).
Immunofluorescence
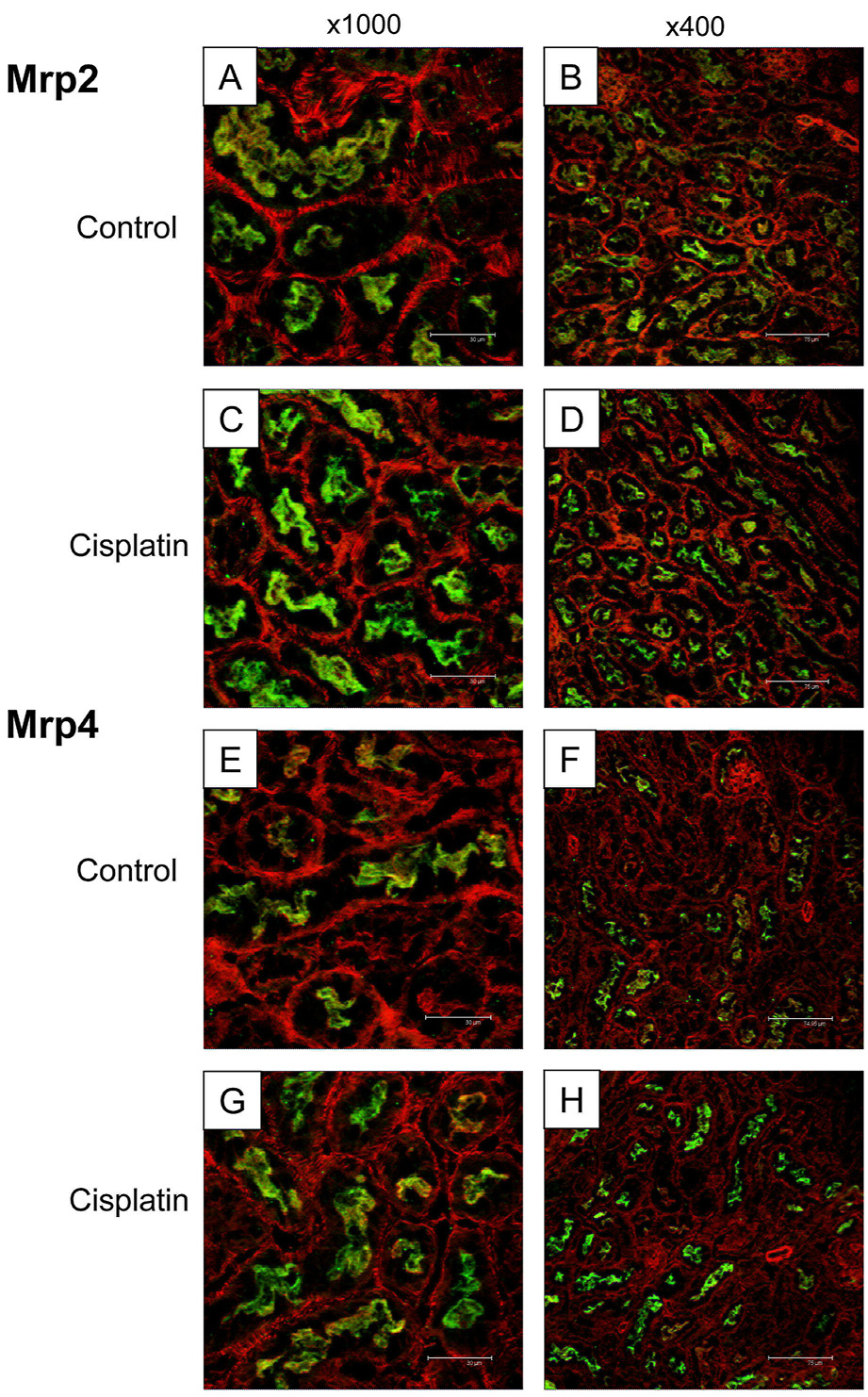
Kidneys were embedded in Optimal Cutting Temperature compound and brought to −20°C. Cryosections (5 µm) were thaw-mounted onto Superfrost glass slides (Fisher Scientific) and stored at −80°C until use. Tissue sections were fixed with 4% paraformaldehyde for 5 minutes. All antibody solutions were filtered through 0.22 µm membrane syringe-driven filters (Osmonics Inc., Minnetonka, MN) prior to use. Briefly, cryosections were blocked with 5% serum/phosphate-buffered saline with 0.1% Triton X (PBS-Tx) for 1 h and then incubated with primary antibody diluted 1:100 in 5% serum/PBS-Tx for 2 h at room temperature. The M2III-5 Mrp2 antibody used for westerns does not work for immunofluorescence staining. In turn, a different anti-Mrp2 antibody was obtained from Bruno Stieger (University of Zurich, Switzerland) for these experiments. Mrp4 protein was detected using the M4I-10 antibody from George Scheffer (VU Medical Center, Amsterdam, The Netherlands). Sections were subsequently washed three times in PBS-Tx, and incubated for 1 h with goat anti-rat Alexa 488 IgG (Invitrogen Corporation, Carlsbad, CA) for Mrp4 detection and fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA) for Mrp2 detection. Secondary antibodies were diluted 1:200 in 5% goat serum/PBS-Tx containing 1:200 rhodamine-labeled phalloidin (Invitrogen, Carlsbad, CA). Sections were air dried and mounted in Prolong Gold (Invitrogen Corp.). Sections were visualized and images were captured using a Leica SP2 Spectral Confocal microscope (Leica Microsystems AG, Wetzlar, Germany) at excitation wavelengths of 488 and 633 nm for detection of Alexa 488/FITC and rhodamine, respectively. Each fluorescent channel was acquired sequentially and then overlaid to create the final image. Representative images are shown at x400 and x1000 magnifications. All sections were both stained and imaged under uniform conditions for each antibody. Negative control staining was performed by incubating cryosections without primary antibody (data not shown). Regions of tissue containing autofluorescent necrotic cells were identified by negative control staining and excluded from imaging.
Statistical Analysis
Quantitative results were expressed as mean ± standard error (S.E.) (n = 4–6 mice). Data were analyzed using unpaired t-test with GraphPad Prism (v4) software (GraphPad Software, Inc, San Diego, CA). Asterisks (*) represent a statistical difference between control and cisplatin-treated groups. Significance was set at p < 0.05.
RESULTS
Cisplatin-induced nephrotoxicity
Administration of cisplatin (18 mg/kg) to male C57BL/6J mice resulted in renal injury 4 days after treatment. Kim-1 is a novel renal biomarker of tubular damage that is measured in kidney or urine in addition to conventional serum chemistry markers. In the present study, cisplatin treatment increased renal Kim-1 mRNA levels by 42-fold over control values (Fig. 1A). This is in agreement with elevated plasma BUN levels (63 mg/dl) in cisplatin-treated mice compared to controls (26 mg/dl) (Fig. 1B). Histologic evaluation of kidneys from cisplatin-treated mice demonstrated degeneration, necrosis and sloughing of S3 segment proximal tubule epithelium at the outer layer of the outer medulla, (Fig. 1D). In addition, necrotic tubules contained eosinophilic amorphous material and pyknotic and karyorhectic debris (depicted by arrows in Fig. 1D). A representative kidney section from a vehicle-treated control mouse is shown in Fig. 1C.
Figure 1. Markers of cisplatin-induced nephrotoxicity.
(A) Renal Kim-1 mRNA expression. Total RNA was isolated from kidneys of mice 4 days following injection with cisplatin (18 mg/kg, i.p.) or saline vehicle. RNA was analyzed by branched DNA assay for expression of Kim-1. The data are presented as mean relative light units (RLU) ± SE. (B) Plasma BUN Level. Plasma was isolated from mice treated with cisplatin or saline vehicle. The data are presented as mean plasma BUN (mg/dl) ± SE. (C and D) Kidneys from control (C) and cisplatin-treated (D) mice were fixed in formalin and stained with hematoxylin and eosin (x200). Asterisks (*) represent a statistical difference (p<0.05) from control.
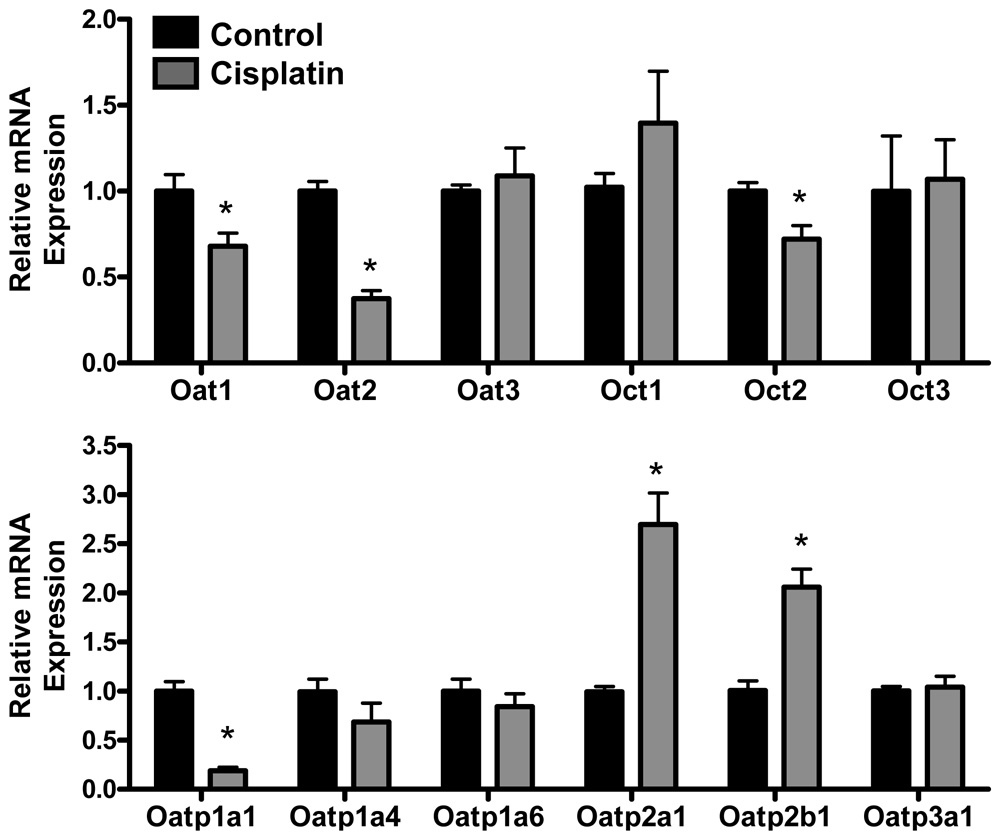
Uptake Transporters: Renal mRNA expression of Oat, Oct and Oatp transporters in cisplatin-treated mice
Cisplatin treatment reduced mRNA expression of Oat1, Oat2, Oct2 and Oatp1a1 to 68, 37, 72 and 21% of controls, respectively. By contrast, cisplatin treatment increased mRNA levels of Oatp2a1 (2.7-fold) and Oatp2b1 (2-fold). No changes in Oat3, Oct1, Oct3, Oatp1a4, Oatp1a6 or Oatp3a1 mRNA were observed in cisplatin-treated mice.
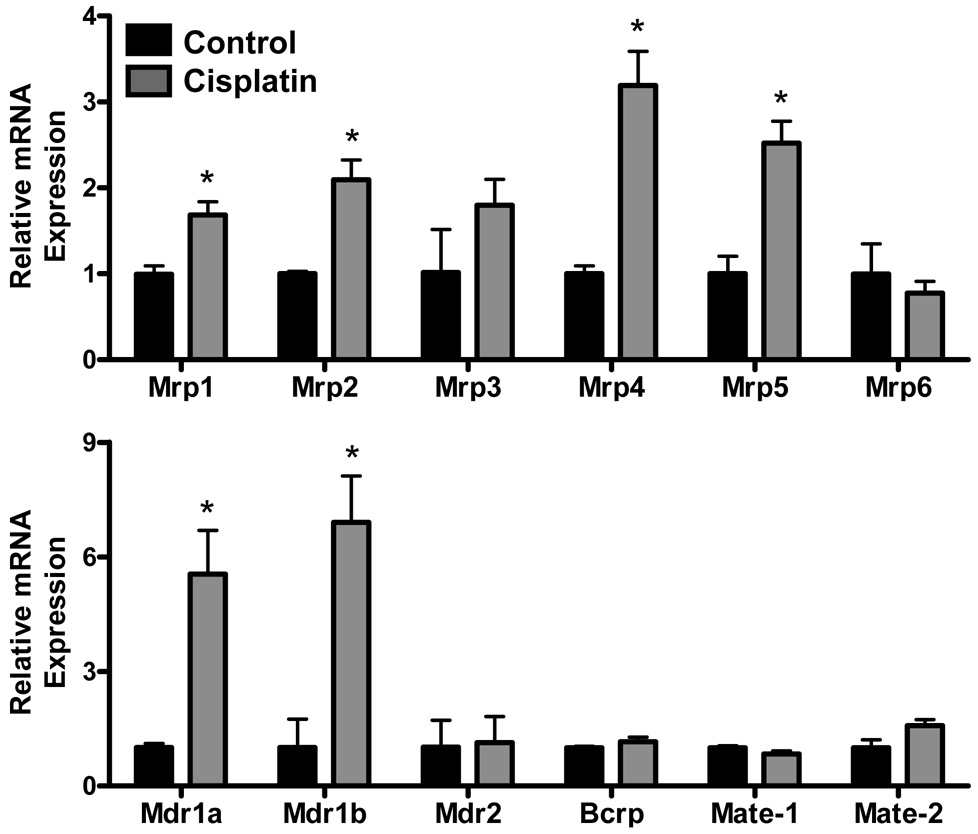
Efflux Transporters: Renal mRNA expression of Mrp, Mdr, Bcrp and Mate transporters in cisplatin-treated mice
Cisplatin treatment increased mRNA expression of Mrp1 (1.7-fold), Mrp2 (2.1-fold), Mrp4 (3.2-fold), Mrp5 (2.5-fold), Mdr1a (5.5-fold) and Mdr1b (6.9-fold). No significant changes in Mrp3, Mrp6, Mdr2, Bcrp, Mate-1 or Mate-2 mRNA were observed in cisplatin-treated mice.
Renal protein expression of Oct, Mrp, Pgp and Bcrp transporters in cisplatin-treated mice
Western blots were performed to determine whether changes in transporter mRNA levels corresponded with altered protein expression. Representative blots are shown in Fig. 4A. Similar to mRNA expression, Oct2 protein levels were reduced by cisplatin administration to 28% of control mice (Fig 4B). By contrast, kidneys from cisplatin-treated mice had increased expression of Mrp2 (2.7-fold), Mrp4 (2.2-fold), Mrp5 (4.4-fold) and Pgp (4.5-fold) proteins. Of note, the Pgp antibody (C219) does not discriminate different Mdr isoforms. Levels of Mrp1, Mrp6 and Bcrp proteins were unchanged by cisplatin exposure. Protein analysis of other transporters assessed by branched DNA was not performed because there are no specific antibodies commercially available to detect these proteins in mice.
Figure 4. Renal protein expression of Oct, Mrp, Bcrp and Pgp transporters in cisplatin-treated mice.
Western immunoblots were performed using kidney crude membranes (60 µg protein/well) from mice at 4 days after treatment with cisplatin (18 mg/kg, i.p.) or saline vehicle. The data are presented as representative blots (A) and as mean relative Oct2, Mrp1, Mrp2, Mrp4, Mrp5, Mrp6, Pgp and Bcrp expression ± S.E. Blots were probed for β-actin staining to confirm equal protein loading. Asterisks (*) represent a statistical difference (p<0.05) from control.
Immunofluorescent staining of Mrp2 and Mrp4 transporters in cisplatin-treated mice
Immunofluorescent staining of Mrp2 and Mrp4 proteins was performed on kidney sections obtained from vehicle and cisplatin-treated mice to determine patterns and localization of altered protein expression. Mrp2 and Mrp4 were selected for immunofluorescent analysis due to the substantial increase in their expression in Western blots. These two transporters are typically localized to the apical brush border membrane of proximal tubule cells. Antibodies used for western blot immunostaining of Oct2, Mrp5 and Pgp were initially tested and were not suitable for immunofluorescent detection in mouse kidney. Two magnifications are provided to highlight changes within the cortex (x400) and in a subset of proximal tubules (x1000). Mrp2 and Mrp4 immunofluorescence (green) are shown in Fig. 5. The actin cytoskeleton is shown in red. Immunostaining of Mrp2 and Mrp4 proteins was observed on the apical membrane of proximal tubules in control-treated mice (Figs. 5A and B, Mrp2; Figs. 5E and F, Mrp4). Increased apical staining of Mrp2 and Mrp4 proteins was observed in kidney sections from cisplatin-treated mice (Figs. 5C and D, Mrp2; Figs 5G and 5H, Mrp4). No intracellular or basolateral staining of Mrp2 and Mrp4 was detected in any of the kidneys from mice treated with cisplatin. This indicates that induction of Mrp2 and Mrp4 protein expression as seen by western blotting was confined to the apical membrane and not due to alterations in cellular localization.
Figure 5. Immunofluorescent staining of Mrp2 and Mrp4 transporters in kidneys from cisplatin-treated mice.
Indirect immunofluorescence against apical Mrp2 protein (green) and actin (red) was conducted on kidney sections obtained at 4 days from control (A, B) and cisplatin-treated (C, D) mice. Indirect immunofluorescence against apical Mrp4 protein (green) and actin (red) was conducted on kidney sections obtained at 4 days from control (E, F) and cisplatin-treated (G, H) mice. Two magnifications are shown; x1000 (A, C, E, G) and x400 (B, D, F, H).
DISCUSSION
The present study investigated the effect of cisplatin toxicity (18 mg/kg) on the renal expression of numerous uptake and efflux xenobiotic transporters. Rodent models of acute renal failure by cisplatin are well-characterized. Injury is typically observed between 3 and 5 days following single dose exposure. Based on previous reports and our own pilot time-course studies, we selected 4 days following treatment as the time point to assess potential changes in expression of transport proteins. At this time point, proximal tubule injury was evident in cisplatin-treated mice and accompanied by marked increases in plasma BUN and renal Kim-1 mRNA levels.
Using branched DNA and western blot analysis, we observed increased mRNA and protein levels of Mrp2, Mrp4, Mrp5, Mdr1a, Mdr1b, Oatp2a1 (mRNA only) and Oatp2b1 (mRNA only) following cisplatin treatment. By contrast, expression of Oat1, Oat2, Oct2 and Oatp1a1 mRNA in kidney was reduced in cisplatin-treated mice. There was no change in levels of Mrp3, Mrp6, Mdr2, Bcrp, Mate-1, Mate-2, Oat3, Oct1, Oct3, Oatp1a6 and Oatp3a1 mRNA. The final studies in this manuscript document the enhanced apical immunofluorescent staining of Mrp2 and Mrp4 proteins in proximal tubules from cisplatin-treated mice.
Differential expression of transporters involved in the uptake and clearance of cisplatin and its conjugates may be one means to limit intracellular accumulation of cisplatin upon re-exposure. Similar to published data in rats, Oct2 protein levels were decreased 72% in cisplatin-treated mice (Huang et al. 2001). Previous reports demonstrate uptake of cisplatin via rat and human Oct2 (Ciarimboli et al. 2005; Yonezawa et al. 2005). Multiple lines of evidence suggest that Mrp2 is responsible for cisplatin efflux from cancer cells. First, high levels of MRP2 along with decreased cisplatin accumulation and decreased DNA adduct formation are linked to cisplatin resistance in tumor cells (Cui et al. 1999; Kool et al. 1997). Second, fluorescein-labeled cisplatin has been shown to associate with MRP2 in cytoplasmic vesicles in carcinoma cells (Safaei et al. 2005). Third, reduction of MRP2 protein using RNA interference, antisense transfection and anti-MRP2 hammerhead ribozymes restores sensitivity of tumor cells to cisplatin (Materna et al. 2005; 2006). In addition, early work performed by Ishikawa et al. in 1993 demonstrate that the monoplatinum-diglutathione conjugate is transported by a glutathione S-conjugate pump (Ishikawa and Ali-Osman 1993). This glutathione S-conjugate pump was subsequently identified as Mrp2/MRP2 (Muller et al. 1994). Taken together, researchers have proposed that Mrp2 is a likely transporter for removal of cisplatin from cells. Mate isoforms at the brush border membrane have gained recent attention as possible efflux transporters of platinum-based chemotherapy drugs. The cisplatin analog, oxaliplatin, is a good in vitro substrate for rat Mate-1 and human Mate2-K (Yokoo et al. 2007; Yonezawa et al. 2006). These transporters are less efficient in transporting cisplatin. Of note, Mate expression was unchanged in the current study.
Reductions in the expression of basolateral uptake transporters will decrease renal clearance of drugs co-administered with cisplatin resulting in higher plasma concentrations and increased potential for adverse events. Similarly, enhanced renal clearance of pharmaceuticals via Mrp2, Mrp4 and Pgp across the brush border membrane into the urine may reduce plasma concentrations and in turn, the therapeutic efficacy of these agents. This may be of particular importance when considering that these transporters are responsible for the excretion of numerous anticancer drugs which may be administered in conjunction with cisplatin. In addition, expression of apical uptake carriers (Oat2 and Oatp1a1) was decreased by cisplatin, favoring reduced reabsorption of filtered substrates.
Enhanced expression of efflux transporters along with concomitant decreases in uptake carrier proteins may also be a defense mechanism to promote proximal tubule cell recovery and survival. This response is expected to reduce uptake of substrates from blood into renal tubule cells and to increase chemical efflux to filtrate, thereby limiting intracellular accumulation not only of xenobiotics, but also endogenous substances and uremic toxins. For example, Mrp2 can excrete oxidized glutathione and lipid peroxides generated during renal injury, and in turn, restore intracellular redox balance (Ji et al. 2002; Reichard et al. 2003; Suzuki and Sugiyama 1998). Increased levels of Mrp4, Mrp5, Pgp, Oatp2a1 and Oatp2b1 mRNA 4 days after cisplatin may also promote tissue repair by reducing chemical burden of proximal tubules and/or promoting paracrine communication of signaling molecules involved in regeneration. Mrp4 transports cyclic nucleotides (i.e., cAMP and cGMP), prostanoids (PGE1, PGE2, TXB2), steroids, bile salts, folate, glutathione and urate (Chen et al. 2001; 2002; Rius et al. 2006; 2005). Some of these chemicals may work as signaling molecules to mediate tissue recovery. Mrp5 can similarly transport cyclic nucleotides and folate (Jedlitschky et al. 2000; Wielinga et al. 2003). Oatp2a1 (also known as prostaglandin transporter) is localized to the apical surface of rat collecting ducts, glomerular endothelial and mesangial cells and is responsible for reabsorption of prostanoids from filtrate into the renal tubule (Bao et al. 2002). Increased expression of this transporter in collecting ducts, glomerular endothelial and mesangial cells can also relieve the chemical burden of proximal tubule cells undergoing injury and repair following cisplatin exposure and some of the effluxed molecules may signal neighboring cells as part of the tissue recovery response.
In vivo functional assays of transporter function in cisplatin-treated mice are difficult since there are few isoforms-specific substrates. Instead, transporters tend to have broad and overlapping substrate profiles. Furthermore, expression of Pgp is altered in the liver in response to cisplatin (Demeule et al. 1999). Taken together, a lack of specific substrates as well as extrarenal expression changes make functional studies difficult to interpret.
Our data suggest that tissue injury by cisplatin is a requirement for these changes in renal transporters. In support of this, the inactive and non-nephrotoxic isomer of cisplatin, transplatin, failed to induce expression of Mdr1a/1b mRNA in rat kidney despite active uptake into proximal tubules similar to cisplatin (Huang et al. 2001). In addition, differential regulation of renal transporters is observed in killifish and rat models of chemical- and surgical-induced nephrotoxicity including exposure to arsenic and the aminoglycoside gentamicin (Miller et al. 2007; Notenboom et al. 2005). Collectively, these data support the hypothesis that induction of efflux transporters and reduction of uptake transporters following chemical renal injury is an adaptive recovery mechanism to reduce accumulation of xenobiotics, uremic toxins and endogenous molecules.
Differential expression of renal transporters as observed in this study is likely due to specific regulatory signaling pathways rather than cell loss in response to cisplatin exposure. For example, preliminary time-course studies reveal down-regulation of Oct2 mRNA at 3 days following cisplatin treatment when there is minimal histopathological cell loss. Similarly, it is important to note that alterations in transporter expression are family and isoform specific and not due to global disruption of plasma membrane integrity since no change in Bcrp and Mrp6 mRNA and protein levels were observed in this study.
In summary, this study is the first to report the gene and protein regulation of xenobiotic transporters in mouse kidney after cisplatin exposure. Down-regulation of Oat1, Oat2, Oct2 and Oatp1a1 and up-regulation of Mrp2, Mrp4, Mrp5, Mdr1a, Mdr1b, Oatp2a1 and Oatp2b1 in kidneys were observed. Decreased uptake of organic anions and cations into the kidneys may limit the burden of proximal tubule cells attempting to repair from damage. Similarly, increased renal Mrp2, Mrp4 and Pgp expression may protect the kidneys against injury from cisplatin nephrotoxicity by excreting toxic substrates generated during cellular stress.
Supplementary Material
Figure 2. Renal mRNA expression of Oat, Oct and Oatp transporters in cisplatin-treated mice.
Total RNA was isolated from kidneys of mice 4 days following injection with cisplatin (18 mg/kg, i.p.) or saline vehicle. RNA was analyzed by branched DNA assay for expression of Oat1-3, Oct1-3, Oatp1a1, 14, 1a6, 2a1, 2b1 and 3a1. The data are presented as mean relative mRNA expression ± SE. Asterisks (*) represent a statistical difference (p<0.05) from control.
Figure 3. Renal mRNA expression of Mrp, Mdr, Bcrp and Mate transporters in cisplatin-treated mice.
Total RNA was isolated from kidneys of mice 4 days following injection with cisplatin (18 mg/kg, i.p.) or saline vehicle. RNA was analyzed by branched DNA assay for expression of Mrp1-6, Mdr1a, 1b, 2, Bcrp, Mate-1 and Mate-2. The data are presented as mean relative mRNA expression ± SE. Asterisks (*) represent a statistical difference (p<0.05) from control.
ACKNOWLEDGEMENTS
The authors would like to thank Steven Cohen and Angela Slitt for assistance with preliminary dose and time response studies. Lauren Aleksunes was a Howard Hughes Medical Institute Predoctoral Fellow. This work was supported by National Institutes of Health Grant DK069557.
ABBREVIATIONS
- Bcrp
breast cancer resistance protein
- BUN
blood urea nitrogen
- FITC
fluorescein isothiocyanate
- Kim-1
kidney injury molecule-1
- Mate
multidrug and toxin extrusion proteins
- Mdr
multidrug resistance proteins
- Mrp
multidrug resistance-associated proteins
- Oat
organic anion transporters
- Oatp
organic anion transporting polypeptides
- Oct
organic cation transporters
- PBS-Tx
phosphate-buffered saline with 0.1% Triton X
- Pgp
P-glycoprotein
- RLU
relative light units
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest to report for the authors.
Contributor Information
Lauren M Aleksunes, Email: lauren.aleksunes@gmail.com.
Lisa M Augustine, Email: augustin@pharmacy.arizona.edu.
George L Scheffer, Email: GL.Scheffer@vumc.nl.
Nathan J Cherrington, Email: cherrington@pharmacy.arizona.edu.
José E Manautou, Email: jose.manautou@uconn.edu.
REFERENCES
- Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci. 2005;83:44–52. doi: 10.1093/toxsci/kfi013. [DOI] [PubMed] [Google Scholar]
- Alnouti Y, Petrick JS, Klaassen CD. Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos. 2006;34:477–482. doi: 10.1124/dmd.105.006932. [DOI] [PubMed] [Google Scholar]
- Bao Y, Pucci ML, Chan BS, Lu R, Ito S, Schuster VL. Prostaglandin transporter PGT is expressed in cell types that synthesize and release prostanoids. Am J Physiol Renal Physiol. 2002;282:F1103–F1110. doi: 10.1152/ajprenal.00152.2001. [DOI] [PubMed] [Google Scholar]
- Buist SC, Klaassen CD. Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1-3; Slc22a6-8) mRNA levels. Drug Metab Dispos. 2004;32:620–625. doi: 10.1124/dmd.32.6.620. [DOI] [PubMed] [Google Scholar]
- Chen ZS, Lee K, Kruh GD. Transport of cyclic nucleotides and estradiol 17-beta-D-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem. 2001;276:33747–33754. doi: 10.1074/jbc.M104833200. [DOI] [PubMed] [Google Scholar]
- Chen ZS, Lee K, Walther S, Raftogianis RB, Kuwano M, Zeng H, Kruh GD. Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer research. 2002;62:3144–3150. [PubMed] [Google Scholar]
- Cheng X, Maher J, Chen C, Klaassen CD. Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps) Drug Metab Dispos. 2005;33:1062–1073. doi: 10.1124/dmd.105.003640. [DOI] [PubMed] [Google Scholar]
- Ciarimboli G, Ludwig T, Lang D, Pavenstadt H, Koepsell H, Piechota HJ, Haier J, Jaehde U, Zisowsky J, Schlatter E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol. 2005;167:1477–1484. doi: 10.1016/S0002-9440(10)61234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Konig J, Buchholz JK, Spring H, Leier I, Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol. 1999;55:929–937. [PubMed] [Google Scholar]
- Demeule M, Brossard M, Beliveau R. Cisplatin induces renal expression of P-glycoprotein and canalicular multispecific organic anion transporter. Am J Physiol. 1999;277:F832–F840. doi: 10.1152/ajprenal.1999.277.6.F832. [DOI] [PubMed] [Google Scholar]
- Dobyan DC, Levi J, Jacobs C, Kosek J, Weiner MW. Mechanism of cis-platinum nephrotoxicity: II. Morphologic observations. J Pharmacol Exp Ther. 1980;213:551–556. [PubMed] [Google Scholar]
- Huang Q, Dunn RT, 2nd, Jayadev S, DiSorbo O, Pack FD, Farr SB, Stoll RE, Blanchard KT. Assessment of cisplatin-induced nephrotoxicity by microarray technology. Toxicol Sci. 2001;63:196–207. doi: 10.1093/toxsci/63.2.196. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem. 1993;268:20116–20125. [PubMed] [Google Scholar]
- Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275:30069–30074. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- Ji B, Ito K, Suzuki H, Sugiyama Y, Horie T. Multidrug resistance-associated protein2 (MRP2) plays an important role in the biliary excretion of glutathione conjugates of 4-hydroxynonenal. Free Radic Biol Med. 2002;33:370–378. doi: 10.1016/s0891-5849(02)00906-1. [DOI] [PubMed] [Google Scholar]
- Karbach U, Kricke J, Meyer-Wentrup F, Gorboulev V, Volk C, Loffing-Cueni D, Kaissling B, Bachmann S, Koepsell H. Localization of organic cation transporters OCT1 and OCT2 in rat kidney. Am J Physiol Renal Physiol. 2000;279:F679–F687. doi: 10.1152/ajprenal.2000.279.4.F679. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Lu H. Xenobiotic transporters: ascribing function from gene knockout and mutation studies. Toxicol Sci. 2008;103:35–45. doi: 10.1093/toxsci/kfm214. [DOI] [PubMed] [Google Scholar]
- Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA, Baas F, Borst P. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer research. 1997;57:3537–3547. [PubMed] [Google Scholar]
- Materna V, Liedert B, Thomale J, Lage H. Protection of platinum-DNA adduct formation and reversal of cisplatin resistance by anti-MRP2 hammerhead ribozymes in human cancer cells. Int J Cancer. 2005;115:393–402. doi: 10.1002/ijc.20899. [DOI] [PubMed] [Google Scholar]
- Materna V, Stege A, Surowiak P, Priebsch A, Lage H. RNA interference-triggered reversal of ABCC2-dependent cisplatin resistance in human cancer cells. Biochem Biophys Res Commun. 2006;348:153–157. doi: 10.1016/j.bbrc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Miller DS, Shaw JR, Stanton CR, Barnaby R, Karlson KH, Hamilton JW, Stanton BA. MRP2 and Acquired Tolerance to Inorganic Arsenic in the Kidney of Killifish (Fundulus heteroclitus) Toxicol Sci. 2007;97:103–110. doi: 10.1093/toxsci/kfm030. [DOI] [PubMed] [Google Scholar]
- Muller M, Meijer C, Zaman GJ, Borst P, Scheper RJ, Mulder NH, de Vries EG, Jansen PL. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci U S A. 1994;91:13033–13037. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notenboom S, Miller DS, Kuik LH, Smits P, Russel FG, Masereeuw R. Short-term exposure of renal proximal tubules to gentamicin increases long-term multidrug resistance protein 2 (Abcc2) transport function and reduces nephrotoxicant sensitivity. J Pharmacol Exp Ther. 2005;315:912–920. doi: 10.1124/jpet.105.089094. [DOI] [PubMed] [Google Scholar]
- Reichard JF, Doorn JA, Simon F, Taylor MS, Petersen DR. Characterization of multidrug resistance-associated protein 2 in the hepatocellular disposition of 4-hydroxynonenal. Archives of biochemistry and biophysics. 2003;411:243–250. doi: 10.1016/s0003-9861(03)00002-x. [DOI] [PubMed] [Google Scholar]
- Rius M, Hummel-Eisenbeiss J, Hofmann AF, Keppler D. Substrate specificity of human ABCC4 (MRP4)-mediated cotransport of bile acids and reduced glutathione. American journal of physiology. 2006;290:G640–G649. doi: 10.1152/ajpgi.00354.2005. [DOI] [PubMed] [Google Scholar]
- Rius M, Thon WF, Keppler D, Nies AT. Prostanoid transport by multidrug resistance protein 4 (MRP4/ABCC4) localized in tissues of the human urogenital tract. The Journal of urology. 2005;174:2409–2414. doi: 10.1097/01.ju.0000180411.03808.cb. [DOI] [PubMed] [Google Scholar]
- Safaei R, Katano K, Larson BJ, Samimi G, Holzer AK, Naerdemann W, Tomioka M, Goodman M, Howell SB. Intracellular localization and trafficking of fluorescein-labeled cisplatin in human ovarian carcinoma cells. Clin Cancer Res. 2005;11:756–767. [PubMed] [Google Scholar]
- Shord SS, Thompson DM, Krempl GA, Hanigan MH. Effect of concurrent medications on cisplatin-induced nephrotoxicity in patients with head and neck cancer. Anticancer Drugs. 2006;17:207–215. doi: 10.1097/00001813-200602000-00013. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sugiyama Y. Excretion of GSSG and glutathione conjugates mediated by MRP1 and cMOAT/MRP2. Seminars in liver disease. 1998;18:359–376. doi: 10.1055/s-2007-1007170. [DOI] [PubMed] [Google Scholar]
- Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003;278:17664–17671. doi: 10.1074/jbc.M212723200. [DOI] [PubMed] [Google Scholar]
- Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui K. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol. 2007;74:477–487. doi: 10.1016/j.bcp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Yonezawa A, Masuda S, Nishihara K, Yano I, Katsura T, Inui K. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem Pharmacol. 2005;70:1823–1831. doi: 10.1016/j.bcp.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Yonezawa A, Masuda S, Yokoo S, Katsura T, Inui K. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family) J Pharmacol Exp Ther. 2006;319:879–886. doi: 10.1124/jpet.106.110346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.