Abstract
Ascl3, also know as Sgn1, is a member of the mammalian achaete scute (Mash) gene family of transcription factors, which have been implicated in cell fate specification and differentiation. In the mouse salivary gland, expression of Ascl3 is restricted to a subset of duct cells. Salivary gland function depends on the secretory acinar cells, which are responsible for saliva formation, and duct cells, which modify the saliva and conduct it to the oral cavity. The salivary gland ducts are also the putative site of progenitor cells in the adult gland. Using a Cre recombinase-mediated reporter system, we followed the fate of Ascl3-expressing cells after the introduction of an EGFP-Cre expression cassette into the Ascl3 locus by homologous recombination. Lineage tracing shows that these cells are progenitors of both acinar and ductal cell types in all three major salivary glands. In the differentiated progeny, expression of Ascl3 is down-regulated. These data directly demonstrate a progenitor-progeny relationship between duct cells and the acinar cell compartment, and identify a population of multipotent progenitor cells, marked by expression of Ascl3, which is capable of generating both gland cell types. We conclude that Ascl3-expressing cells contribute to the maintenance of the adult salivary glands.
Keywords: salivary gland, progenitor cells, submandibular, sublingual, Ascl3, Demilune cell and parotid protein, duct cells, Cre recombinase, mouse, lineage tracing
Introduction
Salivary glands are essential for oral health. Radiation treatment of head and neck cancer patients, as well as autoimmune diseases, such as Sjögren’s syndrome, inflict cellular damage that is usually severe and irreversible, causing salivary gland atrophy and its pathological consequences. The ability to restore salivary function in an impaired or damaged gland would be a significant medical achievement. A prerequisite, however, is an understanding of the mechanisms by which these cells differentiate during development and are maintained and regenerated in adulthood.
There are three major pairs of salivary glands in mammals known as the submandibular, sublingual and parotid glands. Each gland is distinct in its structural architecture, cellular composition, and secretory products, but all are comprised of two predominant cell types (reviewed in Young and Van Lennep 1978). The secretory acinar cells, arranged in clusters, generate the initial saliva, which drains into ducts arranged in a branching network. The duct cells modify and conduct the saliva to the oral cavity.
The salivary glands develop initially as epithelial invaginations, which form buds, and then undergo branching morphogenesis (reviewed in Tucker 2007). In rodents, both the submandibular and parotid glands are immature at birth. Final cytodifferentiation of the acinar and duct cells occurs postnatally, while cytodifferentiation in the sublingual gland occurs prior to birth (Redman and Sreebny 1971; Chang 1974; Cutler and Chaudhry 1974; Redman and Ball 1978; Gresik 1980). The acinar cells are classified as either mucous or serous, based on the secretory proteins they produce. The mouse sublingual glands are composed predominantly of mucous-secreting acinar cells, capped by serous demilune cells; the parotid gland consists only of serous acinar cells, while the submandibular gland is made up of both (reviewed in Young and Van Lennep 1978).
The mature salivary glands turn over slowly (Zajicek et al. 1985; Redman 1995) and all cell types in the parenchyma are found to undergo cell division (Redman and Sreebny 1970; Dardick et al. 1990; Denny et al. 1990; Denny et al. 1993; Redman 1995). However, there is also evidence for a population of stem cells, which apparently contributes to maintenance and regeneration in the adult salivary gland (Zajicek et al. 1985; Denny et al. 1993; Denny and Denny 1999; Man et al. 2001). Clonal analysis has been used to demonstrate that multipotent progenitor cells from the submandibular gland are able to generate acinar, ductal and myoepithelial cells in vitro (Kishi et al. 2006). Results of indirect labeling experiments suggest that the progenitor cells are located within the intercalated ducts, the smallest elements of the salivary ductal system (Zajicek et al. 1985; Chai et al. 1993; Denny et al. 1993; Denny and Denny 1999; Man et al. 2001; Kimoto et al. 2008). The regeneration of adult rat submandibular gland following ligation-induced atrophy supports the assertion that multipotential cells are located within the ducts, the only remaining structures in the atrophic glands (Takahashi et al. 1998). In fact, a subpopulation of cells isolated from regenerating salivary glands has been reported to differentiate into hepatic and pancreatic lineages, suggesting that they may be adult stem cells (Okumura et al. 2003). To date, however, direct identification of ductal progenitor cells has not been demonstrated.
Ascl3, (achaete scute homolog like 3), also known as Sgn1, is a basic helix-loop-helix transcription factor, and a member of the mammalian achaete scute homolog (Mash) gene family. Members of this gene family implicated in cell fate specification and differentiation events. Mash1 (Ascl1) is expressed in subsets of neural progenitors in both the central and peripheral nervous system (Guillemot et al. 1993), and Mash2 (Ascl2) is involved in Schwann cell differentiation and control of proliferation in adult peripheral nerves (Küry et al. 2002).
Ascl3 was originally characterized as a transcription factor specifically localized in the duct cells of the salivary glands (Yoshida et al. 2001). The expression of Ascl3 is barely detectable at birth, but is increased during the postnatal period of cell differentiation (Yoshida et al. 2001). Using targeted homologous recombination, we have inserted an EGFP-Cre expression cassette into the Ascl3 locus. In combination with the Rosa 26R strain (Soriano 1999) as a Cre-inducible lineage tracer, the cassette labels cells in which Ascl3 is expressed, as well as lineage descendants of such cells. Using this tool, we show that descendants of Ascl3-expressing cells, as detected by LacZ expression, include both duct and acinar cell types, but that expression of the Ascl3 protein is confined to duct cells. Characterization of the LacZ-positive cell types reveals that in the sublingual gland, Ascl3-expressing cells are progenitors of serous demilune cells. The EGFP-Cre cassette thus labels a population of multipotent progenitor cells within the salivary glands, which is capable of generating at least two cell lineages. Our data suggest that Ascl3-expressing progenitor cells contribute to the maintenance of mature salivary glands.
Results
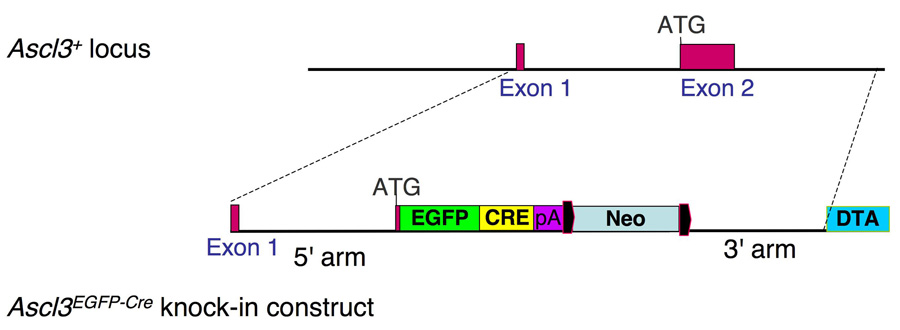
Generation of the Ascl3EGFP-Cre knock-in allele in the mouse
The Ascl3 gene is comprised of only two exons separated by an intron of 2.3 kilobases. A targeting vector was constructed to insert an EGFP-Cre recombinase fusion expression cassette into this locus by homologous recombination, replacing the entire Ascl3 coding sequence encoded by exon 2 (Figure 1). The Ascl3 promoter thereby drives expression of the EGFP-Cre recombinase fusion protein. This recombination generates a loss-of-function allele. Characterization of the homozygous mutant phenotype, and of Ascl3 function in vivo, is ongoing. Based on all analyses conducted so far, the Ascl3EGFP-Cre allele is completely recessive. Heterozygous animals are born at expected frequencies and are indistinguishable in growth and fertility, and show no signs of increased morbidity compared to their wild type littermates. We detect no change in salivary gland function in Ascl3EGFP-Cre/+ heterozygous mice (data not shown). In this report we have used the Ascl3EGFP-Cre allele as a lineage tracing tool to mark and analyze a defined subpopulation of salivary gland cells.
Figure 1.
Introduction of the EGFP-Cre expression cassette into the Ascl3 locus. The Ascl3 gene locus is comprised of only two exons. A knock-in construct was generated which replaces the second exon with an expression cassette encoding EGFP and Cre recombinase as a fusion protein. This construct was targeted to the Ascl3 locus by homologous recombination, placing EGFP-Cre under control of the Ascl3 promoter. The neomycin gene used for ES cell targeting is flanked by LoxP sites (black) and was subsequently removed by Cre recombination. The diphtheria toxin A (DTA) gene was used for negative selection, and is not retained in the recombined Ascl3EGFP-Cre allele.
Expression of the Ascl3EGFP-Cre knock-in construct recapitulates endogenous Ascl3 expression
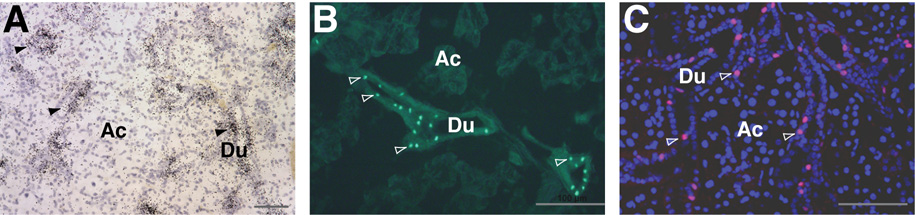
Expression of endogenous Ascl3 is localized to the duct cells in the three major salivary glands (Yoshida et al. 2001), as confirmed by in situ hybridization (Figure 2A). The expression pattern of the Ascl3EGFP-Cre knock-in allele can be tracked by the fluorescence of the EGFP gene product or by immunohistochemistry using an antibody against Cre recombinase. Ascl3EGFP-Cre/+ heterozygous mice show expression of EGFP in the ducts of submandibular (Figure 2B), sublingual and parotid glands (not shown). To confirm these results, we also used a polyclonal antibody against Cre recombinase to localize expression of the EGFP-Cre fusion protein on paraffin sections of submandibular, sublingual and parotid glands. As expected, Cre recombinase is present in duct cells of the submandibular (Figure 2C), sublingual (Figure 6D), and parotid salivary glands (not shown). This pattern reflects that of endogenous Ascl3 mRNA detected through in situ hybridization (Figure 2A). To confirm the duct cell-specificity of the Cre expression, we performed double-immunohistochemical labeling, using an antibody to aquaporin 5, which is a membrane channel localized to the apical surface of acinar cells (Matsuzaki et al. 1999). There is no detectable Cre recombinase or EGFP expression in acinar cells in any of the three major salivary glands (Figures 2B,C and data not shown). We therefore conclude that expression of the EGFP-Cre fusion protein faithfully recapitulates the duct cell-specific pattern of the endogenous Ascl3 gene.
Figure 2.
EGFP-Cre recombinase expression recapitulates endogenous Ascl3 expression. AIn situ hybridization on a paraffin section of submandibular gland from Ascl3EGFP-Cre/+ female using a radioactively labeled antisense probe to Ascl3 coding sequence. Positive signal from endogenous Ascl3 mRNA expression (black grains) is concentrated in the ducts. Hematoxylin staining was used to stain all cells. Arrowheads indicate labeled duct structures. Ac, acinar cells; Du, duct cells. Scale bar is 50 µm.
B. Fluorescent image of a frozen section from Ascl3EGFP-Cre/+ female submandibular gland. EGFP expression driven by the Ascl3 promoter is detectable in the nuclei of a subset of ductal cells (arrowheads). Ac, acinar cells; Du, duct cells. Scale bar is 100 µm.
C. Immunohistochemical staining of paraffin section from Ascl3EGFP-Cre/+ submandibular gland examined using antibodies to Cre recombinase and aquaporin 5. Ascl3-expressing Cre+ cells are labeled red. Antibody to aquaporin 5 labels apical membranes of acinar cells and is shown in green. All nuclei are stained with ToPro3 (blue). Large round nuclei are acinar cells; smaller aligned nuclei are from duct cells. Arrowheads indicate duct cells in which Ascl3-driven Cre recombinase expression is detected. Ac, acinar cells; Du, duct cells. Scale bar is 50 µm.
Figure 6.
Lineage tracing of Ascl3-expressing cells reveals continued contribution to the sublingual acinar cell population with age. Sublingual glands were removed from females of the Ascl3EGFP-Cre/+/Rosa26R strain at 3 weeks (A), 3 months (B), and 1 year of age (C), embedded, sectioned and analyzed for LacZ expression. With increasing age, there is a growing contribution of Ascl3-expressing progeny to the acinar cell compartment of the gland. The arrow in (B) indicates LacZ expression in an acinar cell, while the arrowhead points to a LacZ-positive demilune cell. D. Immunohistochemical staining using antibodies to Cre recombinase and aquaporin 5 on paraffin section of 5-month-old female Ascl3EGFP-Cre/+/Rosa26R mouse reconfirms that the endogenous Ascl3 expression in sublingual gland is restricted to the ducts, and is not ectopically activated in LacZ-positive acinar cells. Cre-expressing cells are red (arrows). Antibody to aquaporin 5 stains the apical membranes of acinar cells green. All nuclei are stained with ToPro. Du, ducts; Ac, acinar cells. Scale bars are 50 µm.
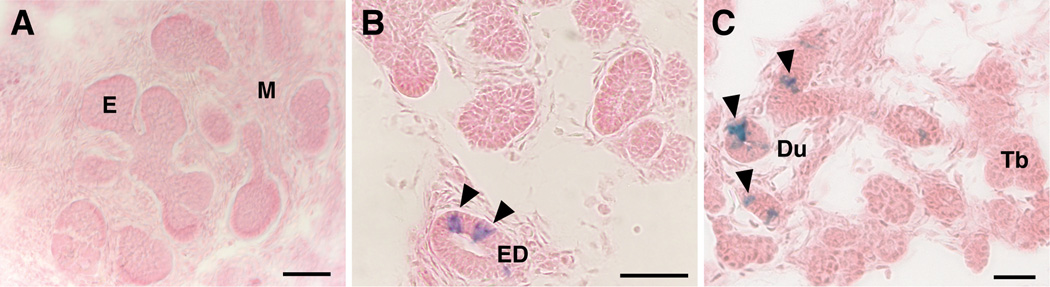
Ascl3 expression is activated in cells of the embryonic salivary gland ducts
To determine the contribution of Ascl3-expressing cells to salivary gland development and maintenance, we examined the time course of EGFP-Cre expression in Ascl3EGFP-Cre/+ /Rosa embryos, using lineage tracing. Ascl3EGFP-Cre/+ heterozygotes were crossed with the Rosa26R reporter mouse strain (Gt(ROSA)26Sortm1Sor; Soriano 1999). This strain carries a silenced ß-galactosidase (LacZ) gene under the control of the ubiquitously active Rosa26 locus. In the presence of Cre recombinase, the silencing sequence is removed by recombination, activating LacZ expression. Sections from salivary glands at different stages of development were stained for LacZ expression to assess the timing of Ascl3 promoter activation. At the early bud or pseudoglandular stage of submandibular gland development, we see no evidence of Ascl3 expression, as detected through the activation of LacZ (Figure 3A). However, at the canalicular stage, occurring at E15.5, LacZ-positive cells are observed in the large excretory duct (Figure 3B). By the terminal bud stage, just prior to birth (E17.5), a small number of LacZ-positive cells are detected in ductal structures of all three major glands (Figure 3C). However, there is no evidence of LacZ expression in cells of the terminal end buds, the presumptive acinar cells. Ascl3 expression in the duct cells of all three salivary glands increases during the postnatal differentiation phase (Yoshida et al. 2001). In agreement, we observe that the number of LacZ-positive cells increases by postnatal day 5, in the salivary glands of Ascl3EGFP-Cre/+/Rosa mice (not shown). Significantly, all LacZ-labeled cells are located within the ducts. Thus, during embryonic salivary gland development, the Ascl3-expressing cells and their progeny are restricted to the ducts.
Figure 3.
Ascl3 expression is initiated during embryogenesis and is confined to the presumptive duct cells of the developing salivary gland. Ascl3EGFP-Cre/+/Rosa embryos taken at A) embryonic (E) day 13.5, B) E15.5 and C) E17.5 were fixed, sectioned and stained for LacZ expression in order to determine the Ascl3 expression pattern during salivary gland development. A. At the pseudoglandular stage, there is no detectable expression of Ascl3 in the embryonic submandibular gland. B. In the canalicular stage, at E15.5, limited expression of Ascl3 is observed only in the large excretory duct (arrowheads), based on the detection of LacZ activation. C. At the terminal bud stage Ascl3-expressing cells are limited to the cells of the presumptive ducts (arrowheads). E, epithelial buds; M, mesenchyme; ED, excretory duct; Du, presumptive ducts; Tb, terminal end buds. Scale bars are 50 µm.
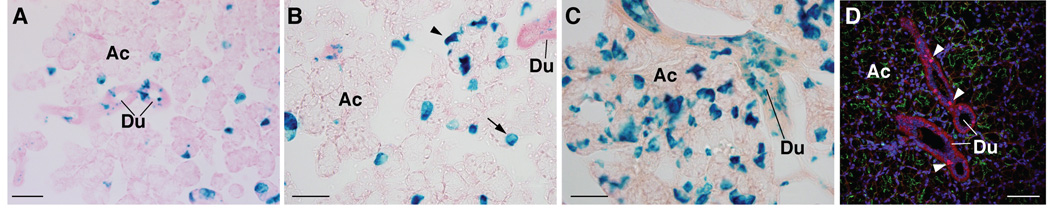
Lineage tracing reveals progeny of Ascl3-expressing cells in both duct and acinar cell compartments
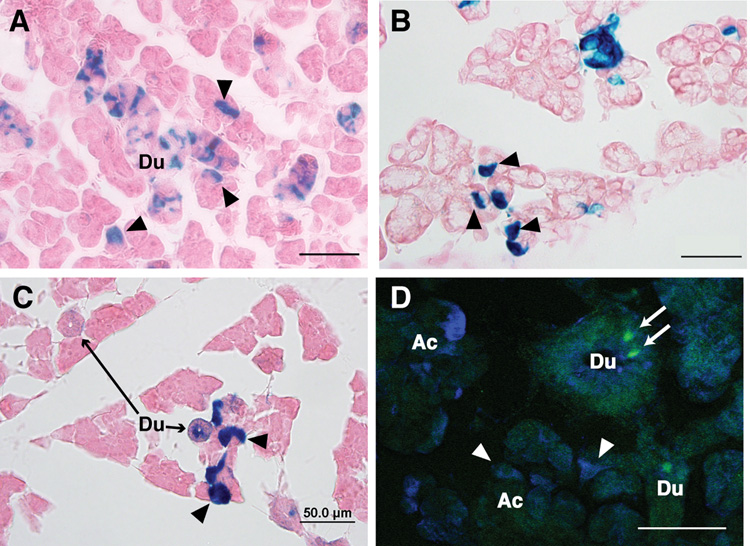
The prevailing hypothesis is that the ducts are the source of progenitor cells for the salivary gland. We have shown that both endogenous Ascl3 and the EGFP-Cre fusion protein are only detected in a subset of duct cells. Intriguingly, the expression of two related members of the achaete-scute gene family, Mash1 and Mash2, is found in progenitors of several neuronal lineages (Guillemot et al. 1993; Cau et al. 1997; Küry et al. 2002). To investigate whether Ascl3-expressing cells might act as progenitors in the salivary gland, we took advantage of the Cre recombinase activity to trace cells in which the EGFP-Cre cassette is active, as well as their descendants. Salivary glands were isolated from 3-week-old Ascl3EGFP-Cre/+ /Rosa 26R mice, fixed and sectioned. Frozen sections from submandibular, sublingual and parotid glands were stained for LacZ activity (Figure 4A–C). Consistent with the pattern of expression observed for Cre recombinase (Figure 2C), we observe LacZ-positive duct cells in all three glands, indicating that they are derived from Ascl3-expressing cells. No staining was observed in salivary gland tissue taken from control littermates that did not carry the Ascl3EGFP-Cre allele. The endogenous ß-galactosidase activity present in the salivary gland (Nowroozi et al. 1998) is therefore not detected with the staining conditions used.
Figure 4.
Lineage tracing of progeny from Ascl3-expressing cells in salivary glands. Frozen sections of salivary glands from 3-week-old female Ascl3EGFP-Cre/+/Rosa26R mice, stained for LacZ expression, which marks the progeny of cells in which Cre recombinase, driven by the Ascl3 promoter, has been expressed. A. Submandibular B. Sublingual C. Parotid gland. Arrowheads point to acinar cells in which the LacZ gene has been activated. D. Immunohistochemistry of Ascl3EGFP-Cre/+ submandibular gland section probed with antibody to beta-galactosidase (blue). Expression of the EGFP-Cre fusion protein, as detected by EGFP (green, arrows), is limited to cells in the duct and is not ectopically activated in LacZ-positive cells in the acinar compartment (arrowheads). Scale bars are 50 µm.
Most notably, although expression of the Ascl3EGFP-Cre gene is restricted to duct cells (Figure 2), progeny of these cells in the Ascl3EGFP-Cre/+/Rosa 26R mice are not located only in the ducts. Acinar cells are also labeled with LacZ (arrows, Figure 4A–C). The submandibular and parotid glands and, most prominently, the sublingual glands exhibit significant numbers of LacZ-positive acinar cells, derived from Ascl3-expressing cells. In sections from Rosa26R littermates, which do not carry the Ascl3EGFP-Cre allele, there is no detectable LacZ staining of any cell type (data not shown).
In order to establish that the LacZ expression is not due to ectopic activation of the EGFP-Cre recombinase fusion protein in acinar cells, we examined sections for co-localization of EGFP and beta-galactosidase, using an antibody against the latter. As expected, duct cells in which EGFP-Cre is detected (arrows, Figure 4D) are also LacZ-positive. However, LacZ-positive cells within the acinar clusters (arrowheads, Figure 4D) show no evidence of ectopic expression of the EGFP-Cre fusion protein. Both EGFP fluorescence and immunohistochemistry for Cre recombinase indicate that expression of the EGFP-Cre fusion protein remains restricted to a subset of duct cells (Figure 2B,C and Figure 4D). We conclude that Ascl3-expressing cells, located in the ducts, are progenitors of LacZ-positive cells found in both duct and acinar compartments, and that the Ascl3 promoter is active in the precursor cells, and down-regulated in the descendants.
Duct and acinar cells are derived from a common precursor expressing Ascl3
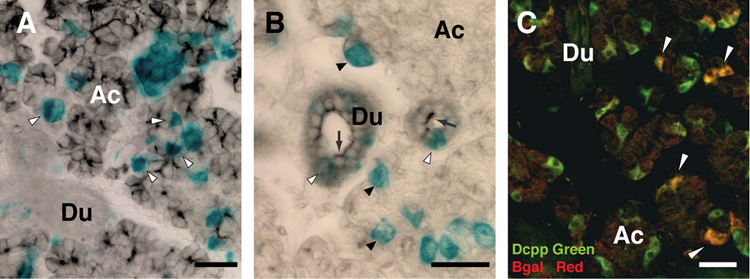
To characterize the LacZ-positive descendants of Ascl3-expressing precursors, LacZ staining was combined with immunohistochemistry, using antibodies to aquaporin 5 and cytokeratin 19, acinar and duct-specific proteins, respectively. Submandibular gland tissue was isolated from an Ascl3EGFP-Cre/+/Rosa26 adult female, fixed and stained for LacZ in whole mount (Figure 5A; blue). The tissue was then embedded in paraffin, sectioned and stained with antibody to aquaporin 5 (Figure 5A; black), a membrane protein specifically localized to the apical surface of acinar cells. The LacZ-positive cells residing in the acinar compartment are co-localized with the antibody to aquaporin 5 (Figure 5A, arrowheads), confirming that progeny of Ascl3-expressing cells include differentiated acinar cells.
Figure 5.
Ascl3-expressing cells generate both duct and acinar cell progeny. A. Co-localization of LacZ expression with the acinar cell-specific protein aquaporin 5. Submandibular gland isolated from 6-week-old female Ascl3EGFP-Cre+/Rosa26 mouse was fixed and stained for LacZ in whole mount (blue). Following post-fixation, the tissue was embedded in paraffin, sectioned and stained with antibody to aquaporin 5, which labels apical membranes of acinar cells (black). Arrows indicate cells in which aquaporin 5 protein is co-localized with LacZ, demonstrating that differentiated acinar cells are among the descendants of Ascl3-expressing cells. Duct cells (Du) are not positive for aquaporin 5. Ac, acinar cells. B. Co-localization of LacZ-positive progeny with duct cell-specific marker, cytokeratin 19. Submandibular gland tissue stained for LacZ expression (blue), was embedded, sectioned and probed with antibody to cytokeratin 19 (black), which is localized to the apical membrane of duct cells (arrows). LacZ-positive cells in the ducts show co-localization with cytokeratin 19 (white arrowheads); LacZ-positive cells in the acinar (Ac) compartment (black arrowheads) do not react with duct cell-specific antibodies. C. Co-localization of LacZ expression with Dcpp, a secretory protein expressed in serous demilune cells of the sublingual gland. Dcpp antibody localizes to serous demilune cells (green). Antibody to beta-galactosidase is shown in red. Co-localization of the two antibodies (yellow), is indicated by arrowheads, and demonstrates that Ascl3-expressing cells are precursors of demilune cells in the sublingual gland. Du, duct cells; Ac, acinar cells. Scale bars are 50 µm.
Similar staining was carried out combined with a duct cell-specific antibody to cytokeratin 19 (Figure 5B; arrows). The cytokeratin 19 antibody co-localizes with LacZ-positive cells in the ducts, which include both Ascl3-expressing cells and their ductal descendants (Figure 5B, white arrowheads). In contrast, LacZ-positive acinar cells (black arrowheads, Figure 5B) do not react with antibody to cytokeratin 19. These data clearly show that cells expressing the Ascl3 gene can generate both acinar and ductal cell types. An antibody to smooth muscle alpha actin, which marks myoepithelial cells, showed no co-localization with the LacZ stained cells, ruling out a contribution of Ascl3-expressing cells to the myoepithelial cell type (data not shown). We conclude that Ascl3 is expressed in progenitor cells that can differentiate to ductal and acinar, but not myoepithelial, cell lineages.
Ascl3-expressing cells are precursors of demilune cells in the sublingual gland
The mouse sublingual gland is primarily composed of mucous acini, capped by terminal serous demilune cells (reviewed in Young and Van Lennep 1978). These cells are distinguished by their production and secretion of demilune cell and parotid protein (Dcpp). Dcpp is initially expressed by immature acinar cells, but in the adult mouse is restricted to intercalated ducts of the parotid glands, and demilune cells in the sublingual gland (Bekhor et al. 1994). The LacZ-labeled cells in the sublingual gland appear to include both serous demilune and mucous acinar cells (see Figure 6B). To confirm the identity of these cells, we examined sections of sublingual glands from Ascl3EGFP-Cre/+/ Rosa26 mice, using antibodies to beta-galactosidase and Dcpp. Co-localization of these antibodies confirms that a significant number of the LacZ-positive cells are serous demilune cells (Figure 5C, arrowheads). This establishes that Ascl3-expressing cells of the sublingual duct are progenitors of the serous demilune cell type.
In contrast to the sublingual gland, the parotid gland has very few LacZ-positive cells outside the ductal compartment (Figure 4C). Dcpp is expressed in intercalated duct cells of the parotid gland, but double immunohistochemistry on parotid sections shows that there is little co-localization of LacZ-positive cells with Dcpp expression in these glands (data not shown).
Ascl3 expression marks a pool of adult progenitor cells that contribute to salivary gland maintenance
Differentiation in the sublingual gland is apparently complete by birth (Redman and Ball 1978; Wolff et al. 2002), and cells in the mature salivary glands turnover slowly (Zajicek et al. 1985; Redman 1995). However, in sublingual glands from 3-week- to 1-year-old Ascl3EGFP-Cre/+ /Rosa 26R mice, we observe a steady and significant increase in the number of LacZ-positive cells in the acinar compartment (Figure 6A–C). LacZ-positive progeny include acinar cells, serous demilune cells, and duct cells. However, immunohistochemistry for Cre recombinase demonstrates that Ascl3-expressing cells remain sequestered in the ducts over this time period (Figure 6D). These observations support our conclusion that progeny of Ascl3-expressing cells move from the duct to the acinar compartment and that, as they do so, the expression of Ascl3 is turned off.
In contrast to the sublingual gland, Ascl3-expressing cells in the parotid and submandibular glands yield predominantly duct cells and small numbers of acinar cells. Even in older adults, only small clusters of LacZ-positive acinar cells are detected in these glands (data not shown). Given the difference in cell composition between sublingual, submandibular and parotid glands, this observation indicates that Ascl3-expressing cells are progenitors of mucous and/or seromucous, but not serous, acinar cells. The LacZ-expressing progeny never completely populate the sublingual gland. This could reflect the inherent inefficiency of Cre recombinase, but may also indicate that maintenance of the salivary glands involves more than one type of progenitor cell.
Discussion
Tissue maintenance in the adult salivary glands has been proposed to occur both through division of fully differentiated cells (Redman and Sreebny 1970; Dardick et al. 1990; Denny et al. 1990; Redman 1995) and through the contribution of a stem cell-like progenitor pool (Zajicek et al. 1985; Ball et al. 1988; Denny et al. 1993; Chai et al. 1993; Takahashi et al. 1998; Denny and Denny 1999; Man et al. 2001). Long-term labeling studies have suggested that acinar and granulated duct cells in the rat and mouse submandibular glands apparently differentiate from intercalated duct cells (Zajicek et al. 1985; Chai et al. 1993; Denny and Denny 1999; Man et al. 2001), and that parotid intercalated ducts are the source of acinar and striated duct cells (Schwartz-Arad et al. 1988). Here we directly demonstrate the existence of a progenitor cell pool and establish the transcription factor Ascl3 as a marker for this population of cells. We show that these cells act as progenitors for both acinar and ductal descendants. Interestingly, the Ascl3-expressing cells are found not only in the intercalated ducts, but in all mature ducts of the mouse salivary glands. This is consistent with findings by other investigators. Recently, a small population of BrdU label-retaining cells was detected in intercalated and large excretory ducts, as well as in striated and granular ducts, of adult rat submandibular glands following an 8-week chase period (Kimoto et al. 2008). In addition, the intercalated duct cells demonstrate the most proliferation in the submandibular glands of young female mice, while in males the striated duct cells are more proliferative (Denny et al. 1993; Chai et al. 1993). Our data concur with these results, suggesting that progenitor cells may be more widely localized throughout the ductal system.
The Ascl3 gene is a member of the mammalian achaete scute homolog (Mash) gene family (Yoshida et al. 2001). Mash1 (Ascl1), a closely related member of the same family, is expressed in adult progenitors of both neurons and oligodendrocytes, and functions in the specification of these two lineages (Parras et al. 2004; Battiste et al. 2007). In adult brain, Mash1-expressing cells resemble undifferentiated transit amplifying cells and, following cell-cycle exit, Mash1 expression is transient in differentiating neuroblasts (Parras et al. 2004). Based on the lineage tracing of Ascl3-expressing cells and on the absence of detectable Ascl3 expression in differentiated LacZ-positive acinar cells, we speculate that Ascl3 may similarly distinguish adult progenitors in the salivary gland, which are committed to differentiate either to acinar or ductal cells.
Although Ascl3 is expressed in the embryonic salivary glands, there is no contribution to the acinar cell population before birth. However, postnatally, we observe a continuous increase in the number of LacZ-positive progeny, in both the duct and acinar cell compartments. The most significant contribution is observed in the sublingual gland, although development and final differentiation of the gland is reported to be complete at birth (Redman and Ball 1978; Wolff et al. 2002). We conclude that the LacZ-positive cells represent proliferating cell replacements, derived from Ascl3-expressing progenitors.
Clonal analysis has demonstrated the presence of multipotent cells in the submandibular gland that are capable of generating acinar, ductal and myoepithelial cell types (Kishi et al. 2006). Our data show that Ascl3-expressing cells represent progenitor cells of serous demilune cells, as well as some ductal and acinar cell progeny. However, not all cells in either the acini or the ducts of any salivary gland type are positive for LacZ. This indicates that Ascl3-expressing cells are clearly not progenitors of all salivary gland cell types, and are therefore not stem cells. The numbers of LacZ-negative serous and mucous acinar cells, as well as the unlabeled duct cells, suggests that Ascl3- expressing cells represent only a subset of the total progenitor cell population.
Maintenance of the salivary gland parenchyma is derived from the division of fully differentiated cells with age (Redman 1995; Denny and Denny 1999). Because the activation of LacZ is a heritable genetic event (Soriano 1999), division of differentiated LacZ-labeled acinar and duct cells will serve to increase the number of LacZ-positive cells in the acinar compartment. It is reasonable to assume that LacZ-positive acinar cells originate from Ascl3-expressing progenitors, and subsequently undergo further cell division in the acinar compartment, generating additional LacZ-positive progeny. Our results are consistent with the model of the salivary gland as a “slowly renewing” organ (Schwartz-Arad et al. 1988), in which cell replenishment is derived partially from progenitor cells, while the remainder can be attributed to division of differentiated cells.
The identification and isolation of stem cells in the salivary gland has been hampered in the past by a lack of specific markers. We have demonstrated that Ascl3-expressing progenitor cells located in the ducts contribute to both acinar and ductal cells in the mature gland. The identification of Ascl3 as a marker for a progenitor cell population is a critical step in the progress toward identifying the source of cells that are responsible for salivary gland maintenance and regeneration.
Materials and methods
Insertion of EGFP-Cre cassette by gene targeting
A gene targeting vector was constructed to include 5′ and 3′ homologous arms of Ascl3 sequence that were generated by PCR using an isolated BAC clone (BPRC, Oakland, CA.) containing 129S6/SvEvTac mouse genomic DNA as template. Design of the PCR primers was based on available sequence (GenBank accession no. NM_020051). The 5′ arm of homology extends 3.2 kb from the start of the first exon to the ATG initiation codon located within the second exon. Primers used for amplifying this sequence were: forward 5′-CATCTGGTGCCAGCCTTGCTGC-3′ and reverse 5′- AAATATGCGGCCGCCGTTTCCTTTCACCTAGAAAC-3′, which includes a NotI restriction site for cloning. The 3′ arm of homology (2.8 kb) extends into the 3′ untranslated region of the Ascl3 gene. Primers for this arm were: forward 5′- ACAGCTAGCGTAGCTCATGTAGAC-3′ and reverse 5′- GATAAGCTGCTTGCTGTGAAAGC-3′. The EGFP-Cre expression cassette was a gift from Dr. William Bowers (University of Rochester). The coding sequences for both EGFP and Cre recombinase are fused, and a β-globin polyadenylation site was added at the 3′ end of the sequence. The EGFP-Cre expression cassette was inserted in-frame with the ATG codon of the Ascl3 coding sequence at the introduced NotI site. The backbone of the targeting vector also includes the neomycin resistance gene driven by the phosphoglycerate kinase promoter, and the gene encoding alpha subunit of diptheria toxin (DTA) for negative selection. The targeting construct was introduced into CJ7 ES cells (a gift from Dr. Rulang Jiang, University of Rochester) by electroporation, and four positive clones were isolated. The targeted clones were injected into blastocysts and the recombined allele was passed through the germline. Two strains were generated from separate targeted ES clones, and both were used for this study.
Mouse colony maintenance and genotyping
Ascl3EGFP-Cre/+ animals were maintained on a C57Bl/6 background. Heterozygotes were crossed with homozygotes from the Rosa26R reporter mouse strain (Gt(ROSA)26Sortm1Sor; Soriano 1999). Genotyping was performed with the following PCR primers: Ascl3: forward 5′-CCACCCCAGTGCCTCTACACAAAT-3′, reverse 5′- GTCGCTGGAGAAGGGCAGCAGA-3′, and Cre reverse 5′- GGTGTACGGTCAGTAAATTGGAC-3; LacZ: forward 5′- GCGCCCATCTACACCAACGTG-3′, and reverse 5′- CCAGCGCAGCACCATCACCGC-3′. Double heterozygotes were identified and used for the analysis of ß-galactosidase (LacZ) expression. Mice were maintained on a 12-h light, 12-h dark schedule with ad libitum access to food and water. All procedures and protocols were approved by the University Committee on Animal Resources at the University of Rochester.
Staining for B-galactosidase activity
Animals were euthanized and the salivary glands were isolated. For whole mount staining, glands were fixed overnight at 4°C in 0.2% glutaraldehyde, 2 mM MgCl2, 5mM EGTA, in 0.1M potassium phosphate (KPO4) buffer, pH 7.3. Tissue was rinsed 3 times 15 minutes in detergent rinse [2mM MgCl2, 0.01% sodium deoxycholate, 0.02% IGEPAL (Sigma Chemical Co.), in 0.1 M potassium phosphate (KPO4) buffer, pH 7.3]. Staining was performed overnight as described (Nagy et al. 2003). The stained tissues were post-fixed in 4% paraformaldehyde, processed through ethanol gradations, embedded in paraffin and sectioned for immunohistochemistry. For frozen sections, glands were isolated and fixed in 0.2% paraformaldehyde in 0.1M PIPES (pH 6.9) at 4°C. Tissues were rinsed in 1xPBS, equilibrated in 30% sucrose overnight and embedded in OCT compound (Tissue-Tek) for sectioning. Sections of 15 microns were post-fixed in 0.2% paraformaldehyde on ice for 10 minutes, rinsed in PBS with 2mM MgCl2 and processed for staining as described (Nagy et al. 2003).
In situ hybridization
The coding sequence of Ascl3 was generated by PCR using the following primers: Ascl3F: 5’-GGAAACGATGGACACCAGAAGC-3’; Ascl3R: 5’- CCCAGGCAAGCAACATTTAATG-3’, and cloned into Topo 2.1 vector (Invitrogen). In situ hybridization was performed on paraffin sections of submandibular gland isolated from a female Ascl3EGFP-Cre/+ mouse 3 months of age. Slides were processed for in situ hybridization as described (Palis and Kingsley 1995) using in vitro transcribed sense and antisense strand RNA probes labeled with 33P. Darkfield and brightfield images were obtained with a Nikon Optiphot microscope (Nikon, Melville, New York, United States) and SPOT RT-Slider digital camera (Diagnostic Instruments, Sterling Heights, Michigan, United States).
Immunohistochemistry
Submandibular, sublingual and parotid glands were collected and fixed in 4% paraformaldehyde in PBS for 2 – 12 hours, embedded in paraffin, and sectioned at 7 microns. Tissue sections were deparaffinized, and subjected to antigen retrieval in 1mM EDTA buffer, pH 8.0 for 10 minutes with heating. Sections were allowed to cool, washed in PBS and subjected to blocking and primary antibody incubation. For Cre recombinase immunohistochemistry, sections were blocked in 10% normal donkey serum with 10% milk in PBS for 1 hour at room temperature and then incubated in a polyclonal anti-Cre recombinase antibody (1:600; Covance) diluted in antibody diluent (Dako Cytomation), for 2 hours at room temperature. All other sections were blocked in 5% normal donkey serum for 45 minutes at room temperature, washed in 3 changes of PBS and incubated in the primary antibody for 1 hour at room temperature, or overnight at 4°C. The primary antibodies used were goat polyclonal cytokeratin 19 (1:100; Santa Cruz) and goat polyclonal aquaporin 5 (1:100; Santa Cruz). Secondary antibodies used were CY3-labelled donkey anti-chicken or CY2-labelled donkey anti-goat (Jackson ImmunoResearch). Nuclei were labeled with ToPro3 (Invitrogen). For beta-galactosidase antibody staining, frozen sections were prepared as for LacZ enzymatic staining, described above. Sections were cut at 16 microns, post-fixed, washed in detergent rinse, and incubated overnignt at 4°C with chicken polyclonal beta-galactosidase antibody (1:1500; Abcam). CY3-labelled donkey anti-chicken was used as the secondary antibody (Jackson ImmunoResearch). Double-labeling immunohistochemistry with antibodies to beta-galactosidase (chicken polyclonal) and Dcpp (rabbit polyclonal) was performed on frozen sections. Secondary antibodies used were CY3-labelled donkey anti-chicken, and CY2-labeled donkey anti-rabbit (Jackson ImmunoResearch).
Imaging
Fluorescent images were collected using a Leica TCS SPII confocal microscope and Leica confocal software. Images of Lac-Z stained tissue were collected using an Olympus DX41 microscope connected to a DSL camera, and using DSL Manager software. Double-labeled images were generated using Adobe Photoshop.
Acknowledgments
The authors gratefully thank Dr. James Melvin for support, valuable discussion, and critical reading of the manuscript. We also thank Dr. Dirk Bohmann for comments and critical reading of the manuscript. The technical assistance of Jennifer Scantlin, Mark Wagner, Jamie Littleton and Pamela McPherson is gratefully acknowledged. This work was supported by NIH/NIDCR grant R21 DE017386-01 to C.E.O. T.B. was supported by NIH/NIDCR Training grant 2T32DE007202-17A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ball W, et al. Secretory proteins as markers for cellular phenotypes in rat salivary glands. Dev. Biol. 1988;125:265–279. doi: 10.1016/0012-1606(88)90210-2. [DOI] [PubMed] [Google Scholar]
- Battiste J, et al. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134:285–293. doi: 10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- Bekhor I, et al. cDNA cloning, sequencing and in situ localization of a transcript specific to both sublingual demilune cells and parotid intercalated duct cells in mouse salivary glands. Arch. Oral Biol. 1994;39:1011–1022. doi: 10.1016/0003-9969(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Cau E, et al. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. doi: 10.1242/dev.124.8.1611. [DOI] [PubMed] [Google Scholar]
- Chai Y, et al. Proliferative and structural differences between male and female mouse submandibular glands. Anat. Rec. 1993;235:303–311. doi: 10.1002/ar.1092350214. [DOI] [PubMed] [Google Scholar]
- Chang WWL. Cell population changes during acinus formation in the postnatal rat submandibular gland. Anat. Rec. 1974;178:187–202. doi: 10.1002/ar.1091780204. [DOI] [PubMed] [Google Scholar]
- Cutler LS, Chaudhry AP. Cytodifferentiation of the acinar cells of the rat submandibular gland. Dev. Biol. 1974;41:31–41. doi: 10.1016/0012-1606(74)90280-2. [DOI] [PubMed] [Google Scholar]
- Dardick I, et al. A review of the proliferative capacity of major salivary glands and the relationship to current concepts of neoplasia in salivary glands. Oral Surg. Oral Med. Oral Path. 1990;69:53–67. doi: 10.1016/0030-4220(90)90269-x. [DOI] [PubMed] [Google Scholar]
- Denny PC, et al. Three-dimensional reconstruction of adult female mouse submandibular gland secretory structures. Anat. Rec. 1990;226:489–500. doi: 10.1002/ar.1092260411. [DOI] [PubMed] [Google Scholar]
- Denny PC, et al. Parenchymal cell proliferation and mechanisms for maintenance of granular duct and acinar cell populations in adult male mouse submandibular gland. Anat. Rec. 1993;235:475–485. doi: 10.1002/ar.1092350316. [DOI] [PubMed] [Google Scholar]
- Denny PC, Denny PA. Dynamics of parenchymal cell division, differentiation, and apoptosis in the young adult female mouse submandibular gland. Anat. Rec. 1999;254:408–417. doi: 10.1002/(SICI)1097-0185(19990301)254:3<408::AID-AR12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Gresik EW. Postnatal developmental changes in submandibular glands of rats and mice. J. Histochem. Cytochem. 1980;28:860–870. doi: 10.1177/28.8.6160181. [DOI] [PubMed] [Google Scholar]
- Guillemot F, et al. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Kimoto M, et al. Label-retaining cells in the rat submandibular gland. J. Histochem. Cytochem. 2008;56:15–24. doi: 10.1369/jhc.7A7269.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, et al. Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem. Biophys. Res. Comm. 2006;340:544–552. doi: 10.1016/j.bbrc.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Küry P, et al. Mammalian achaete scute homolog 2 is expressed in the adult sciatic nerve and regulates the expression of Krox24, Mob-1, CXCR4, and p57kip2 in Schwann cells. J. Neurosci. 2002;22:7586–7595. doi: 10.1523/JNEUROSCI.22-17-07586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man YG, et al. Contributions of intercalated duct cells to the normal parenchyma of submandibular glands of adult rats. Anat. Rec. 2001;263:202–214. doi: 10.1002/ar.1098. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, et al. Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: immunolocalization and effect of secretory stimulation. Cell Tissue Res. 1999;295:513–521. doi: 10.1007/s004410051257. [DOI] [PubMed] [Google Scholar]
- Nagy A, et al. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- Nowroozi N, et al. Two gene products for beta-galactosidase are differentially expressed in the mouse salivary glands. J. Craniofac. Genet. Dev. Biol. 1998;18:51–57. [PubMed] [Google Scholar]
- Okumura , et al. Salivary gland progenitor cells induced by duct ligation differentiate into hepatic and pancreatic lineages. Hepatology. 2003;38:104–113. doi: 10.1053/jhep.2003.50259. [DOI] [PubMed] [Google Scholar]
- Palis J, Kingsley PD. Differential gene expression during early murine yolk sac development. Mol. Reprod. Dev. 1995;42:19–27. doi: 10.1002/mrd.1080420104. [DOI] [PubMed] [Google Scholar]
- Parras CM, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman RS. Proliferative activity by cell type in the developing rat parotid gland. Anat. Rec. 1995;241:529–540. doi: 10.1002/ar.1092410411. [DOI] [PubMed] [Google Scholar]
- Redman RS, Ball WD. Cytodifferentiation of secretory cells in the sublingual gland of the prenatal rat: a histological, histochemical and ultrastructural study. Am. J. Anat. 1978;153:367–390. doi: 10.1002/aja.1001530304. [DOI] [PubMed] [Google Scholar]
- Redman RS, Sreebny LM. Proliferative behavior of differentiating cells in the developing rat parotid gland. J. Cell Biol. 1970;46:81–87. doi: 10.1083/jcb.46.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman RS, Sreebny LM. Morphologic and biochemical observations on the development of the rat parotid gland. Dev. Biol. 1971;25:248–279. doi: 10.1016/0012-1606(71)90030-3. [DOI] [PubMed] [Google Scholar]
- Schwartz-Arad D, et al. The rat parotid gland – a renewing cell population. J. Anat. 1988;161:143–151. [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Takahashi S, et al. Origin of acinar cell regeneration after atrophy of the rat parotid induced by duct obstruction. Int. J. Exp. Pathol. 1998;79:293–301. doi: 10.1046/j.1365-2613.1998.710405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AS. Salivary gland development. Sem. Cell Dev. Biol. 2007;18:237–244. doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Wolff MS, et al. Development of the rat sublingual gland: a light and electron microscopic immunocytochemical study. Anat. Rec. 2002;266:30–42. doi: 10.1002/ar.10027. [DOI] [PubMed] [Google Scholar]
- Yoshida S, et al. Sgn1, a basic helix-loop-helix transcription factor delineates the salivary gland duct cell lineage in mice. Dev. Biol. 2001;240:517–530. doi: 10.1006/dbio.2001.0473. [DOI] [PubMed] [Google Scholar]
- Young JA, Van Lennep EW. The Morphology of Salivary Glands. New York: Academic Press; 1978. [Google Scholar]
- Zajicek G, et al. The streaming submandibular gland. Anat. Rec. 1985;213:150–158. doi: 10.1002/ar.1092130206. [DOI] [PubMed] [Google Scholar]