Abstract
The hippocampal formation is generally considered essential for processing episodic memory. However, the structural organization of hippocampal afferent and efferent axonal connections is still not completely understood, although such information is critical to support functional hypotheses. The full extent of axonal projections from field CA1 to the interbrain (diencephalon) is analyzed here with the Phaseolus vulgaris-leucoagglutinin (PHAL) method. The ventral pole of field CA1 establishes direct pathways to, and terminal fields within, the anterior hypothalamic nucleus, ventromedial hypothalamic nucleus, lateral hypothalamic and lateral preoptic areas, medial preoptic area, and certain other hypothalamic regions, as well as particular midline thalamic nuclei. These results suggest that hippocampal field CA1 modulates motivated or goal-directed behaviors, and physiological responses, associated with the targeted hypothalamic neuron populations.
Indexing terms: hypothalamus; lateral hypothalamic area; subiculum, thalamus
Introduction
Field CA1 of Ammon's horn is an integral component of the hippocampal formation (as defined by Blackstad, 1956; see Swanson et al, 1987), and it displays unique structural, physiological, and molecular features among the various hippocampal cortical areas (Zhao et al., 2001). Although the fundamental organization of intrahippocampal circuitry has been relatively well known for some time (Blackstad, 1956; Anderson et al., 1971), the scope and depth of extrahippocampal projections are not yet fully appreciated. With the autoradiographic method Swanson and Cowan (1975, 1977a,b) established that the century old notion of Ammon's horn (fields CA1-CA3) as the source mammillary body inputs via the descending column of the fornix is incorrect—they arise in the subicular complex—and confirmed earlier reports of field CA1 axonal projections directly to the subiculum, entorhinal area, and lateral septal nucleus (Cajal, 1909-1911; Lorente de Nó, 1934; Blackstad, 1956; Nauta, 1956, 1958; Raisman et al., 1966; Hjorth-Simonsen, 1973; Meibach and Siegel, 1977). They also demonstrated that the general projection pattern of field CA1 is not homogenous throughout. Instead, there is a differential, topographic origin of specific projections along field CA1's longitudinal axis, commonly referred to as the dorsoventral or septotemporal axis. Further evidence for this connectional heterogeneity was provided by the discovery of a ventral field CA1 axonal projection to rostroventral regions of the medial prefrontal cortex using anterograde and retrograde tracers (Swanson, 1981).
Thus, through the 1980s known outputs of field CA1 could be divided into two major classes, one descending through the fimbria and precommissural fornix directly to the lateral septal nucleus, and the other consisting of association projections to other cortical areas—directly to the subiculum and entorhinal area of the hippocampal formation, and to the medial prefrontal cortex; and indirectly via multsynaptic pathways to broad regions of the temporal lobe, starting with “relays” in parahippocampal regions of the temporal lobe adjacent to the hippocampal formation (see Swanson et al., 1987). This view was expanded in the first neuroanatomical study devoted exclusively to field CA1 projections (van Groen and Wyss, 1990a). Using PHAL, they described field CA1 projections directly to the subiculum, postsubiculum, parasubiculum, and entorhinal area of the hippocampal formation; the retrosplenial area, perirhinal area, and medial prefrontal region of the cerebral cortex; the olfactory bulb and anterior olfactory nucleus; parts of the amygdala and hypothalamus; and even some cortical commissural projections.
The analysis presented here represents the first systematic reexamination of field CA1 projections since then, and focuses on interbrain targets. Inputs to the cerebral nuclei and cerebral cortex are presented in following papers. The pioneering study of van Groen and Wyss (1990a) has been improved in three ways: considerably smaller injection sites, many of which are clearly restricted entirely to field CA1 as they and we define it, were made; many more experiments, which together involve virtually all regions of field CA1, were prepared; and more comprehensive series of histological sections throughout the forebrain were collected for a higher resolution analysis. The results indicate that field CA1 extrinsic projections are even more extensive, and topographically organized, than recognized previously—and that some projections reported by van Groen and Wyss (1990a) arise in the two cortical areas on either side of field CA1—field CA3 and the subiculum.
Materials and Methods
The materials and methods were similar to those described previously (Gerfen and Sawchenko, 1984; Groenewegen and Wouterlood, 1990; Canteras and Swanson, 1992; Smith, 1992). Experiments were performed according to the NIH Guidelines for the Care and Use of Laboratory Animals, and all protocols were approved by the University of Southern California Institutional Animal Care and Use Committee. The experiments described here were chosen from a collection of over 60 PHAL injections throughout field CA1. Adult male Harlan Sprague-Dawley rats (250-325 g) received a single, stereotaxically placed iontophoretic injection of a 2.5% solution of PHAL (Vector Laboratories, Burlingame, CA), prepared in 0.01 M sodium phosphate-buffered saline (NaPBS), pH 7.4, into various regions of field CA1 through a glass micropipette (10-15 μm tip diameter) by applying a positive current (5 μA, 7 second on/off intervals) for 7-10 minutes. Animals were anesthetized for stereotaxic surgery with an equal mixture of ketamine and xylazine solutions (100 mg/ml ketamine and xylazine) given 1 ml/kg body weight, intramuscularly as needed.
After surviving 14-17 days, the rats were deeply anesthetized with pentobarbital (35-50 mg/kg body weight, intraperitoneally) and perfused transcardially with 150 ml of 0.9% NaCl followed by 400 ml of ice-cold 4% paraformaldehyde in 0.1 M borate buffer (pH 9.5). Brains were removed, postfixed overnight at 4°C in the same fixative containing 10% sucrose, and frozen. Then, serial 30 μm-thick sections (1-in-4) were cut in the transverse plane on a sliding microtome. One complete series of sections was processed to detect PHAL using the immunohistochemical procedure described elsewhere (Gerfen and Sawchenko, 1984). An adjacent series of sections was stained with thionin to determine quickly cytological boundaries. Brightfield and darkfield photomicrographs were taken with a CCD camera (Diagnostics Instruments, Sterling Heights, MI). Darkfield photomicrographs also were taken with a Wild Leitz 35 mm camera mounted on a Wild M3Z stereozoom microscope; film was scanned with a Nikon 35 mm Film Scanner (LS-1000) into Adobe PhotoShop 5, where standard methods were used to compose and adjust brightness and contrast on a Mac PowerPC G4. Parceling of the rat brain, terminology for describing morphologic features of PHAL-labeled axons, and mapping strategies and procedures follow Swanson (2004), unless indicated otherwise. More information on the new lateral hypothalamic area (LHA) parceling scheme was recently published (Swanson et al., 2005).
The overall spatial distribution of each injection site is plotted onto an unfolded map or “flatmap” of the hippocampal formation (Fig. 1). The unfolded map represents the surface of a solid model of the hippocampal formation that was reconstructed from a series of transverse sections (Swanson et al., 1978; Petrovich et al., 2001). A plot display of injection sites on the unfolded map allows rapid, qualitative analysis and comparison of injection site distribution.
Fig. 1.
Distribution of injection sites centered in hippocampal field CA1. Relative locations of PHAL injections from 62 experiments, plotted onto an unfolded map of the rat hippocampal formation (adapted from Petrovich et al., 2001). Injection sites filled with black illustrate experiments with injection sites limited to, or contained within, the ventral pole of field CA1. This triangular region occupies about 10% of field CA1's total surface area.
Results
A major finding is the presence of PHAL-labeled axons in broad regions of the hypothalamus. These projections are labeled solely by injections that label neurons in the ventral pole of what has traditionally been regarded as field CA1 in the rat. Inputs to the hypothalamus are represented by an iontophoretic injection of PHAL (Experiment HIPPO103) confined to the ventral pole of field CA1, and centered next to the medial border with field CA2 (Figs. 1-3), away from the lateral border with the subiculum. Labeled hypothalamic projections are predominantly ipsilateral, with contralateral exceptions as noted.
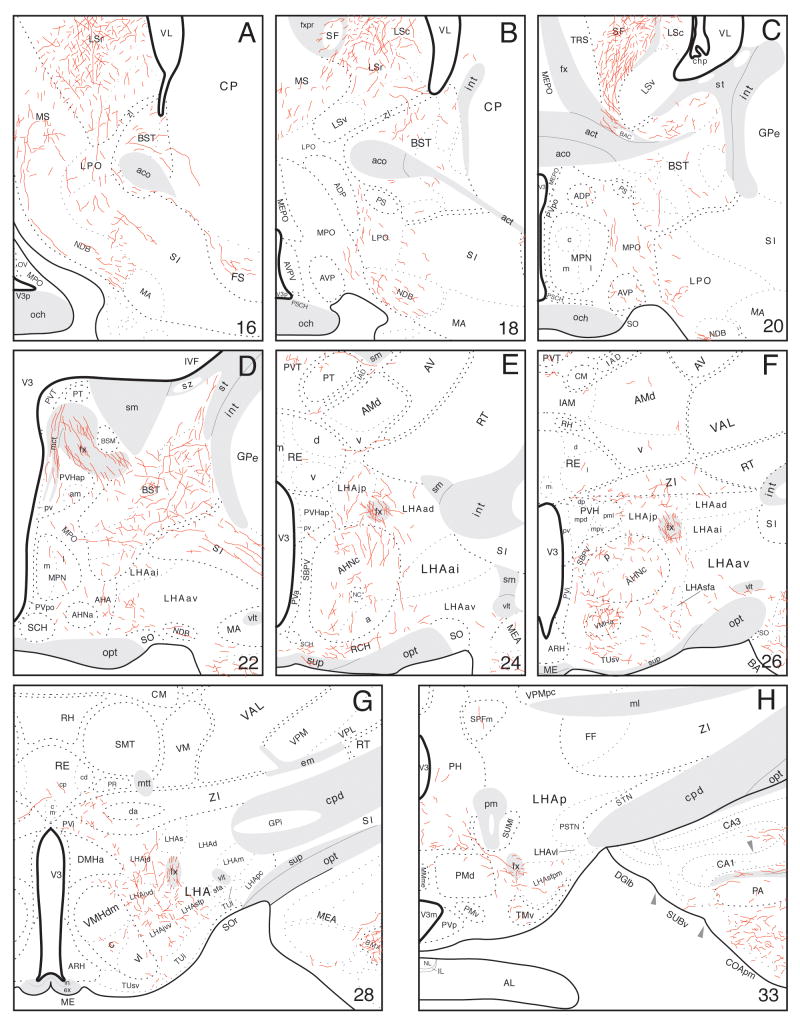
Fig. 3.
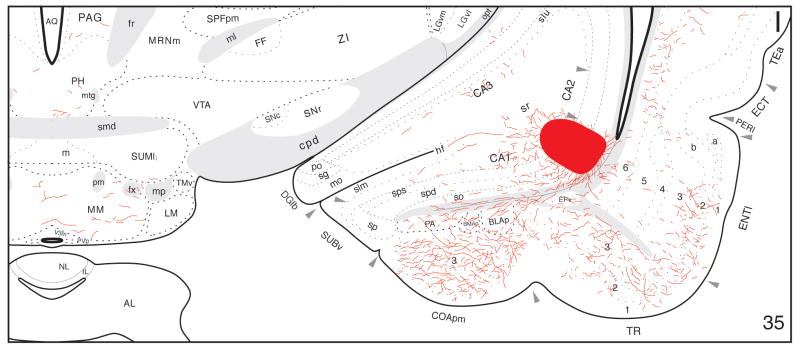
A-I: Distribution of PHAL-labeled axons (red lines) plotted onto a series of rat brain reference drawings (adapted from Swanson, 2004), cut in the transverse plane and arranged from rostral (A) to caudal (I)—after an injection centered in the ventral pole of hippocampal field CA1 (red area in I).
From the injection site, PHAL-immunoreactive axons enter the alveus and extend rostrally through the fimbria, eventually to innervate mainly the hypothalamic lateral zone and medial nuclei. The most prominent group of labeled axons travels through the postcommissural column of the fornix in a fairly tight bundle, with fibers exiting at various levels to innervate particular hypothalamic cell groups (Fig. 3D-I). These fibers may represent collaterals of parent axons continuing farther caudally through the column of the fornix, although this cannot be stated unequivocally with the present method. Near the rostral pole of the thalamus, a small group of axons diverges from the postcommissural fornix to enter the medial corticohypothalamic tract and innervate regions near the third ventricle. The second most prominent group of labeled axons extends ventrally from the lateral septal nucleus—a major striatal target of field CA1 by way of the precommissural fornix—to enter the lateral preoptic area (Fig. 3A). These fibers appear to generate a terminal field in the lateral preoptic area, and to be part of a larger group that also innervates laterally adjacent regions of the substantia innominata.
Inputs through the column of the fornix
The lateral zone
The LHA receives the strongest field CA1 projection of any hypothalamic component. LHA medial regions receive a moderate projection of varicose fibers and terminal boutons from the rostral end of the LHA to about mid-rostrocaudal levels of the ventromedial nucleus, where the projection becomes considerably denser (Fig. 3D-G). Rostroventrally, a tight group of PHAL-labeled fibers is observed in the ventromedial corner of the LHA anterior region's ventral zone, and a few labeled fibers are also seen in the intermediate zone (Fig. 3D-F). Rostrodorsally, a moderate plexus of varicose fibers with boutons occupies the LHA juxtaparaventricular region and an adjacent medial expanse of the anterior region's dorsal zone, dorsal to the anterior hypothalamic nucleus (Fig. 3E,F). At the level of the ventromedial nucleus, the terminal field becomes significantly denser (Fig. 3G). Here, the LHA's juxtadorsomedial region, and dorsal and ventral zones of the juxtaventromedial region, all contain a moderately dense terminal field.
Innervation of the LHA's perifornical tier includes a moderate terminal field in the posterior zone, a light terminal field in the premammillary zone, and a few labeled axons in the anterior zone of the subfornical region (Fig. 3F-H).
Overall, field CA1 inputs tend to avoid LHA regions lateral to the fornix. However, it is worth noting that in the anterior group a few labeled axons extend ventrolaterally from the fornix to end with a small number of boutons in lateral expanses of the anterior region's ventral zone, ventral to the substantia innominata and medial to the medial amygdalar nucleus (Fig. 3E,F). Medially, there is also a light projection to the retrochiasmatic area, between the anterior nucleus and the optic tract and supraoptic commissures (Fig. 3E); in fact, some of these fibers are in the supraoptic commissures and cross the midline to innervate the contralateral retrochiasmatic area. At this same level, a small plexus of varicose fibers is also observed in the subparaventricular zone of the periventricular region. Caudal to the retrochiasmatic area, rostral regions of the tuberal nucleus's subventromedial part are moderately innervated, whereas the terminal field diminishes to just a few scattered fibers toward the caudal pole (Fig. 3F-G).
The medial nuclei
At anterior hypothalamic levels, labeled axons are dispersed in the anterior hypothalamic area ventral to the bed nuclei of the stria terminalis. The central part of the anterior hypothalamic nucleus is moderately innervated with varicose fibers and a small number of terminal boutons (Fig. 3E,F). A few axons enter the anterior and posterior parts of the nucleus, but none are observed in the dorsal part.
The anterior part of the ventromedial nucleus is moderately innervated, whereas the central part is lightly innervated, and only a few isolated fibers are observed in the ventrolateral part (Fig. 3G,H). In experiments with larger injection sites, significantly denser terminal fields in the anterior and central parts of the ventromedial nucleus are observed (Fig. 4). Interestingly, labeled fibers are never observed in the dorsomedial part of the ventromedial nucleus (or in the prominent, overlying dorsomedial nucleus of the periventricular region).
Fig. 4.
A-B: Darkfield photomicrographs showing the distribution of PHAL-labeled axons in the hypothalamus at the level of the anterior hypothalamic nucleus (A), and the ventromedial hypothalamic nucleus (B). C-D: Brightfield photomicrographs demonstrating the location of the injection site (C) and an adjacent thionin-stained section (D) in Experiment HIPPO161. Scale bars: 200 μm.
The field CA1 projection to hypothalamus ends with the column of the fornix in the mammillary body. The medial mammillary nucleus receives a light input that extends as a few axons across the midline to medial regions of the contralateral medial nucleus. A few labeled axons also extend into adjacent cell groups, including the ventral tuberomammillary, dorsal premammillary, and lateral supramammillary nuclei. Finally, ventral regions of the posterior hypothalamic nucleus receive a light input, and a few scattered axons are also observed in the contralateral posterior nucleus (Fig. 3H-F).
Inputs through the medial corticohypothalamic tract
In addition to its projection through the column of the fornix, field CA1 sends a tiny bundle of axons into the medial corticohypothalamic tract, a divergence that occurs just after the fornix descends caudal to the anterior commissure (Figs. 3D and 5). These axons appear to innervate lightly the hypothalamic periventricular region, and the rostral thalamus (see below). Because neurons in ventral regions of the adjacent subiculum are the only reported source of the medial corticohypothalamic tract (see Canteras and Swanson, 1992), this is indeed a new observation. On leaving the medial corticohypothalamic tract, a few labeled axons traverse the anterior parvicellular and anterior magnocellular parts of the paraventricular hypothalamic nucleus (Fig. 3D). Axons in the former are virtually free of boutons and varicosities, whereas those in the latter appear to generate small numbers of varicosities, terminal boutons, and boutons of passage. Fibers continue rostroventrally from the paraventricular nucleus to innervate lightly lateral regions of the medial preoptic area (Fig. 3B,C). This pathway generates a light projection with terminal boutons to the anteroventral preoptic nucleus, and sends a few solitary fibers to the anterodorsal preoptic nucleus.
Fig. 5.
Darkfield photomicrograph showing the divergence of PHAL-labeled axons from the fimbria to the medial corticohypothalamic tract, in a transverse section from an experiment with an injection confined to the ventral pole of hippocampal field CA1. Figure 3D is at approximately the level of this photomicrograph. Scale bar: 200 μm.
In some experiments, sparse labeling is observed in the lateral part of the medial preoptic nucleus, and in the anteroventral periventricular nucleus. In addition, a few scattered, varicose fibers are observed in the lateral zone of the paraventricular nucleus's posterior magnocellular part. The latter fibers actually appear to arrive via the descending column of the fornix at the level of the anterior hypothalamic nucleus, rather than via the medial corticohypothalamic tract (Fig. 3E).
Inputs through the precommissural fornix
There is a relatively moderate projection in the lateral preoptic area, adjacent to fibers in the medial preoptic area (Fig. 3B,C). While many fibers in the lateral preoptic area generate terminal boutons and boutons of passage, roughly half of them continue laterally into the adjacent substantia innominata. It appears likely that this projection to and through the lateral preoptic area—or some fraction of it—represents a separate projection pathway arising from axons that extend ventrally from the lateral septal nucleus; other methods will be needed to determine the precise structural organization of this complex part of the descending projection system from ventral field CA1. In any event, whereas labeled field CA1 axons in caudal levels of the preoptic region typically run parallel to one another, fibers projecting specifically through the lateral preoptic area appear to descend caudoventrally from the septal region instead of rostroventrally through the medial preoptic area (from the column of the fornix; see above). Many fibers in the lateral preoptic region end abruptly in its caudoventromedial tip. Cajal (1909-1911) referred to axons descending from the region of the lateral septal nucleus to the preoptic region as lateral arcuate fibers.
Inputs to the thalamus
Field CA1 inputs to the thalamus arrive through two routes and are very light—often appearing only as scattered fibers—but are distinctive enough to warrant attention. The paraventricular thalamic, paratenial, and interanteromedial nuclei receive very light projections that extend caudally from the medial corticohypothalamic tract, and some of these fibers extend across the midline into the contralateral paraventricular and interanteromedial nuclei. In addition, scattered fibers are seen in the nucleus reuniens and anteromedial nucleus (Fig. 3E,F). A second projection extends dorsally from the column of the fornix, at the level of the anterior hypothalamic nucleus, lightly to innervate rostromedial regions of the zona incerta (Fig. 3E-G). This projection selectively avoids the zona incerta region with a concentration of dopaminergic cell bodies (the A13 cell group). Caudal and lateral regions of the zona incerta are devoid of labeled axons. At this level, scattered fibers are also observed bilaterally in the nucleus reuniens and anteromedial nucleus of the thalamus.
Additional observations
Eight experiments involving ventral regions of field CA1 label projections to the hypothalamus (Fig. 1), and all of those confined to field CA1 label the same overall projection pattern. For example, Experiment HIPPO161 has a large injection site centered in the most ventral tip of field CA1 (Fig. 1). The projection pattern observed in this experiment is virtually identical to that observed in Experiment HIPPO103 (described above), although the density of terminal fields in the former is clearly greater than in the latter.
The anatomical boundaries of field CA1 used in the present study are derived from the original work of Blackstad (1956) and discussed further in Swanson and colleagues (1987). The ventral end of field CA1 appears to be triangular, and comes to a point with no transverse dimension. Therefore, only differences along the longitudinal axis are possible in the ventral end of field CA1 with the method used here. No reliable topographic organization of projections was detected from the ventral region of field CA1 indicated in Figure 1, which occupies about 10% of field CA1's surface area as measured from the flatmap.
Discussion
The present results document for the first time axonal projections from the ventral end of hippocampal field CA1 to widespread hypothalamic cell groups, as well as the existence of inputs directly to the thalamus. Novel observations include moderate inputs to the lateral preoptic area, medial regions of the LHA, and the anterior part of the ventromedial nucleus. Light terminal fields are also generated in other regions of the LHA (including its retrochiasmatic area and tuberal nucleus, and the ventral region's lateral zone), and the medial preoptic and anteroventral periventricular nuclei, central part of the ventromedial nucleus and subparaventricular zone, and the medial mammillary and posterior hypothalamic nuclei. Very sparse projections are observed consistently in the anterodorsal preoptic, anteroventral periventricular, paraventricular hypothalamic, ventrolateral part of the ventromedial, dorsal premammillary, and lateral mammillary nuclei. Another novel observation is the presence of PHAL-labeled fibers from ventral field CA1 in the medial corticohypothalamic tract.
The only previously described input from ventral field CA1 to the interbrain is a dense cluster of labeled fibers caudally in the anterior hypothalamic region (van Groen and Wyss, 1990a), which is confirmed here as a moderate projection to the central and posterior parts of the anterior hypothalamic nucleus. However, we did not confirm their report of an input to the anterior part of the dorsomedial hypothalamic nucleus. Instead, a terminal field in the laterally adjacent juxtadorsomedial region of the LHA is labeled, and it selectively avoids the dorsomedial nucleus. This minor difference is probably explained by their much larger injection sites (30 minutes of continuous current was used to deposit tracer), which may well have spread to include adjacent regions of the ventral subiculum (Fig. 1)—an area known to innervate strongly the dorsomedial nucleus (Canteras and Swanson, 1992; Kishi et al., 2000). Kishi and colleagues (2000) also illustrated, but did not comment on, retrogradely labeled neurons in ventral field CA1 after cholera toxin b injections in the anterior hypothalamic nucleus, which we confirmed with PHAL. Many parts of the hypothalamus identified here as receiving inputs from ventral field CA1 may have been identified because the analysis was at much higher resolution (1-in-4 vs. 1-in-10 series of sections) than previous work (van Groen and Wyss, 1990a).
This is also the first explicit description of a field CA1 projection to thalamus. Risold and colleagues (1997) illustrated but did not comment on retrogradely labeled neurons in ventral field CA1 after fluorogold injections into the region of the thalamus containing the paratenial, paraventricular, and reuniens nuclei. In the present material, light inputs were also noted to the interanteromedial and anteromedial nuclei, and to rostromedial regions of the zona incerta (apparently avoiding the dopaminergic A13 group).
Whereas the very small PHAL injections employed here are useful for labeling neuron populations restricted to very small areas like ventral field CA1, and for analyzing the topographic organization of projections, a disadvantage is that the overall densities of pathways and terminal fields are under-estimated. All projections described here arise from only a very small (currently unknown) percentage of the total neuron population in ventral field CA1. Thus, the total output of ventral field CA1 to hypothalamic cell groups is relatively substantial compared to the rather sparse distributions labeled by the current experiments. This is confirmed by the considerably denser projection to anterior hypothalamus reported by van Groen and Wyss (1990a) and by the relatively denser terminal fields labeled by our larger injections (see Fig. 4).
Previously, neurons in ventral subiculum were the only known source of fibers projecting through the medial corticohypothalamic tract (see Swanson and Cowan, 1975; Canteras and Swanson, 1992). The origin of some medial corticohypothalamic tract fibers in ventral field CA1—along with the other interbrain projections described here—is supported by the precise localization of PHAL injections sites, and by at least four critical, obvious differences between projections from the ventral poles of field CA1 and the subiculum (see Table 1). First, the dorsomedial hypothalamic nucleus is clearly innervated the latter but not former (Canteras and Swanson, 1992; Kishi et al., 2000). Second, the ventromedial hypothalamic nucleus and ventromedial LHA are more densely innervated by field CA1 than by the subiculum (Kishi et al., 2000). Indeed, the LHA is the predominant target of ventral field CA1, whereas the hypothalamic medial nuclei are the chief targets of the subiculum (Canteras and Swanson, 1992; Kishi et al., 2000). Third, the latter (Canteras and Swanson, 1992) but not the former (Fig. 2B,C) very densely innervates the ventral part of the lateral septal nucleus (Fig. 2B,C; Canteras and Swanson, 1992). And fourth, axons from ventral field CA1 travel mostly through the postcommissural fornix to innervate hypothalamic targets with fewer axons traveling through the medial corticohypothalamic tract, whereas in contrast, axons from the ventral subiculum travel mostly through the medial corticohypothalamic tract with fewer axons traveling through the postcommissural fornix (Canteras and Swanson, 1992; Kishi et al., 2000). Because there are no published molecular markers to distinguish unambiguously ventral field CA1 from ventral subiculum (Zhao et al., 2001; Lein et al., 2004), previous connection reports combined with injection placement of the injections here (Figs. 1, 2, and 3J) provide the best assurance that only cells contained within field CA1 were labeled.
Table 1.
Comparison of projections from ventral field CA1 in the present study and projections from the ventral subiculum as reported previously, in the rat1
| SUBv [Canteras & Swanson (1992)] | SUBv [Kishi et al. (2000)] | CA1v [Cenquizca & Swanson (2005)] | |
|---|---|---|---|
| HYPO | |||
| AHNa | * | ||
| AHNc | * | ||
| AHNd | * | ||
| AHNp | * | * | * |
| ARH | * | * | |
| ADP | * | ||
| AVP | * | * | |
| AVPV | * | ||
| DMH | * | * | |
| LHA (all parts) | * | ||
| LPO | * | ||
| MePO | * | ||
| MM | * | * | * |
| MPO | * | * | * |
| MPNl | * | * | |
| PH | * | * | * |
| PMd | * | ||
| PMv | * | * | |
| PVp | * | ||
| PVH (all parts) | * | ||
| RCH | * | * | |
| SCH | * | ||
| SPVZ | * | * | * |
| SUMl | * | ||
| SUMm | * | * | |
| TMd | * | * | |
| TMv | * | * | |
| TUsv | * | * | |
| VMHa | * | ||
| VMHc | * | * | * |
| VMHdm | * | ||
| VMHvl | * | * | * |
| THAL | |||
| AM | * | ||
| IAM | * | ||
| LH | * | ||
| PVT | * | * | |
| PT | * | * | |
| RE | * | * | |
| ZI | * |
Clear differences between projections of field CA1 and subiculum are emphasized by italicized abbreviations.
Fig. 2.
The ventral field CA1 PHAL injection site (black oval regions) in Experiment HIPPO103 (A-C). Panel A is a photomicrograph through the rostral end of the injection site, panel B through the middle, and panel C through the caudal end of the injection site. Panels D-F are photomicrographs of nearby thionin (Nissl)-stained sections to show cytoarchitectonic features of the transverse histological sections (paired with panels A-C, respectively). As currently understood, the major architectonic feature that distinguishes field CA1 from the subiculum is the presence of a clear stratum oriens in the former. See Figures 1 and 3-I for relative location of this injection site. Scale = 200 μm.
Moreover, the present study compares the projection patterns of 8 experiments in the ventral one-third of field CA1. All of the experiments with an injection restricted to field CA1 exhibited similar projection patterns. In experiments where the deposit of tracer spread to the adjacent subiculum, the projection pattern exhibited clear differences (not illustrated) that have been described in previous reports. In addition, we obtained by chance a PHAL injection site strictly confined to the ventral pole of field CA3, and no hypothalamic projections were labeled (not illustrated). Nevertheless, new molecular markers may eventually help determine—together with additional functional and structural evidence—whether the ventral tenth of what is now regarded as field CA1 is in fact part of field CA1, whether it is part of the ventral subiculum, or whether it is a combination of the two, perhaps with partial interdigitation of layers or cell types. Currently, the most unambiguous distinction between ventral regions of field CA1 and the subiculum is the presence of a stratum oriens in the former and not the latter (Blackstad, 1956; Swanson et al., 1987).
To begin understanding the functional significance of field CA1-hypothalamus projections, it is helpful to review briefly what is known about the possible function of each innervated cell group. Overall, hypothalamic cell groups, like many in the lower brainstem, initiate behavior in response to sensory or to cerebral cortex inputs, which may arrive directly or via the cerebral nuclei (basal ganglia). Characteristically, hypothalamic cell groups coordinate somatomotor and visceral (autonomic and neuroendocrine) motor responses typical of various classes of motivated or goal-oriented behavior (see Risold et al., 1997). The cerebral hemispheres (including Ammon's horn) modulate and regulate hypothalamic output in conjunction with initiating and directing the foraging or exploratory behavior associated with obtaining specific goal objects (Risold et al., 1997; Swanson, 2000).
Historically, views of the LHA's role in behavior have been complex and somewhat vague. In golden hamsters, dorsomedial regions—roughly equivalent to dorsal and intermediate zones of the LHA anterior region that receive a clear ventral field CA1 input—are heavily connected with the anterior hypothalamic and lateral septal nuclei, positioning them with defensive behavior control circuitry (Ferris et al., 1990; Canteras, 2002). In contrast, ventromedial LHA regions—including the juxtadorsomedial, juxtaventromedial, and posterior zone of the subfornical region—appear to be involved in feeding behavior (Khan et al., 1999; Watts et al., 1999; Mullett et al., 2000; Holland et al., 2002; Petrovich et al., 2002; Goto et al., 2005). And finally, LHA regions near the supraoptic and paraventricular nucleus that receive direct inputs from ventral field CA1 may influence magnocellular neuroendocrine system responses (Simerly and Swanson, 1988; Risold et al., 1997; Thompson and Swanson, 1998).
The hypothalamic medial nuclei recently were included in a behavior control column playing a critical role in the expression of ingestive (eating and drinking), reproductive (sexual and parental), and defensive (fight or flight) behaviors (Swanson, 2000). The anterior hypothalamic nucleus clearly is essential for aspects of defensive behavior (see Ferris et al., 1990; Ferris et al., 1994; Bamshad and Albers, 1996; Bamshad et al., 1997; Ferris et al., 1997; Kollack-Walker et al., 1997; Risold et al., 1997; Canteras et al., 2001; Canteras, 2002) and previous anatomical work demonstrated a massive field CA1 to lateral septal nucleus to anterior hypothalamic nucleus projection (Risold et al., 1994, 1997) as well as a considerably more massive field CA1 to subiculum to anterior hypothalamic nucleus projection (Hjorth-Simonsen, 1973; van Groen and Wyss, 1990a; Canteras and Swanson, 1992; Kishi et al., 2000). The present results indicate that the ventral end of field CA1 also projects directly to the anterior hypothalamic nucleus. Because the septo-hypothalamic projection is GABAergic, and field CA1 projections are glutamatergic, the anatomical results suggest that field CA1 sends a direct excitatory projection to the anterior hypothalamic nucleus, and two indirect, much more substantial, projections to the nucleus—one excitatory, by way of the subiculum; and one inhibitory, by way of a “relay” in the lateral septal nucleus.
Ventral field CA1 also projects directly to the anterior part of the ventromedial nucleus, although there appears to be no clear evidence about its functional role. However, the dorsomedial part is involved in defensive behavior and the ventrolateral part in reproductive behavior (see Swanson, 2000; Canteras, 2002). Because the ventrolateral and central parts are lightly innervated, and the dorsomedial part is selectively avoided, by field CA1 inputs, it might be suggested that the anterior part is involved in reproductive behavior control networks (Canteras et al., 1994; Simerly, 1996; Petrovich et al., 2001; Simerly, 2002), although this hypothesis must be tested experimentally. Another possible route for field CA1 influences on reproductive behavior is by way of direct inputs to medial preoptic regions, some of which are sexually dimorphic (Simerly and Swanson, 1986; Albers and Rawls, 1989; Ibañez et al., 2001; Sheehan and Numan, 2002; Simerly, 2002; Stack et al., 2002). Again, this possibility requires experimental analysis.
Caudally, the mammillary nuclei (medial, lateral, and supramammillary) maintain very dense bi-directional connections with the septo-hippocampal system, and many studies have demonstrated mammillary effects on septo-hippocampal physiology and vice versa (see Wyss et al., 1979; Swanson et al., 1987; Allen and Hopkins, 1989; Pan and McNaughton, 1997; Vertes and Kocsis, 1997; Naber and Witter, 1998; Stackman and Taube, 1998; Kiss et al., 2000; Vertes and McKenna, 2000). Up until the 1970s it was universally believed that field CA1 projects massively to the mammillary body through the fornix—in fact, that Ammon's horn is the sole origin of the postcommissural fornix (see Nauta, 1958; Raisman et al., 1966). Use of anterograde and retrograde tracing methods radically altered this view: instead it was claimed that the postcommissural fornix arises from the ventral subiculum not Ammon's horn (see Swanson and Cowan, 1975; Swanson et al., 1987). The present results indicate that the ventral pole of field CA1, representing about 10% of its total surface area, does indeed contribute to the postcommissural fornix input to the mammillary body—and to the medial corticohypothalamic tract as well, also like the ventral subiculum—although these projections of ventral field CA1 are relatively light. As can be appreciated from Figure 1, the region of field CA1's ventral pole that generates hypothalamic inputs is exceptionally small and difficult to approach stereotaxically (in three dimensions its surface is a curving triangle, with two sides about three mm long, and the other side about one mm long), and it was not examined specifically in any of the earlier structural analyses. For further documentation of the hippocampal flatmap in terms of transverse section analysis, see Figure 6 in Petrovich and colleagues (2001).
Several other hypothalamic regions in receipt of direct field CA1 inputs also project to the cerebral cortex, directly and/or indirectly via the thalamus. It is premature even to speculate about the functional significance of this circuitry, but such regions include the ventromedial nucleus (Canteras et al., 1994; Risold et al., 1997; McDonald, 1998; Pikkarainen et al., 1999; Pitkänen et al., 2000; Petrovich et al., 2001); lateral hypothalamic area (Haglund et al., 1984; Saper, 1985), tuberomammillary nucleus (Köhler et al., 1985; Panula et al., 1989), and posterior hypothalamic nucleus (Vertes et al., 1995; Rempel-Clower and Barbas, 1998; Gerashchenko et al., 2001). In fact, virtually the entire hypothalamus projects topographically upon anterior, midline, and medial regions of the dorsal thalamus, and those parts of the latter receiving any sign of direct inputs from ventral field CA1 (the anteromedial, interanteromedial, paratenial, paraventricular, and reuniens nuclei) all project to the hippocampal formation of the cerebral cortex (reviewed in Risold et al., 1997).
Our results thus suggest that ventral field CA1 influences hypothalamic neural activity and associated behaviors by way of direct axonal projections. However, these influences are probably minor compared to a multitude of stronger disynaptic pathways from field CA1 to hypothalamus. First, by far the densest projection of field CA1 is to the adjacent subiculum (Hjorth-Simonsen, 1973), including ventral field CA1 to ventral subiculum (in preparation). As already mentioned, the ventral subiculum projects densely to widespread parts of the hypothalamus through the column of the fornix and medial corticohypothalamic tract (see Swanson and Cowan, 1975; Canteras and Swanson, 1992). Indeed, the possible functional significance of hippocampal influences on hypothalamic function is exemplified by the observation that non-cortical projections of the ventral subiculum are essentially the same as those of the suprachiasmatic nucleus (Canteras and Swanson, 1992). Second, ventral field CA1 projects to the parasubiculum (Swanson and Cowan, 1977; van Groen and Wyss, 1990a; in preparation), which in turn projects massively to the mammillary body (Swanson and Cowan, 1975; van Groen and Wyss, 1990b). Third, ventral field CA1 projects heavily to ventral regions of the medial prefrontal cortex (Swanson, 1981; Gabbott et al., 2002), in particular the infralimbic area (Brodmann's area 25), which in turn has by far the densest hypothalamic projections of any prefrontal or insular cortical area (Brittain, 1988; Hurley et al., 1991). Fourth, field CA1 also innervates parts of the insular and amygdalar regions that in turn innervate the hypothalamus (van Groen and Wyss, 1990a; Wyss and van Groen, 1992; in preparation). And fifth, the major descending projection from field CA1 as a whole is to the lateral septal nucleus—and the latter establishes a massive set of bi-directional connections with the hypothalamus, as discussed above.
Field CA1 is thus positioned to modulate hypothalamic neural activity through direct projections (Fig. 6) and through cortical regions that in turn project directly to the hypothalamus. Furthermore, an extensive literature indicates that cortical areas receiving direct field CA1 inputs are strongly interconnected—and that the cerebral nuclei (basal ganglia) parts they innervate in turn massively innervate the hypothalamus (see McDonald et al., 1999; Swanson, 2000). In short, a major part of the cerebral hemisphere is devoted to regulating and modulating hypothalamic activity and associated behaviors. This is not surprising in view of the fact that the hypothalamus is essential for life and for maintaining the species—with hippocampal field CA1 playing a critical role in elaborating cerebral cortical influences.
Fig. 6.
Summary diagram to indicate the general organization of projections from the ventral pole of hippocampal field CA1 to the interbrain (diencephalon). Projections to other parts of the forebrain are not shown. The flatmap of the rat central nervous system is based on Swanson (2004); line thickness is roughly proportional to relative projection strength.
There is some rather indirect, recent evidence supporting the hypothesis that motivated behavior is regulated by the hippocampal formation (Andrade et al., 2000; Tracy et al., 2001), and more direct evidence for hippocampal influences on hypothalamic functions like pituitary-adrenal control (Jacobson and Sapolsky, 1991; de Kloet et al., 1993; Joëls and de Kloet, 1994; Bhatnagar et al., 1997; Herman and Cullinan, 1997; Lee and Davis, 1997), pituitary-gonadal control (Jennes et al., 1995; Lathe, 2001), penile erection (Chen and Chang, 2001), and ovulation (Lathe, 2001). The structural data presented here concerning the topographic organization of direct field CA1 axonal pathways to specific hypothalamic neuron populations can be used to formulate more incisive experimental analyses of hippocampo-hypothalamic functions. How current models of hippocampal involvement in episodic memory and navigation—with presumed underlying mechanisms involving LTP and LTD—interface with the “function” of field CA1 (that is, its descending output to influence physiology and behavior) remains a tantalizing mystery.
Acknowledgments
Grant sponsor: National Institutes of Health (2R01NS16686 and MH12765).
Abbreviations
- aco
anterior commissure, olfactory limb
- act
anterior commissure, temporal limb
- AHA
anterior hypothalamic area
- AHN
anterior hypothalamic nucleus
- AHNa
anterior hypothalamic nucleus, anterior part
- AHNc
anterior hypothalamic nucleus, central part
- AHNd
anterior hypothalamic nucleus, dorsal part
- AHNp
anterior hypothalamic nucleus, posterior part
- AL
pituitary gland, anterior lobe
- AMd
anteromedial nucleus thalamus, dorsal part
- AMv
anteromedial nucleus thalamus, ventral part
- AQ
cerebral aqueduct
- ARH
arcuate nucleus hypothalamus
- AV
anteroventral nucleus thalamus
- AVP
anteroventral preoptic nucleus
- AVPV
anteroventral periventricular nucleus
- BA
bed nucleus accessory olfactory tract
- BAC
bed nucleus anterior commissure
- BMA
basomedial nucleus amygdala
- BSM
bed nucleus of the stria medullaris
- BST
bed nuclei of the stria terminalis
- BSTif
bed nuclei of the stria terminalis, posterior division, interfascicular nucleus
- BSTpr
bed nuclei of the stria terminalis, posterior division, principal nucleus
- BSTsz
bed nuclei of the stria terminalis, posterior division, cell-sparse zone
- CA1
field CA1, Ammon's horn
- CA1slm
field CA1, stratum lacunosum-moleculare
- CA1so
field CA1, stratum oriens
- CA1spd
field CA1, pyramidal layer, deep
- CA1sps
field CA1, pyramidal layer, superficial
- CA1sr
field CA1, stratum radiatum
- CA2
field CA2, Ammon's horn
- CA3
field CA3, Ammon's horn
- CA3slu
field CA3, stratum lucidum
- chp
choroid plexus
- CM
medial nucleus thalamus
- COApm
cortical nucleus amygdala, posterior part, medial zone
- CP
caudoputamen
- cpd
cerebral peduncle
- DGlb
dentate gyrus, lateral blade
- DGlb-mo
dentate gyrus, lateral blade-molecular layer
- DGlb-po
dentate gyrus, lateral blade-polymorph layer
- DGlb-sg
dentate gyrus, lateral blade-granule cell layer
- DMHa
dorsomedial nucleus hypothalamus, anterior part
- ECT
ectorhinal area
- em
external medullary lamina thalamus
- ENT
entorhinal area
- ENTl1-6
entorhinal area, lateral part, layers 1-6
- EPv
endopiriform nucleus, ventral part
- fi
fimbria
- FF
fields of Forel
- fr
fasciculus retroflexus
- FS
fundus of the striatum
- fx
columns of the fornix
- fxpo
postcommissural fornix
- fxpr
precommissural fornix
- GPe
globus pallidus, external segment
- GPi
globus pallidus, internal segment
- IAD
interanterodorsal nucleus thalamus
- IAM
interanteromedial nucleus thalamus
- IL
pituitary gland, intermediate lobe
- int
internal capsule
- IVF
interventricular foramen
- laf
lateral arcuate fibers
- LGvl
lateral geniculate complex, ventral part, lateral zone
- LGvm
lateral geniculate complex, ventral part, medial zone
- LHA
lateral hypothalamic area
- LHAa
lateral hypothalamic area, anterior region
- LHAad
lateral hypothalamic area, anterior region, dorsal zone
- LHAai
lateral hypothalamic area, anterior region, intermediate zone
- LHAav
lateral hypothalamic area, anterior region, ventral zone
- LHAd
lateral hypothalamic area, dorsal region
- LHAjd
lateral hypothalamic area, juxtadorsomedial region
- LHAjp
lateral hypothalamic area, juxtaparaventricular region
- LHAjvd
lateral hypothalamic area, juxtaventromedial region, dorsal zone
- LHAjvv
lateral hypothalamic area, juxtaventromedial region, ventral zone
- LHAm
lateral hypothalamic area, magnocellular nucleus
- LHAp
lateral hypothalamic area, posterior region
- LHApc
lateral hypothalamic area, parvicellular region
- LHAs
lateral hypothalamic area, suprafornical region
- LHAsf
lateral hypothalamic area, subfornical region
- LHAsfa
lateral hypothalamic area, subfornical region, rostral zone
- LHAsfp
lateral hypothalamic area, subfornical region, posterior zone
- LHAsfpm
lateral hypothalamic area, subfornical region, premammillary zone
- LHAvl
lateral hypothalamic area, ventral region, lateral zone
- LM
lateral mammillary nucleus
- LPO
lateral preoptic area
- LSc
lateral septal nucleus, caudal part
- LSr
lateral septal nucleus, rostral part
- LSv
lateral septal nucleus, ventral part
- MA
magnocellular preoptic nucleus
- mct
medial corticohypothalamic tract
- ME
median eminence
- MEex
median eminence, external lamina
- MEin
median eminence, internal lamina
- MEA
medial nucleus amygdala
- MEPO
median preoptic nucleus
- ml
medial lemniscus
- MM
medial mammillary nucleus
- MMme
medial mammillary nucleus, median part
- mp
mammillary peduncle
- MPN
medial preoptic nucleus
- MPNc
medial preoptic nucleus, central part
- MPNl
medial preoptic nucleus, lateral part
- MPNm
medial preoptic nucleus, medial part
- MPO
medial preoptic area
- MRNm
midbrain reticular nucleus, magnocellular part
- MS
medial septal nucleus
- mtg
mammillotegmental tract
- mtt
mammillothalamic tract
- NC
nucleus circularis
- NDB
nucleus of the diagonal band
- NL
pituitary gland, neural lobe
- och
optic chiasm
- opt
optic tract
- OV
vascular organ of the lamina terminalis
- PA
posterior nucleus amygdala
- PAG
periaqueductal gray
- PERI
perirhinal area
- PH
posterior hypothalamic nucleus
- pm
principal mammillary tract
- PMd
dorsal premammillary nucleus
- PMv
ventral premammillary nucleus
- PR
perireuniens nucleus
- PRE
presubiculum
- PS
parastrial nucleus
- PSCH
suprachiasmatic preoptic nucleus
- PSTN
parasubthalamic nucleus
- PT
paratenial nucleus
- PVa
anterior periventricular nucleus hypothalamus
- PVH
paraventricular nucleus hypothalamus
- PVHam
paraventricular nucleus hypothalamus, anterior magnocellular part
- PVHap
paraventricular nucleus hypothalamus, anterior parvicellular part
- PVHdp
paraventricular nucleus hypothalamus, dorsal parvicellular part
- PVHmpd
paraventricular nucleus hypothalamus, medial parvicellular part, dorsal zone
- PVHmpv
paraventricular nucleus hypothalamus, medial parvicellular part, ventral zone
- PVHpml
paraventricular nucleus hypothalamus, posterior magnocellular part, lateral zone
- PVHpv
paraventricular nucleus hypothalamus, periventricular part
- PVi
intermediate periventricular nucleus hypothalamus
- PVp
posterior periventricular nucleus hypothalamus
- PVpo
preoptic periventricular nucleus
- PVT
paraventricular nucleus thalamus
- RCH
retrochiasmatic area
- RE
nucleus reuniens
- REcd
nucleus reuniens, caudal division, dorsal part
- REcm
nucleus reuniens, caudal division, median part
- REcp
nucleus reuniens, caudal division, posterior part
- REd
nucleus reuniens, rostral division, dorsal part
- REl
nucleus reuniens, rostral division, lateral part
- REm
nucleus reuniens, rostral division, medial part
- REv
nucleus reuniens, rostral division, ventral part
- RH
rhomboid nucleus
- RT
reticular nucleus thalamus
- SBPV
subparaventricular zone hypothalamus
- SCH
suprachiasmatic nucleus
- SF
septofimbrial nucleus
- SI
substantia innominata
- sm
stria medullaris
- smd
supramammillary decussation
- SMT
submedial nucleus thalamus
- SNc
substantia nigra, compact part
- SNr
substantia nigra, reticular part
- SO
supraoptic nucleus
- SOr
supraoptic nucleus, retrochiasmatic part
- SPFm
subparafascicular nucleus thalamus, magnocellular part
- SPFpm
subparafascicular nucleus thalamus, parvicellular part, medial division
- st
stria terminalis
- STN
subthalamic nucleus
- SUB
subiculum
- SUBv
subiculum, ventral part
- SUMl
supramammillary nucleus, lateral part
- SUMm
supramammillary nucleus, medial part
- sup
supraoptic commissures
- TEa
temporal association areas
- TMv
tuberomammillary nucleus, ventral part
- TR
postpiriform transition area
- TUi
tuberal nucleus, intermediate part
- TUl
tuberal nucleus, lateral part
- TUsv
tuberal nucleus, subventricular part
- V3
third ventricle
- V3m
third ventricle, mammillary recess
- V3p
third ventricle, preoptic recess
- VAL
ventral anterior-lateral complex thalamus
- VISC
visceral area
- VL
lateral ventricle
- vlt
ventrolateral hypothalamic tract
- VM
ventral medial nucleus thalamus
- VMH
ventromedial nucleus hypothalamus
- VMHa
ventromedial nucleus hypothalamus, anterior part
- VMHc
ventromedial nucleus hypothalamus, central part
- VMHdm
ventromedial nucleus hypothalamus, dorsomedial part
- VMHvl
ventromedial nucleus hypothalamus, ventrolateral part
- VPL
ventral posterolateral nucleus thalamus
- VPLpc
ventral posterolateral nucleus thalamus, parvicellular part
- VPM
ventral posteromedial nucleus thalamus
- VPMpc
ventral posteromedial nucleus thalamus, parvicellular part
- ZI
zona incerta
- ZIda
zona incerta, dopaminergic group
- zl
zona limitans
Footnotes
Associate Editor: Paul Sawchenko
Literature Cited
- Albers HE, Rawls S. Coordination of hamster lordosis and flank marking behavior: role of arginine vasopressin within the medial preoptic-anterior hypothalamus. Brain Res Bull. 1989;23:105–109. doi: 10.1016/0361-9230(89)90168-8. [DOI] [PubMed] [Google Scholar]
- Allen GV, Hopkins DA. Mamillary body in the rat: topography and synaptology of projections from the subicular complex, prefrontal cortex, and midbrain tegmentum. J Comp Neurol. 1989;286:311–336. doi: 10.1002/cne.902860303. [DOI] [PubMed] [Google Scholar]
- Anderson P, Bliss TV, Skrede KK. Lamellar organization of hippocampal pathways. Exp Brain Res. 1971;13:571–591. doi: 10.1007/BF00234087. [DOI] [PubMed] [Google Scholar]
- Andrade JP, Madeira MD, Paula-Barbosa MM. Sexual dimorphism in the subiculum of the rat hippocampal formation. Brain Res. 2000;875:125–137. doi: 10.1016/s0006-8993(00)02605-6. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Albers HE. Neural circuitry controlling vasopressin-stimulated scent marking in Syrian hamsters (Mesocricetus auratus) J Comp Neurol. 1996;369:252–263. doi: 10.1002/(SICI)1096-9861(19960527)369:2<252::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Karom M, Pallier P, Albers HE. Role of the central amygdala in social communication in Syrian hamsters (Mesocricetus auratus) Brain Res. 1997;744:15–22. doi: 10.1016/s0006-8993(96)01061-x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Costall B, Smythe JW. Hippocampal cholinergic blockade enhances hypothalamic-pituitary-adrenal responses to stress. Brain Res. 1997;766:244–248. doi: 10.1016/s0006-8993(97)00684-7. [DOI] [PubMed] [Google Scholar]
- Blackstad TW. Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J Comp Neurol. 1956;105:417–537. doi: 10.1002/cne.901050305. [DOI] [PubMed] [Google Scholar]
- Brittain DA. PhD thesis. Department of Neurosciences, University of California at San Diego: 1988. The efferent connections of the infralimbic area in the rat. [Google Scholar]
- Cajal S Ramon y. Histologie du système nerveux de l'homme & des vertébrés. Madrid: Maloine; 1909-1911. [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Ribeiro-Barbosa ER, Comoli E. Tracing from the dorsal premammillary nucleus prosencephalic systems involved in the organization of innate fear responses. Neurosci Biobehav Rev. 2001;25:661–668. doi: 10.1016/s0149-7634(01)00048-3. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Chen K, Chang LS. Oxytocinergic neurotransmission at the hippocampus in the central neural regulation of penile erection in the rat. Urology. 2001;58:107–112. doi: 10.1016/s0090-4295(01)01000-7. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joëls M. Functional implications of brain corticosteroid receptor diversity. Cell Mol Neurobiol. 1993;13:433–455. doi: 10.1007/BF00711582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Delville Y, Irvin RW, Potegal M. Septo-hypothalamic organization of a stereotyped behavior controlled by vasopressin in golden hamsters. Physiol Behav. 1994;55:755–759. doi: 10.1016/0031-9384(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Gold L, De Vries GJ, Potegal M. Evidence for a functional and anatomical relationship between the lateral septum and the hypothalamus in the control of flank marking behavior in Golden hamsters. J Comp Neurol. 1990;293:476–485. doi: 10.1002/cne.902930310. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott P, Headlam A, Bushby S. Morphological evidence that CA1 hippocampal afferents monosynaptically innervate PV-containing neurons and NADPH-diaphorase reactive cells in the medial prefrontal cortex (Areas 25/32) of the rat. Brain Res. 2002;946:314–322. doi: 10.1016/s0006-8993(02)02487-3. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Salin-Pascual R, Shiromani PJ. Effects of hypocretin-saporin injections into the medial septum on sleep and hippocampal theta. Brain Res. 2001;913:106–115. doi: 10.1016/s0006-8993(01)02792-5. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Canteras NS, Burns G, Swanson LW. Projections from the subfornical region of the lateral hypothalamic area. J Comp Neurol. 2005 doi: 10.1002/cne.20764. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wouterlood FG. Light and electron microscopic tracing of neuronal connections with Phaseolus vulgaris leucoagglutinin (PHA-L), and combinations with other neuroanatomical techniques. In: Björklund A, editor. Analysis of neuronal microcircuits and synaptic interactions. Amsterdam, New York: 1990. pp. 47–123. [Google Scholar]
- Haglund L, Swanson LW, Kohler C. The projection of the supramammillary nucleus to the hippocampal formation: an immunohistochemical and anterograde transport study with the lectin PHA-L in the rat. J Comp Neurol. 1984;229:171–185. doi: 10.1002/cne.902290204. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hjorth-Simonsen A. Some intrinsic connections of the hippocampus in the rat: an experimental analysis. J Comp Neurol. 1973;147:145–161. doi: 10.1002/cne.901470202. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Ibañez MA, Gu G, Simerly RB. Target-dependent sexual differentiation of a limbic-hypothalamic neural pathway. J Neurosci. 2001;21:5652–5659. doi: 10.1523/JNEUROSCI.21-15-05652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jennes L, Brame B, Centers A, Janovick JA, Conn PM. Regulation of hippocampal gonadotropin releasing hormone (GnRH) receptor mRNA and GnRH-stimulated inositol phosphate production by gonadal steroid hormones. Mol Brain Res. 1995;33:104–110. doi: 10.1016/0169-328x(95)00113-7. [DOI] [PubMed] [Google Scholar]
- Joëls M, de Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain: implications for ion permeability and transmitter systems. Prog Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Khan AM, Curras MC, Dao J, Jamal FA, Turkowski CA, Goel RK, Gillard ER, Wolfsohn SD, Stanley BG. Lateral hypothalamic NMDA receptor subunits NR2A and/or NR2B mediate eating: immunochemical/behavioral evidence. Am J Physiol. 1999;276:R880–891. doi: 10.1152/ajpregu.1999.276.3.R880. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Ono K, Yokota S, Ishino H, Yasui Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. J Comp Neurol. 2000;419:205–222. doi: 10.1002/(sici)1096-9861(20000403)419:2<205::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kiss J, Csaki A, Bokor H, Shanabrough M, Leranth C. The supramammillo-hippocampal and supramammillo-septal glutamatergic/aspartatergic projections in the rat: a combined [3H]D-aspartate autoradiographic and immunohistochemical study. Neuroscience. 2000;97:657–669. doi: 10.1016/s0306-4522(00)00127-5. [DOI] [PubMed] [Google Scholar]
- Köhler C, Swanson LW, Haglund L, Wu JY. The cytoarchitecture, histochemistry and projections of the tuberomammillary nucleus in the rat. Neuroscience. 1985;16:85–110. doi: 10.1016/0306-4522(85)90049-1. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. Hormones and the hippocampus. J Endocrinol. 2001;169:205–231. doi: 10.1677/joe.0.1690205. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Zhao X, Gage FH. Defining a molecular atlas of the hippocampus using DNA microarrays and high-throughput in situ hybridization. J Neurosci. 2004;24:3879–3889. doi: 10.1523/JNEUROSCI.4710-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de Nó R. Studies on the structure of the cerebral cortex II. Continuation of the study of the ammonic system. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann NY Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Meibach RC, Siegel A. Efferent connections of the hippocampal formation in the rat. Brain Res. 1977;124:197–224. doi: 10.1016/0006-8993(77)90880-0. [DOI] [PubMed] [Google Scholar]
- Mullett MA, Billington CJ, Levine AS, Kotz CM. Hypocretin I in the lateral hypothalamus activates key feeding-regulatory brain sites. Neuroreport. 2000;11:103–108. doi: 10.1097/00001756-200001170-00021. [DOI] [PubMed] [Google Scholar]
- Naber PA, Witter MP. Subicular efferents are organized mostly as parallel projections: a double-labeling, retrograde-tracing study in the rat. J Comp Neurol. 1998;393:284–297. [PubMed] [Google Scholar]
- Nauta WJH. An experimental study of the fornix system in the rat. J Comp Neurol. 1956;104:247–271. doi: 10.1002/cne.901040205. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. Hippocampal projections and related neural pathways to the midbrain in the cat. Brain. 1958;81:319–340. doi: 10.1093/brain/81.3.319. [DOI] [PubMed] [Google Scholar]
- Pan WX, McNaughton N. The medial supramammillary nucleus, spatial learning and the frequency of hippocampal theta activity. Brain Res. 1997;764:101–108. doi: 10.1016/s0006-8993(97)00431-9. [DOI] [PubMed] [Google Scholar]
- Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Setlow B, Holland PC, Gallagher M. Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci. 2002;22:8748–8753. doi: 10.1523/JNEUROSCI.22-19-08748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Raisman G, Cowan WM, Powell TP. An experimental analysis of the efferent projection of the hippocampus. Brain. 1966;89:83–108. doi: 10.1093/brain/89.1.83. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Barbas H. Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey. J Comp Neurol. 1998;398:393–419. doi: 10.1002/(sici)1096-9861(19980831)398:3<393::aid-cne7>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Risold PY, Canteras NS, Swanson LW. Organization of projections from the anterior hypothalamic nucleus: a Phaseolus vulgaris-Leucoagglutinin study in the rat. J Comp Neurol. 1994;348:1–40. doi: 10.1002/cne.903480102. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Hypothalamocortical projections. J Comp Neurol. 1985;237:21–46. doi: 10.1002/cne.902370103. [DOI] [PubMed] [Google Scholar]
- Sheehan T, Numan M. Estrogen, progesterone, and pregnancy termination alter neural activity in brain regions that control maternal behavior in rats. Neuroendocrinology. 2002;75:12–23. doi: 10.1159/000048217. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Sexually differentiated neural systems controlling the preovulatory release of gonadotropin. Curr Opin Endocrinol Diabetes. 1996;3:171–177. [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Ann Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986;246:312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Smith Y. Anterograde tracing with PHA-L and biocytin at the electron microscopic level. In: Bolam JP, editor. Experimental neuroanatomy: a practical approach. Oxford: IRL Press; 1992. pp. 61–79. [Google Scholar]
- Stack EC, Balakrishnan R, Numan MJ, Numan M. A functional neuroanatomical investigation of the role of the medial preoptic area in neural circuits regulating maternal behavior. Behav Brain Res. 2002;131:17–36. doi: 10.1016/s0166-4328(01)00370-9. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Taube JS. Firing properties of rat lateral mammillary single units: head direction, head pitch, and angular head velocity. J Neurosci. 1998;18:9020–9037. doi: 10.1523/JNEUROSCI.18-21-09020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain An atlas with printed and electronic templates for data, models, and schematics. 3rd. Amsterdam: Elsevier Academic Press; 2004. [Google Scholar]
- Swanson LW, Cowan WM. Hippocampo-hypothalamic connections: origin in subicular cortex not Ammon's horn. Science. 1975;189:303–304. doi: 10.1126/science.49928. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. Autoradiographic studies of the development and connections of the septal area in the rat. In: DeFrance JF, editor. The septal nuclei. New York: Plenum Press; 1977a. pp. 37–64. [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977b;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Köhler C, Björklund A. The limbic region. I: The septohippocampal system. In: Björklund A, Hökfelt T, Swanson LW, editors. Integrated systems of the CNS, Part I. Amsterdam: Elsevier; 1987. pp. 125–277. [Google Scholar]
- Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parcelling scheme of the lateral hypothalamic zone. Neurosci Lett. 2005;387:80–84. doi: 10.1016/j.neulet.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–715. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Tracy AL, Jarrard LE, Davidson TL. The hippocampus and motivation revisited: appetite and activity. Behav Brain Res. 2001;127:13–23. doi: 10.1016/s0166-4328(01)00364-3. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol. 1990a;302:515–528. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. The connections of presubiculum and parasubiculum in the rat. Brain Res. 1990b;518:227–243. doi: 10.1016/0006-8993(90)90976-i. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Crane AM, Colom LV, Bland BH. Ascending projections of the posterior nucleus of the hypothalamus: PHA-L analysis in the rat. J Comp Neurol. 1995;359:90–116. doi: 10.1002/cne.903590107. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Vertes RP, McKenna JT. Collateral projections from the supramammillary nucleus to the medial septum and hippocampus. Synapse. 2000;38:281–293. doi: 10.1002/1098-2396(20001201)38:3<281::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G, Kelly AB. Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration-induced anorexia. J Neurosci. 1999;19:6111–6121. doi: 10.1523/JNEUROSCI.19-14-06111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM, Swanson LW, Cowan WM. A study of subcortical afferents to the hippocampal formation in the rat. Neuroscience. 1979;4:463–476. doi: 10.1016/0306-4522(79)90124-6. [DOI] [PubMed] [Google Scholar]
- Wyss JM, van Groen T. Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus. 1992;2:1–11. doi: 10.1002/hipo.450020102. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lein ES, He A, Smith SC, Aston C, Gage FH. Transcriptional profiling reveals strict boundaries between hippocampal subregions. J Comp Neurol. 2001;441:187–196. doi: 10.1002/cne.1406. [DOI] [PubMed] [Google Scholar]